Abstract
Two critical stages of mammalian oocyte regulation are prophase I arrest, which is important for sustaining the oocyte pool, and meiosis I (MI), which produces fertilisable eggs. Here we find that the spindle assembly checkpoint protein, BubR1, regulates both stages in mouse oocytes. We show that oocytes depleted of BubR1 cannot sustain prophase I arrest and readily undergo germinal vesicle breakdown (GVBD), a marker for re-entry into MI. BubR1-depleted oocytes then arrest prior to completing MI, marked by failure of polar body extrusion. Both meiotic defects in BubR1-depleted oocytes are due to reduced activi ty of the master regulator known as the anaphase-promoting complex (APC), brought about through diminished levels of the APC co-activator, Cdh1.
Mammalian oocytes arrest at prophase I from birth until puberty when hormonal signals induce the resumption o f meiosis I (MI) and progression to meiosis II (MII), the stage at which fertilisation occurs. Prophase I-arrested oocytes possess an intact nucleus referred to as the germinal vesicle (GV), with GV breakdown (GVBD) and first polar body extrusion (PBE) sig nifying the resumption and conclusion of MI respectively (Fig. 1A). Cell-cycle progression is driven by a proteolytic machinery known as the anaphase-promoting complex (APC) acting in concert with one of two co-activators, Cdc20 and Cdh1(1). Unlike mitotic prometaphase in which APCCdc20 is the principal APC species(1), in mammalian oocytes, APCCdh1 is active during prophase I(2, 3) and early prometaphase I prior to APCCdc20 in late MI(4)(Fig. 1A). In both systems however, the anaphase-trigger is APCCdc20-mediated securin and cyclin B1 degradation(1, 4, 5). The key inhibitor of APCCdc20-dependent anaphase-onset is the spindle assembly checkpoint (SAC), the core components of which are drawn from the Mad and Bub protein families(6). Mad2 and Bub1 have predicted APCCdc20-directed SAC roles in mouse oocytes and consequently regulate late MI coincident with APCCdc20 activity(7, 8). Here, we examine the role of another key SAC protein, BubR1, in the unique environment in mouse oocytes where APCCdh1 is active prior to activation of APCCdc20.
Fig. 1.
Prophase I arrest is compromised after BubR1-depletion. (A) Schematic of MI. (B) Mock-depleted (+ControlMO), BubR1-depleted (+BubR1MO), Mad2-depleted (+Mad2MO)(7), Cdh1-depleted (+Cdh1MO)(9) and uninjected oocytes were scored for GVBD after 24 h in IBMX. Note that Cdh1-depleted oocytes escape prophase I arrest as shown previously(2). Increased GVBD following BubR1-depletion could be prevented by expressing either BubR1 from an hBubR1 cRNA (+BubR1MO+hBubR1 cRNA) or Cdh1 from a Cdh1 cRNA (+BubR1MO+Cdh1 cRNA)(9). Error bars, mean ± SEM; N ≥ 3. Asterisks denote a significant difference from uninjected controls (P < 0.0001; Student’s t-test). (C and D) Samples (50 oocytes) of GV-stage oocytes from each of the groups depicted were immunoblotted either for Cdh1 and actin (C) or for BubR1, Cdh1 and GAPDH (D) (N = 2). (*), non-specific band.
We employed a morpholino-based gene-silencing approach(9) to deplete BubR1 in oocytes(2, 7). Using a BubR1-targeting morpholino (termed BubR1MO)(9), we depleted ~80% of endogenous BubR1 (fig. S1). Unexpectedly, during the course of evaluating BubR1MO, we found that 25% of BubR1-depleted oocytes spontaneously underwent GVBD in 3-isobutyl-1-methylxanthine (IBMX), a drug which maintains prophase I arrest in almost 98% of control and Mad2-depleted oocytes (Fig. 1B)(2, 9). This indicated that BubR1-depleted oocytes have reduced capacity for sustaining prophase I arrest. In mouse oocytes, the prophase I arrest-state is dependent upon APCCdh1 activity(2, 3). We therefore examined Cdh1 in BubR1-depleted oocytes and found that it was reduced by ~60% (Fig. 1C). This reduction in Cdh1 levels was specific to BubR1-depletion as Cdh1 was unaffected by Mad2-depletion (Fig. 1C) and Cdh1 levels could be restored in BubR1MO-injected oocytes by co-expressing human BubR1 (hBubR1) from hBubR1 cRNA (Fig . 1D). This suggested that the fragility of prophase I arrest following BubR1-depletion was due to reduced APCCdh1 activity. In support of this , GVBD rates in BubR1-depleted oocytes declined dramatically after restoring Cdh1 l evels by injecting either Cdh1 cRNA or hBubR1 cRNA (Fig. 1B) . Thus, by maintaining APCCdh1 activity, BubR1 is important for prophase I arrest in mouse oocytes consistent with a prophase I role for homologues of BubR1 in yeast and flies(10, 11).
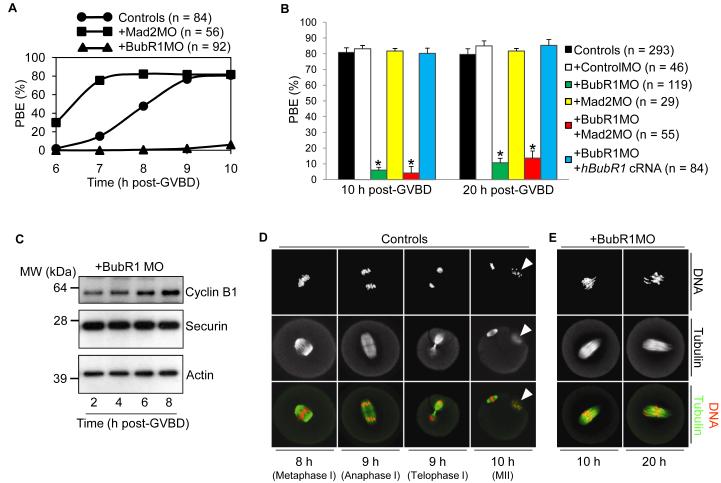
Although BubR1-depleted oocytes readily resumed MI, there wa s a marked reduction in PBE rates . By 10 h post-GVBD, 80% of control oocytes undergo PBE contrasting sharply with only 6% of BubR1-depleted oocytes (Fig. 2, A and B). This effect was not anticipated as BubR1, like Mad2, is widely known to inhibit APCCdc20(1, 6). Indeed, we find that in oocytes, BubR1 exhibits properties typical of an SAC protein, including the capacity for APCCdc20 inhibition (fig. S2). Hence, BubR1-depletion would be expected to prematurely activate APCCdc20 and to advance MI exit as occurs after Mad2-depletion (Fig. 2A)(7). Instead, BubR1-depleted oocytes undergo an MI arrest (Fig. 2, A and B) implying that BubR1 has additional functions during MI separate from its SAC role.
Fig. 2.
BubR1-depletion induces a prometaphase I arrest. (A) Timeline of PBE for uninjected controls, Mad2-depleted and BubR1-depleted oocytes. Oocytes were scored for the presence of polar bodies at 6 h, 7 h , 8 h, 9 h and 10 h post-GVBD. Note the contrasting effects of Mad2- and BubR1-depletion on PBE. (B) PBE is inhibited after BubR1-depletion. PBE rates were determined at 10 h and 20 h post-GVBD. Note that prometaphase I arrest is robust after BubR1-depleti on so that PBE increases only modestly (from 6% to 11%) during an additional 10 h of culture (from 10 h post-GVBD to 20 h post-GVBD) . Furthermore, co-depletion of Mad2 does not restore PBE rates indicating that MI arrest is not SAC-mediated. Error bars, me an + SEM; N ≥ 3. Asterisks denote a significant difference from uninjected controls (P < 0.0001; Student’s t test). (C) Samples (50 oocytes) of BubR1-depleted oocytes at 2 h, 4 h, 6 h and 8 h post-GVBD were blotted for cyclin B1, securin and actin. (D and E) Control and BubR1-depleted oocytes were immunostained for tubulin and DNA at the times post-GVBD indicated in the figure (n ≥ 15 oocytes per time-point). Note that by 10 h post-GVBD in controls, a polar body (white arrowhead) with associated chromosomes is present.
In order to explore the mechanism of MI arrest in BubR1-depleted oocytes, we examined the levels of securin and cyclin B1. We observed that after BubR1-depletion, securin and cyclin B1 levels remained stable by 8 h post-GVBD whereas both proteins had been destroyed in controls (Fig. 2C and fig. S3) . Given that securin and cyclin B1 destruction are required for anaphase I(4, 5), this indicated that BubR1-depleted oocytes arrest before anaphase I. We confirmed this by immunostaining spindles and chromosomes. By 10 h post-GVBD and beyond, BubR1-depleted oocytes show no evidence of anaphase I, telophase I or MII configurations all of which are apparent in controls between 8 h and 10 h post-GVBD (Fig. 2, D and E). Together, these data show that BubR1-depleted oocytes arrest in prometaphase I.
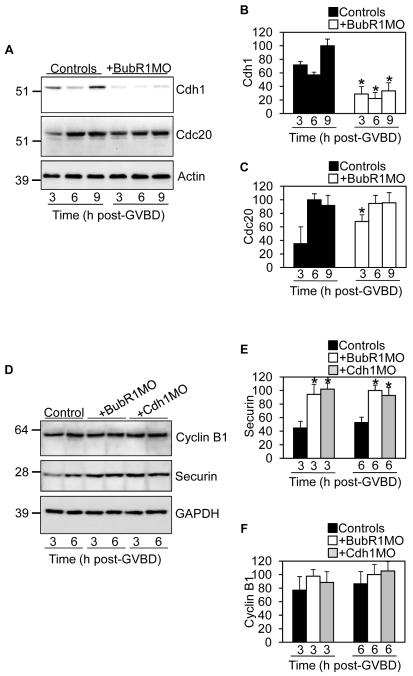
APCCdh1 is active during prometaphase I (Fig. 1A), the stage at which BubR1-depleted oocytes arrest. Given that we have found BubR1 to be important for Cdh1 stability in prophase I (Fig. 1, C and D), a similar BubR1-dependent effect on APCCdh1 in prometaphase I could plausibly underpin prometaphase I arrest after BubR1-depletion. This prompted us to examine Cdh1 levels during MI. Strikingly, as with prophase I, following BubR1-depletion , Cdh1 was again reduced by 50-70% throughout prometaphase I whereas Cdc20 was unaffected (Fig. 3, A to C). In the reverse experiment, BubR1 over-expression stabilised Cdh1 (fig. S4, A and B). This supported our hypothesis that deregulated APCCdh1 is central to prometaphase I arrest following BubR1-depletion. Moreover, since Mad2 does n ot share BubR1’s influence on Cdh1 levels (Fig. 1C), this explains why BubR1-and Mad2-depletion produce contrasting phenotypes.
Fig. 3.
(A to C) Cdh1 is reduced after BubR1-depletion. Samples (30 oocytes) of BubR1-depleted oocytes were immunoblotted along with uninjected controls at 3 h, 6 h and 9 h post-GVBD for Cdh1, Cdc20 and actin (A). Band intensities of (B) Cdh1 and (C) Cdc20 on blots were quantified and normalised to values found in controls. (D to F) Securin is preferentially stabilised after either BubR1- or Cdh1-depletion. Samples (30 oocytes) of BubR1-depleted and Cdh1-depleted oocytes were immunoblotted along with uninjected controls at 3 h and 6 h post-GVBD for securin, cyclin B1 and GAPDH (D). Band intensities of (E) securin and (F) cyclin B1 on blots were quantified and normalised to values found in controls. Error bars, mean + SEM; N = 3. Asterisks (B and C and E) denote a significant difference from uninjected controls (P < 0.0001; Student’s t test).
We therefore focused on the first 6 hours of MI when APCCdh1 is active (Fig. 1A) and examined securin and cyclin B1, two recognised APCCdh1 substrates that must be meticulously regulated for proper M-phase progression(1). We observed that during early prometaphase I, securin was 2-fold higher in BubR1-depleted oocytes compared to controls whereas cyclin B1 was unaffected (Fig. 3, D to F). Thus, after BubR1-depletion, Cdh1 is reduced and securin is increased. The inverse relationship between Cdh1 and securin was consistent with securin being a preferential APCCdh1 substrate during early prometaphase I as is the case during prophase I(2) and in mitosis(12). In support of this we found that securin, but not cyclin B1, was stabilised during early prometaphase I in oocytes depleted of Cdh1 (Fig . 3, D to F, fig. S5 and fig. S11). The converse was also true as Cdh1 over-expression led to a reduction in securin (fig. S6). Hence, these data show that low-level APCCdh1-mediated securin destruction occurs during prometaphase I and is BubR1-dependent. As further confirmation of BubR1-dependency, we found that the extent of the Cdh1 decrease and securin increase was proportional to the severity of BubR1-depletion (fig. S7). Moreover, BubR1 over-expression increased Cdh1 and decreased securin (fig. S4, A to C), changes that were exactly opposite to those induced by BubR1-depletion. Thus, APCCdh1-mediated securin destruction during early prometaphase I is BubR1-dependent and is required for preventing securin over-accumulation.
Next we asked whether failure of anaphase I in BubR1-depleted oocytes (Fig. 2) might be attributable to high securin levels. We now turned our attention to APCCdc20 as anaphase I is mediated by late-acting APCCdc20 and not by APCCdh1(4)(Fig. 1A). Anaphase I is exquisitely vulnerable to any perturbation of the natural balance between APCCdc20 and either securin or cyclin B1. For instance, over-expression of securin from an exogenous Securin-GFP cRNA invokes a prometaphase I arrest(5) by overwhelming APCCdc20. Similarly, prometaphase I arrest after BubR1-depletion could arise if APCCdc20 was being outstripped by elevated secur in. If this were so, one clear prediction is that redressing the presumed APCCdc20/securin mismatch should enable BubR1-depleted oocytes to undergo anaphase I and to exit MI. Entirely consistent with this notion, PBE rates were significantly increased in BubR1-depleted oocytes either by over-expressing Cdc20 or by restraining securin expression (from 6% to 40% and 66% respectively; fig. S8).
Notably however, PBE rates in BubR1-depleted oocytes were not fully restored to wild-type levels after reinstating a favourable APCCdc20/securin balance (fig. S8). This suggested that other deficits following BubR1-knockdown such as kinetochore-microtubule attachment defects, known to be BubR1-dependent in mitosis(13), might also hinder meiotic progression. To address this possibility, we investigated two readouts of kinetochore-microtubule attachment status: Mad2, which localises to unattached kinetochores(14), and the presence of cold-stable microtubules since microtubules are unstable at 4 °C unless attached to kinet ochores(13). At 4 h post-GVBD, kinetochore-microtubule attachments have not yet formed in control oocytes(15), which consequently exhibit strong Mad2 staining (fig. S2B) and virtually no cold-stable microtubules (Fig. 4D). By 8 h post-GVBD, when kinetochores become fully attached, chromosomes are well-aligned, Mad2 becomes undetectable (Fig. 4A and fig. S2C) and a metaphase I spindle persists after cold-treatment (Fig. 4E). In stark contrast, by 8 h post-GVBD in BubR1-depleted oocytes, chromosomes are misaligned, remain strongly positive for Mad2 (Fig. 4, B and C) and have very few cold-stable microtubules (Fig. 4F). Kinetochore-microtubule attachment defects are not a consequence of the Cdc20/securin imbalance brought about by BubR1-depletion as they persist when securin expression is restrained or Cdc20 is co-expressed in BubR1-depleted oocytes (fig. S9). Thus, microtubule attachments form less efficiently after BubR1-depletion and likely explain previous observations of chromosome alignment defects in MII oocytes from BubR1-deficient mutant mice(16).
Fig. 4.
BubR1-depletion impairs kinetochore-microtubule attachments. (A to C) At 8 h post-GVB D, control oocytes (A) and BubR1-depleted oocytes (B and C) were immunostained for tubulin, DNA and Mad2. (D to F) Control oocytes were cold-treated(9) and immunostained at 4 h post-GVBD (D) and 8 h post-GVBD (E). Note the change in spindle morphology afte r cold-treatment (compare A and E). BubR1-depleted oocytes were cold-treated and immunostained at 8 h post-GVBD (F) (n ≥ 20 oocytes per group). Note that chromosomes are extended although kinetochore-microtubule attachments are lacking (B and C), implicating direct contacts between microtubules and chromatin as demonstrated previously(15). Conversely, chromosomes become compacted when microtubules are lacking (F, white arrow).
We show here that BubR1 sustains Cdh1 levels in prophase I and prometaphase I before inhibiting APCCdc20 in late MI. Interestingly, Cdh1 is also required for sustaining BubR1 levels revealing a co-dependency between BubR1 and Cdh1 that could be relevant to the mechanism by which BubR1 stabilises Cdh1 (fig. S10). In prophase I , BubR1-dependent Cdh1 stabilisation is important for preventing unscheduled re-entry into MI. Following GVBD, BubR1 promotes prometaphase I progression by preventing indiscriminate securin accumulation and by contributing to the establishment of kinetochore-microtubule attachments . As part of its SAC role, BubR1 then modulates the metaphase I-to-anaphase I transition. BubR1’s Cdh1-directed role appears to predominate as BubR1-depleted oocytes experience MI arrest whilst BubR1 over-expression accelerates MI (Fig. S4D).
These data uncover a striking contrast between somatic cells and oocytes; during APCCdh1-dominated prometaphase I in oocytes , BubR1 promotes M-phase progression by stabilising Cdh1 whereas in mitotic prometaphase where APCCdc20 predominates, BubR1 delays anaphase-onset by inhibiting APCCdc20. Moreover, cyclin B1 is the APC-substrate that is preferentially regulated by BubR1 during mitosis(12) whereas during MI, it is securin (these data). BubR1 deficiency could have grave consequences for fertility by reducing the prophase I-arrested oocyte reservoir and compromising the yield of fertilisable eggs.
Supplementary Material
Acknowledgements
This work was supported by a Wellcome Trust Clinical Fellowship (082587/Z/07/Z) to H.H. J.C. is funded by an MRC grant. We thank Stephen Taylor, William Earnshaw and Katja Wassmann for reagents.
Footnotes
Supporting Online Material
www.sciencemag.org, Materials and Methods, Figs. S1 to S11, References
References and Notes
- 1.Peters J. Nat Rev Mol Cell Biol. 2006;7:644. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 2.Marangos P, Carroll J. Nat Cell Biol. 2008;10:445. doi: 10.1038/ncb1707. [DOI] [PubMed] [Google Scholar]
- 3.Reis A, Chang H, Levasseur M, Jones K. Nat Cell Biol. 2006;8:539. doi: 10.1038/ncb1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis A, et al. Nat Cell Biol. 2007;9:1192. doi: 10.1038/ncb1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbert M, et al. Nat Cell Biol. 2003;5:1023. doi: 10.1038/ncb1062. [DOI] [PubMed] [Google Scholar]
- 6.Musacchio A, Salmon ED. Nat Rev Mol Cell Biol. 2007;8:379. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 7.Homer H, et al. Genes Dev. 2005;19:202. doi: 10.1101/gad.328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuinness BE, et al. Curr Biol. 2009;19:369. doi: 10.1016/j.cub.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 9.Materials and methods are available as supporting material on Science Online.
- 10.Cheslock P, Kemp B, Boumil R, Dawson D. Nat Genet. 2005;37:756. doi: 10.1038/ng1588. [DOI] [PubMed] [Google Scholar]
- 11.Malmanche N, et al. Curr Biol. 2007;17:1489. doi: 10.1016/j.cub.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeganathan KB, Malureanu L, van Deursen JM. Nature. 2005;438:1036. doi: 10.1038/nature04221. [DOI] [PubMed] [Google Scholar]
- 13.Lampson M, Kapoor T. Nat Cell Biol. 2005;7:93. doi: 10.1038/ncb1208. [DOI] [PubMed] [Google Scholar]
- 14.Waters JC, Chen RH, Murray AW, Salmon ED. J Cell Biol. 1998;141:1181. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunet S, et al. J Cell Biol. 1999;146:1. doi: 10.1083/jcb.146.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker D, et al. Nat Genet. 2004;36:744. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.