Abstract
Increased consumption of cruciferous vegetables is associated with a reduced risk of developing prostate cancer. Indole-3-carbinol (I3C) and 3,3′-diindolylmethane (DIM) are phytochemicals derived from cruciferous vegetables that have shown promise in inhibiting prostate cancer in experimental models. Histone deacetylase (HDAC) inhibition is an emerging target for cancer prevention and therapy. We sought to examine the effects of I3C and DIM on HDACs in human prostate cancer cell lines: androgen insensitive PC-3cells and androgen sensitive LNCaP cells. I3C modestly inhibited HDAC activity in LNCaP cells by 25% but no inhibition of HDAC activity was detected in PC-3 cells. In contrast, DIM significantly inhibited HDAC activity in both cell lines by as much as 66%. Decreases in HDAC activity correlated with increased expression of p21, a known target of HDAC inhibitors. DIM treatment caused a significant decrease in the expression of HDAC2 protein in both cancer cell lines but no significant change in the protein levels of HDAC1, HDAC3, HDAC4, HDAC6 or HDAC8 were detected. Taken together, these results show that inhibition of HDAC activity by DIM may contribute to the phytochemicals anti-proliferative effects in the prostate. The ability of DIM to target aberrant epigenetic patterns, in addition to its effects on detoxification of carcinogens, may make it an effective chemopreventive agent by targeting multiple stages of prostate carcinogenesis.
Keywords: 3,3′-diindolylmethane; histone deacetylase; indole-3-carbinol; prostate cancer; chemoprevention; epigenetics
Introduction
Prostate cancer is the second most frequently diagnosed cancer in men (International Agency for Research on Cancer, 2010; Jemal et al., 2011). Nutrition and diet are important modifiable risk factors for prostate cancer development. Epidemiological studies have shown an inverse association between cruciferous vegetable intake and cancer risk in many tissues including the prostate (Liu et al., 2012). In particular, increased consumption of glucosinolates, sulfur-containing compounds found in cruciferous vegetables like Brussels sprouts, have been shown to significantly reduce the risk of prostate cancer (Steinbrecher et al., 2009). When raw vegetables are chopped or chewed the glucosinolate glucobrassicin is broken down into indole-3-carbinol (I3C) (Fig. 1A) by an enzyme that is found in the food, myrosinase (Aggarwal and Ichikawa, 2005). During digestion, I3C undergoes extensive and rapid self condensation in the acidic environment, producing many oligomeric products including a major product 3,3′-diindolylmethane (DIM) (Fig. 1A) (Sarkar and Li, 2004; Aggarwal and Ichikawa, 2005). DIM is formed from 2 molecules of I3C (Sarkar and Li, 2004).
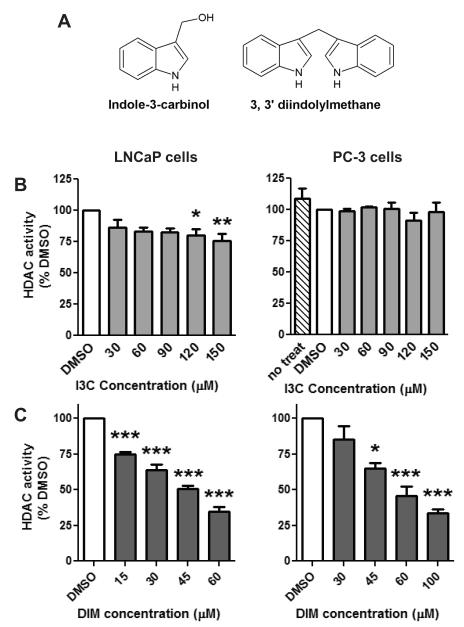
Fig. 1.
Effect of I3C and DIM on HDAC activity. A) Chemical structures of indole-3-carbinol (I3C) and 3, 3′ diindolylmethane (DIM). LNCaP and PC-3 prostate cancer cells were treated with increasing concentrations of I3C (B) or DIM (C) or vehicle control, DMSO, for 48 hours. Data are expressed as a percentage of the DMSO control treatment and represent an average of 3-8 independent experiments ± SEM. Statistical significance: *p<0.05, **p<0.01, ***p<0.001 compared to DMSO control samples.
Studies in prostate cancer models and cells have concluded that both I3C and DIM inhibit prostate carcinogenesis, decrease cell proliferation, increase apoptosis and induce G1 cell cycle arrest (Chinni et al., 2001; Nachshon-Kedmi et al., 2004b; Garikapaty et al., 2006; Hsu et al., 2006; Souli et al., 2008; Vivar et al., 2009; Fares et al., 2010). While both I3C and DIM have been established as chemopreventive agents, there is growing evidence that the acid condensation products, such as DIM, have distinct targets and greater bioactivity. For example, DIM was more effective at lower concentrations than I3C in inducing apoptosis in prostate cancer cells (Garikapaty et al., 2006). Furthermore, DIM has been shown to be more effective than I3C at down regulating the Akt survival pathway in prostate cancer cells (Garikapaty et al., 2006). While I3C and DIM have the potential to be therapeutic and chemopreventative phytochemicals, the complete mechanisms by which they induce the anti-carcinogenic properties is not well understood.
The role of epigenetics in controlling gene expression and prostate cancer development is an emerging area of research. In particular, the identification of agents that can target epigenetic dysregulation during cancer development is of keen interest. We have previously identified phytochemicals, derived from cruciferous vegetables such as sulforaphane, to act as dietary histone deacetylases (HDACs) inhibitors and limit prostate cancer cell growth (Myzak et al., 2006a; Myzak et al., 2006b; Clarke et al., 2011). HDACs regulate gene expression by removing acetyl groups from histones (reviewed in (Perry et al., 2010)). HDACs can be divided into classes based on their structure and sequence homology: class I consists of HDAC 1,2,3,8, class II includes HDAC 4,5,6,7,9 and 10. Increased HDAC activity and expression can result in repression of genes, such as p21, that regulate cell cycle and apoptotic mechanisms (Abbas and Gupta, 2008). In cancer patients, global decreases in histone acetylation corresponded with an increased grade of cancer and risk of prostate cancer recurrence (Seligson et al., 2005). Increased expression of HDAC1, HDAC2 and HDAC3 has been reported in prostate cancers and is associated with tumor cell proliferation (Halkidou et al., 2004; Weichert et al., 2008). Taken together these findings support the hypothesis that upregulation of HDACs and hypoacetylation of histones may contribute to prostate cancer progression. We hypothesized that I3C and DIM may have anti-carcinogenic activity in prostate cancer cells through the inhibition of HDACs. Our goals were to examine the effects of I3C and DIM on HDAC activity, protein expression, acetylation of HDAC target proteins, and expression of subsequent markers of cell cycle arrest in prostate cancer cells.
Material and methods
Culturing and Treatment of Cells
Androgen-dependent prostate cancer epithelial cells (LNCaP) and androgen-independent prostate cancer epithelial cells (PC-3) were obtained from American Type Tissue Collection (Manassas, VA). Cells were cultured at 5% CO2 and 37°C in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with glutamine plus 10% fetal bovine serum (Cellgro, Manassas, VA). Cells were treated with dimethylsulfoxide (DMSO) (vehicle control), I3C or DIM (Sigma-Aldrich, St. Louis, MO) at μM concentrations dissolved in DMSO. Based on pharmacokinetic studies in animals and previous studies in prostate cancer cell lines, we treated cells with increasing concentrations of I3C or DIM, up to 150 μM or 100 μM respectively (Chinni et al., 2001; Anderton et al., 2004a; Anderton et al., 2004b; Nachshon-Kedmi et al., 2004b; Garikapaty et al., 2006; Hsu et al., 2006; Souli et al., 2008; Vivar et al., 2009; Fares et al., 2010). A different range of DIM doses was used in each cell line because DIM is more potent at inducing anti-proliferative effects in LNCaP cells as compared to PC-3 cells (Wang et al., 2012). Cells were harvested using an immunoprecipitation lysis buffer containing protease inhibitors (Thermo Scientific, Waltham, MA) 24 and 48 hrs post treatment. In the cell proliferation assay, LNCaP and PC-3 cells were treated with 60 μM I3C, DIM or DMSO in quadruplicate. The number of viable cells was quantified 48 hours later using a standard trypan blue exclusion assay (Sigma-Aldrich) and a hemacytometer. All cells and chemicals were handled using appropriate personal protective equipment.
HDAC activity
We used HDAC substrate and deacetylated standard from Enzo Life Sciences, (Farmingdale, NY) to perform HDAC activity assays. Cells were treated with I3C or DIM before cell lysates were collected. The lysates were diluted 10 times with HDAC buffer and protein levels of diluted lysates were determined using Pierce BCA protein assay kit (Thermo Scientific). Fifteen μl of a diluted lysate was mixed with 10 μl of assay buffer in a well of a 96-well white plate for fluorescent assays. To initiate the HDAC reaction, 25 μl of 100 μM HDAC substrate was add to each well. After 30 minute incubation at 37°C, 50 μl of assay developer was added to each well to terminate the enzymatic reaction and release the fluorophore from the reaction product. After 15 min of developing fluorescence at room temperature, fluorescence was measured at 460 nm with a Spectra MAX Gemini XS Plate Reader (Molecular Devices, Sunnyvale, CA) using an excitation wavelength of 360 nm. HDAC activity was expressed relative to total protein concentration and expressed relative to the DMSO control. Negative controls, positive controls and standards were assayed in parallel with the samples. The direct inhibitory effects of DIM and I3C on HDAC proteins were studied using HeLa cell nuclear extracts from Enzo HDAC assay kits. We also included trichostatin A (TSA) and DMSO as positive and negative controls, respectively.
HDAC, acetylated histone and p21 protein expression
Protein expression was determined by standard western blotting techniques. Proteins were separated by SDS-PAGE on 4-12 % Bis-Tris gels (Life Technologies, Grand Island, NY) and transferred to nitrocellulose (Bio-Rad, Hercules, CA). Blots were blocked in a solution of phosphate-buffered saline with 2% Tween containing 2% BSA for 1 h and then incubated overnight with primary antibodies specific against HDAC1, AcH3, AcH4 (Millipore, Billerica, MA) HDAC2-4, HDAC6, HDAC8, (Santa Cruz Biotechnology) H3, H4, (Bethyl Laboratories, Montgomery, TX), [g533]-actin, (Sigma-Aldrich), or p21 (Cell Signaling, Danvers, MA). Membranes were then washed and incubated with goat anti-rabbit or goat anti-mouse secondary antibodies using standard conditions (Santa Cruz Biotechnology, Santa Cruz, CA). To visualize proteins, membranes were incubated with Super Signal Femto Substrate (Thermo Scientific) and the chemiluminescent signal was detected on an Alpha Innotech system (Protein Simple, Santa Clara, CA). Densitometry of each band was determined using Image J (Bethesda, MD), normalized to [g533]-actin and expressed as a percentage of the DMSO control.
Evaluation of HDAC2 mRNA expression
Total RNA was isolated from cells using a standard Trizol extraction (Life Technologies). One μg of total RNA was reverse transcribed into cDNA using SuperScript III First-Strand Synthesis SuperMix for qRT-PCR and diluted (Life Technologies). Real time PCR was performed using the following PCR primers: HDAC2 (forward: 5′-ATGACAAACCAGAACACTCC-3′, reverse: 5′-TCCTTCTCCTTCATCCTCAG-3′), GAPDH (forward: 5′-CGAGATCCCTCCAAAATCAA-3′, reverse: 5′-TTCACACCCATGACGAACAT-3′). Real time PCR reactions were performed using Fast SYBR Green Mastermix (Life Technologies) on 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Gene copies were determined using the standard curve method. A standard curve was generated from serial dilutions of purified plasmid DNA that encoded for each gene of interest. Data represent the copy number of the gene of interest normalized to the copy number of the GAPDH housekeeping gene.
HDAC2 gene silencing
LNCaP cells were trypsinized and transfected with 30 nM HDAC2-specific siRNA (Ambion Silencer siRNA s6493) using siPORT NeoFX transfection agent (Life Technologies). Control cells were transfected with an equal concentration of scrambled siRNA (Ambion Silencer Select Negative Control No. 1) or an equal volume of buffer alone. Transfected cells were seeded in a 6-well tissue culture plates at 2.3×105 cells per well. RNA and protein was collected at 48 and 72h post transfection respectively.
Statistics
Graphs were created and statistical significance was determined with GraphPad Prism software (La Jolla, CA). Each bar represents an average result calculated from at least 3 independent experiments ± the standard error of the mean. Significant differences between control and treatment groups was calculated using a one way ANOVA and Dunnett’s post-hoc test where p < 0.05.
Results
The effects of both I3C and DIM were tested in both androgen-dependent (LNCaP) and androgen-independent (PC-3) prostate cancer cells respectively. I3C did not significantly inhibit HDAC activity at doses < 100 μM in LNCaP cells. At concentrations >120 μM, I3C treatment produced a modest 25% reduction in HDAC activity (Fig. 1B). I3C exposure had no effect on HDAC activity in PC-3 cells at any concentration tested (Fig. 1B). In striking contrast to I3C, DIM was a much more potent inhibitor of HDAC activity (Fig. 1C). Specifically, a significant and dose dependent decrease was observed in HDAC activity in both types of prostate cancer cells. A 55-65% reduction in HDAC activity was detected with 60 μM DIM at 48 hours. The increased potency of DIM to inhibit HDAC activity was correlated with greater inhibition of cell growth. A significant 50% decrease in viable cells was detected in both LNCaP and PC-3 cells treated with 60 μM DIM for 48 hours. In contrast, 60 μM I3C did not significantly inhibit cell growth in either cell line (data not shown). Taken together, these results show that DIM is an effective inhibitor of HDAC activity and this may contribute to the enhanced anti-proliferative effect observed with DIM in prostate cancer cells.
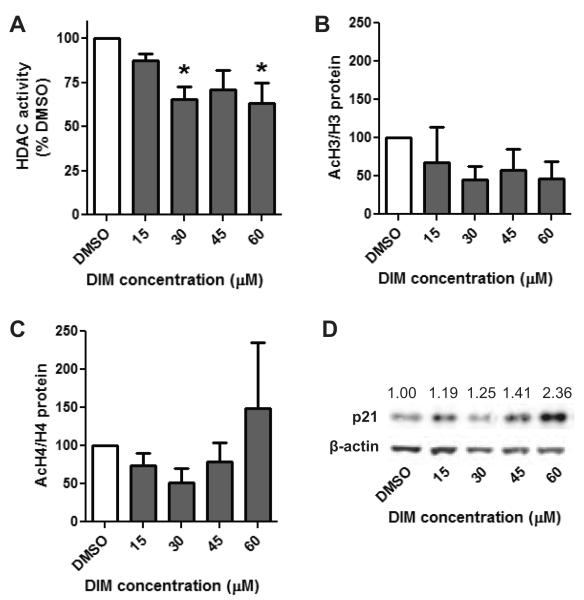
There was a marked difference in the concentration of DIM that was necessary to induce significant inhibition of HDAC activity in LNCaP cells as compared to PC-3 cells. Only a 15 μM concentration of DIM was necessary to significantly inhibit HDAC activity in LNCaP cells while a 45 μM concentration was needed in PC-3 cells. This increased sensitivity of androgen sensitive cells was associated with down regulation of the AR protein (data not shown). Since this cell line was more sensitive we also evaluated if DIM could inhibit HDAC activity at an earlier time point. A significant 35% reduction in HDAC activity was found at the 30 and 60 μM concentrations in LNCaP cells 24 hours after treatment (Fig. 2A).
Fig. 2.
Inhibition of HDAC activity is associated with the upregulation of p21 protein. LNCaP cells were treated with increasing concentrations of DIM, or vehicle control, for 24 hours and evaluated for A) HDAC activity B) acetylation of H3 C) acetylation of H4 and D) p21 protein expression. A-C) Data are expressed as a percentage of the DMSO control treatment and represent an average of 4 independent experiments ± SEM. Statistical significance: *p<0.05 compared to DMSO control samples. D) Representative blot of p21 expression 24 hours post DIM treatment. Numbers indicate densitometry results of p21 expression normalized to [g533]-actin, n=4.
We next determined if inhibition of HDAC activity in DIM treated samples resulted in an increase in the acetylation of histones. No significant change in the global acetylation of histones H3 or H4 was seen with DIM treatment (Fig. 2B and C). We also verified that I3C treatment of PC-3 cells did not cause a significant change in the acetylation of H3 nor H4 (data not shown). To further explore if HDAC inhibition had any downstream effect we assessed if DIM treatment was associated with an increase in p21 expression. A marked increase in p21 protein expression was found in LNCaP cells treated with DIM (Fig. 2D). These data show that DIM inhibits HDAC activity and this may be a cause for the DIM-induced increase in p21 expression.
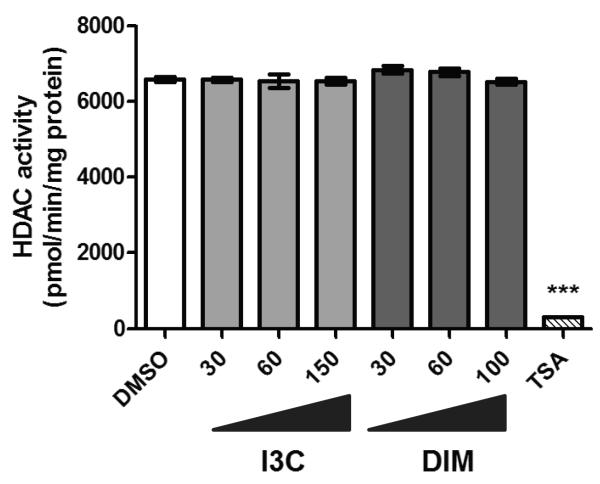
Pharmacological HDAC inhibitors and sulforaphane have been shown to inhibit HDAC activity through competitive inhibition of HDAC enzymes. For this reason we sought to determine if DIM also acts as a direct HDAC inhibitor. Using a cell-free system with known elevated HDAC activities, we found that neither DIM nor I3C decreased HDAC activity as compared to the vehicle (DMSO) control (Fig. 3). As a positive control the competitive HDAC inhibitor TSA (20 nM) was shown to decrease HDAC activity in this system by over 95%. These results suggests that DIM is not likely to act as a direct competitive inhibitor of HDAC activity.
Fig. 3.
I3C and DIM do not directly inhibit HDAC activity. Nuclear extracts containing HDAC enzymes were incubated with increasing concentrations of I3C or DIM. The vehicle (DMSO), 20 nM TSA, and no treatment controls were also included. Data represent an average of 3 independent measurements ± SEM. *** indicates statistical significance of p<0.001 compared to DMSO control samples.
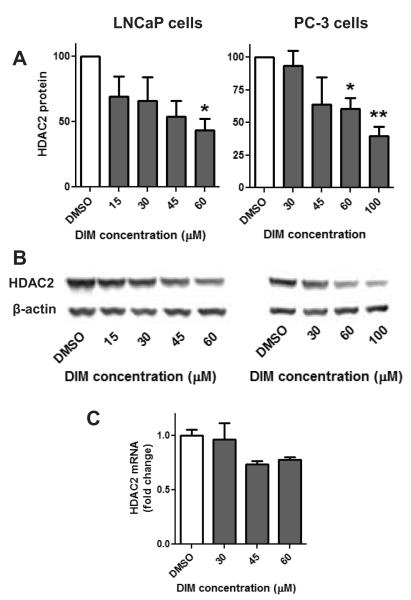
Another possible mechanism by which HDAC activity can be decreased in the cell is through down regulation of specific HDAC proteins (Bhatnagar et al., 2009; Li et al., 2010). Among the class I HDACs, a dose dependent decrease in HDAC2 expression was detected in both PC-3 and LNCaP cells with DIM treatment (Fig. 4A-B). A statistically significant decrease in HDAC2 protein expression was only detected at doses at, or above 60 μM DIM at 48h (Fig. 4A-B). At this same concentration and time point HDAC2 mRNA levels were not significantly decreased by DIM treatment in either cell line (data not shown). At 24 hours after DIM treatment HDAC2 mRNA levels were examined in LNCaP cells and a trend for a decrease in HDAC2 mRNA of 30% was found at 45 and 60 μM concentrations but this was not statistically significant (Fig. 4C, p-value of 0.1133).
Fig. 4.
DIM-induced decrease in HDAC2 protein. LNCaP and PC-3 cells were treated with increasing concentrations of DIM or DMSO. A) Data are densitometry results of Western blots for HDAC2 protein levels normalized to [g533]-actin and the DMSO control following 48h treatment. Data are expressed as a percentage of the DMSO control treatment and represent an average of 3-7 independent experiments ± SEM. Statistical significance: *p<0.05, **p<0.01. B) Representative blot of HDAC2 and [g533]-actin expression 48 hours post DIM treatment. C) HDAC2 transcript levels in LNCaP cells at 24h post DIM, or DMSO control treatment.
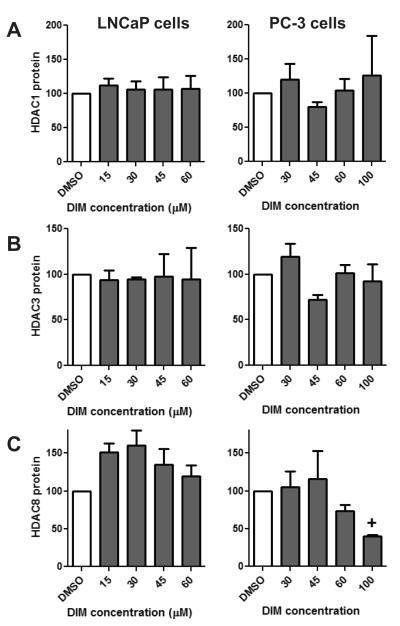
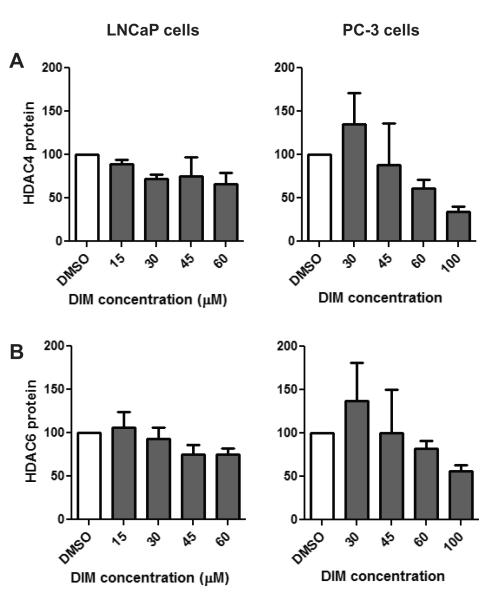
Examination of other class I HDACs revealed a decrease in HDAC8 protein in PC-3 cells which approached statistical significance at the 100 μM concentration (Fig. 5C, p=0.0582). In contrast, HDAC 8 expression did not decrease in LNCaP cells treated with DIM (Fig. 5C). No difference in the expression of HDAC1 or HDAC3 proteins was detected with DIM treatment in either cell type (Fig. 5A and 5B). Among the class II HDACs, we show that DIM treatment of prostate cancer cells did not result in a significant reduction of HDAC4 (p = 0.0857) nor HDAC6 (p = 0.4169) protein (Fig. 6). While no significant change was observed, it is worth noting that a trend in decreasing HDAC4 and 6 was observed in PC-3 cells, however, only at the high 100 μM concentration (Fig. 6). We also examined if I3C inhibited any of the HDAC proteins in prostate cancer cells. Treatment of PC-3 cells with up to 150 μM concentrations of I3C did not significantly change the levels of HDAC1-4, HDAC6 nor HDAC8 (data not shown). These results illustrate that inhibition of HDAC activity by DIM, but not I3C, is primarily achieved through down regulation of HDAC2 protein levels in prostate cancer cells.
Fig. 5.
Effect of DIM on class I HDACs. LNCaP and PC-3 cells were treated with increasing concentrations of DIM or DMSO for 48 hours. Data are densitometry results of Western blots for A) HDAC1 B) HDAC3 and C) HDAC8 protein levels normalized to [g533]-actin and the DMSO control. Data are expressed as a percentage of the DMSO control treatment and represent an average of 3-7 independent experiments ± SEM. Statistical significance: + p=0.0582, compared to DMSO control samples.
Fig. 6.
Effect of DIM on class II HDACs. LNCaP and PC-3 cells were treated with increasing concentrations of DIM or DMSO for 48 hours. Data are densitometry results of Western blots for A) HDAC4 and B) HDAC6 protein levels normalized to [g533]-actin and the DMSO control. Data are expressed as a percentage of the DMSO control treatment and represent an average of 3-7 independent experiments ± SEM. No significance difference was detected with DIM treatment.
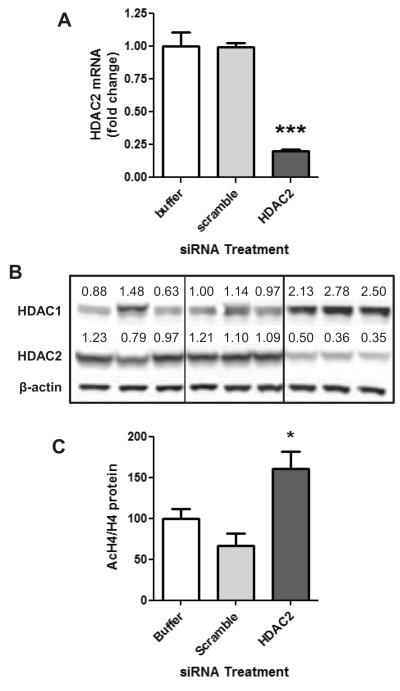
In order to determine if decreases in HDAC2 protein alone was sufficient to produce changes in epigenetic endpoints, LNCaP cells were transfected with siRNA specific to HDAC2. HDAC2 siRNA caused a significant decrease in HDAC2 mRNA and protein levels following transfection (Fig. 7A-B). We observed that HDAC2 knockdown also resulted in increases in HDAC1 expression (Fig7B). Despite the increases in HDAC1 protein, the decreased HDAC2 expression was associated with an increase in acetylated H4 levels (Fig. 7C). Thus, decreasing HDAC2 proteins has relevant epigenetic consequences even under compensatory actions of HDAC1.
Fig. 7.
HDAC2 knockdown in LNCaP cells results in increases in acetylated histone levels. LNCaP cells were transfected with HDAC2 siRNA, control scrambled siRNA, or vehicle control. A) HDAC2 mRNA expression was evaluated 48h following transfection. B) HDAC1 and HDAC2 protein expression was determined 72h following transfection. C) Acetylated histone H4 protein levels normalized to [g533]-actin and H4 protein levels 72 hours following HDAC2 siRNA knockdown. Statistical significance as compared to scrambled control siRNA: *p<0.05, ***p<0.001.
Discussion
The chemopreventive effects of DIM are well–established and have been linked to proliferation/survival events and the induction of apoptosis (reviewed in (Banerjee et al., 2012). Despite this intense study, the precise molecular mechanisms by which DIM inhibits proliferation and induces apoptosis remains unclear. Here we show for the first time that DIM, but not I3C, inhibits HDAC activity in cancer cells. This inhibition of HDACs by DIM is associated with an up-regulation of the cell cycle regulator p21 in prostate cancer cells. We further show that DIM induces the down regulation of HDAC2 which is a protein that is upregulated in prostate cancers and associated with decreased patient survival (Seligson et al., 2005). Taken together, these results identify DIM as a potential dietary epigenetic modulator in prostate cancer cells. The use of DIM alone, or in combination with established treatment regimens, may increase efficacy of anti-cancer therapies and prevention strategies, without serious side effects ((Nachshon-Kedmi et al., 2004a; Li et al., 2005; Fares et al., 2010) and reviewed in (Banerjee et al., 2012)).
Many HDAC inhibitors act by inhibiting catalytic activity of HDAC enzymes. We have previously shown that treatment of cancer cells with sulforaphane decreased HDAC activity by both competitive inhibition and a decrease in the expression of HDAC 3, 4 and 6 proteins (Myzak et al., 2004; Clarke et al., 2011). In the current study, we show that unlike sulforaphane, DIM does not appear to act as a direct inhibitor, but caused a selective decrease in HDAC expression, primarily at the protein level. It is possible that DIM may specifically alter protein turnover pathways that specifically control HDAC2 protein levels post-translationally. In colon cancer cells, the phytochemical sulforaphane has been shown to specifically decrease HDAC3 protein levels, with no change in mRNA levels by inducing HDAC3 degradation through post-translational phosphorylation of the protein (Rajendran et al., 2011). DIM-induced down regulation of HDAC2 in prostate cancer cells is consistent with a previous report in colon cancer cells that also observed a DIM-induced down regulation of HDAC2 (Bhatnagar et al., 2009; Li et al., 2010). The mechanism leading to cell specific degradation of HDAC2, a trend for decreasing HDAC4, 6 and 8, and the involvement of the proteasome, is an important area for future research. DIM has also been shown to inhibit proteasome activity (Chinnakannu et al., 2009) and in colon cancer cells decreases in HDAC protein expression were linked to proteasomal degradation (Li et al., 2010). In contrast to colon cancer cells, we did not detect down regulation of the other class I HDACs, HDAC1 and HDAC3, in prostate cancer cells. This difference highlights that DIM may inhibit specific HDACs in a cell specific manner. Further investigation in the mechanisms by which DIM alter HDACs, and their contribution to the anti-proliferative effects of DIM is warranted.
DIM was effective at inhibiting HDAC activity at lower concentrations in androgen-dependent LNCaP cells compared to androgen-independent PC-3 prostate cancer cells. This is consistent with the literature showing that DIM induces anti-proliferative and pro-apoptotic effects at lower concentrations in androgen sensitive prostate cancer cells (Wang et al., 2012). Importantly, the dose of DIM that was necessary to achieve HDAC inhibition in androgen-dependent cells is approaching concentrations that have been estimated to be achievable through consumption of cruciferous vegetables, such as 200 grams of broccoli (Chang et al., 2006). There are several reasons why androgen-dependent cells are likely more sensitive than androgen-independent cells, one of which relates to the ability of DIM to regulate the androgen receptor (AR) pathway (Li et al., 2007; Chinnakannu et al., 2009). We confirmed the down regulation of the AR protein by DIM. It is possible that this decrease in AR can occur through HDAC inhibition as the HDAC inhibitor TSA, has been shown to down regulate AR gene expression in prostate cancer cells (Rokhlin et al., 2006). Additionally, DIM has been shown to influence the metabolism of testosterone (Wortelboer et al., 1992), alter binding of testosterone to the AR receptor (Bovee et al., 2008; Abdelbaqi et al., 2011), and thus alters the AR signaling pathway (Le et al., 2003). More research is needed to clarify the role of HDAC inhibition in the down regulation of AR signaling in androgen sensitive prostate cancer cells.
Inhibition of HDAC activity is generally associated with an increase in the acetylation of histones (Abbas and Gupta, 2008). We did not observe a significant change in the global acetylation of histones H3 or H4 under conditions of DIM treatment. Despite this, we observed an increase in p21 expression which is often transcriptionally repressed in cancer cells through up-regulation of class I HDACs (Abbas and Gupta, 2008). Identification of specific histone targets following DIM induced alterations in HDAC activity are an important area for future study. Our p21 results are consistent with a report in colon cancer cells where DIM treatment inhibited HDAC protein expression and was associated with an increase in p21 expression but no significant change in the global acetylation of H3 was detected (Li et al., 2010). More specific examination in colon cells revealed increased acetylation of H3 in the p21 promoter. A discrepancy between HDAC inhibition and acetylation of histones was also noted in breast cancer cells treated with sulforaphane (Pledgie-Tracy et al., 2007). HDACs act in complexes with many other proteins including histone acetyltransferases and DNA methyltransferases (Bantscheff et al., 2011). Further investigation will be needed to understand the complex interplay between DIM-induced HDAC inhibition, alterations of other epigenetic marks, and changes in expression of targets affecting cancer cell growth.
HDAC inhibition is emerging as a promising field in cancer chemoprevention and therapy. Several clinical trials aimed at establishing the chemotherapeutic efficacy of HDAC inhibitors are currently ongoing. They are based on evidence that cancer cells undergo cell cycle arrest, differentiation and apoptosis in vitro, and that tumor volume and/or tumor number may be reduced in animal models treated with HDAC inhibitors. DIM has been shown to be well tolerated in humans and inhibits growth of prostate cancer both in vitro and in mouse models (Cho et al., 2011). Despite the fact that I3C did not strongly affect HDAC activity, I3C has been shown to be effective in limiting cancer growth in several other models in vitro and in vivo, and still may be an important chemopreventive agent through alternate mechanisms (Wang et al., 2006; Yu et al., 2006; Lubet et al., 2011; Wang et al., 2012). The chemopreventive properties of DIM are likely attributed to targeting multiple mechanisms. Herein, we establish that HDAC inhibition by DIM, possibly by decreasing specific class I HDACs, may be an additional novel target for chemoprevention. Regardless, the potential for DIM, and other chemicals derived from cruciferous vegetables, to target aberrant epigenetic patterns, in addition to their effects on detoxification and carcinogen metabolism, may make them effective chemoprevention agents at multiple stages of the prostate carcinogenesis pathway.
Highlights.
DIM inhibits HDAC activity and decreases HDAC2 expression in prostate cancer cells.
DIM is significantly more effective than I3C in inhibiting HDAC activity.
I3C has no effect on HDAC protein expression.
Inhibition of HDAC activity by DIM is associated with increased p21 expression.
HDAC inhibition may be a novel epigenetic mechanism for cancer prevention with DIM.
Acknowledgements
We thank Lyndsey Shorey from D. Williams lab for providing I3C and DIM. We thank Drs. John Clarke, Anna Hsu, Mansi Parasramka, Praveen Rajendran and Carmen Wong for technical assistance and helpful conversations. This work was supported by NIH grants CA90890, CA65525, CA122906, CA122959, CA80176, and by NIEHS Center grant P30 ES00210.
Abbreviations
- DIM
3,3′-diindolylmethane
- LNCaP
androgen-dependent prostate cancer cells
- PC-3
androgen-independent prostate cancer cells
- AR
androgen receptor
- DMSO
dimethylsulfoxide
- H3
histone 3
- H4
histone 4
- HDAC
histone deacetylase
- I3
Cindole-3-carbinol
- TSA
trichostatin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement The authors have no conflict of interest to disclose.
References
- Abbas A, Gupta S. The role of histone deacetylases in prostate cancer. Epigenetics. 2008;3:300–309. doi: 10.4161/epi.3.6.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelbaqi K, Lack N, Guns ET, Kotha L, Safe S, Sanderson JT. Antiandrogenic and growth inhibitory effects of ring-substituted analogs of 3,3′-diindolylmethane (Ring-DIMs) in hormone-responsive LNCaP human prostate cancer cells. The Prostate. 2011:1401–1412. doi: 10.1002/pros.21356. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, Mager DE. Physiological modeling of formulated and crystalline 3,3′-diindolylmethane pharmacokinetics following oral administration in mice. Drug. Metab. Dispos. 2004a;32:632–638. doi: 10.1124/dmd.32.6.632. [DOI] [PubMed] [Google Scholar]
- Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, Steward WP, Williams ML. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer. Res. 2004b;10:5233–5241. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Parasramaka MA, Sarkar FH. Cellular, molecular and biological insight into chemopreventive and therapeutic potential of 3,3′-diindolmethane (DIM) In: Sarkar FH, editor. Nuetraceuticals and Cancer. Springer; New York: 2012. pp. 111–133. [Google Scholar]
- Bantscheff M, Hopf C, Savitski MM, Dittmann A, Grandi P, Michon AM, Schlegl J, Abraham Y, Becher I, Bergamini G, Boesche M, Delling M, Dumpelfeld B, Eberhard D, Huthmacher C, Mathieson T, Poeckel D, Reader V, Strunk K, Sweetman G, Kruse U, Neubauer G, Ramsden NG, Drewes G. Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nat. Biotechnol. 2011;29:255–265. doi: 10.1038/nbt.1759. [DOI] [PubMed] [Google Scholar]
- Bhatnagar N, Li X, Chen Y, Zhou X, Garrett SH, Guo B. 3,3′-diindolylmethane enhances the efficacy of butyrate in colon cancer prevention through down-regulation of survivin. Cancer Prevention Research. 2009;2:581–589. doi: 10.1158/1940-6207.CAPR-08-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee TF, Schoonen WG, Hamers AR, Bento MJ, Peijnenburg AA. Screening of synthetic and plant-derived compounds for (anti)estrogenic and (anti)androgenic activities. Anal. Bioanal. Chem. 2008;390:1111–1119. doi: 10.1007/s00216-007-1772-3. [DOI] [PubMed] [Google Scholar]
- Chang X, Firestone GL, Bjeldanes LF. Inhibition of growth factor-induced Ras signaling in vascular endothelial cells and angiogenesis by 3,3′-diindolylmethane. Carcinogenesis. 2006;27:541–550. doi: 10.1093/carcin/bgi230. [DOI] [PubMed] [Google Scholar]
- Chinnakannu K, Chen D, Li Y, Wang Z, Dou QP, Reddy GP, Sarkar FH. Cell cycle-dependent effects of 3,3′-diindolylmethane on proliferation and apoptosis of prostate cancer cells. J. Cell. Physiol. 2009;219:94–99. doi: 10.1002/jcp.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–2936. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Park SY, Kim EJ, Kim JK, Park JH. 3,3′-Diindolylmethane inhibits prostate cancer development in the transgenic adenocarcinoma mouse prostate model. Mol. Carcinog. 2011;50:100–112. doi: 10.1002/mc.20698. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Hsu A, Yu Z, Dashwood RH, Ho E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol. Nutri. Food Res. 2011:999–1009. doi: 10.1002/mnfr.201000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares F, Azzam N, Appel B, Fares B, Stein A. The potential efficacy of 3,3′-diindolylmethane in prevention of prostate cancer development. Eur. J. Cancer Prev. 2010;19:199–203. doi: 10.1097/CEJ.0b013e328333fbce. [DOI] [PubMed] [Google Scholar]
- Garikapaty VP, Ashok BT, Tadi K, Mittelman A, Tiwari RK. 3,3′-Diindolylmethane downregulates pro-survival pathway in hormone independent prostate cancer. Biochem. Biophys. Res. Commun. 2006;340:718–725. doi: 10.1016/j.bbrc.2005.12.059. [DOI] [PubMed] [Google Scholar]
- Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. The Prostate. 2004;59:177–189. doi: 10.1002/pros.20022. [DOI] [PubMed] [Google Scholar]
- Hsu JC, Dev A, Wing A, Brew CT, Bjeldanes LF, Firestone GL. Indole-3-carbinol mediated cell cycle arrest of LNCaP human prostate cancer cells requires the induced production of activated p53 tumor suppressor protein. Biochem. Pharmacol. 2006;72:1714–1723. doi: 10.1016/j.bcp.2006.08.012. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer GLOBOCAN 2008, Cancer Fact Sheet: Prostate Cancer. 2010.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. C.A. Cancer. J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3′-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J. Biol. Chem. 2003;278:21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- Li Y, Chinni SR, Sarkar FH. Selective growth regulatory and pro-apoptotic effects of DIM is mediated by AKT and NF-kappaB pathways in prostate cancer cells. Front. Biosci. 2005;10:236–243. doi: 10.2741/1523. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Guo B. Chemopreventive agent 3,3′-diindolylmethane selectively induces proteasomal degradation of class I histone deacetylases. Cancer Research. 2010;70:646–654. doi: 10.1158/0008-5472.CAN-09-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J. Biol. Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- Liu B, Mao Q, Cao M, Xie L. Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int. J. Urol. 2012;19:134–141. doi: 10.1111/j.1442-2042.2011.02906.x. [DOI] [PubMed] [Google Scholar]
- Lubet RA, Heckman BM, De Flora SL, Steele VE, Crowell JA, Juliana MM, Grubbs CJ. Effects of 5,6-benzoflavone, indole-3-carbinol (I3C) and diindolylmethane (DIM) on chemically-induced mammary carcinogenesis: is DIM a substitute for I3C? Oncol. Rep. 2011;26:731–736. doi: 10.3892/or.2011.1316. [DOI] [PubMed] [Google Scholar]
- Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB. J. 2006a;20:506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006b;27:811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- Nachshon-Kedmi M, Fares FA, Yannai S. Therapeutic activity of 3,3′-diindolylmethane on prostate cancer in an in vivo model. Prostate. 2004a;61:153–160. doi: 10.1002/pros.20092. [DOI] [PubMed] [Google Scholar]
- Nachshon-Kedmi M, Yannai S, Fares FA. Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3′-diindolylmethane through the mitochondrial pathway. Br. J. Cancer. 2004b;91:1358–1363. doi: 10.1038/sj.bjc.6602145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AS, Watson RW, Lawler M, Hollywood D. The epigenome as a therapeutic target in prostate cancer. Nat. Rev. Urol. 2010;7:668–680. doi: 10.1038/nrurol.2010.185. [DOI] [PubMed] [Google Scholar]
- Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol. Cancer Ther. 2007;6:1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Delage B, Dashwood WM, Yu TW, Wuth B, Willia,s DE, Ho E, Dashwood RH. Histone deacetylase turnover and recovery in sulforaphane-treated colon cancer cells: competing actions of 14-3-3 and Pin1 in HDAC3/SMRT corepresor compex dissociation/reassembly. Mol. Cancer. 2011;10:68. doi: 10.1186/1476-4598-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokhlin OW, Glover RB, Guseva NV, Taghiyev AF, Kohlgraf KG, Cohen MB. Mechanisms of cell death induced by histone deacetylase inhibitors in androgen receptor-positive prostate cancer cells. Mol. Cancer Res. 2006;4:113–123. doi: 10.1158/1541-7786.MCR-05-0085. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Li Y. Indole-3-carbinol and prostate cancer. J. Nutr. 2004;134:3493S–3498S. doi: 10.1093/jn/134.12.3493S. [DOI] [PubMed] [Google Scholar]
- Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–1266. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- Souli E, Machluf M, Morgenstern A, Sabo E, Yannai S. Indole-3-carbinol (I3C) exhibits inhibitory and preventive effects on prostate tumors in mice. Food Chem. Toxicol. 2008;46:863–870. doi: 10.1016/j.fct.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Steinbrecher A, Nimptsch K, Husing A, Rohrmann S, Linseisen J. Dietary glucosinolate intake and risk of prostate cancer in the EPIC-Heidelberg cohort study. Int. J. Cancer. 2009;125:2179–2186. doi: 10.1002/ijc.24555. [DOI] [PubMed] [Google Scholar]
- Vivar OI, Lin CL, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane induces a G(1) arrest in human prostate cancer cells irrespective of androgen receptor and p53 status. Biochem. Pharmacol. 2009;78:469–476. doi: 10.1016/j.bcp.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dashwood WM, Bailey GS, Williams DE, Dashwood RH. Tumors from rats given 1,2-dimethylhydrazine plus chlorophyllin or indole-3-carbinol contain transcriptional changes in beta-catenin that are independent of beta-catenin mutation status. Mutat. Res. 2006;601:11–18. doi: 10.1016/j.mrfmmm.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Schoene NW, Milner JA, Kim YS. Broccoli-derived phytochemicals indole-3-carbinol and 3,3′-diindolylmethane exerts concentration-dependent pleiotropic effects on prostate cancer cells: comparison with other cancer preventive phytochemicals. Mol. Carcinog. 2012;51:244–256. doi: 10.1002/mc.20774. [DOI] [PubMed] [Google Scholar]
- Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, Kristiansen G. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br. J. Cancer. 2008;98:604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortelboer HM, de Kruif CA, van Iersel AA, Falke HE, Noordhoek J, Blaauboer BJ. Acid reaction products of indole-3-carbinol and their effects on cytochrome P450 and phase II enzymes in rat and monkey hepatocytes. Biochem. Pharmacol. 1992;43:1439–1447. doi: 10.1016/0006-2952(92)90200-3. [DOI] [PubMed] [Google Scholar]
- Yu Z, Mahadevan B, Lohr CV, Fischer KA, Louderback MA, Krueger SK, Pereira CB, Albershardt DJ, Baird WM, Bailey GS, Williams DE. Indole-3-carbinol in the maternal diet provides chemoprotection for the fetus against transplacental carcinogenesis by the polycyclic aromatic hydrocarbon dibenzo[a,l]pyrene. Carcinogenesis. 2006;27:2116–2123. doi: 10.1093/carcin/bgl072. [DOI] [PubMed] [Google Scholar]