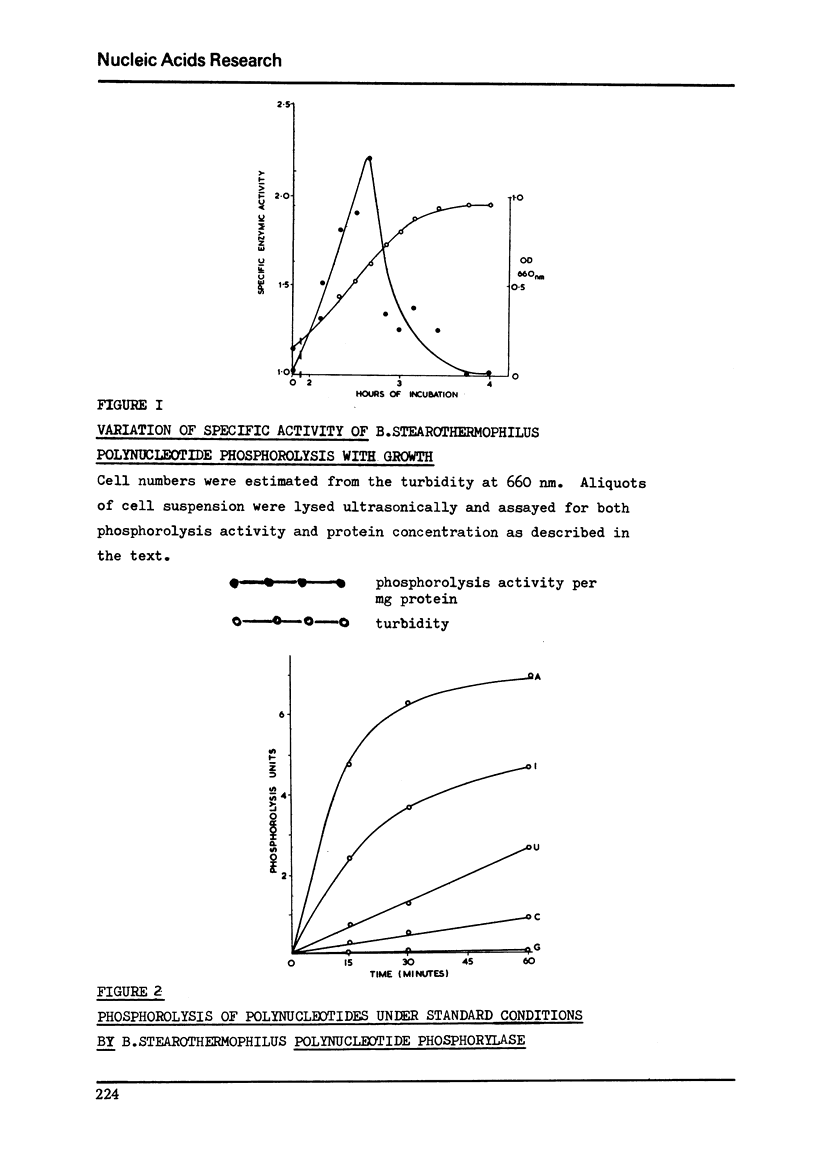
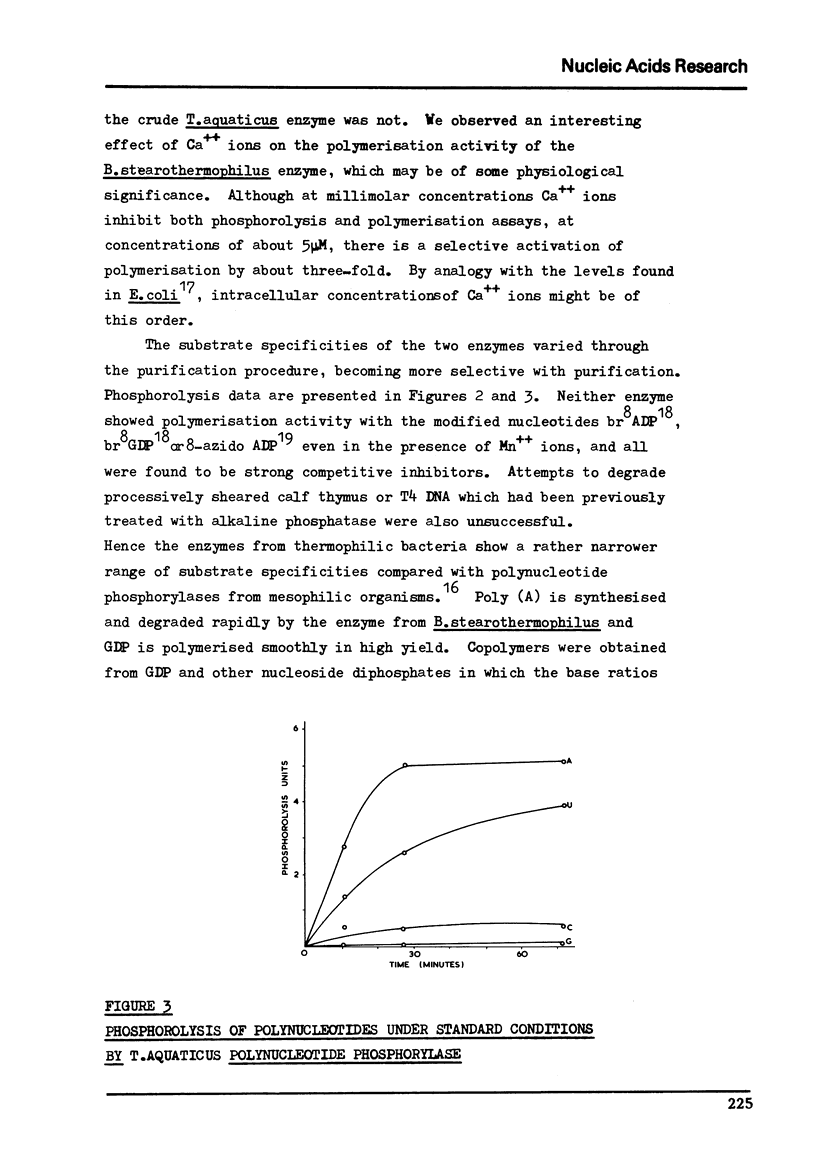
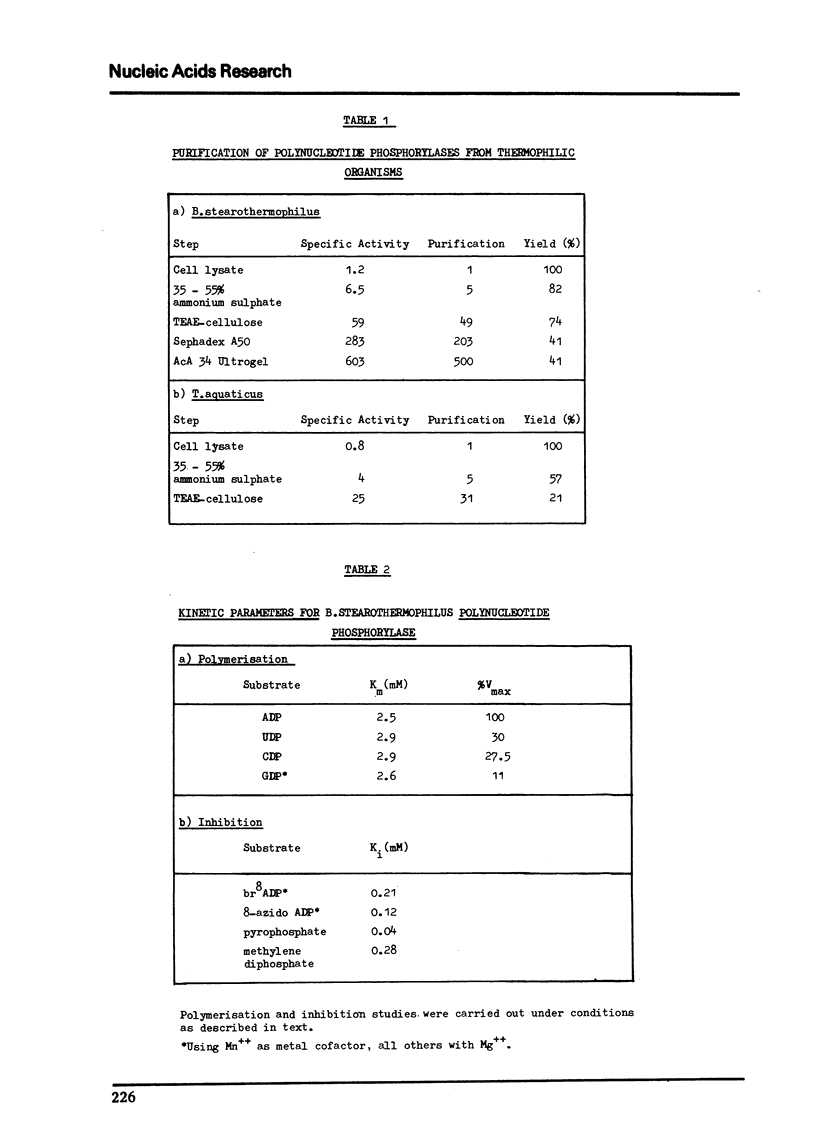
Abstract
Polynucleotide phosphorylase from Bacillus stearothermophilus has been purified to homogeneity. Polyacrylamide gel electrophoresis run under denaturing conditions indicates that the enzyme is a tetramer with subunits of apparent molecular weight 51,000 daltons. A partial purification of polynucleotide phosphorylase from Thermus aquaticus has also been effected. The two enzymes show similar catalytic properties, which differ little from those of mesophilic polynucleotide phosphorylases. The use of thermostable polynucleotide phosphorylases for in vitro nucleic acid synthesis is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D., Freeze H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol. 1969 Apr;98(1):289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachimori A., Muramatsu N., Noso Y. Studies on an ATPase of thermophilic bacteria. I. Purification and properties. Biochim Biophys Acta. 1970 Jun 10;206(3):426–437. doi: 10.1016/0005-2744(70)90158-0. [DOI] [PubMed] [Google Scholar]
- Howard F. B., Frazier J., Miles H. T. Formation of polynucleotide helices having purine nucleotide residues in a syn configuration. J Biol Chem. 1972 Oct 25;247(20):6733–6735. [PubMed] [Google Scholar]
- Ikehara M., Tazawa I., Fukui T. Polynucleotides. VII. Synthesis of ribopolynucleotides containing 8-substituted purine nucleotides by polynucleotide phosphorylase. Biochemistry. 1969 Feb;8(2):736–743. doi: 10.1021/bi00830a040. [DOI] [PubMed] [Google Scholar]
- Kapuler A. M., Monny C., Michelson A. M. The relationship of mono- and polynucleotide conformation to catalysis by polynucleotide phosphorylase. Biochim Biophys Acta. 1970 Sep 17;217(1):18–29. doi: 10.1016/0005-2787(70)90118-8. [DOI] [PubMed] [Google Scholar]
- Letendre C. H., Singer M. F. Further characterization of the polynucleotide phosphorylase of Micrococcus luteus. Nucleic Acids Res. 1975 Feb;2(2):149–164. doi: 10.1093/nar/2.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Portier C., van Rapenbusch R., Minh-Nguy-Thang, Grunberg-Manago M. Quaternary structure of polynucleotide phosphorylase from Escherichia coli. Eur J Biochem. 1973 Dec 3;40(1):77–87. doi: 10.1111/j.1432-1033.1973.tb03170.x. [DOI] [PubMed] [Google Scholar]
- Silver S., Kralovic M. L. Manganese accumulation by Escherichia coli: evidence for a specific transport system. Biochem Biophys Res Commun. 1969 Mar 10;34(5):640–645. doi: 10.1016/0006-291x(69)90786-4. [DOI] [PubMed] [Google Scholar]
- Tazawa S., Tazawa I., Alderfer J. L., Ts'o P. O. Conformation of oligoinosinates: chain-length dependence and comparison to other oligonucleotides. Biochemistry. 1972 Sep 12;11(19):3544–3558. doi: 10.1021/bi00769a009. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]


