Abstract
The protective actions of estrogen have been well evaluated in various models of neurodegeneration. These neuroprotective mechanisms may include a direct neuronal anti-apoptotic effect as estrogen modulates actions of key regulators of the mitochondrial/intrinsic apoptotic cascade. We tested the ability of estrogen to protect against apoptotic signaling in cortical cell cultures exposed to Tat 1-86 (50nM), and additionally, whether the beneficial actions of estrogen involved an estrogen receptor sensitive mechanism. We demonstrated that estrogen pretreatment significantly delayed Tat-induced cell death in primary cortical cultures. Pretreatment with 17β-estradiol (10nM) attenuated the increased expression of anti-apoptotic protein Bcl-2, pro-apoptotic protein Bax and activation of caspases linked to mitochondrial apoptotic pathway following Tat exposure. In addition, select components of apoptotic pathway signaling appear more sensitive to estrogen receptor (ER) activation, as the addition of ER antagonist ICI 182,780 reversed estrogen downregulation of Bax and caspase 3, while estrogen effects on Tat-induced Bcl-2 and caspase 9 expression were maintained. Moreover, the addition of preferential ERα and ERβ antagonists (MPP dihydrochloride and PHTPP) indicated that estrogen effects on caspase 3 may be mediated by both receptor subtypes, while ERβ was more involved in estrogen effects on Bax. Our data suggest that estrogen intervenes against HIV Tat-induced cortical neuronal dysfunction via intersecting mitochondrial apoptotic pathway signaling in an ER-sensitive manner.
Introduction
HIV-1 infection of the central nervous system (CNS) is accompanied by neurological impairments, including cognitive dysfunction and loss of motor capacity. HIV-1 infection in the brain is characterized by reduced synaptic density and major neuronal loss in the basal ganglia, hippocampus, and cortex (Weiss et al, 1993, Stout et al., 1998). Such neuropathological features have been associated with the development of HIV associated dementia (HAD) (Jones & Power, 2006). Although HAART therapy has reduced the prevalence of HAD, it has not been able to prevent its development. Additionally, with the increased life expectancy of HIV infected patients, incidences of HAD are actually rising. Moreover, HAD is the most common form of dementia in people 40 years of age or less and is a significant independent risk factor for death due to AIDS ( Sevigny et al., 2007; Ances & Ellis, 2007; McArthur et al., 2005;Ellis et al.,1997).
Neuronal injury is a primary basis/component of many neurodegenerative diseases, including HAD. Given that the HIV-1 virus does not directly infect neurons, it has been determined that detrimental effects in the brain are mediated by released toxic viral products (King et al., 2006; van de Bovenkamp et al., 2002). HIV-1 viral protein Tat can be released by HIV-infected cells to the extracellular space, cerebrospinal fluid (CSF) and sera. The HIV Tat protein is thought to play a significant role in HIV related neuropathology. Tat has been detected in the brains of people with HIVE by mRNA and Western blotting analyses (Wiley et al., 1996; Hudson et al, 2000). Tat is taken up by CNS cells, and often results in toxic consequences, such as neuronal death (Aksenov 2009, 2006;2003). Cerebral atrophy and white matter abnormalities are common neuroimaging findings among HIV-infected individuals, and are evident in both cortical and subcortical regions of the brain (Paul et al., 2002). Several reports have shown large neuronal loss in cortical regions in AIDS (Everall et al., 1991; Everall et al., 1993; Fischer et al., 1999; Moore et al., 2006).
The involvement of apoptotic signaling cascades has been implicated in the dysfunction and death of neurons in neurodegenerative disorders, including HAD. Extracellular Tat exposure has been shown to induce calcium release and subsequent calcium overload, alterations in gene transcription, dysregulation of glutamate and excitotoxicity, and increase expression of apoptotic proteins (Bonavia et al., 2001;Haughey et al., 2001; Bruce-Keller et al; Perez et al, 2001; Wallace, 2006; King et al., 2006). Prior research has found that Tat induced apoptotic death in primary neuronal cultures isolated from cortex, hippocampus and midbrain (New et al., 1997; Bonavia et al., 2001, Aksenova et al., 2009). Additionally studies have demonstrated the neurotoxic potential of Tat involves a decrease of mitochondrial membrane potential, accumulation of reactive oxygen species and caspase activation (Aksenov et al., 2001; 2003 Aksenov et al., 2006; Aksenova et al., 2006; Kruman et al., 1998).
Hormone levels are thought to play an important role in the susceptibility and immune responses to HIV-1 infection in women. There is evidence that HIV-1 infected women have a lower plasma viral load and a higher CD4 cell count compared to HIV infected men and differ from men in their response to anti-retroviral therapy, suggesting that gender differences play a role in the progression of HIV-1 (Gandi et al., 2002; Gilad et al., 2003; Umeh and Currier, 2006; Sterling et al., 2001; Rezza et al., 2000). Reports have also demonstrated that women have a greater risk of developing AIDS with a comparable viral load and CD4 count as men (Farazadegan et al., 1998). Low levels of estrogen have been associated with a prolonged disease course, and with an increase incidence of AIDS-related dementia and Parkinson’s like symptoms, suggesting that estrogen replacement may attenuate the cognitive and motor dysfunction associated with HIV-1 disease progression (Wallace, 2006; Wilson et al., 2006).
Accumulating evidence suggests that estrogen is able to protect against various neurodegenerative insults. For example, estrogen reduces the extent of cell death in response to a variety of noxious disease-related stimuli such as excitatory amino acids (Sribnick et al., 2004), oxidative stress (Nilsen, 2008; Wallace et al., 2006) and β-amyloid toxicity (Nilsen et al., 2006; Hosoda et al., 2001). These cellular actions may explain its ability to ameliorate cognitive decline and reduce the incidence and progression of neurodegenerative disease. Furthermore, estrogen was shown effective in preventing the neuronal damage associated with Tat-induced toxicity (Kendall et al., 2005; Wallace et al., 2006). 17 β-estradiol treatment was also shown to reduce the number of apoptotic neurons and modulate the expression of anti-apoptotic proteins including Bcl-2 in primary neuronal cultures (Nilsen et. al., 2006).
Both in vitro and in vivo studies have demonstrated that estrogens may promote neuronal survival through estrogen receptor (ER) activity (Vanoye-Carlo et al., 2009; Morissette et al., 2008; Miller et al., 2005; Amantea et al., 2005; Turchan et al., 2001). The mechanism(s) of ER-dependent estrogen neuroprotection are not fully understood, but may be linked to regulation of proteins that either promote cell survival or induce cell death. In the current study, we examined the regulatory effects of estrogen on the expression of apoptosis regulating proteins in cultured cortical neurons following exposure to HIV-1 Tat. We evaluated if estrogen protection against Tat neurotoxicity involves regulation of the pro- and anti-apoptotic proteins Bax and Bcl-2, respectively and caspase activity, and the role of ER subtypes in neuronal death from HIV-1 Tat exposure.
Material and Methods
Neuronal Cell Culture
Neuronal cultures were prepared from 18-day-old Sprague-Dawley rat fetuses. Rat cortices were dissected and incubated for 15 min in a solution of 2 mg/mL trypsin in Ca2+ and Mg2+-free Hanks balanced salt solution (HBSS) buffered with 10 mM HEPES (Invitrogen, Carlsbad, CA). The tissue was then exposed for 2 min to soybean trypsin inhibitor and rinsed three times with HBSS. Cells were dissociated by trituration and distributed to poly-L-lysine coated culture plates. At the time of plating each well contained DMEM/F12 medium supplemented with 100 mL/L fetal bovine serum. After a 24-h period, the DMEM/F12 medium was replaced with 2% v/v B-27 Neurobasal medium supplemented with 2 mM GlutaMax and 0.5% w/v D-(+) glucose. Cultures were used for experiments after 12 days in culture.
Experimental treatment of cultures
Recombinant Tat 1-86 (50 nM) (Diatheva, Italy) was added to culture medium. To assess neurotoxicity, cell cultures were exposed to Tat for 4, 24, 48, or 72 h for cell viability assays and 4, 16, and 24h before cell harvesting for ELISA experiments. In assessing estrogen effects against Tat toxicity, 17 β-estradiol (10 nM, Sigma) was added to neuronal culture 24 h prior to Tat exposure. Cells were also treated with the estrogen receptor antagonist ICI 182,780, the ERα specific antagonist, MPP dihydrocloride, or the ERβ specific antagonist PHTPP (100 nM, Tocris Cookson Inc, Ellisville, MO) 1h before estrogen treatment to determine if estrogen effects against Tat toxicity were receptor mediated.
Cell viability test
Neuronal survival was determined using a Live/Dead viability/cytotoxicity kit (Molecular Probes, Eugene, OR) in rat fetal cortical cell cultures prepared in 96-well plates. In accordance with the manufacturer’s protocol, neurons were exposed to cell-permeant calcein AM (2 μM), which is hydrolyzed by intracellular esterases, and to ethidium homodimer-1 (4 μM), which binds to nucleic acids. The cleavage product of calcein AM produces a green fluorescence (F530 nm) when exposed to 494-nm light and is used to identify live cells. Bound ethidium homodimer-1 produces a red fluorescence (F645 nm) when exposed to 528-nm light, allowing the identification of dead cells. Fluorescence was measured using a Bio-Tek Synergy HT microplate reader (Bio-Tek Instruments, Inc., Winooski, VT). Each individual F530 nm and F645 nm value on a plate was corrected for background fluorescence (readings obtained from cell cultures (wells) that were not exposed to calcein AM and ethidium bromide) by the microplate reader KC4 software package (Bio-Tek Instruments, Inc., Winooski, VT). For each individual cell culture (well) on a plate, ratios between corrected green and red fluorescence (F530 nm/F645 nm, Live/Dead ratios) were calculated. All individual relative numbers of live and dead cells were expressed in terms of percentages of average maximum Live/Dead ratio determined for the set of non-treated control cell cultures (8–16 wells) from the same plate: [F530 nm/F645 nm]well n/[F530 nm/F645 nm]average max × 100%.
ELISA detection of Apoptotic proteins
Expression of apoptotic signaling proteins in cell lysates was determined by ELISA. Cell lysates were prepared from cultures grown in 24-well plates. Tat treated cell cultures were exposed to 50nM Tat 1-86 for 4, 16, or 24 hours before harvesting. At the time of harvesting, medium was removed and cells were washed three times with Dulbecco phosphate-buffered saline, D-PBS, (8 mM Na2HPO4, 1.5 mM KH2PO4, 0.137 M NaCl and 2.7 mM KCL at pH 7.4) and lysed with CellLytic ™- M mammalian cell lysis buffer (Sigma Chemicals) containing protease inhibitors (protease inhibitors cocktail, Sigma Chemicals). All samples in a group (6 sister culture wells) were pooled together and protein concentration was determined by BCA method (Pierce). Each well of Costar 96-well ELISA plates (Corning Inc, PA) was coated overnight at 4 °C using 100 μl of 20 mM carbonate coating buffer, pH 9.6. Cortical cell lysate samples were diluted 1:10 with D-PBS and 20ug of each sample were added to the plate wells. After overnight incubation at 4 °C, plates were rinsed 5 times with PBST (0.05% Tween 20 in PBS, pH = 7.4) and blocked with 1% BSA in PBS for 2 h at room temperature. After blocking, plates were washed again as described above and primary anti-Bax, anti-Bcl-2, anti- active Caspase 9 and anti- active Caspase 3 antibodies (all primary antibodies, Abcam, Cambridge, MA) diluted 1:5000 or 1:7500 (caspase 3) in 0.1% BSA-PBST were added to each well except for blanks and no-primary antibody control wells. Plates were kept overnight at 4 °C. When the incubation with primary antibodies was completed, plates were again washed 5 times with PBST and secondary antibodies (goat anti-rabbit alkaline phosphatase conjugated, Sigma) diluted 1: 2000 in 0.1% BSA-PBST were added to each well except for blank and no-secondary antibody control wells. After 2 h of incubation, secondary antibody solution was removed, plates were washed 5 times with PBST and 100 μl of BluePhos phosphatase substrate mixture (KPL Research, Gaithersburg, MD) was added to the plate wells. After 30 min of incubation, the absorbance at 650 nm was determined using a Bio-Tek Synergy HT microplate reader. Multiple readings were taken within a 1-h time period.
Statistical Analysis
Statistical comparisons were made using ANOVA and planned comparisons were used to determine specific treatment effects. Significant differences were set at P<0.05. Data represent percent of control values.
Results
Estrogen delays cell viability changes in primary neuronal cultures exposed to Tat 1-86
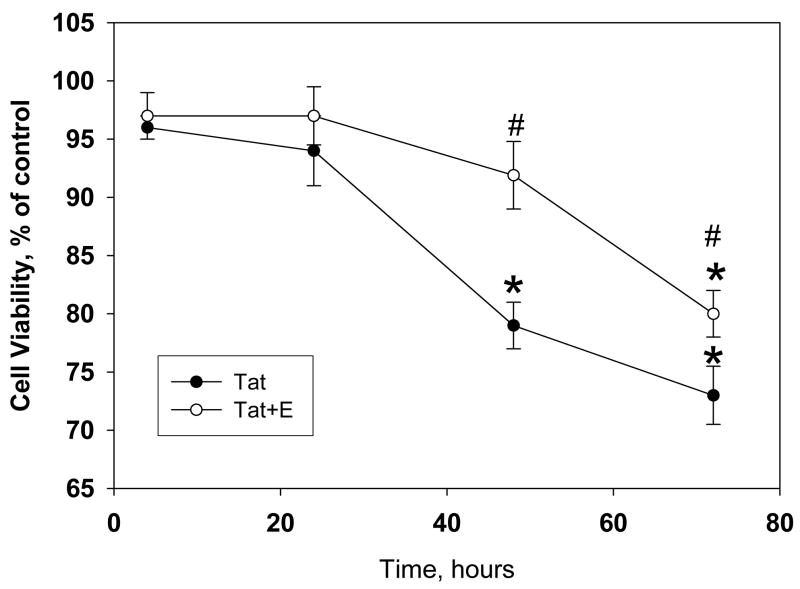
Previous studies have demonstrated that 17 β-estradiol was protective against neurotoxicity induced by HIV-1 viral proteins in cell culture (Kendall et al., 2005; Turchan et al., 2001), specifically using Tat 1-72. We evaluated the ability of 17 β-estradiol to protect against HIV-1-86 Tat-induced toxicity. Preliminary dose response studies demonstrated no significant differences in protective efficacy of doses ranging from 10nM–100nM of 17β-estradiol (data not shown). Additionally, prior studies have shown that 10nM dose of 17 β-estradiol was effective against HIV protein toxic insults in vitro (Kendall et al., 2005). Significant decreases in cell viability were detected in Tat-treated cultures after 48h and 72h, P<0.05. Pre-incubation with 10 nM 17 β-estradiol attenuated the neurotoxic effects of HIV-1 Tat 1-86 (Figure 1). Treatment with estradiol prevented the 20% reduction in cell viability observed in cultures treated with Tat alone. Repeated measures ANOVA revealed significant main effects of time [F(3,27)=29.65, p<0.05] and treatment [F(1,9)=7.69, p<0.05] and a significant time and treatment interaction [F(3,27)=7.41, p<0.05]. Specifically, 17 β-estradiol was effective in delaying the onset of Tat-induced cell death, as a significant difference in cell viability between Tat-treated vs. Tat + 17 β-estradiol cultures was detected at 48h and 72h, p<0.05.
Figure 1. Estrogen treatment delays HIV-1 Tat 1-86 mediated decrease of cell viability in primary rat cortical cultures.
The graph demonstrates the decrease in live cells in primary cultures following exposure to 50 nM Tat 1-86. Addition of 10nM 17β-estradiol 24h prior to Tat 1-86 exposure was able to significantly delay onset of cell death in primary neuronal cultures. Data presented as mean values, n of sister cultures analyzed 5–10 per each time point. *P<0.05 as compared to non-treated controls, #P<0.05 as compared to Tat-treated vs. Tat+E treated cultures
HIV/Tat exposure increases Bax, Bcl-2, and caspase activation in neuronal culture
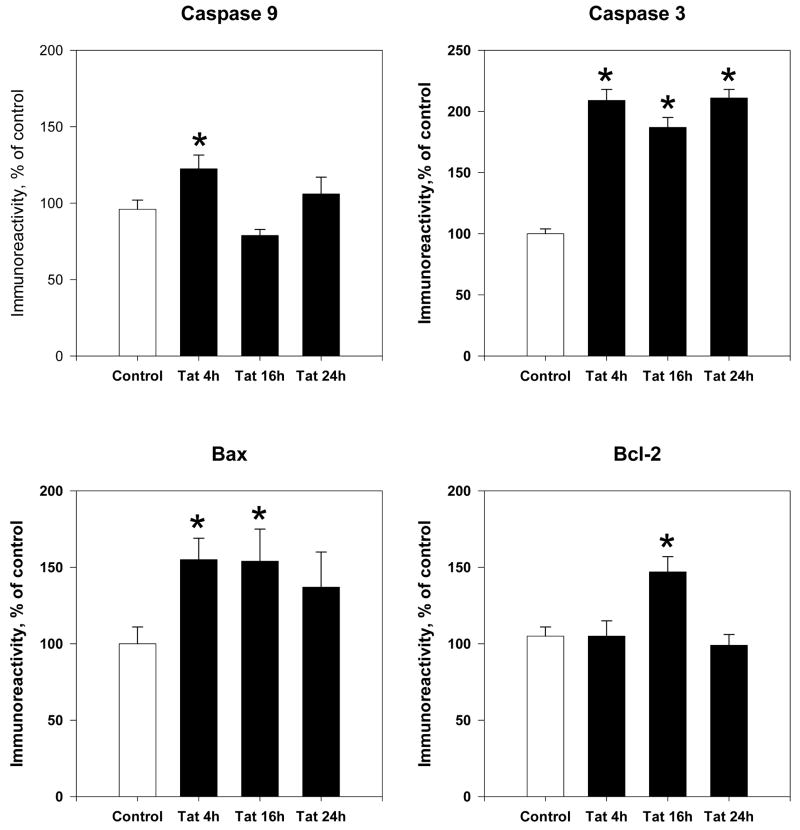
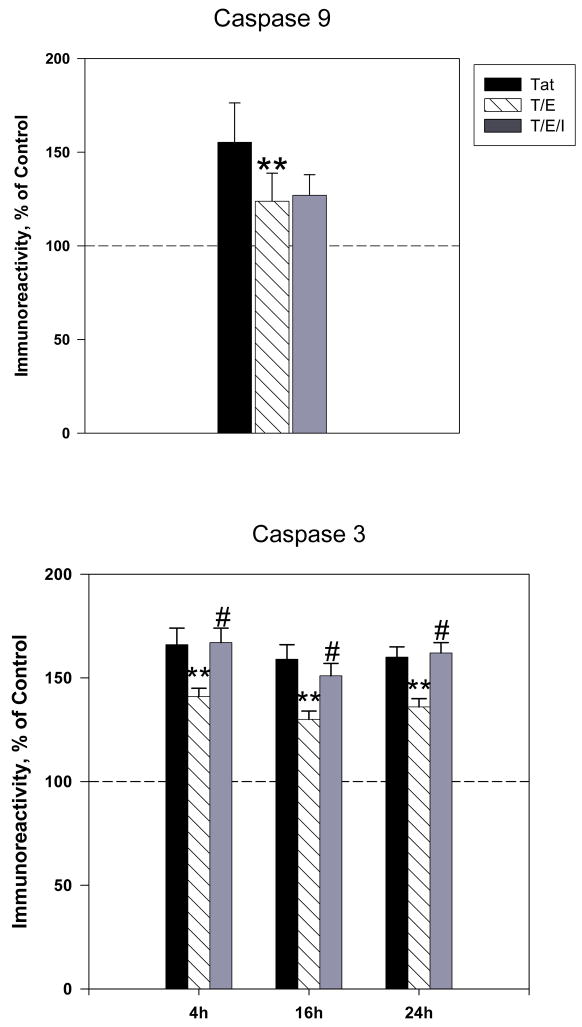
Prior experiments have demonstrated that Tat treatment induced apoptosis in primary cell cultures (Aksenova et al., 2009; Bonavia et al., 2001). Therefore, we assessed active caspase expression and pro- and anti-apoptotic proteins, Bax and Bcl-2 at different time points following exposure to 50nM Tat 1-86 (Figure 2). Results indicate that active caspase 9 protein levels were significantly increased early (4h) after Tat exposure (122±9%, P<0.05); levels at later time points decreased and were not significantly different from untreated controls. Tat also induced a significant increase in active caspase 3 expression early (4h) (208 ± 9%, P<0.05) after treatment, and the increase was maintained throughout later time points, 16h (187± 7%, P<0.05) and 24h (211±7, P<0.05).
Figure 2. Time course of apoptotic protein expression following Tat exposure.
Cortical lysates were collected at 4h, 16h and 24h following Tat 1-86 (50nM) exposure. Expression of Bcl-2, Bax, active Caspase 9 and active Caspase 3 were assessed by ELISA experiments. Results are presented as % of control values, experiments performed in triplicate, *P< 0.05 as compared to controls
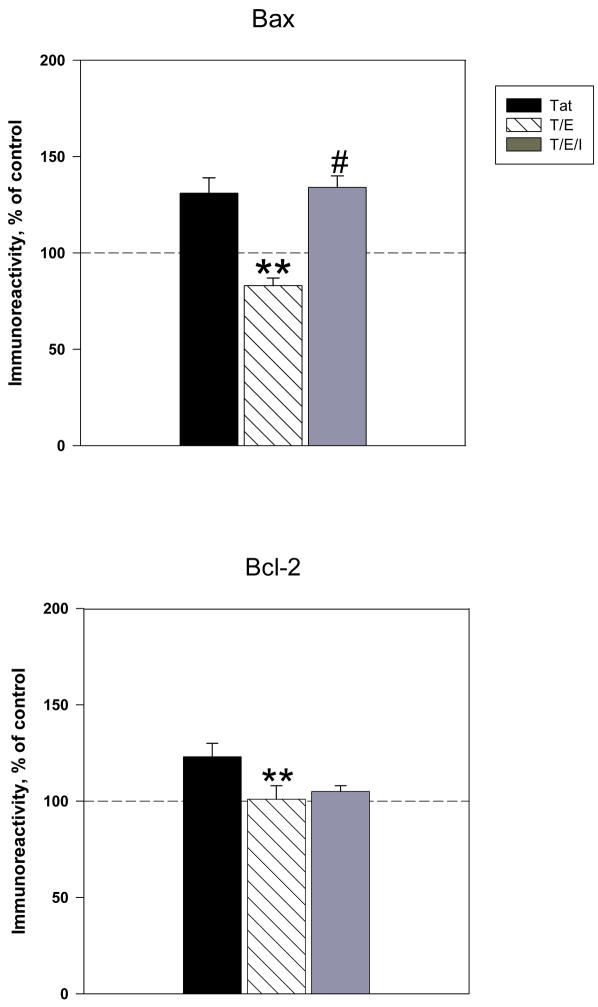
We also observed the expression of upstream apoptotic regulatory proteins Bcl-2 and Bax, which monitor mitochondrial membrane permeability and thus the release of apoptotic factors from mitochondria involved in caspase activation. Results indicated that Tat treatment caused a ~60% increase in pro-apoptotic Bax expression at 4h (P<0.05) that was maintained until 16h of exposure (P< 0.05) (Figure 2). Additionally, anti-apoptotic Bcl-2 protein levels reached significantly high levels only after 16h of Tat exposure (147±10%, P<0.05). No significant changes were seen at 4h or 24h of Tat exposure, as levels were similar to that of controls.
17 β-estradiol attenuates Tat-induced expression of Bcl-2, Bax and active caspases in neuronal cultures
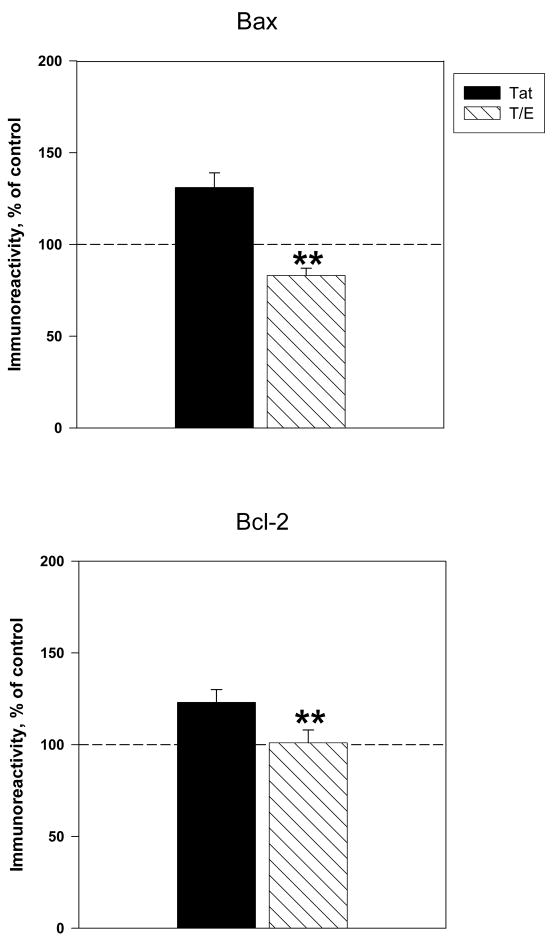
We examined the effects of estrogen against Tat –induced Bcl-2 and Bax protein expression. Our findings demonstrate that pretreatment with estrogen caused a significant reduction in Tat-induced expression of Bax vs. cultures treated with Tat alone (83 ± 4% vs 131±8%, P<0.001) (Figure 3A). Addition of 17 β-estradiol completely reversed Tat-induced changes in Bax, as exhibited by an approximately 45% reduction in Bax protein levels, relative to those generated by Tat alone. Results indicated that estrogen administration attenuated the Tat-induced increase in Bcl-2 observed at 16h of exposure (Figure 3B). Compared to cultures treated with Tat alone, cultures treated with estradiol (10nM) 24h before Tat exposure displayed a significant 20% decrease in Bcl-2 expression (103±7% vs. 123±7% tat-treated cultures, P < 0.05).
Figure 3. 17β-estradiol attenuates Tat-induced expression of Bcl-2 and Bax protein levels.
Cortical cultures were treated with 10nM 17β-estradiol 24h prior to Tat exposure. Expression of apoptotic proteins A. Bax (4h Tat exposure) and B. Bcl-2 (16h of Tat exposure) were assessed by ELISA experiments. Results are presented as % of control value, with experiments performed in triplicate, *P< 0.05 as compared to Tat-treated cultures. Legend box: Tat (T), Tat+Estrogen (T/E). Reference line in graph represents control group.
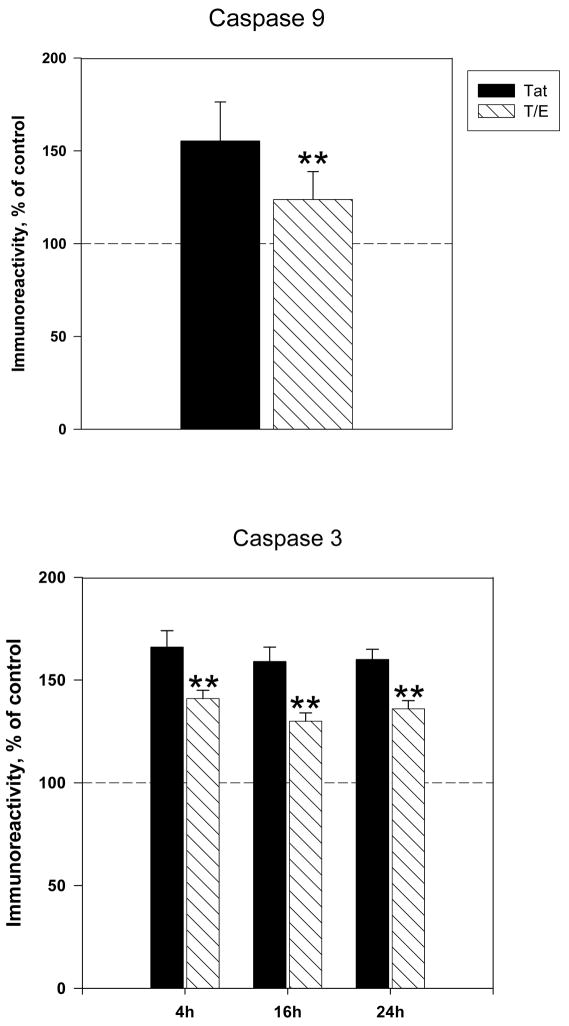
Caspase activation is known to be a critical phase in the cell death process. Activation of initiator caspases, such as caspase 9 leads to the further activation of effector caspase 3, which triggers the final proteolytic phase of apoptosis (Green, 1998; Haeberlein, 2004; Spierings et al., 2005). We examined if the addition of estradiol could eliminate Tat-induced increases in active caspase 9 and active caspase 3 expression. Results indicated that pretreatment with 10nM 17 β-estradiol significantly attenuated early (4h) Tat-induced caspase 9 expression (P<0.05) (Figure 4A). We then determined if the inhibition of Tat-mediated caspase 9 activation by estrogen corresponded with reduced active caspase 3 immunoreactivity. Our results show that estrogen treated cultures displayed modest though significant attenuation of caspase 3 expression at 4h (141±4 vs. 166±8 tat-treated cultures, P<0.05), 16h (130±4 vs. 159 tat-treated cultures, P<0.05) and 24h (136±4 vs. 160±5 tat-treated cultures, P<0.05) (Figure 4B). However, estrogen’s down regulation of caspase 3 was not complete to the level of untreated controls.
Figure 4. 17β-estradiol attenuates Tat-induced increases in active caspase expression.
Cortical cultures were treated with 10nM 17β-estradiol 24h prior to Tat exposure. Expression of active A. Caspase 9 and B. Caspase 3 were assessed by ELISA experiments. Results are presented as % of control value, with experiments performed in triplicate, *P< 0.05 as compared to Tat-treated cultures. Legend box: Tat (T), Tat+Estrogen (T/E). Reference line in graph represents control group.
Estrogen receptors mediate 17β-estradiol effects against Tat-induced Bax and Caspase 3 expression
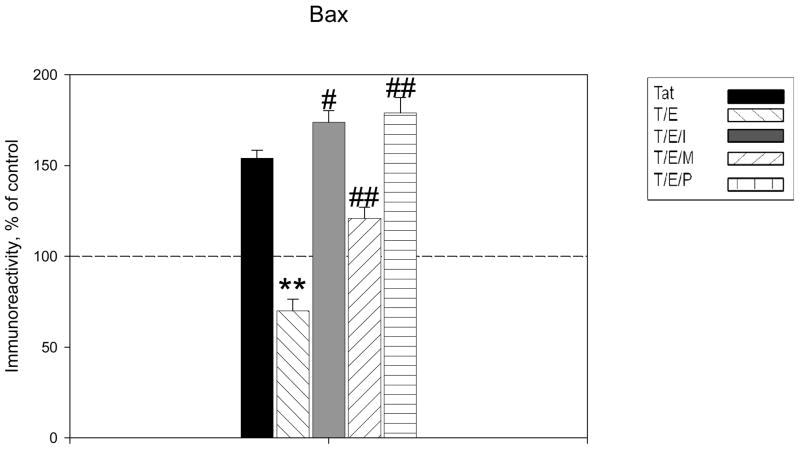
To determine potential mechanisms of estrogenic action, we investigated the effects of ICI 182780, a specific ER antagonist, on 17β-estradiol- inhibition of apoptotic protein expression in Tat treated cell cultures. Cell cultures were incubated for 1 hr with ICI 182,780 (100nM) before the addition of 17β-estradiol and then exposed to 50nM Tat 1-86 for 4, 16, and 24h. The addition of ER antagonist was able to block estrogen protective effects on Tat-induced Bax protein expression, as Bax levels were similar to that of cultures treated with Tat alone (134±6%, P<0.05, compared to Tat+E treated cultures) (Figure 5A). Results indicate that ICI 182,780 was not able to block estrogen actions on Bcl-2 expression with Tat exposure (Figure 5B). There was no significant difference in Bcl-2 levels in 17β-estradiol treated and ICI 182,780 treated cortical lysates. We then used ER subtype-selective antagonists to further define whether ER effects were mediated by either ERα and/or ERβ. ERα and ERβ specific antagonists, MPP and PHTPP respectively, were added to cultures prior to 17 β-estradiol treatment. The addition of MPP only partially attenuated estrogen effects on Bax expression, while the addition of PHTPP markedly abrogated estrogen downregulation of Bax (Figure 5C).
Figure 5. Tat-induced Bax expression sensitive to estrogen receptor -mediated estrogenic actions.
A. Estrogen receptor antagonist, ICI 182,780, was added to cultures 1h before treatment with 17β-estradiol. Estrogen effects on Tat-induced increased expression of Bax were reversed by ICI 182,780, suggesting that estrogenic actions on this protein are mediated by ER signaling. B. Estrogen effects on Bcl-2 expression were not attenuated by the addition of ER antagonist. C. Specific antagonists for ERα (MPP, 100nM) and ERβ (PHTPP, 100nM) were added to cultures 30 min before treatment with 17β-estradiol. ER subtype specific antagonists reveal that ERβ mediated signaling preferential for estrogen effects on Bax. Experiments performed in triplicate, *P<0.05 as compared to Tat-treated cultures, **P<0.05 compared to Tat+E treated cultures. Legend box: Tat (T), Tat+Estrogen (T/E), T/E+ICI182, 780 (T/E/I), T/E+ MPP (T/E/M), T/E+PHTPP (T/E/P). Reference line in graph represents control group.
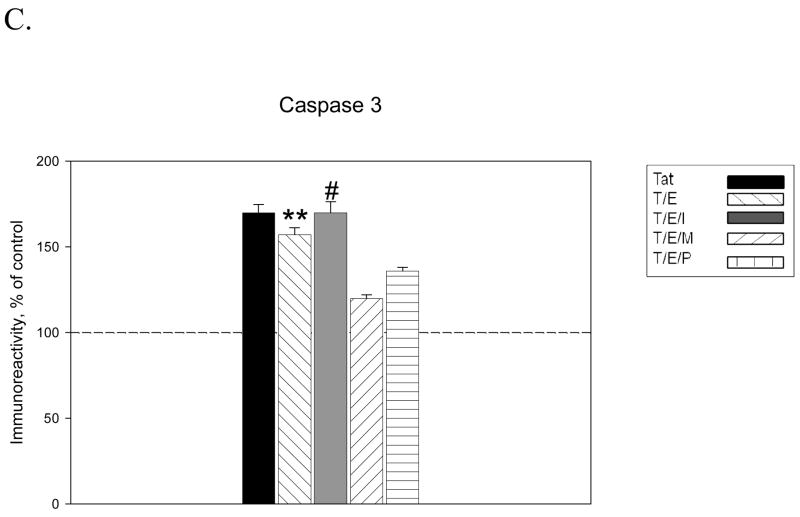
We also evaluated if estrogen receptors play a role in regulation of active caspase expression. ICI treatment displayed no effect on active caspase 9 expression at 4h of Tat exposure, as estrogen effects on caspase 9 were maintained, suggesting the estrogen actions on caspase 9 are not receptor-mediated (Figure 6A). However, estrogen effects on caspase 3 expression were significantly attenuated by ICI treatment. At each time point evaluated, estrogen actions on Tat-induced caspase 3 expression were ICI-sensitive, as caspase levels in ICI treated cultures were not significantly different from those present in cultures treated with Tat alone (Figure 6B). ERα and ERβ specific antagonists, MPP dihydrochloride and PHTPP respectively, were added to cultures prior to 17 β-estradiol treatment. Furthermore, results indicated that the addition of either MPP or PHTPP was unable to attenuate 17 β-estradiol effects on caspase expression, suggesting that 17 β-estradiol actions on Tat-induced caspase expression are mediated by both ER subtypes (Figure 6C).
Figure 6. Estrogen receptor mediates estrogen effects on Tat-induced Caspase 3 not Caspase 9 active expression.
Estrogen receptor antagonist, ICI 182,780, was added to cultures 1h before treatment with 17β-estradiol. A. Estrogen effects on caspase 9 expression were maintained in presence of ER antagonist. B. Estrogen effects on Tat-induced expression of caspase 3 were reversed by ICI 182,780, suggesting that estrogenic actions on caspase 3 are ER mediated. C. Estrogen effects against Tat-induced caspase 3 expression were maintained in presence of specific antagonists for ERα (MPP, 100nM) and ERβ (PHTPP, 100nM). Experiments performed in triplicate, *P<0.05 as compared to Tat-treated cultures, **P<0.05 as compared to Tat+E treated cultures. Legend box: Tat (T), Tat+Estrogen (T/E), T/E+ICI182780 (T/E/I), T/E+ MPP (T/E/M), T/E+PHTPP (T/E/P). Reference line in graph represents control group.
Discussion
Extensive cortical neuron damage and dropout is a significant consequence of NeuroAIDS, likely stemming from susceptibility of cortical cells to the various toxic mechanisms attributed to HIV-1 viral infection, contributed by the protein Tat. Tat may play a role in HIV-1 related neuropathology as prior studies have demonstrated the presence of Tat mRNA and protein in patients with HIVE and HAD. AIDS dementia is characterized by neuronal loss associated with synaptic damage (Weiss et al., 1999) and Tat exposure in vitro has been shown to induce similar pathology (Kim et al., 2008). As HIV-infected individuals are living longer, the rate of CNS disease is actually increasing, as well as the incidence of cognitive impairment, intensifying the need for therapeutic interventions capable of supporting neuronal functioning and viability.
The neuroprotective actions of estrogen have been demonstrated in many experimental models of neurodegenerative disease, including various dementias (Pike et al., 2009; Wilson et al., 2006). However, the mechanisms underlying the beneficial effect of estrogen are not well understood (Green & Simpkins, 2000). In this study, we sought to determine if estrogen, by an ER-sensitive mechanism, attenuates Tat-induced cell death by inhibiting caspase cascade activation and modulating levels of anti-apoptotic and pro-apoptotic Bcl-2 related proteins, key regulators of mitochondrial/intrinsic apoptotic signaling.
Additionally, we determined that Tat –induced cell death was mediated by activation of caspases, a family of enzymes involved in signal transduction of apoptotic stimuli and cellular disassembly (Stennicke and Salvesen, 2000). Caspase 3, the downstream effector of the caspase cascade, is activated in several neurodegenerative disorders (Garden et al., 2002, Namura et al., 1998; Su et al, 2000). Elevated active caspase 3 immunoreactivity was reported in cerebrocortical neurons from patients with HAD and in cultured rodent neurons exposed to HIV viral proteins (Zheng et al., 1999). Our results demonstrated that active caspase 3 expression was significantly increased early following Tat exposure and these elevated levels were maintained for 24h after Tat exposure. These findings support our recent findings (Aksenov et al., 2009) that detected increases in total activated caspases in living cells and demonstrated increases in total caspase activity early after Tat exposure, peaking at 24h.
Previous studies have shown that the neurotoxic capabilities of Tat include inducing oxidative stress and disrupting mitochondrial membrane functioning (Aksenov et al., 2006; Aksenova et al., 2006; Kruman et al., 1998). Moreover, the mitochondria play a central role in mediating apoptotic signaling (Kroemer et al., 2007; Danial &Korsmeyer, 2004; Green& Kroemer, 2004:1998). As such, we determined if Tat-induced caspase 3 activation corresponds with the expression of key regulatory proteins linked to mitochondria mediated apoptotic pathway in cultured cortical neurons. Following the addition of Tat 1-86 to primary cultures, increased expression of active caspase 9, an initiator caspase associated with mitochondria-mediated apoptosis pathway, was apparent after 4h of Tat exposure. The activation of caspase 9 was detected as early as 2h following Tat treatment in living cells (Aksenova et al., 2009). However, active caspase 8 immunoreactivity, associated with extrinsic/death receptor mediated apoptotic cell death has not been detected in Tat exposed cortical cultures (data not shown). Lack of caspase 8 activation in Tat treated cultures suggests that the death- receptor initiated apoptotic cascade may not play a major role in Tat -induced caspase-dependent apoptosis.
Consistent with the suggestion that mitochondrial intrinsic pathway contributes to Tat-mediated apoptosis, our results demonstrated that pro-apoptotic Bax protein levels were significantly increased early after the addition of Tat 1-86 to neuronal cultures. This increase in Bax expression was concurrent with expression of active caspases. Moreover, in our culture model, a ~20–30% reduction in cell viability with Tat exposure has been observed (Aksenova et al., 2009). Even with prolonged exposure and with Tat maintaining its toxicity, only a particular population of neurons appeared to be Tat-sensitive and die, whereas the remaining cells were resistant to Tat-toxicity. We observe that rat fetal neuronal cell cultures contain a subpopulation of neurons in which Tat interactions are able to induce a caspase-dependent apoptotic cascade. It is likely that the early induction of pro-apoptotic signals (Bax, caspase activation) may increase the susceptibility of Tat-sensitive cells to cell death. However, we found that an increase in anti-apoptotic protein Bcl-2 followed this induction of Bax expression and caspase activation. The significant increase in Bcl-2 expression may mediate a neuronal survival promoting response and thus reflect a compensatory mechanism to impede further apoptotic signaling.
Although Tat-induced apoptosis has been associated with caspase activation (Kruman et al., 1998), alternate mechanisms of cell death may also be activated by Tat toxicity. Caspase-independent apoptosis is well established and suggest the induction of alternate execution pathways. NMDA receptors are known to play the key role in the mechanism of Tat-mediated apoptosis (Haughey et al., 2001; Perez et al., 2001; Eugenin et al., 2007; Kim et al., 2008; Aksenova et al., 2009). Activation of NMDA receptors led to the development of apoptosis without involvement of caspases, due to the direct action of apoptosis-inducing factor (AIF) on neuron nuclei (Evstratova et al., 2009). This mechanism involves the release of AIF, which is translocated from the mitochondrial membrane to the nucleus, inducing DNA degradation.
Estrogen deficient states may partly account for declines in cognition and neurodegeneration that are associated with dementia, such as HAD. The current study shows estrogen treatment was able to delay the onset of cell death by attenuating Tat- induced apoptotic signaling. Upstream of caspase activation, we also show that Tat- induced increases in expression of Bcl-2 and Bax was reversed by estrogen. Since the Bcl-2 related proteins are made up of a group of apoptosis regulatory genes of which Bcl-2 is anti-apoptotic and Bax is pro-apoptotic, the ratio of Bcl-2 to Bax may determine whether the cells undergo apoptotic cell death or survive the toxic insult. Perhaps a key mechanism by which estrogens augment apoptotic signaling in Tat-induced cell death is by altering this ratio in favor of anti-apoptotic proteins (Zhang et al., 2003). The significant down regulation of proapoptotic protein Bax by estrogen could serve to balance this ratio and normalize mitochondrial function, preventing the release of apoptotic factors and initiation of the caspase cascade. This estrogen effect on Bax along with its effects on Bcl-2 and caspases, although modest, serves to prolong the compensatory/survival promoting response of cells which corresponds with the significant delay of apoptotic cell death observed with estrogen treatment.
It is widely accepted that the biological actions of estrogen are mediated by two different receptor subtypes, ERα and ERβ. We found the addition of a nonspecific ER antagonist ICI 182,780, which blocks both ERα and ERβ, attenuated the estrogen down-regulation of Bax and caspase 3 with Tat exposure. Using subtype-specific antagonists we sought to further determine if these estrogen effects were selectively mediated by either ERα and/or ERβ. The ERα-specific antagonist MPP and ERβ-specific antagonist PHTPP did not attenuate the estrogen effects on caspase 3 expression. Our results indicated that estrogen receptor-mediated effects on Tat-induced caspase 3 expression are not selective to a specific ER subtype. However, estrogen effects on Bax are preferential for ERβ mediated signaling, although ERα did contribute to these effects as well. Estrogen actions on caspase 9 expression and Bcl-2 levels were maintained in presence of antagonists, implying these estrogen actions are not receptor-dependent. These results suggest that ERs may be involved in select aspects of apoptotic signaling. Indeed, a previous report has shown that HIV-1 Tat toxic effects on mitochondria and neuronal cell survival may be independently regulated (Turchan et al., 2001). It seems that estrogens are protective against both mitochondrial dysfunction and cell death by receptor and non-receptor mediated mechanisms. More evidence indicates that in addition to the genomic ER-mediated effects, many effects of estradiol may involve cross talk with other signal transduction pathways (Mhyre & Dorsa, 2006). Estrogen may act via an indirect, non-genomic response involving activation/phosphorylation of Akt which can mediate the anti-apoptotic signaling pathway and involves regulation of the antiapoptotic protein Bcl-2 (Honda et al.,2000; Singh, 2001).
Recent reports are suggesting the mitochondria as a promising target for estrogen-mediated protection (Arnold and Beyer, 2009; Klinge, 2008; Simpkins and Dykens, 2008). There is evidence that estrogen receptors and estrogen-binding proteins are located in the mitochondrial matrix or mitochondrial membranes. Activation of the nuclear ERs may directly regulate mitochondria by controlling the transcriptional activity of genes coding for mitochondrial associated proteins (Arnold and Beyer, 2009). The substantial effect of estrogen on Bax expression may relate to the mitochondrial localization of ERs, particularly as a recent study reported that ERβ colocalized with a mitochondrial marker in rat primary cortical and hippocampal neurons (Yang et al., 2004).
Exposure to HIV-1 viral proteins initiates a significant toxic cascade that likely involves activation of multiple cell death pathways. It is clear, that estrogen was able to attenuate Tat-induced apoptotic signaling and prolonged cell viability. With the induction of many apoptotic pathways and different factors/mediators of execution, it is difficult to delineate a point of convergence of the various pathways, which would be ideal as a target to interrupt the apoptotic process and support cell survival. However, the ER-β appears to be a primary point for pharmacological targeting.
In summary, the present study suggests the neuroprotective actions of estrogen against HIV-1 Tat-mediated toxic insults involve regulating ER-responsive apoptotic factors. Our results indicated that HIV-1 Tat-mediated cell death induces key regulatory factors of the mitochondria-linked apoptotic pathway. Estrogen treatment was effective in delaying cell death that involved attenuating Tat- induced caspase activity and pro-apoptotic protein expression. The protective estrogenic effect was mediated by an ER-sensitive mechanism specific to Bax and caspase 3. Furthermore, the present study indicated that both ERα and ERβ may contribute to the beneficial actions of estrogen, with some selective effects of ERβ.
Acknowledgments
This work was supported by NIH grants DA1137, DA 13137, HD 43680.
References
- Aksenov MY, Hasselrot U, Bansal Ak, et al. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, et al. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Adams SM, Mactutus CF, Booze RM. Neuronal survival and resistance to HIV-1 Tat toxicity in the primary culture of rat fetal neurons. Exp Neurol. 2009;5(2):253–263. doi: 10.1016/j.expneurol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52:119–132. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Arnold S, Beyer C. Neuroprotection by estrogen in the brain: the mitochondrial compartment as presumed therapeutic target. J Neurochem. 2009;110:1–11. doi: 10.1111/j.1471-4159.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288(2):301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Deutsch R, Heaton RK, Marcotte TD, McCutchan JA, Nelson JA, et al. Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch Neurol. 1997;54:416–424. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- Estratova AA, Mironova EV, Dvoretskova EA, Antonov SM. Apoptosis and the receptor specificity of its mechanisms during the neurotoxic action of glutamate. Neurosci Behav Physiol. 2009;39:353–362. doi: 10.1007/s11055-009-9141-7. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci USA. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL. Neuronal number and volume alterations in the neocortex of HIV infected individuals. J Neurol Neurosurg Psychiatry. 1993;56:481–186. doi: 10.1136/jnnp.56.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzadegan JH, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, Quinn TC, Vlahov D. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–1514. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- Fischer CP, Gundersen HJG, Pakkenberg B. Preferential loss of large neocortical neurons during HIV infection: a study of the size distribution of neocortical neurons in the human brain. Brain Res. 1999;828:119–126. doi: 10.1016/s0006-8993(99)01344-x. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35:313–322. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad J, Walfisch A, Borer A, Schlaeffer F. Gender difference and sex-specific manifestations associated with human immunodeficiency virus infection in women. Eur J Obstet Gynecol Reprod Biol. 2003;109:199–205. doi: 10.1016/s0301-2115(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondria cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci. 2000;18:347–358. doi: 10.1016/s0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 tat through phosphorylation of NMDA receptors potentiates glutamate excitoxicity. J Neurochem. 2001;78(3):457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Honda K, Shimohama S, Sawada H, Kihara T, Nakamizo T, Shibasaki H, Akaike A. Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons. J Neurosci Res. 2001;64:466–475. doi: 10.1002/jnr.1098. [DOI] [PubMed] [Google Scholar]
- Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res. 2000;60:321–327. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Hosoda T, Nakajima H, Honjo H. Estrogen protects neuronal cells from amyloid beta-induced apoptotic cell death. Neuroreport. 2001;12:1965–1970. doi: 10.1097/00001756-200107030-00038. [DOI] [PubMed] [Google Scholar]
- Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis. 2006;21:1–17. doi: 10.1016/j.nbd.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Kendall SL, Anderson CF, Turchan-Cholewo J, Land CL, Mactutus CF, Booze RM. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: testosterone and ICI 182,780 sensitive mechanism. BMC Neurosci. 2005;6:40. doi: 10.1186/1471-2202-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Martemyanov KA, Thayer SA. Human immunodeficiency virus protein Tat induces synapses loss via a reversible process that is distinct from cell death. J Neurosci. 2008;28:12604–12613. doi: 10.1523/JNEUROSCI.2958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem. 2008;105:1342–1351. doi: 10.1002/jcb.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeh JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I HNRC Group. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Myhre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New DR, Ma M, Epstein LG, et al. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol. 1997;3:168–173. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- Nilsen J. Estradiol and neurodegenerative oxidative stress. Front Neuroendocrinology. 2008;29:463–475. doi: 10.1016/j.yfrne.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Nilsen J, et al. Estrogen protects neuronal cells from amyloid β-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci. 2006;7:74. doi: 10.1186/1471-2202-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging finding in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev. 2002;26:353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Perez A, Probert AW, Wang KK, Sharmeen L. Evaluation of HIV-1 tat induced neurotoxicity in rat cortical cell culture. J Neurovirol. 2001;7(1):1–10. doi: 10.1080/135502801300069575. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroids hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza G, Lepri AC, d’Arminio-Monforte A, Pezzotie P, Castelli F, Dianzani F, et al. Plasma viral load concentrations in women and men from different exposure categories and with known duration of HIV infection. ICONA Study Group. J Acquir Immune Defic Syndr. 2000;25:56–62. doi: 10.1097/00042560-200009010-00008. [DOI] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, Schiffitto G, McArthur JC, Sacktor N, et al. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. 2007 doi: 10.1001/archneur.64.1.97. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Brain Res Rev. 2008;57:421–430. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Caspases –controlling intracellular signals by protease zymogen activation. Biochim Biophys Acta. 2000;1477:299–306. doi: 10.1016/s0167-4838(99)00281-2. [DOI] [PubMed] [Google Scholar]
- Sterling TA, Vlahov D, Astermborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-RNA levels and progression to AIDS I women and men. N Engl J Med. 2001;344:720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, et al. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study, HIV Neurobehavioral Research Center Group. Arch Neurol. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- Su JH, Nichol KE, Sitch T, Sheu P, Chubb C, Miller BL, Tomaselli KJ, Kim RC, Cotman CW. DNA damage and activated caspase-3 expression I neurons and astrocytes: evidence for apoptosis in frontotemporal dementia. Exp Neurol. 2000;163:9–19. doi: 10.1006/exnr.2000.7340. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser K, et al. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2(1):3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeh OC, Currier JS. Sex differences in pharmacokinetics and toxicity of antiretroviral therapy. Expert Opin Drug Metab Toxicol. 2006;2:273–283. doi: 10.1517/17425255.2.2.273. [DOI] [PubMed] [Google Scholar]
- van de Bovenkamp M, Nottet HS, Pereira CF. Interactions of human immunodeficiency virus-1 proteins with neurons: possible role in the development of human immunodeficiency virus-1- associated dementia. Eur J Clin Invest. 2002;32:619–627. doi: 10.1046/j.1365-2362.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- Vanoye-Carlo A, Mendoza-Rodriguez CA, Morales T, Langley E, Cerbon M. Estrogen receptors increased expression during hippocampal neuroprotection in lactating rats. J Steroid Biochem Mol Biol. 2009;116:1–7. doi: 10.1016/j.jsbmb.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120 and tat(1-72)-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wallace DR. HIV Neurotoxicity: potential therapeutic interventions. J Biomed Biotechnol. 2006;2006:1–10. doi: 10.1155/JBB/2006/65741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Haug H, Budka H. Neuronal damage in the cerebral cortex of AIDS brains: a morphometric study. Acta Neuropatholo (Berl) 1993;85:185–189. doi: 10.1007/BF00227766. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Baldwin M, Achim CL. Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS. 1996;10:843–847. doi: 10.1097/00002030-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Dimayuga FO, Reed JL, Curry TE, Anderson CF, Nath A, Bruce-Keller AJ. Immune modulation by estrogens: role in CNS HIV-1 infection. Endocrine. 2006;29:289–297. doi: 10.1385/ENDO:29:2:289. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Valencia T, Brun-Zinkernage AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci USA. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goodyer C, LeBlanc A. Selective and protracted apoptosis in human primary neurons microinjected with active caspase -3, -6, -7, and -8. J Neurosci. 2000;20:8384–8389. doi: 10.1523/JNEUROSCI.20-22-08384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, et al. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1 associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]