Abstract
Drug-resistant micro-organisms became widespread in the 20th Century, often with devastating consequences, in response to widespread use of natural and synthetic drugs against infectious diseases. Antimalarial resistance provides one of the earliest examples, following the introduction of new medicines that filled important needs for prophylaxis and treatment around the globe. In the present chapter, we offer a brief synopsis of major antimalarial developments from two natural remedies, the qinghaosu and cinchona bark infusions, and of synthetic drugs inspired by the active components of these remedies. We review some contributions that early efficacy studies of antimalarial treatment brought to clinical pharmacology, including convincing documentation of atebrine-resistant malaria in the 1940s, prior to the launching of what soon became first-choice antimalarials, chloroquine and amodiaquine. Finally, we discuss some new observations on the molecular genetics of drug resistance, including delayed parasite clearances that have been increasingly observed in response to artemisinin derivatives in regions of South-East Asia.
Birth of antimalarial treatments east and west: qinghaosu and cinchona
Two thousand years before the isolation of active ART (artemisinin) from the qinghao plant, the therapeutic benefits of qinghao infusion (qinghaosu) for various illnesses were documented in China. The earliest known description of qinghao use dates back to 168 B.C. In a manuscript written during the Mawangdui Han Dynasty, qinghaosu was described as a treatment for haemorrhoids [1–3]. In the Jin Dynasty, detailed extraction procedures and preparations used against intermittent fevers were impressively described by a Dao philosopher and writer from the 4th Century A.D., Ge Hong, in “The Handbook of Prescriptions for Emergency Treatments” [2]. Centuries later, during the Ming Dynasty, Li Shizhen edited the “Compendium of Materia Medica” in 1596 and reported the use of qinghaosu for the treatment of wounds, boils, sores, ‘intermittent fevers’, ‘lingering heat in joints and bones’ and ‘exhaustion due to heat and fever’s [1–3]. Since many of these symptoms overlapped with those of malaria, it is likely that qinghaosu was serendipitously utilized to treat malaria long before it was specifically recognized as an antimalarial remedy [4].
Carl Linnaeus knew and classified qinghao as Artemisia annua in the 18th Century. However, it was not until the late 20th Century, many years after the isolation, characterization and use of its active component by scientists in China [5], that qinghao was widely accepted and applied against malaria in the west. For centuries the western world had relied on the medicinal properties of QN (quinine) and related alkaloids from the bark of the cinchona tree from South America to cure malarial fevers [6]. Robert Talbor, an apothecary apprentice from Cambridge, defined a safe and effective treatment regimen against malaria using cinchona bark infusions in the late 1600s [6–8]. A self-proclaimed ‘feverologist’, Talbor had no further interest in understanding the cause of the disease, which was later systematically investigated by Francisco Torti [9]. Quality supplies of active remedy were improved in 1820 by the isolation of two major active alkaloids of cinchona, QN and cinchonine [6–8] and, in 1852, of two additional alkaloids, quinidine and cinchinidine [10]. QN, a quinoline methanol (Figure 1), was the most abundant alkaloid in the cinchona barks and received the greatest attention [10].
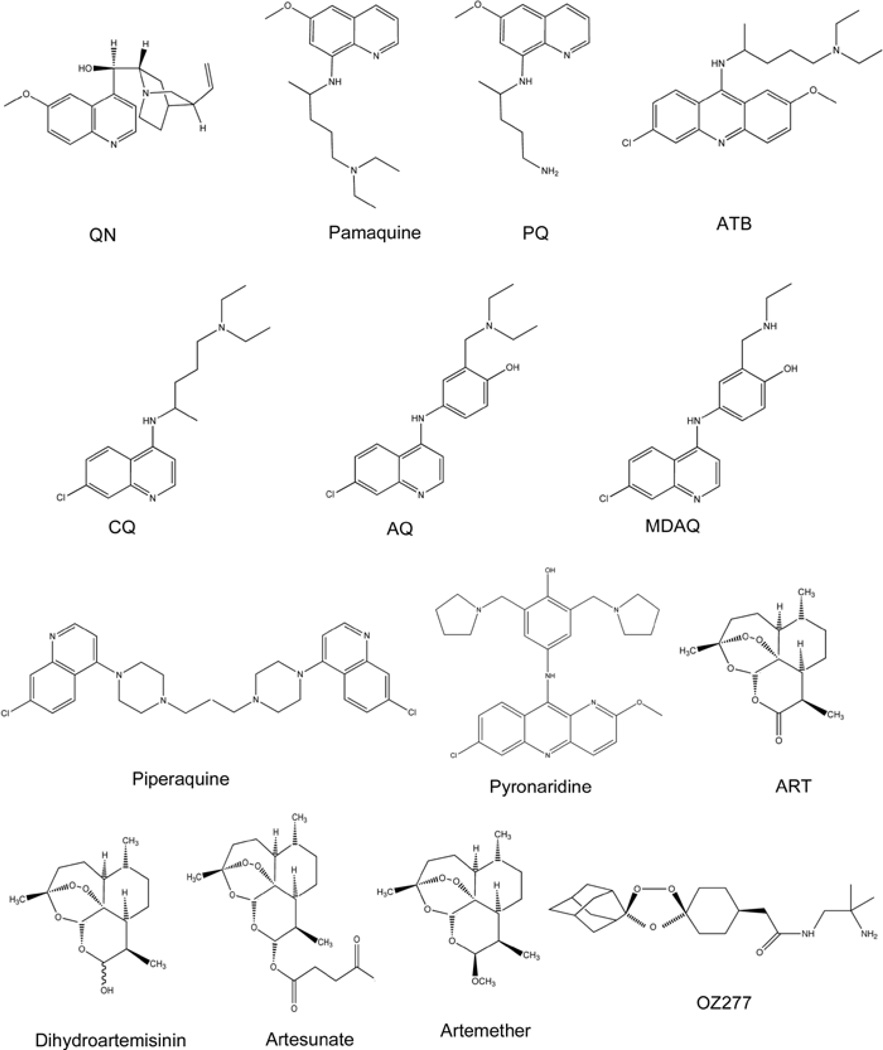
Figure 1. Chemical structures of antimalarial drugs inspired by the active compounds of cinchona bark and qinghao.
Antimalarials inspired by the active compounds of the cinchona bark are characterized by the presence of a quinoline heteroaromatic nucleus. Represented quinoline antimalarials include: QN, a quinoline methanol; the 8-aminoquinolines pamaquine and PQ; the acridine-based compounds ATB and pyronaridine; and the 4-aminoquinolines CQ, AQ, its active metabolite MDAQ, and the bisquinoline piperaquine. It is suggested that the target of most quinoline antimalarials is haematin (aquaferriprotoporphyrin IX), an autoxidized haem released during haemoglobin degradation and found as crystallized dimers in the acidic vacuoles of infected red blood cells of Plasmodium parasites. Most quinoline drugs complex with haematin, which is thought to kill the parasite by an oxidative or osmotic mechanism. Antimalarials inspired by the active compounds of qinghao include the sesquiterpene lactone ART, and its derivatives dihydroartemisinin, artesunate and artemether. The endoperoxide bridge is crucial for its antiparasitic activity and is proposed to cause oxidative stress by the formation of ROS. A recent fully synthetic endoperoxide antimalarial inspired by ART is arterolane (OZ277), which presents a spiroadamantane trixoloane pharmacophore and neutral or basic functional groups designed to improve oral bioavailability and increase its half-life.
From cinchona alkaloids to synthetic antimalarials
Despite aggressive cultivation and horticultural advances, cinchona supplies remained subject to shortages and embargoes during the 19th and early 20th centuries. In 1856, 18-year-old William Henry Perkin, an assistant of August Wilheim von Hofmann, who was responsible for efforts against malaria in a newly created institute at the Royal College of Chemistry in London, attempted to chemically synthesize QN [7]. Although Perkins’ naive efforts were unsuccessful, he serendipitously generated mauvaine, a valuable aniline compound that launched a synthetic dye industry and boosted the emerging field of organic chemistry.
Research to generate novel organic dyes eventually returned the discovery of new antimalarial compounds. Paul Ehrlich pursued the idea that various dyes could stain cells of tissues and microbes via specific interactions, developing the concept that these interactions could be applied to the design of compounds with specific chemotherapeutic effects, including medicines for the treatment of syphilis, trypanosomes and malaria. Efforts to find a synthetic substitute for QN received a great boost from Erlich in 1891, when he successfully treated two malaria patients with Methylene Blue [11].
In addition to the expense of QN and intermittent shortages of supplies, difficulties with QN treatment were reported in Italy and Brazil at the beginning of the 20th Century [1]. These observations stoked worries of QN tolerance, although no full-blown QN resistance was established. The slow development and spread of resistance was perhaps a result of the involvement of multiple genes of the parasite in QN response [12,13].
Antimalarials from synthetic chemistry
The impact of cut-offs in QN supplies during World War I gave a great spur to chemical research on antimalarial discovery [7]. The first fruit of this research was realized in 1926 with the synthesis of the 8-aminoquinoline pamaquine (also known as plasmoquine; Figure 1). Although detrimental side effects restricted its use, pamaquine had an advantage over QN in that it could act against gametocytes and liver stage parasites [14]. In particular, the ability of pamaquine to eliminate persistent liver stage parasites (hypnozoites) of Plasmodium vivax infections boosted the search for alternative drugs with pamaquine-like action. This eventually led to the discovery of PQ (primaquine), a key 8-aminoquinoline antimalarial that remains in use today (Figure 1) [15–17].
Along with expanded efforts in synthetic chemistry, efficient screening systems were also essential to the new efforts in drug discovery. Development of effective bird malaria models supported the evaluation of more than 12 000 synthetic compounds, many based on the primary structure of pamaquine [18]. In 1930, ATB (atebrine, also known as quinacrine or mepacrine; Figure 1), was synthesized on the foundation of an acridine instead of a quinoline ring, and was found to have activity against blood stage malaria parasites. Despite an initial concern about toxicity, ATB proved to be highly successful once its pharmacology and dosing requirements were more fully understood, and it became an important tool against malaria in World War II [1,19].
In 1934, a 4-aminoquinoline compound named resoquine was synthesized by Hans Andersag [20]. For reasons that may have related to predicted toxicity of the compound, the drug was shelved and efforts were directed to an alternative methyl derivative, sontoquine. The formulae of these compounds were received by the U.S. company Winthrop Stearns, but no further action was taken on resoquine and sontoquine until they were included in a large-scale screening programme for new antimalarial drugs organized by the U.S. OSRD (Office of Scientific Research and Development) during World War II [18]. Resoquine, renamed as compound SN 7618 and then CQ (chloroquine; Figure 1), proved to be fast acting against blood stages of all species of malaria parasites, well tolerated, easily administered, readily synthesized, stable and remarkably inexpensive. The OSRD programme identified another important 4-aminoquinoline named compound SN 10751, which is otherwise known as camoquine or AQ (amodiaquine; Figure 1) [21]. AQ is metabolized within a few hours after oral administration, and is considered to be a pro-drug of its major active metabolite, MDAQ (monodesethylamodiaquine; Figure 1) [22]. AQ has been used heavily in many malaria-endemic regions and remains recommended by the WHO (World Health Organization) as a partner drug in ACTs (ART-combination therapies).
In the face of increasing drug-resistant malaria infections (discussed in following sections), additional quinoline and acridine-based compounds have been synthesized, studied and found to be active against Plasmodium falciparum strains that are resistant to CQ and AQ. One important example currently used and recommended in some malaria-endemic areas is piperaquine, a bisquinoline drug synthesized in the 1960s that includes two 4-aminoquinoline moieties (Figure 1). Piperaquine is well tolerated, relatively inexpensive and has been used against P. falciparum and P. vivax malaria in the Indochina region. Combinations of piperaquine with ART or ART derivatives yield high cure rates of multidrug-resistant P. falciparum and CQ-resistant P. vivax infections [23]. However, the piperaquine response varies, and the cause(s) of these variations is/are unclear [24].
Pyronaridine is another important example of an antimalarial structurally related to CQ, but active against CQ-resistant P. falciparum (Figure 1). This synthetic acridine-based drug was investigated in China and has been used officially against malaria in that country since 1980 [25]. Pyronaridine is an azacrine-type Mannich base (1-aza-acridine substitution) that has a naphthyridine nucleus resembling acridine (1,5-napthyridine substitution) and a side chain with an aromatic ring similar to AQ. Its efficacy against vivax and falciparum malaria, including clinically severe cases and infections of strains resistant to other antimalarials, promises a prominent role for this compound in coming years, particularly in ACTs [25,26].
ART, its derivatives and endoperoxide analogues
In the early 1970s, the sesquiterperne lactone ART (Figure 1) was isolated and characterized by Chinese scientists in search of new antimalarial drugs against CQ-resistant malaria during the Vietnam War. The search, known as ‘Program 523,’ was initiated on 23 May 1967 and involved some 600 Chinese scientists from various institutions [2,5].
Although efforts to isolate ART encountered initial difficulties because of its poor solubility in water and oil, Tu Youyou and her team at the China Academy of Traditional Chinese Medicine used insights from Ge Hong’s extraction procedures to obtain stable and consistent preparations of active ART in ether at low temperatures [3]. ART contains an endoperoxide bridge crucial for its antiparasitic activity. Although the molecular targets of ART are not well defined, experimental evidence suggests that ART alkylates multiple targets such as haem and parasite neutral lipid bodies and proteins [27]. It is proposed that this leads to the formation of ROS (reactive oxygen species), which causes oxidative stress and damage to the parasite.
Following the successful isolation of ART, chemical modifications, such as the reduction of its carbonyl group, resulted in the water-soluble derivatives dihydroartemisinin and artesunate and the oil-soluble artemether (Figure 1). These derivatives are widely used today with partner drugs in ACTs. A landmark discovery in the battle against malaria, ART and its derivatives are highly potent and rapid-acting, with a parasite reduction rate of approximately 10 000 parasites per erythrocytic cycle. This is the highest ratio among all licensed antimalarial drugs, including QN [28]. However, these compounds have extremely short half-lives (typically ~1 h in vivo [28]), which may be a reason for high rates of recrudescence after ART monotherapy [28]. To reduce the occurrence of recrudescence seen with ART, new derivates with greater half-lives, such as artemisone, may be useful [29,30].
OZ (ozonide) compounds such as OZ277 (also known as arterolane; Figure 1) and OZ439, fully synthetic endoperoxides, present a spiroadamantane trixoloane pharmacophore and neutral or basic functional groups that improved oral bioavailability and increased half-lives [30,31]. These potent antimalarials with a long half-life are now in clinical trials and may prove valuable in combination therapies to guard against recrudescence and parasite resistance to ART [32].
Plasmodium parasites respond: ATB resistance
The use of ATB came into full force during World War II, particularly in Pacific and Asian theatres where malaria casualties among the troops often greatly exceeded casualties from the war itself. The Australian Army decided to focus on investigations to advance the control of tropical diseases in the Pacific. Neil Hamilton Fairley led these investigations and reported on the malaria situation and tests of new antimalarials, including their use in prophylaxis [19].
Fairley’s studies involved the transfer of soldiers with malaria from battle sites in New Guinea to a hospital in Cairns. Infections from these soldiers were transmitted via bites from laboratory-reared mosquitoes to volunteers who had received drug prophylaxis. He was able to show that several new sulfonamides affected the blood stages of P. vivax and P. falciparum, but were not effective for malaria prophylaxis. He then performed experiments with ATB, alone and in combination with sulfamezathine, and demonstrated remarkable protection against vivax and falciparum malaria when ATB was taken at a dose of 100 mg/day, 6 days per week [19]. Many volunteers were consistently protected despite hundreds of mosquito bites, leading to extensive use of ATB among the Allied troops in late 1944 and 1945. Photofluorimetric assays developed by Brodie et al. [33] established suppressive ATB plasma concentrations and demonstrated that when treatment failures occurred, they correlated with inadequate drug levels, usually from lack of compliance due to complaints of side effects including yellow pigmentation of the skin and gastrointestinal disturbances. For the first time, a drug other than QN succeeded in saving a great number of lives and reduced the tremendous burden of malaria casualties.
In 1945, during the Aitape–Wewak campaign, a falciparum malaria epidemic developed among troops and their medical officers despite rigorous ATB prophylaxis. When increased ATB daily doses of 200 mg did not prevent additional cases, Fairley’s team used parasites from patients to transmit experimental infections and showed that P. falciparum strains had developed heritable resistance [19]. Fairley classified ATB responses as: (I) complete protection; (II) protection while taking the drug, but not when drug-taking ceased; (III) partial suppression, with low parasitaemia; and (IV) no protection against infection. Interestingly, a type IV resistant strain during several sub-passages in volunteers via mosquito bites subsequently switched to a type I response, raising the possibility of a sensitive subpopulation from a mixed infection or of loss of resistance from a genetic reversion.
The mechanism behind the ATB prophylaxis failures and its origin in Aitape–Wewak was not clearly understood. Evidence from Fairley’s experiments pointed to the presence of parasite populations that had evolved resistance to ATB. Further studies by Fairley’s team in 1945 showed that proguanil was able to suppress these ATB-resistant strains. In concluding experiments, Fairley was also able to test the newly developed 4-aminoquinolines CQ and AQ and found no evidence of cross-resistance between these drugs and ATB. Controversy remains about the effects of selective pressure from ATB and pamaquine (which often achieved only sub-therapeutic dosages where they were used) on the later spread of CQ-resistant P. falciparum and PQ-tolerant P. vivax [16]. At the conclusion of his studies, Fairley had established an essential framework for antimalarial chemotherapy assessments including drug quality and stability, verification of proper drug dose administration, absorption, and classifications of drug response levels according to parasite clearance and recrudescence times up to 28 days post-treatment [34].
Rise and fall of CQ and AQ
In the late 1940s and early 1950s, clinical trials for the new and promising 4-aminoquinolines CQ and AQ were reported from India, Brazil, the Philippines, Panama, Ecuador, Taiwan and regions of Africa [35–38]. These trials confirmed the efficacy and potential of both drugs as powerful new weapons against malaria. Cases of drug-resistance were not observed immediately; however, a report of AQ failure was published in 1954 from India [39], and CQ treatment failures were found in South America and South-East Asia between 1957 and 1961 [40–43]. Some studies found cross-resistance between AQ and CQ [44,45]. Today, CQR (CQ resistance) is widespread and CQ has been removed from the WHO recommendations for P. falciparum treatment in all but a few regions. In regions of Africa where CQ-resistant infections still respond to AQ, the WHO lists AQ for use in combination therapies against P. falciparum. AQ should not be used where parasites are effectively resistant to both AQ and CQ in regions such as South America, Oceania, India/South-East Asia and, increasingly, southern and eastern Africa.
The spread of CQR and AQR (AQ resistance) since the 1950s raises important questions and challenges, particularly as they pertain to our ability to discover, develop, deploy and maintain effective drugs against malaria. How did resistance to AQ and CQ originate and spread? Which factors determine levels of resistance and cross-resistance between these drugs in various parasite strains? How stable are the resistance phenotypes? Answers to these questions can be approached by understanding the molecular mechanisms of drug action and resistance, and their influence on clinical outcomes [46].
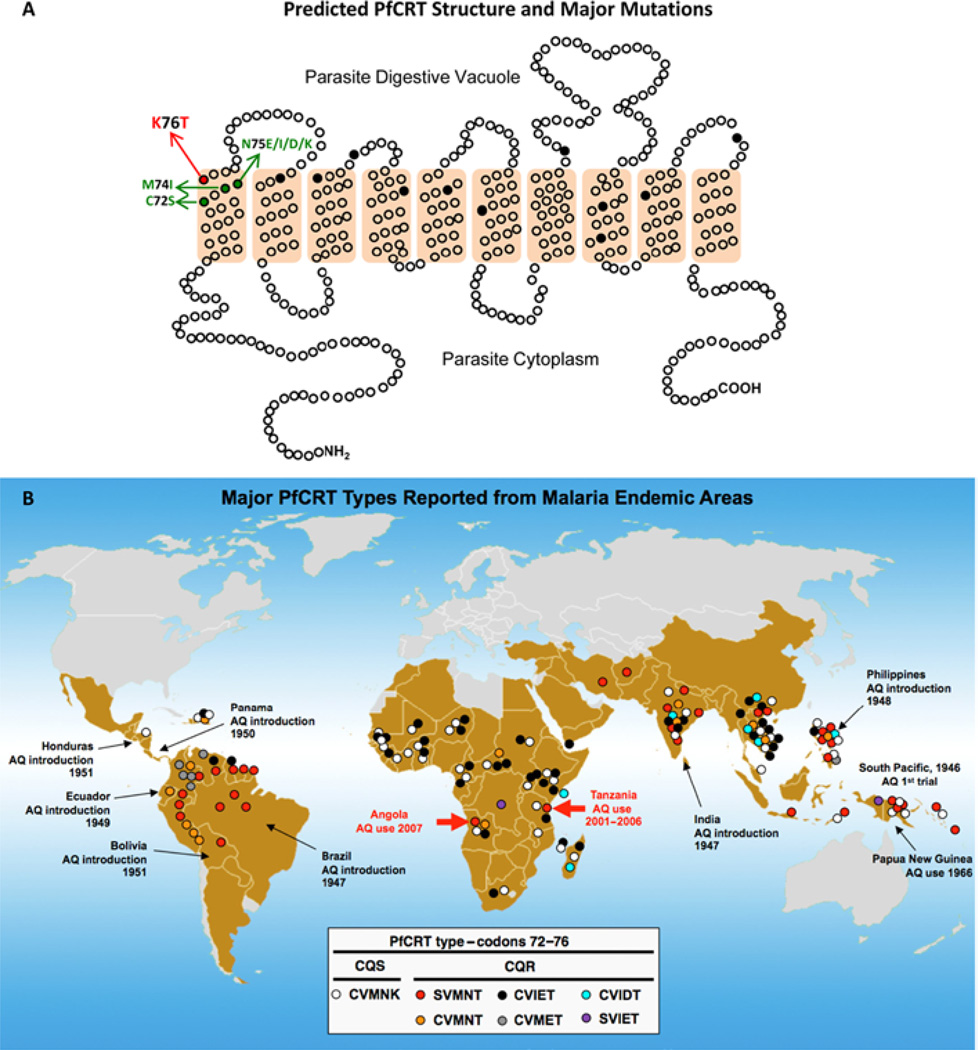
CQ and AQ have been shown to share a similar mode of action by which they accumulate inside the acidic digestive vacuole of the parasite and interfere with the detoxification process of haem, a by-product of haemoglobin degradation [47–49]. Resistant parasites do not accumulate the drug to the extent that sensitive parasites do [50]. Analysis of a genetic cross between a CQ-resistant clone from Indochina, Dd2, and a CQ-sensitive clone from Honduras, HB3, enabled the identification of the genetic determinant of CQR [51,52]. This gene, pfcrt, encodes an essential transporter protein PfCRT (P. falciparum CQ resistance transporter; Figure 2A), which has been classified by bioinformatic analysis as a member of a superfamily of drug/metabolite transporters that lack nucleotide-binding domains [53]. Mutations in pfcrt enable parasites to become resistant by controlling the ability of CQ to accumulate in their digestive vacuoles. A key amino acid mutation is the replacement of lysine by threonine at codon position 76 in the first transmembrane segment [52,54]. Other substitutions at various positions are thought to accommodate or support this key mutation (Table 1). Although these additional mutations occur in most transmembrane segments of PfCRT, CQ-resistant forms are usually classified by the identity of the amino acid residues at positions 72–76: SVMNT, CVIET, CVMNT, CVMET, CVIDT and SVIET (single-letter representation of amino acids; sensitive haplotype, CVMNK). These haplotypes are associated with characteristic geographic distributions and drug resistance phenotypes (Figure 2B).
Figure 2. Predicted protein structure of PfCRT and geographic distribution of major haplotypes based on codon positions 72–76.
(A) Schematic representation of predicted PfCRT structure with ten transmembrane domains and the amino acid positions that have been found to carry mutations in P. falciparum field isolates (black dots). Mutations at positions 163 and 352 that have been selected only in laboratory experiments are not shown [112,113]. Major reported amino acid substitutions at codon positions 72, 74, 75 and 76 are indicated in single letter amino acid code. Reprinted from Current Opinion in Microbiology, vol. 4, Carlton, J.M., Fidock, D.A., Djimde, A., Plowe, C.V. and Wellems, T.E., Conservation of a novel vacuolar transporter in Plasmodium species and its central role in chloroquine resistance of P. falciparum, pp. 415–420, © 2001, with permission from Elsevier. (B) Distribution of reported PfCRT haplotypes (observed in more than a single isolate) from malaria endemic regions. Reprinted with permission from Proceedings of the National Academy of Sciences U.S.A., vol. 106, Sa, J.M., Twu, O., Hayton, K., Reyes, S., Fay, M.P., Ringwald, P. and Wellems, T.E., Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine, pp. 18883–18889, © 2009, National Academy of Sciences, and updated from data in [64,115–119]. CQS, CQ sensitivity.
Table 1. PfCRT and Pgh-1 haplotypes of representative CQ-sensitive & CQ-resistant P. falciparum clones.
Standard single-letter abbreviations indicate the amino acids encoded by polymorphic codons in the different pfcrt and pfmdr1 open reading frames [52,54,55,120–122]. Amino acid residues in bold font show differences from the wild-type HB3 and D10 chloroquine-sensitive sequences.
| (a) Laboratory adapted CQ-sensitive field isolates | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geographic origin | PfCRT polymorphisms | Pgh-1 polymorphisms | ||||||||||||||
| 72 | 74 | 75 | 76 | 97 | 220 | 271 | 326 | 356 | 371 | 86 | 184 | 1034 | 1042 | 1246 | ||
| HB3 | Honduras | C | M | N | K | H | A | Q | N | I | R | N | F | S | D | D |
| D10 | Papua New Guinea | C | M | N | K | H | A | Q | N | I | R | N | Y | S | N | D |
| 106/1 | Sudan | C | I | E | K | H | S | E | S | I | I | Y | Y | S | N | D |
| (b) Laboratory adapted CQ-resistant field isolates | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geographic origin | PfCRT polymorphisms | Pgh-1 polymorphisms | ||||||||||||||
| 72 | 74 | 75 | 76 | 97 | 220 | 271 | 326 | 356 | 371 | 86 | 184 | 1034 | 1042 | 1246 | ||
| Ecu1110 | Ecuador | C | M | N | T | H | S | Q | D | L | R | N | F | S | D | D |
| JAV | Colombia | C | M | E | T | Q | S | Q | N | I | T | N | F | S | D | D |
| Dd2 | Indochina | C | I | E | T | H | S | E | S | T | I | Y | Y | S | N | D |
| GB4* | Ghana | C | I | E | T | H | S | E | N | I | I | Y | F | S | N | D |
| K1 | Thailand | C | I | E | T | H | S | E | S | I | I | Y | Y | S | N | D |
| PH4 | Philippines | C | I | E | T | H | S | E | S | T | I | N | F | C | D | D |
| PH1 | Philippines | C | M | N | T | H | A | Q | D | I | R | Y | Y | S | N | D |
| PH2 | Philippines | S | M | N | T | H | A | Q | D | I | R | Y | Y | S | N | D |
| 7G8 | Brazil | S | M | N | T | H | S | Q | D | L | R | N | F | C | D | Y |
| AN001 | Papua New Guinea | S | M | N | T | H | S | Q | D | L | R | Y | Y | S | N | D |
| N18/N70/S65/S99 | Solomon | S | M | N | T | H | S | Q | D | L | R | Y | Y | S | N | D |
In an analysis of two P. falciparum crosses, one between clones from Central America and South-East Asia (HB3×Dd2) and another between clones from South America and West Africa (7G8×GB4), we recently showed that high levels of AQR in South America derive from particular pfcrt and pfmdr1 alleles presented by the 7G8 clone [55]. The in vitro responses measured by half-maximal inhibitory concentration values (IC50) of the progeny clones showed that the 7G8 PfCRT type SVMNT was linked to higher resistance to MDAQ, the AQ active metabolite (IC50 values 100–200 nM), than to CQ (IC50 values 50–100 nM), whereas the CVIET PfCRT type of Dd2 and GB4 was linked to higher IC50 values for CQ (100–250 nM) than for MDAQ (50–150 nM). These data, together with the reports of clinical outcomes after CQ and AQ treatment, suggest that particular pfcrt haplotypes are associated with different levels of resistance to these drugs, and that the effects of these haplotypes are differentially modulated by pfmdr1 alleles with which they are associated [55].
Drug-resistant parasites of the SVMNT type are prevalent across regions of South America, Iran, Afghanistan, India, Laos and the Pacific Islands [55]. Many of these regions overlap with areas where AQ was used in the 1940s and 1950s, including the area of India from which AQR was reported in 1954 [38,39]. Other regions where the CVIET haplotype is prevalent, e.g. large parts of Africa, areas of South-East Asia and northern countries of South America, may carry populations of drug-resistant P. falciparum with mutant PfCRT types predominantly selected by CQ pressure.
Additional distinguishing features are associated with the SVMNT and CVIET PfCRT types described above. One of these features is the response of parasites to the chemo-sensitizer VP (verapamil), which reduces the CQ IC50 levels of CQ-resistant, but not CQ-sensitive, parasites [56]. This effect of VP chemo-sensitization varies depending on the PfCRT mutant type: CVIET parasites are more readily chemo-sensitized by VP than SVMNT parasites [57]. The mechanism that underlies this difference is not yet understood, but structural differences encoded by polymorphisms in the first transmembrane segment of PfCRT, including a particular association of PfCRT Asn75 with reduced VP chemosensitization of CQ-resistant P. falciparum [55], are probably involved.
The SVMNT and CVIET forms of PfCRT may confer different fitness costs to P. falciparum parasites. After decades of CQ pressure and almost complete selection of resistant CVIET parasites in certain areas of Africa and South-East Asia, drug-sensitive parasites began to return a few years after CQ use was discontinued and other antimalarials such as sulfadoxine/pyrimethamine and ACTs were provided instead [58–60]. In contrast, a return of CQ-sensitive parasites has not been reported from areas where the haplotype SVMNT is prevalent; for example, in Brazil, where SVMNT predominates and drugs other than CQ and AQ have been recommended for many years, only a single sample with the wild-type CQ-sensitive CVMNK sequence was identified by a recent study [61]. Although additional studies are needed to define the fitness costs of the CVIET and SVMNT mutations in drug-resistant parasites, these observations suggest that SVMNT parasites may have a fitness advantage relative to CQ- resistant CVIET parasites in the face of CQ-sensitive CVMNK parasites without drug pressure.
SVMNT P. falciparum parasites have only recently been found in Africa, initially by studies in Tanzania, where the prevalence of this haplotype increased from 0% in 2003 to 19% in 2004 [62]. In the light of increased use of AQ in Tanzania from 2001 to 2004, a role for AQ in the selection of parasites with this haplotype was suggested; when further analysis in the same regions of Tanzania failed to find parasites with this haplotype in 2006 and 2007, the authors suggested that the quick removal of AQ from treatment guidelines after 2006 was involved in the selection of parasites expressing the SVMNT haplotype [63]. The SVMNT haplotype has also been reported from samples collected in Angola in 2007, where the treatment guidelines included AQ in combination with artesunate [64]. Despite the suggestion that this haplotype could have been brought by frequent travellers from Brazil, independent selection of these parasites by AQ pressure is a possibility that cannot be excluded. Interestingly, an ‘intermediate’ SVIET haplotype reported from Papua [65] was also found in the Democratic Republic of Congo [66]. The expansion of SVMNT parasites in Africa presents a direct threat to the efficacy of AQ-containing combination therapies and emphasizes the need for replacement of the AQ component with more effective partner drugs as soon as possible.
When mutant PfCRT is present as the determinant of resistance to CQ and AQ, additional genes are then able to modulate the drug response in P. falciparum [67]. These include the pfmdr1 gene, which encodes a multiple-drug-resistant transporter [Pgh-1 (P-glycoprotein homologue-1)] with twelve transmembrane segments and two nucleotide-binding domains at the digestive vacuole membrane of the parasite [68]. Copy number variation and point mutations at codon positions 86, 184, 1034, 1042 and 1246 (Table 1) have been associated in many cases with greater or lesser levels of resistance in CQ- and AQ-resistant parasites [68–70]. Findings of linkage disequilibrium between pfmdr1 and pfcrt also suggest an interaction between these genes [71]. Analysis of the 7G8×GB4 genetic cross showed that specific allele combinations of pfcrt and pfmdr1 can interact to yield different levels of CQ and MDAQ response [55]. This effect was particularly apparent in recombinant progeny that had inherited the pfcrt allele from the South American clone 7G8; 7G8×GB4 progeny carrying this 7G8 pfcrt allele and the pfmdr1 allele from the African GB4 parent exhibited unusually low in vitro responses to CQ (IC50 values ~50 nM). Comparably low CQ responses were reported from a recent P. falciparum transfection study in which the 7G8 version of pfcrt was introduced by allelic exchange into different CQ-sensitive clones [72]. In that report, the CQR phenotype was strain dependent, and the atypically low CQR was especially clear in a line expressing 7G8 pfcrt and a D10 pfmdr1 type from Papua New Guinea. The features of pfmdr1 as a member of the gene family encoding ABC (ATP-binding cassette) transporters [73] suggest that changes in this protein can affect the responses to a number of structurally unrelated drugs. This is consistent with observations regarding pfmdr1 polymorphisms and variation of copy number associated with responses to a variety of compounds other than CQ and AQ [74–77].
ART tolerance: harbinger of resistance?
Reduced clearance rates of P. falciparum from individuals treated with ART-derived drugs have raised concerns of emerging ART resistance at the Thai–Cambodia border. Median parasite clearance times of 84 h are now prevalent in Palin, Cambodia, in contrast with shorter clearance times that have been documented elsewhere, e.g. 48 h in Wang Pha, Thailand [78,79]. Although the mechanisms responsible for delayed parasite drug clearance are unresolved, recent studies have shown that the delayed clearance phenotype is heritable and that a substantial proportion of the variation in clearance is determined by parasite genetic factors [80,81]. A major research challenge is to identify the genetic determinants that underlie delayed parasite clearance.
Neither patient age nor drug pharmacokinetics have correlated with the delayed parasite clearances in Cambodia, nor have IC50 results from in vitro drug sensitivity tests or proposed molecular markers of drug resistance such as pfmdr1, pfserca, pfcrt, pfatpase6 and ubp-1 [82]. Of these markers, pfmdr1 attracted high initial interest as copy number variations of this gene could be associated with parasite susceptibility to ART in vitro [76,77]. However, pfmdr1 copy number was only weakly correlated with in vivo clearance phenotypes [79]. Additional candidate loci and changes of gene expression that may be associated with ART responses are under further evaluation [83,84].
A possible mechanism by which P. falciparum parasites survive ART exposure may be the entry of a ring-stage subpopulation into a developmentally arrested or ‘dormant’-like state [85]. Alterations in the expression of heat-shock proteins, a cell-cycle regulator and a DNA biosynthesis protein have been reported to occur in ART-tolerant parasites in such a state [84]. ‘Dormant’ parasites (schizont stage) are also reported to occur with atovaquone/proguanil drug combination treatments in vitro, suggesting that developmental arrest may offer a more general defence mechanism of parasites against drugs and other challenges to their survival involving oxidative stress [1].
Careful monitoring of emerging signs of ART resistance requires keen attention to clinical outcomes. The development of new in vitro drug-testing methods and the identification of genetic markers linked to ineffective clearance are needed to support this surveillance. Functional studies of candidate loci along with alternative approaches to the genetics of the ART responses of P. falciparum parasites should improve our understanding of delayed clearances and the threat of ART resistance.
Drug-resistant P. vivax
Although a number of biological and clinical characteristics of P. vivax and P. falciparum differ, many antimalarials that treat asexual blood stage infections have been used with success against both of these parasites [16]. A distinct feature of P. vivax infection, shared with Plasmodium ovale and some other primate malaria parasites, imposes an extra level of effort to achieve complete (radical) cure: the presence of hypnozoites as a latent reservoir of infection in the hepatocytes of the liver [17,86]. After transmission from mosquito bites, only some of the sporozoites that infect hepatocytes undergo immediate tissue schizogony to release merozoites – others become ‘dormant’ hypnozoites which, months to years later, can become active and emerge to cause relapses of malaria after the elimination of blood stage parasites. Different relapse intervals are thought have evolved in the P. vivax strains of tropical and non-tropical regions in association with different seasonal mosquito feeding behaviours [16,87]. Longer relapse intervals are typical of temperate areas where mosquito transmission peaks are severely curtailed during cold seasons; shorter relapse intervals are typical of tropical areas, where mosquito transmission extends through longer periods of the year. Malaria relapses from hypnozoites are a major challenge in the prevention and control of P. vivax infections. In addition to the difficulties for successful treatment of dormant hypnozoites (PQ, the one major drug available for this purpose, is not an easy therapy at the dose schedules required), the lack of biomarkers for hypnozoite detection and the incidence of multiclonal infections often make it difficult to distinguish parasites originating from liver relapse, re-infection by mosquito transmission or recrudescence from incompletely eliminated blood parasites.
Estimates of the global burden of P. vivax vary from 70–390 million cases/year across tropical and temperate regions of the globe [16]. For decades, prevention and treatment of vivax malaria have relied on CQ against asexual blood stages followed by PQ for elimination of liver stages [16]. Unfortunately, failures of CQ+PQ treatment have been reported since 1989 from Indonesia, Myanmar, India and South America [16,88]. In the absence of satisfactory methods for in vitro cultivation for drug response testing or of molecular genetic markers of resistance, Baird et al. [89] developed a 28 day in vivo test for CQR based upon a minimally effective concentration of drug in the blood. In this test, blood concentrations of CQ and its major metabolite MDCQ (monodesethylchloroquine) are determined on the day of recurrent parasitaemia; if an asexual blood stage parasite is detected with CQ+MDCQ concentrations above 100 ng/ml, the P. vivax infection is considered resistant.
The CQR phenotype of P. vivax has been validated in Aotus and Saimiri monkeys under drug treatment, without the confounding factors of re-infection or liver relapses [90–93]. Although the use of these models has provided the means to study some aspects of P. vivax biology in the laboratory, molecular investigations of the drug resistance mechanism have been limited by the lack of satisfactory methods for in vitro parasite cultivation and genetic investigation in the laboratory. A P. vivax orthologue gene of pfcrt, pvcrt-o, was identified and sequenced from the genomic DNA of CQ-sensitive and CQ-resistant isolates, but no mutations were found to be associated with resistance [94]. This result was confirmed in studies that either found a variety of pvcrt-o polymorphisms not associated with CQR [95] or showed fixation of ‘wild-type’ pvcrt-o regardless of drug response [96,97]. In another study, transgenic expression of pvcrt-o was shown to reduce the CQ response of P. falciparum lines, and expression of pvcrt-o with or without a genetically engineered mutation equivalent to pfcrt K76T was able to diminish CQ accumulation in Dictyostelium discoideum [98]. A suggestion that P. vivax CQR is associated with severe disease in Papua New Guinea [99] led to the comparison of pvcrt-o and pvmdr1 transcript levels from one patient with uncomplicated malaria and another with severe clinical manifestations [100]. The authors found increased transcription levels of both genes, especially pvcrt-o, in the case of severe disease. Studies focused on pvmdr1 have not consistently found any clear association between mutations or copy number variation and CQR [96,101–106].
PQ not only acts on liver stage parasites, it is also effective against P. falciparum gametocytes and sporozoites, and has activity against P. vivax blood stages [15–17]. Its antimalarial activity, including its ability to eliminate hypnozoites, has been observed to be enhanced by the presence of partner drugs, including QN and CQ [16]. However, the value of PQ as an antimalarial is compromised by a frequent lack of patient compliance to the course of therapy (typically 14 days) required to clear hypnozoites [15] and by the risk of life-threatening haemolysis in individuals with G6PDH (glucose-6-phosphate dehydrogenase) deficiency [107].
In regions of South-East Asia, South America and the South Pacific, increased dosages of PQ are now often required to clear P. vivax hypnozoites [16]. The term ‘PQ-tolerant’ has been used to define such hypnozoites, which in some examples have required increased doses or prolonged treatments (over 28 days) to eliminate relapses [108]. In the light of this threat to PQ and the continuing public health burden from P. vivax infections, efforts to understand the action of PQ, to understand drug-induced haemolysis in G6PDH deficiency and to discover new therapeutic alternatives warrant high priority in malaria research [109].
Perspectives
Two of the most successful and long-lasting remedies against malaria are derived from natural products of the plant kingdom: the cinchona tree and the qinghao plant. It is striking that both of these compounds, QN and ART, with their different modes of action and particular activities on the blood stages of Plasmodium parasites, are present in abundance in these plants. Little to nothing is known of the natural function of these compounds in the plants, of the protections they might provide against pathogens in the bark and leaves from which they are extracted, or of the selective pressures of evolution that have brought these compounds to prominence in cinchona and qinghao. Their centuries-long efficacy as treatments for malaria, despite heavy use (and abuse) of both remedies on a global scale adds to their mystery. Although evidence has emerged that P. falciparum strains with increased tolerance are present in areas where resistance to other antimalarials is common (e.g. in South-East Asia and the Amazon for QN, and in Cambodia–Thailand for ART), full-blown resistance to QN or ART has not developed and all parasite strains remain treatable today with therapies based on these medicines. With the use of QN and ART in combination therapies and increased efforts to guard against inappropriate use of these drugs, QN and ART derivatives should remain valuable and powerful antimalarial drugs for decades to come.
Searches for synthetic substitutes of QN and, more recently, of ART derivatives, have led to discoveries of remarkable impact and potential. In some cases, disappointment did eventually follow, although other instances of new and highly promising drugs provide hope. CQ and AQ are two examples of synthetic QN substitutes that have succumbed to high levels of drug resistance. Curiously, these failures have occurred while QN still works, even though CQ, AQ and QN all contain the quinoline-ring moiety. An explanation of this observation may lie in the ability of mutant forms of PfCRT to act less efficiently on the complex substituent group of QN than on the simpler substituent groups of CQ and AQ (Figure 1). In light of this explanation, the observation that piperaquine and pyronaridine have remained effective in the face of existing PfCRT-based resistance suggests that these and perhaps other quinoline-containing drugs may hold activity against CQ-resistant malaria parasites and may not readily succumb to a ‘class effect’ mechanism of resistance.
The structure of ART and an understanding of the role of its superoxide moiety have likewise inspired searches for synthetic analogues that are inexpensive, reliable and effective against P. falciparum parasites. Among various chemical series, the OZ compounds have received the greatest attention and are now in advanced clinical trials. It is still too early to know if OZ compounds will be as susceptible as ART to tolerance by P. falciparum, or even if high levels of resistance to these compounds will develop in ART-tolerant parasites. The answer to these considerations will depend upon the mechanism of tolerance to ART and whether another drug-resistance mechanism comes into play against OZ compounds. If P. falciparum can muster no more against OZ than against ART, e.g. brief refuge of ring stages into a developmentally arrested state, OZ compounds with extended half-lives of activity may offer great advantages over ART in the treatment of drug-tolerant strains of P. falciparum.
In the present chapter, we have only briefly discussed the value and limitations of PQ for the elimination of gametocytes in P. falciparum infections and for the elimination of liver hypnozoite stages in P. vivax infections. Likewise, we have not addressed mechanisms of action or resistance involving other common antimalarial drugs, including synthetic compounds such as DHFR (dihydrofolate reductase) inhibitors, quinoline methanols (mefloquine) and sulfa drugs, and compounds from other natural products, such as the tetracyclines and macrolide antibiotics. Various limitations of these drugs in the treatment of severe malaria, such as slow action (e.g. tetracyclines), ready propensity to resistance (e.g. DHFR point mutations) or side effects (e.g. haemolysis in G6PDH-deficient individuals), emphasize the importance of discovery programmes for new medicines against malaria parasites with evolving forms of resistance against existing antimalarial drugs. Recent high-throughput screens of P. falciparum clones with large chemical libraries have identified thousands of compounds with antimalarial activity (<7 µM, in vitro), diverse chemical structures, low cytotoxicity in mammalian cell lines and in vivo antimalarial activity in mouse models of malaria [75,110,111]. Among potential targets, some of these compounds may have activity against P. falciparum protein kinases and proteases that do not contain homologues in the host and therefore may facilitate the discovery of potential drug candidates that avoid effects on the host signalling systems. In the motivation and hope behind these screens, the success stories of QN, ART and their derivative drugs provide tremendous inspiration. The discovery of new classes of compounds with similar antimalarial potential will further enable our efforts to control and eliminate malaria.
Summary.
Malaria drug resistance spread with devastating public health impact in the 20th Century.
Artemisinin from the ancient qinghao plant and quinine from the cinchona tree remain effective against drug-resistant malaria, but these remedies are threatened by increasing tolerance in Plasmodium falciparum parasites
Reduced P. falciparum clearance rates at the Thailand–Cambodia border raise serious concerns of emerging resistance to ACTs
Attempts to synthesize quinine influenced the synthetic dye industry and the emerging field of organic chemistry, which subsequently contributed key synthetic antimalarial drugs including PQ, ATB, AQ and CQ
CQ was a first-line antimalarial in the 20th Century, but eventually it failed against resistant P. falciparum in most endemic areas; AQ is used as a partner drug in ACTs, although it is also compromised by resistance
A better understanding of the actions of antimalarial drugs and mechanisms of drug resistance will lead to more effective therapeutic combinations as well as improved molecular assays to detect and track drug-resistant parasites
Recent high-throughput cell-based screens of large chemical libraries have identified thousands of diverse compounds with antimalarial activity and low cytotoxicity to mammalian cell lines, providing exciting prospects for the discovery and development of novel antimalarial drugs
Acknowledgments
We thank Olivia Twu and Ajay Pilai for helping with figure preparation; Erika Phelps, Michael Krause, Sarah Kaslow and Gloria Tavera for comments on the manuscript; and Julia Knoeckel for help with the translation of an important cited article. This work was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID).
References
- 1.Peters W. Chemotherapy and Drug Resistance in Malaria. 2nd edn. London: Harcourt Brace Jovanich; 1987. [Google Scholar]
- 2.Hsu E. The history of qing hao in the Chinese materia medica. Trans. R. Soc. Trop. Med. Hyg. 2006;100:505–508. doi: 10.1016/j.trstmh.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Cui L, Su XZ. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev. Anti. Infect. Ther. 2009;7:999–1013. doi: 10.1586/eri.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu E. Diverse biologies and experiential continuities: did the ancient Chinese know that qinghao had anti-malarial properties? Can. Bull. Med. Hist. 2009;26:203–213. doi: 10.3138/cbmh.26.1.203. [DOI] [PubMed] [Google Scholar]
- 5.Liang X-T, Fang W-S. Medicinal Chemistry of Bioactive Natural Products. Hoboken: Wiley InterScience; 2006. [Google Scholar]
- 6.Honigsbaum M. The Fever Trail – The Hunt for the Cure for Malaria. London: Pan Macmillan; 2001. [Google Scholar]
- 7.Greenwood D. The quinine connection. J. Antimicrob. Chemother. 1992;30:417–427. doi: 10.1093/jac/30.4.417. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RE, Poynter FN. Robert Talbor, Charles Li, and Cinchona – a contemporary document. Med. Hist. 1962;6:82–85. doi: 10.1017/s0025727300026892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amici RR. The history of Italian parasitology. Vet. Parasltol. 2001;98:3–30. doi: 10.1016/s0304-4017(01)00420-4. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. The Botanical Aspect of the Quinine Question. League of Nations/Health Organization/Malaria Commission, CH./Malaria/16.1. 1924 http://www.who.int/library/collections/historical/en/index4.html.
- 11.Guttman L, Ehrlich P. Ueber die Wirkung des Methylenblau bei Malaria. Berliner Klinische Wochenschrift. 1891;39:953–956. [Google Scholar]
- 12.Ferdig MT, Cooper RA, Mu J, Deng B, Joy DA, Su XZ, Wellems TE. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol. Microbiol. 2004;52:985–997. doi: 10.1111/j.1365-2958.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- 13.Nkrumah LJ, Riegelhaupt PM, Moura P, Johnson DJ, Patel J, Hayton K, Ferdig MT, Wellems TE, Akabas MH, Fidock DA. Probing the multifactorial basis of Plasmodium falciparum quinine resistance: evidence for a strain-specific contribution of the sodium-proton exchanger PfNHE. Mol. Biochem. Parasitol. 2009;165:122–131. doi: 10.1016/j.molbiopara.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manson-Bahr P. The action of plasmochin on malaria. Proc. R. Soc. Med. 1927;20:919–926. doi: 10.1177/003591572702000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin. Infect. Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 16.Baird JK. Resistance to therapies for infection by Plasmodium vivax. Clin. Microbiol. Rev. 2009;22:508–534. doi: 10.1128/CMR.00008-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells TN, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 2010;26:145–151. doi: 10.1016/j.pt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Berliner RW, Blanchard KC, Butler TC, Clark WM, Marshall EK, Schmidt LH, Shannon JA. In: A Survey of Antimalarial Drugs, 1941–1945. Wiselogle FY, editor. vol. II. Ann Arbor: J.W. Edwards; 1946. pp. 31–1607. [Google Scholar]
- 19.Sweeney AW. The possibility of an ‘X’ factor. The first documented drug resistance of human malaria. Int. J. Parasitol. 1996;26:1035–1061. [PubMed] [Google Scholar]
- 20.Jensen M, Mehlhorn H. Seventy-five years of Resochin in the fight against malaria. Parasitol. Res. 2009;105:609–627. doi: 10.1007/s00436-009-1524-8. [DOI] [PubMed] [Google Scholar]
- 21.Burckhalter JH, Tendick FH, Jones EM, Jones PA, Holcomb WF, Rawlins AL. Aminoalkylphenols as antimalarials. II. (Heterocyclic-amino)-α-amino-o-cresols. The synthesis of camoquin. J. Am. Chem. Soc. 1948;70:1363–1373. doi: 10.1021/ja01184a023. [DOI] [PubMed] [Google Scholar]
- 22.Churchill FC, Patchen LC, Campbell CC, Schwartz IK, Nguyen-Dinh P, Dickinson CM. Amodiaquine as a prodrug: importance of metabolite(s) in the antimalarial effect of amodiaquine in humans. Life Sci. 1985;36:53–62. doi: 10.1016/0024-3205(85)90285-1. [DOI] [PubMed] [Google Scholar]
- 23.D’Alessandro U. Progress in the development of piperaquine combinations for the treatment of malaria. Curr. Opin. Infect. Dis. 2009;22:588–592. doi: 10.1097/QCO.0b013e328332674a. [DOI] [PubMed] [Google Scholar]
- 24.Briolant S, Henry M, Oeuvray C, Amalvict R, Baret E, Didillon E, Rogier C, Pradines B. Absence of association between piperaquine in vitro responses and polymorphisms in the genes pfcrt, pfmdr 1, pfmrp and pfnhe in Plasmodium falciparum. Antimicrob. Agents Chemother. 2010;54:3537–3544. doi: 10.1128/AAC.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang C, Lin-Hua T, Jantanavivat C. Studies on a new antimalarial compound: pyronaridine. Trans. R. Soc. Trop. Med. Hyg. 1992;86:7–10. doi: 10.1016/0035-9203(92)90414-8. [DOI] [PubMed] [Google Scholar]
- 26.Tshefu AK, Gaye O, Kayentao K, Thompson R, Bhatt KM, Sesay SS, Bustos DG, Tjitra E, Bedu-Addo G, Borghini-Fuhrer I, et al. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised non-inferiority trial. Lancet. 2010;375:1457–1467. doi: 10.1016/S0140-6736(10)60322-4. [DOI] [PubMed] [Google Scholar]
- 27.Hartwig CL, Rosenthal AS, D’Angelo J, Griffin CE, Posner GH, Cooper RA. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem. Pharmacol. 2009;77:322–336. doi: 10.1016/j.bcp.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 29.Vivas L, Rattray L, Stewart LB, Robinson BL, Fugmann B, Haynes RK, Peters W, Croft SL. Antimalarial efficacy and drug interactions of the novel semi-synthetic endoperoxide artemisone in vitro and in vivo. J. Antimicrob. Chemother. 2007;59:658–665. doi: 10.1093/jac/dkl563. [DOI] [PubMed] [Google Scholar]
- 30.Dong Y, Wittlin S, Sriraghavan K, Chollet J, Charman SA, Charman WN, Scheurer C, Urwyler H, Santo Tomas J, Snyder C, et al. The structure–activity relationship of the antimalarial ozonide arterolane (OZ277) J. Med. Chem. 2009;53:481–491. doi: 10.1021/jm901473s. [DOI] [PubMed] [Google Scholar]
- 31.Vennerstrom JL, Arbe-Barnes S, Brun R, Charman SA, Chiu FC, Chollet J, Dong Y, Dorn A, Hunziker D, Matile H. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430:900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- 32.Eastman RT, Fidock DA. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat. Rev. Microbiol. 2009;7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodie BB, Udenfriend S. The estimation of atebrine in biological fluids and tissues. J. Biol. Chem. 1943;151:299–317. [Google Scholar]
- 34.World Health Organization. Guidelines for the Treatment of Malaria. 2006 http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html.
- 35.Goldsmith K. A controlled field trial of SN 7618-5 (chloroquine) for the suppression of malaria. J. Malar. Inst. India. 1946;6:311–316. [PubMed] [Google Scholar]
- 36.Boldt TH, Goodwine CH. A second year’s field trial with chloroquine suppression of high endemic malaria in a Panamanian village. J. Natl. Malar. Soc. 1949;8:238–246. [PubMed] [Google Scholar]
- 37.Watson RB, Paul JH, Chow LP, P’Eng RY. Field trial of chloroquine (SN-7618-5) for malaria control in central Taiwan (Formosa) Indian. J. Malariol. 1950;4:301–315. [PubMed] [Google Scholar]
- 38.World Health Organization. Summary review of the literature on Camoquin, World Health Organization Expert Committee on Malaria. WHO/MAL/38. 1950 http://whqlibdoc.who.int/malaria/WHO_Mal_38.pdf.
- 39.Patel JC, Dalal SD. Treatment of malaria with a single dose of amodiaquin (Camoquin) Indian J. Malariol. 1954;8:71–76. [PubMed] [Google Scholar]
- 40.Rodrigues DC. Cases of malaria caused by Plasmodium falciparum resistant to treatment with chloroquine. Arq. Hig. Saude Publica. 1961;26:231–235. [PubMed] [Google Scholar]
- 41.Moore DV, Lanier JE. Observations on two Plasmodium falciparum infections with an abnormal response to chloroquine. Am. J. Trop. Med. Hyg. 1961;10:5–9. doi: 10.4269/ajtmh.1961.10.5. [DOI] [PubMed] [Google Scholar]
- 42.Young MD, Moore DV. Chloroquine resistance in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1961;10:317–320. doi: 10.4269/ajtmh.1961.10.317. [DOI] [PubMed] [Google Scholar]
- 43.Young MD, Contacos PG, Stitcher JE, Millar JW. Drug resistance in Plasmodium Falciparum from Thailand. Am. J. Trop. Med. Hyg. 1963;12:305–314. doi: 10.4269/ajtmh.1963.12.305. [DOI] [PubMed] [Google Scholar]
- 44.Young MD. Failure of chloroquine and amodiaquine to suppress Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1962;56:252–256. doi: 10.1016/0035-9203(62)90164-5. [DOI] [PubMed] [Google Scholar]
- 45.Powell RD, Brewer GJ, Alving AS. Studies on a strain of chloroquine-resistant Plasmodium falciparum from Colombia, South America. Am. J. Trop. Med. Hyg. 1963;12:509–512. doi: 10.4269/ajtmh.1963.12.509. [DOI] [PubMed] [Google Scholar]
- 46.Wellems TE, Plowe CV. Chloroquine-resistant malaria. J. Infect. Dis. 2001;184:770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 47.Fitch CD. Mode of action of antimalarial drugs. Ciba Found. Symp. 1983;94:222–232. [PubMed] [Google Scholar]
- 48.Goldberg DE, Slater AF, Cerami A, Henderson GB. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: an ordered process in a unique organelle. Proc. Natl. Acad. Sci. U.S.A. 1990;87:2931–2935. doi: 10.1073/pnas.87.8.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slater AF, Cerami A. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992;355:167–169. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- 50.Krogstad DJ, Gluzman IY, Kyle DE, Oduola AM, Martin SK, Milhous WK, Schlesinger PH. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987;238:1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- 51.Wellems TE, Panton LJ, Gluzman IY, do Rosario VE, Gwadz RW, Walker-Jonah A, Krogstad DJ. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990;345:253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- 52.Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naude B, Deitsch KW, et al. Mutations in the P falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin RE, Kirk K. The malaria parasite’s chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol. Biol. Evol. 2004;21:1938–1949. doi: 10.1093/molbev/msh205. [DOI] [PubMed] [Google Scholar]
- 54.Sidhu AB, Verdier-Pinard D, Fidock DA. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science. 2002;298:210–213. doi: 10.1126/science.1074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sa JM, Twu O, Hayton K, Reyes S, Fay MP, Ringwald P, Wellems TE. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc. Natl. Acad. Sci. U.S.A. 2009;106:18883–18889. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin SK, Oduola AM, Milhous WK. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 57.Mehlotra RK, Fujioka H, Roepe PD, Janneh O, Ursos LM, Jacobs-Lorena V, McNamara DT, Bockarie MJ, Kazura JW, Kyle DE, et al. Evolution of a unique Plasmodium falciparum chloroquine-resistance phenotype in association with pfcrt polymorphism in Papua New Guinea and South America. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12689–12694. doi: 10.1073/pnas.221440898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, Djimde AA, Kouriba B, Taylor TE, Plowe CV. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J. Infect. Dis. 2003;187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Mu J, Li G, Chen P, Guo X, Fu L, Chen L, Su X, Wellems TE. Decreased prevalence of the Plasmodium falciparum chloroquine resistance transporter 76T marker associated with cessation of chloroquine use against P. falciparum malaria in Hainan, People’s Republic of China. Am. J. Trop. Med. Hyg. 2005;72:410–414. [PubMed] [Google Scholar]
- 60.Mwai L, Ochong E, Abdirahman A, Kiara SM, Ward S, Kokwaro G, Sasi P, Marsh K, Borrmann S, Mackinnon M, et al. Chloroquine resistance before and after its withdrawal in Kenya. Malar. J. 2009;8:106. doi: 10.1186/1475-2875-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gama BE, de Oliveira NK, Zalis MG, de Souza JM, Santos F, Daniel-Ribeiro CT, Ferreira-da-Cruz Mde F. Chloroquine and sulphadoxine-pyrimethamine sensitivity of Plasmodium falciparum parasites in a Brazilian endemic area. Malar. J. 2009;8:156. doi: 10.1186/1475-2875-8-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alifrangis M, Dalgaard MB, Lusingu JP, Vestergaard LS, Staalsoe T, Jensen AT, Enevold A, Ronn AM, Khalil IF, Warhurst DC, et al. Occurrence of the Southeast Asian/South American SVMNT haplotype of the chloroquine-resistance transporter gene in Plasmodium falciparum in Tanzania. J. Infect. Dis. 2006;193:1738–1741. doi: 10.1086/504269. [DOI] [PubMed] [Google Scholar]
- 63.Alifrangis M, Lusingu JP, Mmbando B, Dalgaard MB, Vestergaard LS, Ishengoma D, Khalil IF, Theander TG, Lemnge MM, Bygbjerg IC. Five-year surveillance of molecular markers of Plasmodium falciparum antimalarial drug resistance in Korogwe District, Tanzania: accumulation of the 581G mutation in the P. falciparum dihydropteroate synthase gene. Am. J. Trop. Med. Hyg. 2009;80:523–527. [PubMed] [Google Scholar]
- 64.Gama BE, Pereira de Carvalho GA, Lutucuta Kosi FJ, Almeida de Oliveira NK, Fortes F, Rosenthal PJ, Daniel Ribeiro CT, Ferreira da Cruz MD. Plasmodium falciparum isolates from Angola show the StctVMNT haplotype in the pfcrt gene. Malar. J. 2010;9:174. doi: 10.1186/1475-2875-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagesha HS, Casey GJ, Rieckmann KH, Fryauff DJ, Laksana BS, Reeder JC, Maguire JD, Baird JK. New haplotypes of the Plasmodium falciparum chloroquine resistance transporter (pfcrt) gene among chloroquine-resistant parasite isolates. Am. J. Trop. Med. Hyg. 2003;68:398–402. [PubMed] [Google Scholar]
- 66.Severini C, Menegon M, Sannella AR, Paglia MG, Narciso P, Matteelli A, Gulletta M, Caramello P, Canta F, Xayavong MV, et al. Prevalence of pfcr point mutations and level of chloroquine resistance in Plasmodium falciparum isolates from Africa. Infect. Genet. Evol. 2006;6:262–268. doi: 10.1016/j.meegid.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 67.Mu J, Ferdig MT, Feng X, Joy DA, Duan J, Furuya T, Subramanian G, Aravind L, Cooper RA, Wootton JC, et al. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol. Microbiol. 2003;49:977–989. doi: 10.1046/j.1365-2958.2003.03627.x. [DOI] [PubMed] [Google Scholar]
- 68.Foote SJ, Kyle DE, Martin RK, Oduola AM, Forsyth K, Kemp DJ, Cowman AF. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature. 1990;345:255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- 69.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 70.Sidhu AB, Valderramos SG, Fidock DA. pfmdr 1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 2005;57:913–926. doi: 10.1111/j.1365-2958.2005.04729.x. [DOI] [PubMed] [Google Scholar]
- 71.Mehlotra RK, Mattera G, Bockarie MJ, Maguire JD, Baird JK, Sharma YD, Alifrangis M, Dorsey G, Rosenthal PJ, Fryauff DJ, et al. Discordant patterns of genetic variation at two chloroquine resistance loci in worldwide populations of the malaria parasite Plasmodium falciparum. Antimicrob. Agents Chemother. 2008;52:2212–2222. doi: 10.1128/AAC.00089-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valderramos SG, Valderramos JC, Musset L, Purcell LA, Mercereau-Puijalon O, Legrand E, Fidock DA. Identification of a mutant PfCRT-mediated chloroquine tolerance phenotype in Plasmodium falciparum. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000887. e1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peel SA. The ABC transporter genes of Plasmodium falciparum and drug resistance. Drug Resist. Updat. 2001;4:66–74. doi: 10.1054/drup.2001.0183. [DOI] [PubMed] [Google Scholar]
- 74.Wilson CM, Volkman SK, Thaithong S, Martin RK, Kyle DE, Milhous WK, Wirth DF. Amplification of pfmdr 1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from Thailand. Mol. Biochem. Parasitol. 1993;57:151–160. doi: 10.1016/0166-6851(93)90252-s. [DOI] [PubMed] [Google Scholar]
- 75.Yuan J, Johnson RL, Huang R, Wichterman J, Jiang H, Hayton K, Fidock DA, Wellems TE, Inglese J, Austin CP, et al. Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum. Nat. Chem. Biol. 2009;5:765–771. doi: 10.1038/nchembio.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chavchich M, Gerena L, Peters J, Chen N, Cheng Q, Kyle DE. Role of pfmdr 1 amplification and expression in induction of resistance to artemisinin derivatives in Plasmodium falciparum. Antimicrob. Agents Chemother. 2010;54:2455–2464. doi: 10.1128/AAC.00947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen N, Chavchich M, Peters JM, Kyle DE, Gatton ML, Cheng Q. De-amplification of pfmdr 1-containing amplicon on chromosome 5 in Plasmodium falciparum is associated with reduced resistance to artelinic acid in vitro. Antimicrob. Agents Chemother. 2010;54:3395–3401. doi: 10.1128/AAC.01421-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 79.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson TJ, Nair S, Nkhoma S, Williams JT, Imwong M, Yi P, Socheat D, Das D, Chotivanich K, Day NP, et al. High heritability of malaria parasite clearance rate indicates a genetic basis for artemisinin resistance in western Cambodia. J. Infect. Dis. 2010;201:1326–1330. doi: 10.1086/651562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson TJ, Williams JT, Nair S, Sudimack D, Barends M, Jaidee A, Price RN, Nosten F. Inferred relatedness and heritability in malaria parasites. Proc. Biol. Sci. 2010;277:2531–2540. doi: 10.1098/rspb.2010.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Imwong M, Dondorp AM, Nosten F, Yi P, Mungthin M, Hanchana S, Das D, Phyo AP, Lwin KM, Pukrittayakamee S, et al. Exploring the contribution of candidate genes to artemisinin resistance in Plasmodium falciparum. Antimicrob. Agents Chemother. 2010;54:2886–2892. doi: 10.1128/AAC.00032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mu J, Myers RA, Jiang H, Liu S, Ricklefs S, Waisberg M, Chotivanich K, Wilairatana P, Krudsood S, White NJ, et al. Plasmodium falciparum genome-wide scans for positive selection, recombination hot spots and resistance to antimalarial drugs. Nat. Genet. 2010;42:268–271. doi: 10.1038/ng.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Witkowski B, Lelievre J, Barragan MJ, Laurent V, Su XZ, Berry A, Benoit-Vical F. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob. Agents Chemother. 2010;54:1872–1877. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Teuscher F, Gatton ML, Chen N, Peters J, Kyle DE, Cheng Q. Artemisinin-induced dormancy in Plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J. Infect. Dis. 2010;202:1362–1368. doi: 10.1086/656476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krotoski WA, Collins WE, Bray RS, Garnham PC, Cogswell FB, Gwadz RW, Killick-Kendrick R, Wolf R, Sinden R, Koontz LC, et al. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am. J. Trop. Med. Hyg. 1982;31:1291–1293. doi: 10.4269/ajtmh.1982.31.1291. [DOI] [PubMed] [Google Scholar]
- 87.Contacos PG, Collins WE, Jeffery GM, Krotoski WA, Howard WA. Studies on the characterization of Plasmodium vivax strains from Central America. Am. J. Trop. Med. Hyg. 1972;21:707–712. doi: 10.4269/ajtmh.1972.21.707. [DOI] [PubMed] [Google Scholar]
- 88.Baird JK. Chloroquine resistance in Plasmodium vivax. Antimicrob. Agents Chemother. 2004;48:4075–4083. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baird JK, Leksana B, Masbar S, Fryauff DJ, Sutanihardja MA, Suradi, Wignall FS, Hoffman SL. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 1997;56:621–626. doi: 10.4269/ajtmh.1997.56.621. [DOI] [PubMed] [Google Scholar]
- 90.Cooper RD, Rieckmann KH. Efficacy of amodiaquine against a chloroquine-resistant strain of Plasmodium vivax. Trans. R. Soc. Trop. Med. Hyg. 1990;84:473. doi: 10.1016/0035-9203(90)90003-w. [DOI] [PubMed] [Google Scholar]
- 91.Collins WE, Schwartz IK, Skinner JC, Morris C, Filipski VK. The susceptibility of the Indonesian I/CDC strain of Plasmodium vivax to chloroquine. J. Parasitol. 1992;78:344–349. [PubMed] [Google Scholar]
- 92.Cooper RD. Studies of a chloroquine-resistant strain of Plasmodium vivax from Papua New Guinea in Aotus and Anopheles farauti s.l. J. Parasitol. 1994;80:789–795. [PubMed] [Google Scholar]
- 93.Collins WE, Sullivan JS, Fryauff DJ, Kendall J, Jennings V, Galland GG, Morris CL. Adaptation of a chloroquine-resistant strain of Plasmodium vivax from Indonesia to New World monkeys. Am. J. Trop. Med. Hyg. 2000;62:491–495. doi: 10.4269/ajtmh.2000.62.491. [DOI] [PubMed] [Google Scholar]
- 94.Nomura T, Carlton JM, Baird JK, del Portillo HA, Fryauff DJ, Rathore D, Fidock DA, Su X, Collins WE, McCutchan TF, et al. Evidence for different mechanisms of chloroquine resistance in 2 Plasmodium species that cause human malaria. J. Infect. Dis. 2001;183:1653–1661. doi: 10.1086/320707. [DOI] [PubMed] [Google Scholar]
- 95.Suwanarusk R, Russell B, Chavchich M, Chalfein F, Kenangalem E, Kosaisavee V, Prasetyorini B, Piera KA, Barends M, Brockman A, et al. Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS ONE. 2007;2:e1089. doi: 10.1371/journal.pone.0001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barnadas C, Ratsimbasoa A, Tichit M, Bouchier C, Jahevitra M, Picot S, Menard D. Plasmodium vivax resistance to chloroquine in Madagascar: clinical efficacy and polymorphisms in pvmdr 1 and pvcrt-o genes. Antimicrob. Agents Chemother. 2008;52:4233–4240. doi: 10.1128/AAC.00578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Orjuela-Sanchez P, Karunaweera ND, da Silva-Nunes M, da Silva NS, Scopel KK, Goncalves RM, Amaratunga C, Sa JM, Socheat D, Fairhust RM, et al. Single-nucleotide polymorphism, linkage disequilibrium and geographic structure in the malaria parasite Plasmodium vivax: prospects for genome-wide association studies. BMC Genet. 2010;11:65. doi: 10.1186/1471-2156-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sa JM, Yamamoto MM, Fernandez-Becerra C, de Azevedo MF, Papakrivos J, Naude B, Wellems TE, Del Portillo HA. Expression and function of pvcrt-o, a Plasmodium vivax ortholog of pfcrt, in Plasmodium falciparum and Dictyostelium discoideum. Mol. Biochem. Parasitol. 2006;150:219–228. doi: 10.1016/j.molbiopara.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 99.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;5:e128. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fernandez-Becerra C, Pinazo MJ, Gonzalez A, Alonso PL, del Portillo HA, Gascon J. Increased expression levels of the pvcrt-o and pvmdr 1 genes in a patient with severe Plasmodium vivax malaria. Malar. J. 2009;8:55. doi: 10.1186/1475-2875-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sa JM, Nomura T, Neves J, Baird JK, Wellems TE, del Portillo HA. Plasmodium vivax: allele variants of the mdr1 gene do not associate with chloroquine resistance among isolates from Brazil, Papua, and monkey-adapted strains. Exp. Parasitol. 2005;109:256–259. doi: 10.1016/j.exppara.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 102.Brega S, Meslin B, de Monbrison F, Severini C, Gradoni L, Udomsangpetch R, Sutanto I, Peyron F, Picot S. Identification of the Plasmodium vivax mdr-like gene (pvmdr 1) and analysis of single-nucleotide polymorphisms among isolates from different areas of endemicity. J. Infect. Dis. 2005;191:272–277. doi: 10.1086/426830. [DOI] [PubMed] [Google Scholar]
- 103.Marfurt J, de Monbrison F, Brega S, Barbollat L, Muller I, Sie A, Goroti M, Reeder JC, Beck HP, Picot S, et al. Molecular markers of in vivo Plasmodium vivax resistance to amodiaquine plus sulfadoxine-pyrimethamine: mutations in pvdhfr and pvmdr 1. J. Infect. Dis. 2008;198:409–417. doi: 10.1086/589882. [DOI] [PubMed] [Google Scholar]
- 104.Imwong M, Pukrittayakamee S, Pongtavornpinyo W, Nakeesathit S, Nair S, Newton P, Nosten F, Anderson TJ, Dondorp A, Day NP, et al. Gene amplification of the multidrug resistance 1 gene of Plasmodium vivax isolates from Thailand, Laos, and Myanmar. Antimicrob. Agents Chemother. 2008;52:2657–2659. doi: 10.1128/AAC.01459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gama BE, Oliveira NK, Souza JM, Daniel-Ribeiro CT, Ferreira-da-Cruz Mde F. Characterisation of pvmdr 1 and pvdhfr genes associated with chemoresistance in Brazilian Plasmodium vivax isolates. Mem. Inst. Oswaldo Cruz. 2009;104:1009–1011. doi: 10.1590/s0074-02762009000700012. [DOI] [PubMed] [Google Scholar]
- 106.Orjuela-Sanchez P, de Santana Filho FS, Machado-Lima A, Chehuan YF, Costa MR, Alecrim MG, del Portillo HA. Analysis of single-nucleotide polymorphisms in the crt-o and mdr 1 genes of Plasmodium vivax among chloroquine-resistant isolates from the Brazilian Amazon region. Antimicrob. Agents Chemother. 2009;53:3561–3564. doi: 10.1128/AAC.00004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alving AS, Carson PE, Flanagan CL, Ickes CE. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science. 1956;124:484–485. doi: 10.1126/science.124.3220.484-a. [DOI] [PubMed] [Google Scholar]
- 108.Nayar JK, Baker RH, Knight JW, Sullivan JS, Morris CL, Richardson BB, Galland GG, Collins WE. Studies on a primaquine-tolerant strain of Plasmodium vivax from Brazil in Aotus and Saimiri monkeys. J. Parasitol. 1997;83:739–745. [PubMed] [Google Scholar]
- 109.Baird JK, Rieckmann KH. Can primaquine therapy for vivax malaria be improved? Trends Parasitol. 2003;19:115–120. doi: 10.1016/s1471-4922(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 110.Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, et al. Chemical genetics of Plasmodium falciparum. Nature. 2010;465:311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, et al. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 112.Johnson DJ, Fidock DA, Mungthin M, Lakshmanan V, Sidhu AB, Bray PG, Ward SA. Evidence for a central role for PfCRT in conferring Plasmodium falciparum resistance to diverse antimalarial agents. Mol. Cell. 2004;15:867–877. doi: 10.1016/j.molcel.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cooper RA, Lane KD, Deng B, Mu J, Patel JJ, Wellems TE, Su X, Ferdig MT. Mutations in transmembrane domains 1, 4 and 9 of the Plasmodium falciparum chloroquine resistance transporter alter susceptibility to chloroquine, quinine and quinidine. Mol. Microbiol. 2007;64:1139. doi: 10.1111/j.1365-2958.2006.05511.x. [DOI] [PubMed] [Google Scholar]
- 114. Reference deleted. [Google Scholar]
- 115.Thwing JI, Odero CO, Odhiambo FO, Otieno KO, Kariuki S, Ord R, Roper C, McMorrow M, Vulule J, Slutsker L, et al. In-vivo efficacy of amodiaquine-artesunate in children with uncomplicated Plasmodium falciparum malaria in western Kenya. Trop. Med. Int. Health. 2009;14:294–300. doi: 10.1111/j.1365-3156.2009.02222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Griffing S, Syphard L, Sridaran S, McCollum AM, Mixson-Hayden T, Vinayak S, Villegas L, Barnwell JW, Escalante AA, Udhayakumar V. pfmdr 1 amplification and fixation of pfcrt chloroquine resistance alleles in Plasmodium falciparum in Venezuela. Antimicrob. Agents Chemother. 2010;54:1572–1579. doi: 10.1128/AAC.01243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gadalla NB, Elzaki SE, Mukhtar E, Warhurst DC, El-Sayed B, Sutherland CJ. Dynamics of pfcrt alleles CVMNK and CVIET in chloroquine-treated Sudanese patients infected with Plasmodium falciparum. Malar. J. 2010;9:74. doi: 10.1186/1475-2875-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beshir K, Sutherland CJ, Merinopoulos I, Durrani N, Leslie T, Rowland M, Hallett RL. Amodiaquine resistance in Plasmodium falciparum malaria is associated with the pfcrt 72–76 SVMNT allele in Afghanistan. Antimicrob. Agents Chemother. 2010;54:3714–3716. doi: 10.1128/AAC.00358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bharti PK, Alam MT, Boxer R, Shukla MM, Gautam SP, Sharma YD, Singh N. Therapeutic efficacy of chloroquine and sequence variation in pfcrt gene among patients with falciparum malaria in central India. Trop. Med. Int. Health. 2010;15:33–40. doi: 10.1111/j.1365-3156.2009.02425.x. [DOI] [PubMed] [Google Scholar]
- 120.Cooper RA, Ferdig MT, Su XZ, Ursos LM, Mu J, Nomura T, Fujioka H, Fidock DA, Roepe PD, Wellems TE. Alternative mutations at position 76 of the vacuolar transmembrane protein PfCRT are associated with chloroquine resistance and unique stereospecific quinine and quinidine responses in Plasmodium falciparum. Mol. Pharmacol. 2002;61:35–42. doi: 10.1124/mol.61.1.35. [DOI] [PubMed] [Google Scholar]
- 121.Chen N, Kyle DE, Pasay C, Fowler EV, Baker J, Peters JM, Cheng Q. pfcrt allelic types with two novel amino acid mutations in chloroquine-resistant Plasmodium falciparum isolates from the Philippines. Antimicrob. Agents Chemother. 2003;47:3500–3505. doi: 10.1128/AAC.47.11.3500-3505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valderramos SG, Fidock DA. Transporters involved in resistance to antimalarial drugs. Trends Pharmacol. Sci. 2006;27:594–601. doi: 10.1016/j.tips.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]