Abstract
Obesity has been associated with abnormally high expression of the enzyme aromatase in the breast, increased local estrogen production, and predisposition to breast hyperplasia and cancer. Increased adiposity in postmenopausal women may trigger signaling pathways that induce aromatase expression. In breast adipose fibroblasts, increased TNF production may induce the distal aromatase promoter, whereas increased local PGE2 production may induce the proximal promoter region. We review here the mechanisms that control aromatase gene expression in breast adipose tissue, and the paracrine interactions between malignant breast epithelial cells and the surrounding adipose fibroblasts. Systematic characterization of these signaling pathways will facilitate the identification of potential drug targets to selectively reduce aromatase expression and excessive estrogen production, with therapeutic benefit.
Relationship between obesity and estrogen
Approximately two-thirds of adult US women have excess body fat: 36% are obese with a body mass index (BMI) of more than 30, whereas another one-third are overweight (BMI: 25–29) [1,2]. Overweight or obese postmenopausal women exhibit a threefold increased risk for developing breast cancer compared with normal-weight postmenopausal women [3–5]. Based on estimates by the American Cancer Society in 2002, more than 60 000 annual new cases of breast cancer are linked to obesity and increased adiposity in the US. That obesity is a risk factor for breast cancer has been recognized since the 1960s [6]; however, the molecular mechanisms that link obesity to breast cancer are unknown. It has been suggested that products of the subcutaneous adipose tissue in the breast and other peripheral sites may be responsible for the increased breast cancer risk associated with obesity. Studies have shown that estrogen, leptin or other adipose tissue-derived molecules may promote tumor development or growth [7–9]. In the case of estrogen, it might be possible that the hormone triggers development of malignancy and its growth via the induction of several key genes including progesterone receptor (PR), adenosine A1 receptor (ADORA1) and wingless-type MMTV integration-site family, member 11 (WNT11), in neighboring epithelial cells – all of which have been associated with tumor devel-opment or growth [10–16].
In the breast, benign or malignant epithelial cells lie in contact with endothelial cell-lined capillaries, undifferentiated adipose fibroblasts that are also known as preadipocytes, and lipid-filled mature adipocytes [17]. Aromatase, a member of the cytochrome P450 superfamily, is the enzyme responsible for key steps in the synthesis of estrogens [9]. Aromatase is expressed in a several tissues including undifferentiated adipose fibroblasts and breast tumors, but is not expressed in mature adipocytes [9,18]. A greatly increased mass of breast adipose tissue in obese women may locally increase estrogen production within the breast simply because of a higher number of aroma-tase-expressing fibroblasts [9]. In addition to this mass effect, aromatase expression per unit adipose tissue or cell may also increase with weight gain.
Estrogen, a product of the aromatase enzyme in adipose tissue, has long been suspected as the hormone responsible for increasing breast cancer risk in obese postmenopausal women. In fact, the most effective hormonal treatment of postmenopausal breast cancer has been the use of aroma-tase inhibitors that block aromatase activity in the breast and the periphery, thereby reducing the amount of local estrogen production – which in turn helps to suppress recurrence of the breast tumor tissue [19]. A key and unresolved question has been the relative contributions of breast adipose tissue, versus subcutaneous adipose tissue at other body sites, to the formation of estrogen that contributes to increased breast cancer risk and growth. Epidemiologic studies suggested that mildly increased venous blood estrogen levels might account for a portion of the link between obesity and breast cancer incidence [20]. However, the recently performed randomized Women’s Health Initiative study, demonstrating a possible reduction in breast cancer risk in postmenopausal women that were administered estrogen-only hormone replacement, has challenged the role of mildly elevated circulating estrogen levels in breast cancer risk [15,16].
Work from several laboratories over the past two dec-ades has suggested that breast adipose tissue fibroblasts are a crucial site for aromatase expression and estrogen production, and has linked them to the development of breast cancer [17,21,22]. By the same token, the malignant epithelial cells in an existing breast tumor are in direct contact with the surrounding adipose tissue, which is the major supplier of estrogen to cancer [23,24]. It is thus tempting to hypothesize that obesity may be associated with abnormally high expression of aromatase in breast adipose tissue fibroblasts, resulting in elevated levels of local estrogen in the breast and predisposition to breast hyperplasia and cancer. It is plausible that obesity may trigger signaling pathways that induce aromatase expres-sion. In fact, obesity is known to robustly increase adipose tissue levels of tumor necrosis factor (TNF), a known inducer of aromatase expression in adipose fibroblasts[25,26]. Obesity may also increase other local hormones such as prostaglandin E2 (PGE2) that are also known to induce aromatase gene expression in adipose tissue of the breast and elsewhere [26,27]. Aromatase in breast adipose tissue (versus adipose tissue at other body sites) might have a substantially higher impact on carcinogenesis be-cause of its proximity to breast epithelial cells. Therefore, the characterization of signaling pathways, which are activated in obese women and contribute to increased aromatase expression and activity and local estrogen ex-cess in the breast, will facilitate the identification of poten-tial drug targets for reducing the risk of breast cancer. Here we review the mechanisms that control aromatase expression in breast adipose tissue and the paracrine interactions between malignant breast epithelial cells and the surrounding adipose fibroblasts.
Mechanisms of aromatase overexpression in estrogen-responsive breast cancer
A single gene encodes aromatase, the key enzyme for estrogen biosynthesis, the inhibition of which effectively eliminates estrogen production. Today, aromatase inhibitors are the most effective endocrine treatments for estro-xsgen-responsive breast cancer [28]. Several head-to-head randomized clinical trials published since 2000 have dem-onstrated the superiority of aromatase inhibitors to the estradiol antagonist tamoxifen in the treatment of breast cancer [19,29–35]. Thus, inhibiting estrogen formation is therapeutically more efficacious than blocking its action. The ovaries, testes, adipose tissue, skin, the hypothalamus and placenta, all express aromatase, physiologically [36]. By contrast, breast adipose tissue bearing a tumor over-expresses aromatase, leading to local overproduction of estrogen that exerts paracrine and intracrine tumorigenic effects [36]. Although similar promoter regions of aroma-tase may be activated in normal and pathological tissues, the cellular and transcriptional regulatory mechanisms are extremely diverse and cell-specific [9,37]. This has led to a detailed investigation of the mechanisms underly-ing aromatase overexpression in each tissue in an effort to define specific molecular targets for developing new thera-peutics [9,38].
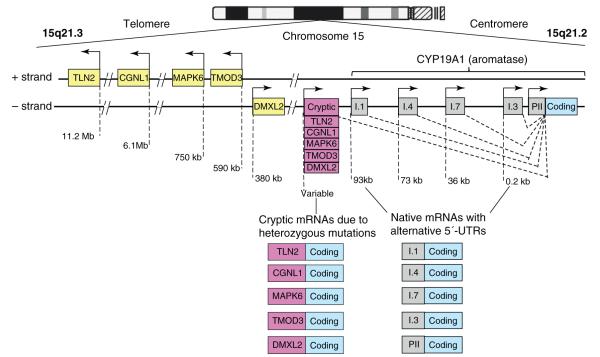
Alternatively used promoters, distributed over a 93 kb regulatory region upstream of a common coding region, control aromatase expression differentially in gonads, adi-pose tissue, bone, brain, skin, fetal liver and placenta [39]. Thus far, ten alternative promoters have been found in humans, including I.1, I.2 in placenta, I.4 in adipose tissue and skin, I.5 in fetal tissues, I.f in brain, I.7 in endothelial cells, I.6 in bone, I.3 in adipose tissue and PII in gonads (Figure 1) [9]. A distinct set of transcription factors reg-ulates each promoter in a signaling pathway- and tissue-specific manner.
Figure 1.
Structure of the CYP19A1 gene. The human CYP19A1 (aromatase) gene is transcribed in the direction from the telomere towards the centromere of chromosome 15 and contains approximately 10 alternatively used native promoters that regulate its expression in a partially tissue-specific fashion. Activation of each promoter transcribes on mRNA species that contains a specific 5′-untranslated region (5′-UTR), which serves as the signature of that particular promoter. Five other genes (TLN2, CGNL1, MAPK6, TMOD3 and DMXL2) clustered in tandem order lie next to aromatase at its telomeric aspect. Heterozygous inversions or deletions change the direction of these genes’ promoters and their 5′-UTRs and move them upstream of the aromatase gene. These cryptic promoters then inappropriately overexpress aromatase in multiple human tissues and cause estrogen excess. The most common manifestation is the feminine growth of breast tissue in young boys (prepubertal gynecomastia) (see [40] for further details).
Follicle-stimulating hormone (FSH) induces the most proximal promoter PII via a cAMP-dependent pathway involving the recruitment of steroidogenic factor-1 and β-catenin in human ovarian granulosa cells [41]. As will be detailed later, PGE2 via cAMP coordinately induces theproximal cluster of promoters PII and I.3, which lie within an approximate 0.2 kb sequence, via recruitment of CCAAT/enhancer-binding protein-β (C/EBPβ), JunD and liver receptor homolog-1 (LRH-1), in human breast adipose fibroblasts [9,42]. By contrast, promoter I.4 that lies some 73 kb upstream of the coding region is induced by gluco-corticoids and the cytokines interleukin -6 or -11, and TNF. This leads to the activation of the c-Jun N-terminal kinase (JNK) activating kinase-1 (Jak1) and recruitment of signal transducer and activator of transcription-3 (STAT3), to-gether with glucocorticoid receptor (GR), in human adipose fibroblasts [9]. In cancer-free breast tissue, adipose fibro-blasts express low levels of aromatase via promoter I.4, whereas the promoter I.3/II region, which is occupied by transcriptional repressors, remains quiescent [43,44].
Distinct cellular and molecular mechanisms are respon-sible for aromatase expression in breast cancer versus disease-free breast tissue [9]. First, cellular composition is altered in breast cancer such that aromatase-expressing undifferentiated adipose fibroblasts accumulate around malignant epithelial cells. Second, molecular alterations in adipose stromal cells favor binding of transcriptional enhancers versus inhibitors to the normally quiescent aromatase promoter I.3/II region. Work from several labo-ratories has suggested that these two mechanisms support local aromatase overexpression in breast cancer and ac-count for the majority of aromatase expression in breast cancer [24,45,46]. A third mechanism has been described and involves increased activity of distal promoter regions such as I.4 and I.7, and this also contributes to aromatase overexpression in breast tumors (Figure 1) [47,48]. Finally, heterozygous mutations, which cause the aromatase cod-ing region to lie adjacent to constitutively active cryptic promoters that normally transcribe other genes, may ac-count for aromatase overexpression in breast and other tissues and excessive estrogen formation (Figure 1) [49]. The clinical significance of this mechanism in cancer de-velopment is not yet known.
Estrogen formation in breast cancer
There are two sources of estrogen in postmenopausal breast cancer. First, estrogen may arise from aromatase activity in extraovarian body sites, such as subcutaneous adipose tissue and skin, and may reach breast cancer in an endocrine manner. Breast cancer incidence correlates pos-itively with body fat content and serum estrogen levels in postmenopausal women, suggesting that this endocrine mechanism stimulates tumor development [50,51]. Sec-ond, an increase in the local concentrations of estrogen may result from aromatase overexpression within the tumor tissue; this mechanism has been demonstrated by several laboratories [52–54]. These groups demonstrated significantly elevated levels of estrone, estrone sulfate and estradiol in breast tumor tissue compared with circulating levels [52–54]. Several groups consistently found increased aromatase enzyme activity and mRNA levels in breast fat adjacent to the cancer tissue compared with distal fat or disease-free breast adipose tissue [17,21,45,46,55–57]. In vivo evidence from a transgenic mouse model revealed that aromatase overexpression in breast tissue was sufficient for maintaining hyperplasia in the absence of circulating estrogen and that aromatase inhibitors abrogated hyper-plasia [58]. These mice developed mammary tumors more rapidly than their wild-type littermates [58].
The biologically active estrogen is estradiol. Aromatase catalyzes the conversion of androstenedione to estrone or testosterone to estradiol. Because the major circulating precursor steroid in postmenopausal women is androstene-dione, reductive enzymes are required for estradiol forma-tion. The enzymes 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1) and aldo–keto reductase family 1, member C3 (AKR1C3), which can convert estrone to estradiol and an-drostenedione to testosterone, respectively, are present in various cell types of malignant and benign breast tissues and complement aromatase activity for the formation of estradiol from circulating androstenedione (Figure 2)[58,59].
Figure 2.
Stromal–epithelial interactions for estrogen production in breast cancer. Breast cancer grows in an environment of adipose tissue. Adipose fibroblasts differentiate to mature adipocytes in the breast. Both cell types lie in close proximity to benign or malignant epithelial cells. The products of malignant and benign cells determine the key biological features of this micro-environment. For example, malignant cells and adipose fibroblasts secrete PGE2 that induces aromatase (CYP19A1) expression in undifferentiated adipose fibroblasts. Aromatase converts androstenedione to estrone, that is further converted to biologically active estradiol, by 17β- hydroxysteroid dehydrogenase type 1 (HSD17B1) in the same cell or in the malignant epithelial cell. Alternatively, the enzyme aldo–keto reductase family 1, member C3 (AKR1C3), which is expressed in myofibroblasts of breast tissue, converts androstenedione to testosterone that may be readily aromatized to estradiol. Thus, PGE2 induces estradiol production directly or indirectly via regulating aromatase enzyme activity in adipose fibroblasts. Once an adipose fibroblast is differentiated into a mature adipocyte it loses its capacity to express aromatase and form estradiol. Malignant breast epithelial cells secrete antiadipogenic cytokines such as TNF and IL-11 to inhibit this differentiation. It appears that malignant epithelial cells maintain neighboring adipose cells in an undifferentiated state to maximize paracrine estradiol biosynthesis.
Cellular localization of aromatase in breast cancer
Approximately 90% of aromatase enzyme activity and mRNA in breast adipose tissue is found in undifferentiated fibroblasts rather than mature adipocytes [60] (Figure 2). Immunoreactive aromatase has been localized to both malignant epithelial cells and surrounding fibroblasts in breast tumor tissues [61–63]. The biologic relevance of immunoreactive aromatase detected by different antibo-dies, however, remained debatable [64]. Markedly high levels of aromatase enzyme activity and gene expression via activation of the promoter I.3/II have been consistently detected in breast adipose tissue or fibroblasts, freshly isolated from breast tissue, with or without cancer[24,60,65]. Aromatase enzyme activity in primary malig-nant breast epithelial cells or cell lines, by contrast, was either undetectable or extremely low [66] (Figure 2).
The dense layer of undifferentiated adipose fibroblasts (desmoplastic reaction) surrounding malignant epithelial cells is essential for the structural and biochemical support of tumor growth [67]. Malignant epithelial cells secrete large quantities of the antiadipogenic cytokines TNF and IL-11, which inhibit the differentiation of fibroblasts to mature adipocytes primarily via suppression of the adipogenic tran-scription factors C/EBPα and peroxisome proliferator-acti-vated receptor-γ (PPARγ) (Figure 2). Thus, large numbers of estrogen-producing adipose fibroblasts are maintained proximal to malignant cells [43,68] (Figure 2).
Signaling pathways that regulate promoter region I.3/II in breast tumor fibroblasts
It has been demonstrated that the use of the proximal promoter I.3/II region accounts for the majority of in vivo aromatase expression in adipose tissue that is located adjacent to breast cancer, and in breast cancer tissue[24,45,46]. In vitro, treatment of undifferentiated adipose fibroblasts with cell-conditioned media derived from ma-lignant breast epithelia was found to induce aromatase expression [43]. This effect could be mimicked by PGE2, which is present in high concentrations in media that have been conditioned with malignant epithelial cells, but not in media conditioned with benign breast epithelial cells [43]. Different actions of PGE2 are mediated via specific G-protein-coupled cell-surface receptors, named E-prosta-noid (EP) receptors, subdivided into four types termed EP1 through EP4 [69]. PGE2 induction of aromatase occurs through the EP1 and EP2 receptor subtypes, which are linked to protein kinase C (PKC) and A (PKA) pathways[70,71]. In fact, this PGE2 effect could be reproduced using a combination of dibutyryl (Bt2) cAMP and phorbol diace-tate (PDA), which also stimulate PKA and PKC, respec-tively [27]. In summary, malignant breast epithelial cells secrete significant quantities of PGE2 that activates PKA and PKC pathways, which in turn induce aromatase ex-pression via the proximal I.3/II promoters in adjacent adipose fibroblasts (Figure 3).
Figure 3.
Signaling pathways for estrogen production and action. PGE2 that originates from malignant epithelial cells or adipose fibroblasts activates specific signaling mechanisms to induce aromatase expression in adipose fibroblasts. The best characterized and most potent components of the PGE2-dependent signaling pathway in the adipose fibroblast are PKA/PKC and p38/JNK. Some of the key transcription factors downstream of this pathway include C/EBPβ, JunD and JunB, and LRH-1 which bind to the tumorigenic aromatase promoter region I.3/II (see text). The key cis-regulatory elements, C/EBPβ-S and CRE and the promoters II and I.3, cluster within a sequence of less than 300 bp. LRH-1 binds to a nuclear receptor half-site located more proximal to promoter II (PII). It is very likely that these key elements regulate the activity of both promoters coordinately. The details of this complex regulation are not well understood. Estrogen produced as a consequence of aromatase activity acts on estrogen receptor-α (ERα) in neighboring epithelial cells to enhance carcinogenesis by transactivating specific genes. PKA, protein kinase A; PKC, protein kinase C; C/EBP, CCAAT/enhancer binding protein; C/EBPβ-S, C/EBPβ site; CRE, cAMP response element; PR, progesterone receptor, Wnt, wingless-type MMTV integration-site family; ADORA, adenosine A1 receptor.
To investigate potential mechanisms for blocking aro-matase activity and estrogen production in breast adipose fibroblasts surrounding malignant epithelial cells, the downstream effectors of the PGE2–cAMP–PKA/PKC path-way have been systemically investigated [27]. These efforts have helped to define the signaling components that con-nect the transcription factors bound to the tumorigenic promoter I.3/II region of the aromatase gene. Studies on adipose fibroblasts pointed to the involvement of twospecific terminal MAP kinases: JNK and p38 (Figure 3) [27]. Detailed dissection of this pathway indicated that PGE2 treatment of breast adipose fibroblasts activated both PKA and PKC and their downstream effectors, JNK and p38, which collectively were necessary for estro-gen production mediated via the I.3/II promoter region of the aromatase gene [27]. These studies have highlighted to role of these terminal MAP kinases in activating aroma-tase gene expression and have pointed to the possibility of using JNK and p38 as potential new drug targets for breast tissue-specific ablation of aromatase expression.
Transcriptional complexes that regulate the I.3/II promoter region in breast tumor fibroblasts
Promoters I.3 and II are located approximately 0.2 kb apart and share several cis-acting elements necessary for promot-er activation, including a nuclear receptor half-site, C/ EBPβ-binding sites, AP-1 binding sites, and cAMP-response elements (CREs) (Figure 3) [43,44]. A large number of transcription factors, which may potentially regulate this complex region, have been reported. Very few of these, however, were demonstrated to have a functional role in human adipose fibroblasts. In this review we focus on transcriptional enhancers that were characterized using in situ techniques such as chromatin immunoprecipitation PCR, or were shown to regulate aromatase mRNA or en-zyme activity using siRNA-based knockdown techniques in primary human adipose fibroblasts. These factors are limit-ed to C/EBPβ, CREB-binding protein (CBP), liver receptor homolog 1 (LRH-1), JunB and JunD [42,44,72–79].
Incubation of breast adipose fibroblasts with malignant epithelial cell-conditioned medium or with PGE2 was shown to lead to recruitment of a stimulatory transcrip-tional complex, comprising C/EBPβ, CBP, JunB, JunD and LRH-1, to the aromatase promoter I.3/II region [44,72–79] (Figure 3). Thus, in undifferentiated adipose fibroblasts in breast tumors, the proximally clustered aromatase promo-ters I.3/II are coordinately regulated by PKA- and PKC-dependent mechanisms (Figure 3). As mentioned above, these promoters are usually quiescent in fibroblasts of normal breast tissue due to the binding of a transcriptional inhibitory complex. In a malignant breast environment, however, the promoter region I.3/II is occupied by several transcriptional enhancers as a result of the activation of multiple signaling pathways that ultimately increase aro-matase expression in breast fibroblasts [43,44] (Figure 3).
The effect of PGE2 on the aromatase promoters is fairly complex. PGE2 has been shown both to activate the proxi-mal aromatase promoters I.3/II and to suppress the distal promoter I.4 activity, in a reciprocal fashion. The net result, however, is a robust increase in total aromatase mRNA levels and enzyme activity in adipose fibroblasts, indicating that PGE2 has a dominant stimulatory effect on the promoter I.3/II region [42]. Because JNK is a crucial MAP kinase that mediates PGE2-dependent aromatase expression in adipose fibroblasts, the roles of its target transcription factors c-Jun, JunB and JunD have recently been examined [42]. Among these, JunD was found to be the crucial transcription factor that mediated PGE2 induc-tion of aromatase, and this involved binding to a cAMP response element (CRE) at the promoter I.3/II region [42]. JunB supported this induction primarily via maintaining steady-state JunD levels. In this context, PGE2 treatment simultaneously suppressed the distal promoter I.4 via the recruitment of c-Jun and JunD which, in this case, para-doxically acted as transcriptional silencers [42]. This line of evidence suggests that targeting JunD may potentially achieve selective ablation of local aromatase expression, and hence estrogen formation in breast cancer, via inhibi-tion of the tumorigenic aromatase promoters I.3/II (Figure 3).
Targeting the aromatase promoter I.3/II region as a therapeutic strategy
Inhibiting the aromatase promoter I.3/II region, which is overactive in adipose fibroblasts associated with breast cancer, is an attractive therapeutic option because it avoids the side effects observed with the currently available nonselective aromatase inhibitors, which cause total estro-gen deprivation. The crucial question is whether ablating this promoter region would lower breast tissue estrogen levels sufficiently to produce a therapeutic effect. This question may be answered via the use of in vivo models in the future. Promoter I.3/II is induced by the common PKA/PKC pathway and may potentially be targeted using several strategies such as the use of PGE2 receptor block-ers, kinase inhibitors, or by knockdown technology such as RNA interference. PGE2 is only one of the hormones that stimulate this pathway, and two of its receptors, EP1 and EP2, may mediate its activity for aromatase induction, introducing redundancy into the system. Thus, suppres-sion of its production or action via targeting its synthetic enzymes or receptors may not be sufficient to achieve a therapeutic effect. The terminal MAP kinases which lie downstream of PKA/PKC, namely p38 and JNK, are other potential targets. JNK inhibition appears to provide near-complete ablation of aromatase activity in adipose fibro-blasts [27]. Several transcriptional enhancers that bind to the promoter I.3/II region were proposed as potential possible therapeutic targets. Very few of these factors, however, were clearly demonstrated to have functional roles. For example, knockdown of LRH-1 or JunD de-creased aromatase expression in human adipose fibro-blasts [42]. JunD knockdown almost completely abolished aromatase activity in primary adipose fibro-blasts, suggesting that targeting of JNK or JunD may produce the most profound aromatase inhibition in breast adipose tissue associated with cancer [27,42]. It should be noted that both JNK and JunD are widely expressed signaling effectors. Thus, their inhibition may cause sys-temic side effects, which may prohibit their widespread use in the treatment of breast cancer. It would be preferable to target the PKA/PKC-dependent transcriptional complex that is assembled at the aromatase promoter I.3/II in breast adipose fibroblasts as a more specific target.
Concluding remarks
Breast cancer is unique in that it develops and thrives in adipose tissue, which provides structural and paracrine support for the growing tumor. The undifferentiated adi-pose fibroblast is the primary cell type that is in contact with the malignant epithelial cell; these two cell typessynergize in favor of tumor growth. A key contribution of adipose fibroblasts to a breast tumor is estrogen produc-tion via aromatase expression [10]. In obese women, in-creased production of TNF may induce the distal aromatase promoter in increased numbers of adipose fibroblasts in the breast and other body sites. Indepen-dently, increased local PGE2 production as a function of obesity and aging may also induce the proximal promoter region of the aromatase gene in breast adipose fibroblasts. In vivo, the proximal promoter region is the primary regulator of aromatase expression in breast adipose tissue proximal to malignant epithelial cells. Identification of the key effectors of this signaling pathway that connects its exogenous stimulator PGE2 and the proximal aromatase promoter is crucial for developing novel and tissue-specific therapeutic strategies [27,42–44,80]. Targeting this path-way may reduce aromatase expression and excessive es-trogen production sufficiently to produce a clinical benefit. Selective inhibition of aromatase expression in the breast may offer a treatment modality with minimal systemic side effects, in contrast to whole-body estrogen depriva-tion that is associated with the currently available nonse-lective aromatase inhibitors.
Acknowledgments
Our work is supported by grants from the National Cancer Institute (CA67167), the Avon Foundation and the Lynn Sage Foundation.
References
- 1.Flegal KM, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Sutton-Tyrrell K, et al. Reproductive hormones and obesity: 9 years of observation from the Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2010;171:1203–1213. doi: 10.1093/aje/kwq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler RG, et al. Relative weight, weight change, height, and breast cancer risk in Asian–American women. J. Natl. Cancer Inst. 1996;88:650–660. doi: 10.1093/jnci/88.10.650. [DOI] [PubMed] [Google Scholar]
- 4.Gunter MJ, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morimoto LM, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States) Cancer Causes Control. 2002;13:741–751. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 6.De Waars F, et al. The biomodal age distribution of patients with mammary carcinoma: evidence for the existence of 2 types of human breast cancer. Cancer. 1964;17:141–151. doi: 10.1002/1097-0142(196402)17:2<141::aid-cncr2820170202>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez RR, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J. Biol. Chem. 2006;281:26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 8.Krause S, et al. The microenvironment determines the breast cancer cells’ phenotype: organization of MCF7 cells in 3D cultures. BMC Cancer. 2010;10:263. doi: 10.1186/1471-2407-10-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulun SE, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol. Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z, et al. Novel estrogen receptor-alpha binding sites and estradiol target genes identified by chromatin immunoprecipitation cloning in breast cancer. Cancer Res. 2007;67:5017–5024. doi: 10.1158/0008-5472.CAN-06-3696. [DOI] [PubMed] [Google Scholar]
- 11.Lin Z, et al. Adenosine A1 receptor, a target and regulator of estrogen receptoralpha action, mediates the proliferative effects of estradiol in breast cancer. Oncogene. 2010;29:1114–1122. doi: 10.1038/onc.2009.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chlebowski RT, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dressing GE, et al. Progesterone receptors act as sensors for mitogenic protein kinases in breast cancer models. Endocr. Relat. Cancer. 2009;16:351–361. doi: 10.1677/ERC-08-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaCroix AZ, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson GL, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 16.Aubuchon M, Santoro N. Lessons learned from the WHI: HRT requires a cautious and individualized approach. Geriatrics. 2004;59:22–26. [PubMed] [Google Scholar]
- 17.Bulun SE, et al. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome P450 transcripts using competitive polymerase chain reaction after reverse transcription. J. Clin. Endocrinol. Metab. 1993;77:1622–1628. doi: 10.1210/jcem.77.6.8117355. [DOI] [PubMed] [Google Scholar]
- 18.Cleland WH, et al. Aromatase activity of membrane fractions of human adipose tissue stromal cells and adipocytes. Endocrinology. 1983;113:2155–2160. doi: 10.1210/endo-113-6-2155. [DOI] [PubMed] [Google Scholar]
- 19.Baum M, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 20.Key TJ, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J. Natl. Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill JS, et al. Aromatase activity in adipose tissue from breast quadrants: a link with tumor site. Br. Med. J. 1988;296:741–743. doi: 10.1136/bmj.296.6624.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulun SE, et al. Distribution of aromatase P450 transcripts and adipose fibroblasts in the human breast. J. Clin. Endocrinol. Metab. 1996;81:1273–1277. doi: 10.1210/jcem.81.3.8772611. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal VR, et al. Alternatively spliced transcripts of the aromatase cytochrome P450 (CYP19) gene in adipose tissue of women. J. Clin. Endocrinol. Metab. 1997;82:70–74. doi: 10.1210/jcem.82.1.3655. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal VR, et al. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. J. Clin. Endocrinol. Metab. 1996;81:3843–3849. doi: 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, et al. Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter I.4. Mol. Endocrinol. 1996;10:1350–1357. doi: 10.1210/mend.10.11.8923461. [DOI] [PubMed] [Google Scholar]
- 27.Chen D, et al. Prostaglandin E2 induces breast cancer related aromatase promoters via activation of p38 and c-Jun NH2-terminal kinase in adipose fibroblasts. Cancer Res. 2007;67:8914–8922. doi: 10.1158/0008-5472.CAN-06-4751. [DOI] [PubMed] [Google Scholar]
- 28.Santen R. To block estrogen’s synthesis or action: that is the question. J. Clin. Endocrinol. Metab. 2002;87:3007–3012. doi: 10.1210/jcem.87.7.8589. [DOI] [PubMed] [Google Scholar]
- 29.Baum M, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98:1802–1810. doi: 10.1002/cncr.11745. [DOI] [PubMed] [Google Scholar]
- 30.Buzdar AU, et al. The impact of hormone receptor status on the clinical efficacy of the new-generation aromatase inhibitors: a review of data from first-line metastatic disease trials in postmenopausal women. Breast J. 2004;10:211–217. doi: 10.1111/j.1075-122X.2004.21320.x. [DOI] [PubMed] [Google Scholar]
- 31.Bonneterre J, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the tamoxifen or Arimidex randomized group efficacy and tolerability study. J. Clin. Oncol. 2000;18:3748–3757. doi: 10.1200/JCO.2000.18.22.3748. [DOI] [PubMed] [Google Scholar]
- 32.Goss PE, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 33.Mouridsen H, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J. Clin. Oncol. 2001;19:2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 34.Milla-Santos A, et al. Anastrozole versus tamoxifen as first-line therapy in postmenopausal patients with hormone-dependent advanced breast cancer: A prospective, randomized, phase III study. Am. J. Clin. Oncol. 2003;26:317–32235. doi: 10.1097/01.COC.0000047126.10522.F9. [DOI] [PubMed] [Google Scholar]
- 35.Paridaens R, et al. Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. Ann. Oncol. 2003;14:1391–1398. doi: 10.1093/annonc/mdg362. [DOI] [PubMed] [Google Scholar]
- 36.Simpson ER, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 37.Simpson ER. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 38.Simpson E, et al. Tissue-specific estrogen biosynthesis and metabolism. Ann. N. Y. Acad. Sci. 2001;949:58–67. doi: 10.1111/j.1749-6632.2001.tb04002.x. [DOI] [PubMed] [Google Scholar]
- 39.Jones ME, et al. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol. Metab. 2006;17:55–64. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Demura M, et al. Regional rearrangements in chromosome 15q21 cause formation of cryptic promoters for the CYP19 (aromatase) gene. Hum. Mol. Genet. 2007;16:2529–2541. doi: 10.1093/hmg/ddm145. [DOI] [PubMed] [Google Scholar]
- 41.Parakh TN, et al. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12435–12440. doi: 10.1073/pnas.0603006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen D, et al. JunD and JunB integrate prostaglandin E2 activation of breast cancer-associated proximal aromatase promoters. Mol. Endocrinol. 2011;25:767–775. doi: 10.1210/me.2010-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, et al. Malignant breast epithelial cells stimulate aromatase expression via promoter II in human adipose fibroblasts: an epithelial–stromal interaction in breast tumors mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2001;61:2328–2334. [PubMed] [Google Scholar]
- 44.Deb S, et al. A novel role of sodium butyrate in the regulation of cancer-associated aromatase promoters I.3 and II by disrupting a transcriptional complex in breast adipose fibroblasts. J. Biol. Chem. 2006;281:2585–2597. doi: 10.1074/jbc.M508498200. [DOI] [PubMed] [Google Scholar]
- 45.Utsumi T, et al. Presence of alternatively spliced transcripts of aromatase gene in human breast cancer. J. Clin. Endocrinol. Metab. 1996;81:2344–2349. doi: 10.1210/jcem.81.6.8964875. [DOI] [PubMed] [Google Scholar]
- 46.Zhou C, et al. Aromatase gene expression and its exon I usage in human breast tumors. Detection of aromatase messenger RNA by reverse transcription-polymerase chain reaction. J. Steroid Biochem. Mol. Biol. 1996;59:163–171. doi: 10.1016/s0960-0760(96)00100-8. [DOI] [PubMed] [Google Scholar]
- 47.Irahara N, et al. Quantitative analysis of aromatase mRNA expression derived from various promoters (I.4, I.3, PII and I.7) and its association with expression of TNF-alpha, IL-6 and COX-2 mRNAs in human breast cancer. Int. J. Cancer. 2006;118:1915–1921. doi: 10.1002/ijc.21562. [DOI] [PubMed] [Google Scholar]
- 48.Sebastian S, et al. Cloning and characterization of a novel endothelial promoter of the human CYP19 (aromatase P450) gene that is up-regulated in breast cancer tissue. Mol. Endocrinol. 2002;16:2243–2254. doi: 10.1210/me.2002-0123. [DOI] [PubMed] [Google Scholar]
- 49.Shozu M, et al. Estrogen excess associated with novel gain-of-function mutations affecting the aromatase gene. N. Engl. J. Med. 2003;348:1855–1865. doi: 10.1056/NEJMoa021559. [DOI] [PubMed] [Google Scholar]
- 50.Hankinson S, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 1999;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 51.Huang Z, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–1411. [PubMed] [Google Scholar]
- 52.Chetrite G, et al. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J. Steroid Biochem. Mol. Biol. 2000;72:23–27. doi: 10.1016/s0960-0760(00)00040-6. [DOI] [PubMed] [Google Scholar]
- 53.Geisler J, et al. A novel HPLC-RIA method for the simultaneous detection of estrone, estradiol and estrone sulphate levels in breast cancer tissue. J. Steroid Biochem. Mol. Biol. 2000;72:259–264. doi: 10.1016/s0960-0760(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 54.van Landeghem AA, et al. Endogenous concentration and subcellular distribution of androgens in normal and malignant human breast tissue. Cancer Res. 1985;45:2907–2912. [PubMed] [Google Scholar]
- 55.Sasano H, et al. Immunolocalization of aromatase and other steroidogenic enzymes in human breast disorders. Hum. Pathol. 1994;25:530–535. doi: 10.1016/0046-8177(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 56.Harada N, et al. Tissue-specific expression of the human aromatase cytochrome P450 gene by alternative use of multiple exons I and promoters, and switching of tissue-specific exons I in carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11312–11316. doi: 10.1073/pnas.90.23.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reed MJ, et al. Control of aromatase activity in breast cancer cells: the role of cytokines and growth factors. J. Steroid Biochem. Mol. Biol. 1993;44:589–596. doi: 10.1016/0960-0760(93)90264-w. [DOI] [PubMed] [Google Scholar]
- 58.Tekmal RR, et al. Aromatase overexpression and breast hyperplasia, an in vivo model – continued overexpression of aromatase is sufficient to maintain hyperplasia without circulating estrogens, and aromatase inhibitors abrogate these preneoplastic changes in mammary glands. Endocr. Relat. Cancer. 1999;6:307–314. doi: 10.1677/erc.0.0060307. [DOI] [PubMed] [Google Scholar]
- 59.Amin SA, et al. Paracrine-stimulated gene expression profile favors estradiol production in breast tumors. Mol. Cell. Endocrinol. 2006;253:44–55. doi: 10.1016/j.mce.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 60.Price T, et al. Determination of aromatase cytochrome P450 messenger ribonucleic acid in human breast tissue by competitive polymerase chain reaction amplification. J. Clin. Endocrinol. Metab. 1992;74:1247–1252. doi: 10.1210/jcem.74.6.1592866. [DOI] [PubMed] [Google Scholar]
- 61.Santen RJ, et al. Stromal spindle cells contain aromatase in human breast tumors. J. Clin. Endocrinol. Metab. 1994;79:627–632. doi: 10.1210/jcem.79.2.8045987. [DOI] [PubMed] [Google Scholar]
- 62.Sasano H, et al. Aromatase and 17beta-hydroxysteroid dehydrogenase type 1 in human breast carcinoma. J. Clin. Endocrinol. Metab. 1996;81:4042–4046. doi: 10.1210/jcem.81.11.8923858. [DOI] [PubMed] [Google Scholar]
- 63.Brodie A, et al. Aromatase expression in the human breast. Breast Cancer Res. Treat. 1998;49(Suppl. 1):S85–S91. doi: 10.1023/a:1006029612990. [DOI] [PubMed] [Google Scholar]
- 64.Sasano H, et al. Validation of new aromatase monoclonal antibodies for immunohistochemistry: progress report. J. Steroid Biochem. Mol. Biol. 2003;86:239–244. doi: 10.1016/s0960-0760(03)00363-7. [DOI] [PubMed] [Google Scholar]
- 65.Ackerman GE, et al. Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J. Clin. Endocrinol. Metab. 1981;53:412–417. doi: 10.1210/jcem-53-2-412. [DOI] [PubMed] [Google Scholar]
- 66.Pauley RJ, et al. Regulated CYP19 aromatase transcription in breast stromal fibroblasts. J. Clin. Endocrinol. Metab. 2000;85:837–846. doi: 10.1210/jcem.85.2.6345. [DOI] [PubMed] [Google Scholar]
- 67.Haagensen CD. Diseases of the Breast. W.B. Saunders Company; 1986. [Google Scholar]
- 68.Meng L, et al. TNFalpha and IL-11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively downregulating C/EBPalpha and PPARgamma: mechanism of desmoplastic reaction. Cancer Res. 2001;61:2250–2255. [PubMed] [Google Scholar]
- 69.Coleman RA, et al. VIII. International Union of Pharmacology. Classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 70.Zhao Y, et al. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- 71.Brueggemeier RW, et al. Aromatase and cyclooxygenases: enzymes in breast cancer. J. Steroid Biochem. Mol. Biol. 2003;86:501–507. doi: 10.1016/s0960-0760(03)00380-7. [DOI] [PubMed] [Google Scholar]
- 72.Clyne CD, et al. Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. J. Biol. Chem. 2002;277:20591–20597. doi: 10.1074/jbc.M201117200. [DOI] [PubMed] [Google Scholar]
- 73.Safi R, et al. Coactivation of liver receptor homologue-1 by peroxisome proliferator-activated receptor gamma coactivator-1alpha on aromatase promoter II and its inhibition by activated retinoid X receptor suggest a novel target for breast-specific antiestrogen therapy. Cancer Res. 2005;65:11762–11770. doi: 10.1158/0008-5472.CAN-05-2792. [DOI] [PubMed] [Google Scholar]
- 74.Lee YK, et al. Phosphorylation of the hinge domain of the orphan nuclear hormone receptor LRH-1 stimulates transactivation. J. Biol. Chem. 2006;281:7850–7855. doi: 10.1074/jbc.M509115200. [DOI] [PubMed] [Google Scholar]
- 75.Hinshelwood MM, et al. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol. Cell. Endocrinol. 2003;207:39–45. doi: 10.1016/s0303-7207(03)00257-0. [DOI] [PubMed] [Google Scholar]
- 76.Krylova IN, et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343–355. doi: 10.1016/j.cell.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 77.Sablin EP, et al. Structural basis for ligand-independent activation of the orphan nuclear receptor LRH-1. Mol. Cell. 2003;11:1575–1585. doi: 10.1016/s1097-2765(03)00236-3. [DOI] [PubMed] [Google Scholar]
- 78.Chand AL, et al. Tissue-specific regulation of aromatase promoter II by the orphan nuclear receptor LRH-1 in breast adipose stromal fibroblasts. Steroids. 2011;76:741–744. doi: 10.1016/j.steroids.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 79.Brown KA, et al. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 2009;69:5392–5399. doi: 10.1158/0008-5472.CAN-09-0108. [DOI] [PubMed] [Google Scholar]
- 80.Ghosh S, et al. IKKbeta mediates cell shape-induced aromatase expression and estrogen biosynthesis in adipose stromal cells. Mol. Endocrinol. 2009;23:662–670. doi: 10.1210/me.2008-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]