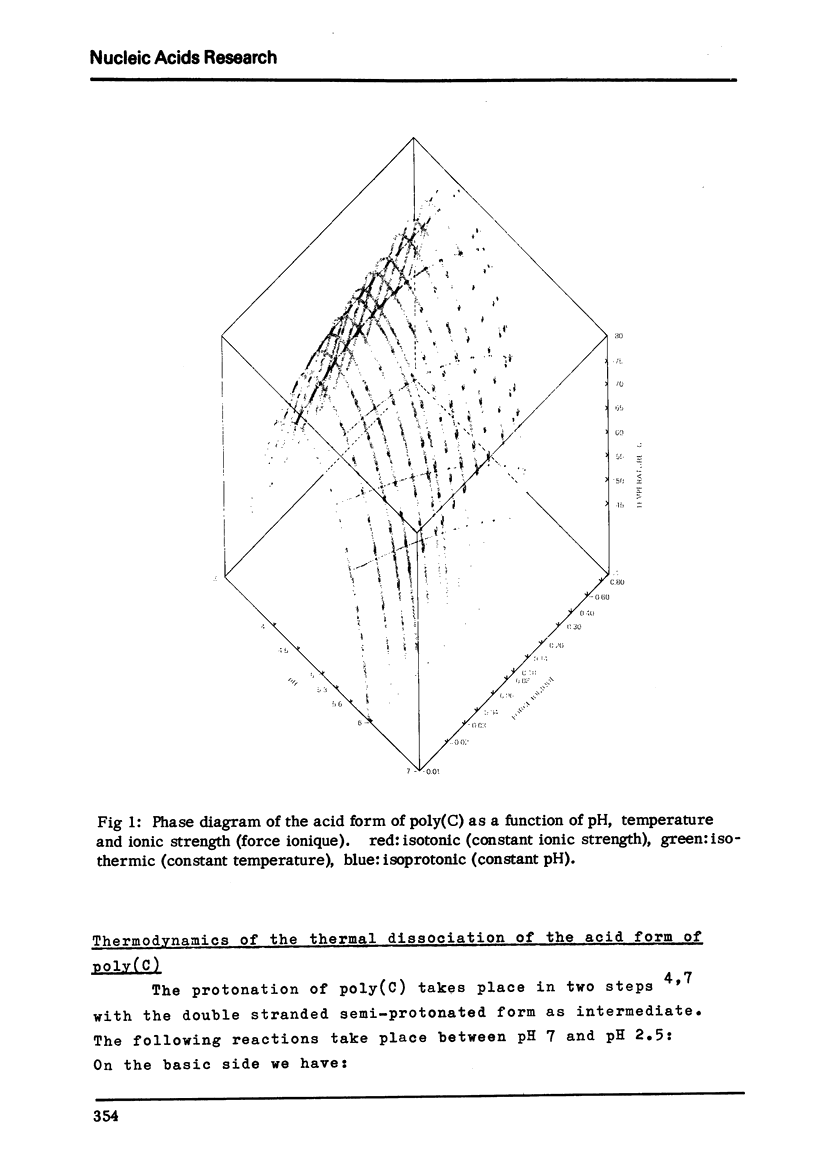
Abstract
A phase diagram (pH, ionic strength, temperature) for the double helical form of poly(C) is presented. The thermodynamic analysis of these data shows that poly(C) behaves essentially as cytidine, if the electrostatic (ionic strength) contributions and the free energy of double helix formation are considered and taken into account.
Full text
PDF

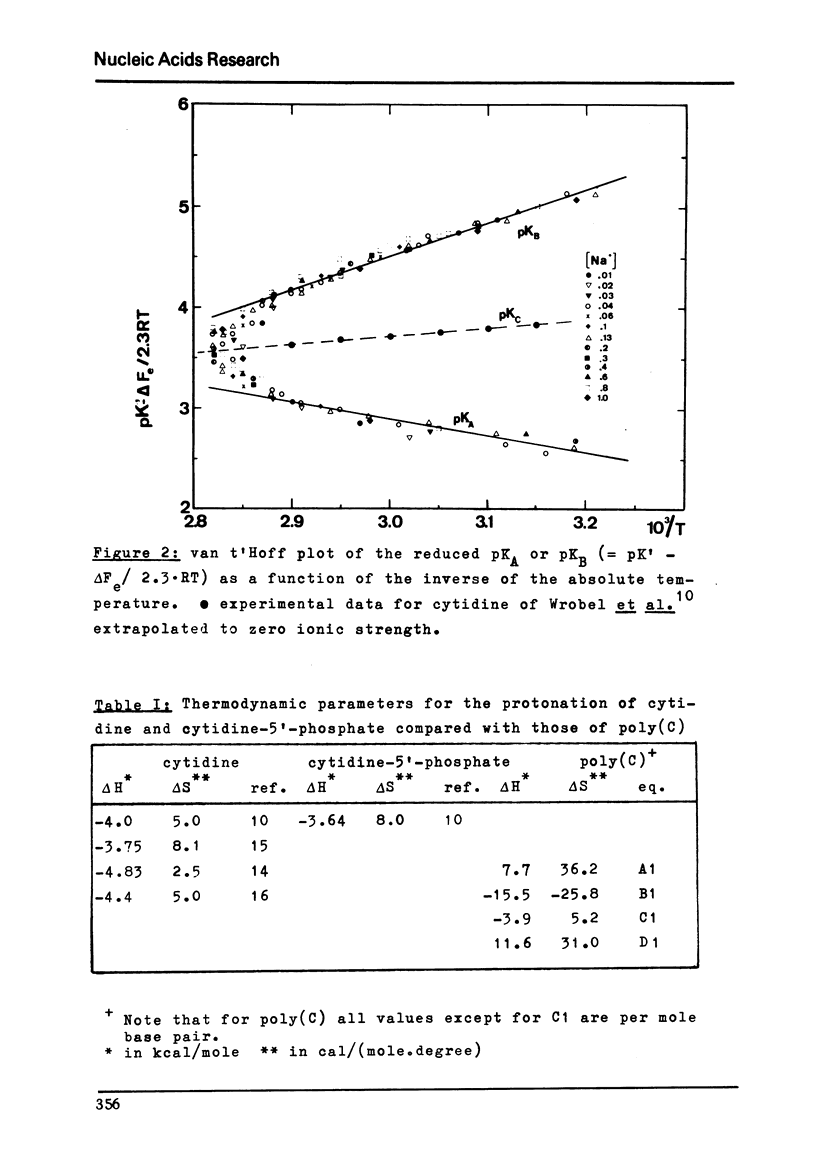

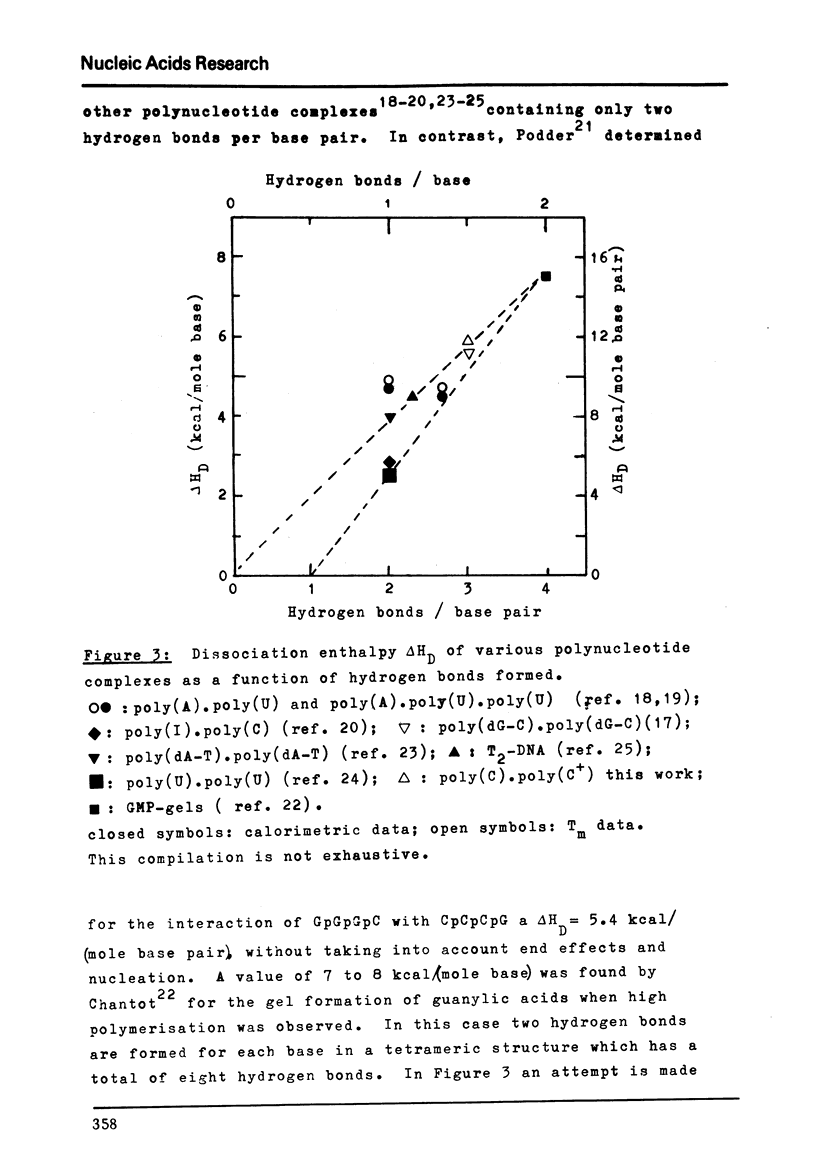


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKINRIMISI E. O., SANDER C., TS'O P. O. Properties of helical polycytidylic acid. Biochemistry. 1963 Mar-Apr;2:340–344. doi: 10.1021/bi00902a028. [DOI] [PubMed] [Google Scholar]
- Chantot J. F. Nucleoside conformations. IX. A calorimetric study of gel formation by guanylic acids. Arch Biochem Biophys. 1972 Nov;153(1):347–356. doi: 10.1016/0003-9861(72)90455-9. [DOI] [PubMed] [Google Scholar]
- Chen G. C., Yang J. T. Some hydrodynamic and optical properties of polyribonucleotides. Biophys Chem. 1973 Dec;1(2):62–72. doi: 10.1016/0301-4622(73)80002-x. [DOI] [PubMed] [Google Scholar]
- Guschlbauer W. Protonated polynucleotide structures. I. The thermal denaturation of polycytidylic acid in acid solution. Proc Natl Acad Sci U S A. 1967 May;57(5):1441–1448. doi: 10.1073/pnas.57.5.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guschlbauer W., Vetterl V. Protonated polynucleotide structures Thermodynamics of the melting of the acid form of polyadenylic acid. FEBS Lett. 1969 Jul;4(1):57–60. doi: 10.1016/0014-5793(69)80196-1. [DOI] [PubMed] [Google Scholar]
- HARTMAN K. A., Jr, RICH A. THE TAUTOMERIC FORM OF HELICAL POLYRIBOCYTIDYLIC ACID. J Am Chem Soc. 1965 May 5;87:2033–2039. doi: 10.1021/ja01087a031. [DOI] [PubMed] [Google Scholar]
- Heinecke M., Bode D., Schernau U. Calorimetric investigation of the helix-coil conversion of polyuridylic acid. Biopolymers. 1974 Jan;13(1):227–235. doi: 10.1002/bip.1974.360130116. [DOI] [PubMed] [Google Scholar]
- KOTIN L. ON THE EFFECT OF IONIC STRENGTH ON THE MELTING TEMPERATURE OF DNA. J Mol Biol. 1963 Sep;7:309–311. doi: 10.1016/s0022-2836(63)80009-1. [DOI] [PubMed] [Google Scholar]
- LANGRIDGE R., RICH A. Molecular structure of helical polycytidylic acid. Nature. 1963 May 25;198:725–728. doi: 10.1038/198725a0. [DOI] [PubMed] [Google Scholar]
- Podder S. K. Co-operative non-enzymic base recognition. A kinetic study of interaction between GpGpGpC and GpCpCpC and of self-association of GpGpGpC. Eur J Biochem. 1971 Oct 26;22(4):467–477. doi: 10.1111/j.1432-1033.1971.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Thiele D., Guschlbauer W. Polynucléotides protonés. VII. Transitions thermiques entre differents complexes de l'acide polyinosinique et de l'acide polycytidylique en milieu acide. Biopolymers. 1969;8(3):361–378. doi: 10.1002/bip.1969.360080307. [DOI] [PubMed] [Google Scholar]
- Wróbel A., Rabczenko A., Shugar D. Conformation of acid forms of poly C: temperature and ionic strength dependence of protonation of cytidine and cytidine-5'-phosphate. Acta Biochim Pol. 1970;17(4):339–349. [PubMed] [Google Scholar]