Abstract
One of the key roles of the immune system is the identification of potentially dangerous pathogens or tumour cells, and raising a wide range of mechanisms to eliminate them from the organism. One of these mechanisms is activation and expansion of antigen-specific cytotoxic T cells, after recognition of antigenic peptides on the surface of antigen presenting cells such as dendritic cells (DCs). However, DCs also process and present autoantigens. Therefore, antigen presentation has to occur in the appropriate context to either trigger immune responses or establishing immunological tolerance. This is achieved by co-stimulation of T cells during antigen presentation. Co-stimulation consists on the simultaneous binding of ligand-receptor molecules at the immunological synapse which will determine the type and extent of T cell responses. In addition, the type of cytokines/chemokines present during antigen presentation will influence the polarisation of T cell responses, whether they lead to tolerance, antibody responses or cytotoxicity. In this review, we will focus on approaches manipulating co-stimulation during antigen presentation, and the role of cytokine stimulation on effective T cell responses. More specifically, we will address the experimental strategies to interfere with negative co-stimulation such as that mediated by PD-L1 (Programmed cell death 1 ligand 1)/PD-1 (Programmed death 1) to enhance anti-tumour immunity.
Keywords: Cancer, Co-stimulation, Dendritic cell, Immunotherapy, Signalling, T cell, B7, PD-L1, PD-L2, CTLA4, PD-1, CD80, MAPK, NF-κB
INTRODUCTION
A key drawback for effective immunotherapy of cancer is to stimulate effective T cell responses against tumour antigens. This is mainly caused by the natural tolerance towards tumour-associated antigens (TAAs), which are in most cases mutated forms of self-proteins. Therefore, a plausible strategy leading to effective cancer immunotherapy would be to present TAAs to antigen-specific T cells in a context that can overcome the endogenous tolerogenic mechanisms. TAA-specific T cells are present in physiological conditions, but their effector activities are strongly suppressed. However, these T cells can be activated and effectively exert therapeutic anti-tumour immune responses [1]. Therefore, cancer immunotherapy is currently being developed in two fronts. The first one, induction of T cells with high affinity TAA-specific T cell receptors (TCRs), and the second, the enhancement of antigen presentation by manipulating the immunological synapse between antigen presenting cells and T cells.
Dendritic cells and antigen presentation
DCs comprise a heterogeneous population of antigen-presenting cells (APCs), which regulate the priming of innate and adaptive immune responses [2]. DCs derive from cluster of differentiation (CD)34 hemapoietic progenitors, and so far, 3 main sub-populations have been described according to the expression of characteristic surface markers [3]. Generally, DCs are classified as myeloid (or conventional) DCs which express CD11c, or CD11c negative plasmacytoid DCs. There is evidence that these two linages derive from myeloid or lymphoid progenitor cells, respectively [4]. In addition, a third DC sub-population originates from differentiated monocytes, which have lost the expression of the monocyte marker CD14 [5].
DCs are generated in the bone marrow and remain in peripheral tissues in an immature stage, where they phagocytize and process a wide range of antigens by proteosomal or endosomal pathways, depending on the nature of the antigen. These antigens also include self-proteins or innocuous substances, but several endogenous tolerogenic mechanisms are in place to prevent autoimmune responses. However, when these DCs encounter pathogens, “danger molecules” such as cellular heat shock proteins or inflammatory mediators, they migrate to secondary lymphoid organs and acquire strong antigen presenting capacities (DC maturation) [6-12]. There, DCs present antigenic peptides to either CD4 or CD8 T cells, depending on the major histocompatibility complex (MHC) context in which these peptides are presented. For example, intracellular antigens (such as viral and tumor antigens) are degraded in the proteasome, and the resulting peptides are preferentially loaded into MHC class I molecules (p-MCH) at the endoplasmic reticulum. These p-MHC I complexes are transported and exposed on the cell membrane [9]. Antigen-specific CD8 T cells recognise these p-MHC I molecules, followed by activation and differentation to cytotoxic T cells. On the other hand, to activate CD4 T cells, antigenic peptides must be presented in the context of MHC class II molecules. This is usually (but not exclusively) accomplished after antigen internalisation by phagocytosis and degradation in endosomes. These CD4 T cells will then differentiate into specific subsets which regulate immune responses in different ways. Thus, these T cells are usually called “helper” (Th cells) and can either stimulate cytotoxic responses (Th1, Th17), antibody responses (Th2), or inhibit immune responses (regulatory T cells, or Tregs). The fate of differentiation will strongly depend on the type of cytokines and chemokines present during antigen presentation.
The “three-signal” hypothesis of T cell activation
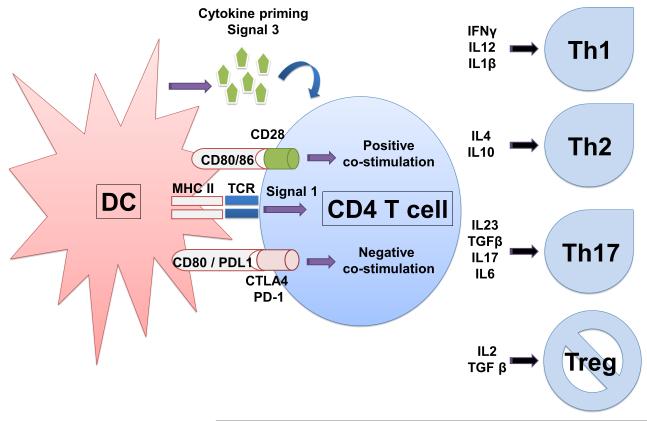
Possibly, one of the key roles of DCs is to control and direct T cell activation at the level of antigen presentation, as they are capable of priming naïve T cells [10]. As explained above, antigens have to be processed into peptides and presented to specific T cells in the immunological synapse [13-16]. It is within the immunological synapse where certain signals are delivered to the T cells resulting in their activation, expansion and acquisition of effector activities. The first signal, or signal 1, refers to the engagement between the TCR and p-MHC complexes on the surface of the APC (Figure 1). This engagement is a necessary, but not sufficient, requirement for T cell activation. In fact, triggering of the TCR alone usually leads to T cell anergy, that is, limited expansion followed by unresponsiveness after reencounter with antigen [17, 18]. To effectively activate T cells, further signals have to be provided to T cells in the immunological synapse. These signals are generally called “co-stimulation” and they are delivered by the binding of certain receptors on the T cell surface with their ligands on the DC surface. Overall, co-stimulation is considered as a second signal or “signal 2” (Figure 1).
Figure 1. Antigen presentation in the immunological sypnase.
The scheme depicts a DC (left) presenting antigen in the context of MHC molecules to a CD4 T cells. The peptide-MHC engages to the T cell TCR delivering signal 1 as shown within the T cells. Simultaneously, co-stimulatory or co-inhibitory ligands on the DC surface (CD80/86, or CD80/PDL1) engage with their receptors on the T cell, leading to either positive stimulation or negative stimulation. The resulting signal from the integration of these co-stimulatory interactions will provide a second signal to T cells. In this scheme, the DC produces cytokines within the immunological synapse, giving a third T cell polarising signal. On the right, the most representative T helper subtypes are shown, and the cytokines promoting these T cell subtypes are also indicated.
Co-stimulation plays a fundamental role in regulating T cell functions at multiple levels, particularly during antigen presentation. Antigen-presenting DCs can engage naïve T cells and provide “positive” (activatory) or “negative” (inhibitory) co-stimulation. Ultimately, these signals are provided by a wide panel of co-stimulatory receptors expressed by DCs. For example, binding between CD80/CD86 in DCs and CD28/CD27 in T cells causes T cell proliferation and acquisition of cytotoxic activities [19]. On the other hand, CD80/86 can also engage inhibitory receptors on T cells such as the cytotoxic T-lymphocyte antigen 4 (CTLA4), and deliver inhibitory signals leading to anergic T cells or Tregs (Figure 1) [15]. As another example, programmed death-ligand 1 (PD-L1) in DCs binds to its receptor PD-1 on T cells. This binding inhibits T cell activities at multiple levels [20-22]. Thus, it is not surprising that tolerogenic immature DCs exhibit low levels of co-stimulatory molecules and up-regulate inhibitory molecules [23, 24]. After encounter with pathogen-derived molecules which trigger pathogen pattern recognition receptors such as toll-like receptors (TLRs), DCs strongly up-regulate co-stimulatory molecules and p-MHC complexes. As both “positive” and “negative” co-stimulation takes place during antigen presentation, there is a downstream integration of these signals, which ultimately determines the “strength” of the second signal and controls T cell activation [19].
As discussed above, two distinct signals need to be delivered for T cell activation; signal 1 (antigen recognition) and signal 2 (co-stimulation). However, although these two signals may activate T cells, an additional signal has to be provided which will polarise T cell differentiation. This is particularly important for CD4 T cell activation, since they can regulate CD8 T and B cell activities.
Signal 3 or cytokine priming
DCs amongst other cell types secrete different cytokines while undergoing antigen presentation to T cells, depending on the particular stimuli that they encountered at the site of infection or inflammation. These cytokines will influence the differentiation profile of T cells activated by signals 1 and 2. This is generally referred to as the “third signal”, “signal 3”, or “cytokine priming” (Figure 1) [25-27]. When this occurs, T cells differentiate into different effector T cells such as CD4 Th1, Th2, Th17, Treg cells or enhance CD8 T cell responses to weakly immunogenic antigens (Figure 1) [28]. As a matter of fact, extensive T cell proliferation can only occur if Ag levels are high in the absence of signal 3. The third signal contributes most to T cell proliferation when Ag levels are low [28]. Moreover, these naïve T cells that proliferate in the absence of a third signal fail to develop cytolytic effector functions. Thus, the presence or absence of a third signal is essential in determining whether stimulation of DCs by Ag results into tolerance or into development of effector function [29].
The detailed characterization of the polarization potential of cytokines allows the rational design of new vaccination protocols for cancer immunotherapy (Figure 1). For example, cytokines interleukin (IL)1 and IL-12 stimulate differentiation towards Th1 and enhancement of CD8 cytotoxic activities [29-35]. Accordingly, CD8α DCs produce high levels of IL12 and they are particularly effective for CD8 T cell priming [36], and stimulation of clonal expansion via expression of Bcl-3 in proliferating T cells, an anti-apoptotic molecule [37, 38]. In certain situations, IL1-β, IL17, IL6, IL23 and transforming growth factor (TGF)-β can polarize T cell differentiation in a pro-inflammatory T helper subset named Th17 [39, 40]. These T cells express IL17, which triggers an accelerated and strong inflammatory reaction. This subset of T cells has been implicated in the development of autoimmune diseases since it seems to promote the loss of tolerance towards allergens and auto-antigens [41-44]. On the other hand, IL10 and TGF-β will drive T cell differentiation towards Th2 or immunosuppressive Tr1 or Foxp3+ Tregs [23, 24, 45-48].
Requirement of the third signal in cis during antigen presentation
The third signal can be provided by DCs to antigen presenting cells in two distinct situations. The first one, by direct activation (activation in cis) by triggering pattern recognition receptors such as TLRs (Figure 2). This recognition strongly activates DCs leading to their phenotypic maturation and secretion of different cytokine patterns. The type of expressed cytokines will depend on the particular TLR that is triggered, which will differentially activate distinct intracellular signaling pathways. For example, TLR2 activation by yeast zymosan activates extracellularly regulated protein kinase (ERK) and stimulates secretion of IL10 that will result in immune suppression [49, 50]. On the other hand, TLR4 stimulation by a wide variety of ligands results in DC maturation, IL12 production and immune stimulation with anti-tumour properties [3, 51-53].
Figure 2. Effects of directly or indirectly activated DCs on antigen presentation and T cell polarisation.
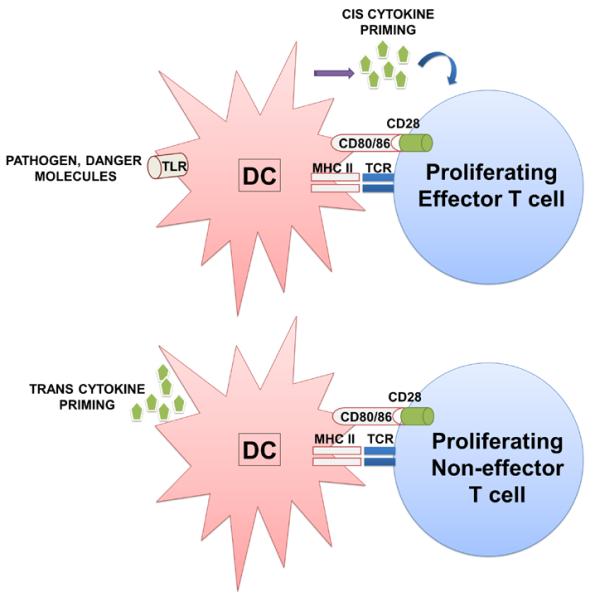
On top, a directly activated DC through TLR engagement (left) presents antigen to T cells and provide the three activatory signals to the T cells (right). In this case, cytokine priming occurs in cis, leading to T cell proliferation and acquisition of effector activities. On the bottom, an indirectly activated DC through cytokine priming in trans (i.e. cytokines produced by neighbouring cells within an inflammatory environment, left of the scheme). In this situation, cytokines are not produced within the immunological synapse and the resulting T cell proliferates, but does not acquire significant effector activities or a distinct differentiation profile.
The second mechanism for providing signal 3 is indirectly in trans through the exposure to inflammatory mediators by neighboring cells during an immune response (Figure 2). This suggests that inflammation itself could substitute pathogen recognition for the induction immune responses [6, 54, 55]. Although from a theoretical point of view this concept could be effectively applied to immunotherapy, there is increasing evidence that indirectly activated APCs after cytokine exposure behave very differently compared to cytokine-secreting, directly activated APCs [56-58]. Indirectly activated DCs up-regulate MHC molecules and are capable of providing co-stimulatory signals, leading to T cell clonal expansion. However, as indirectly activated DCs do not provide the third signal in the immunological synapse, the engaged T cells do not differentiate to particular subsets (Figure 2) [56, 59]. Therefore, inflammation can amplify immune responses, DCs have to provide inflammatory mediators themselves to initiate effective immune responses [56, 60, 61].
These observations demonstrate the importance of developing the right adjuvants to optimize the efficacy of vaccines for immunotherapy [59]. In fact, this could explain the disappointing outcomes of certain cancer immunotherapy clinical trials using CpG as an adjuvant. CpG is recognized by TLR9 and it is a potent inflammatory mediator, although it is absent in conventional human DCs [62, 63]. In addition, CD8α DCs provide strong third signals during antigen presentation, they express TLR3 but not TLR7 [59, 64-66]. Consequently, the right choice of adjuvants could potentiate the current formulations of vaccines for immunotherapy by specifically targeting particular DC subsets.
Modulation of co-stimulation to enhance immunotherapy
The manipulation of the immunological synapsis opens attractive possibilities to control T cell activation and differentiation for the treatment of cancer and autoimmune disorders. To manipulate co-stimulation, the expression levels of co-stimulatory molecules in DCs can be modified. An effective way to achieve this is to specifically activate intracellular signalling pathways in DCs belonging to the TLR signal transduction pathways. The main pathways involved in DC maturation are the nuclear factor (NF)-κB and mitogen activated protein kinases (MAPKs) ERK, p38 and JNK1 [67-73]. This strategy ensures the up-regulation of co-stimulatory, adhesion and major histocompatibility molecules together with cytokine expression, which will provide strong signals 1, 2 and 3.
Most pro-inflammatory genes are controlled by promoters responding to NF-κB dimmers, and thus, this pathway is one of the main controllers of pro-inflammatory responses [8, 74-76]. Its activation is required for up-regulation of co-stimulatory molecules, MHC and pro-inflammatory cytokines, particularly IL6, IL12, tumour necrosis factor (TNF)-α [74, 77-81]. There is also a considerable body of evidence linking MAPKs to enhancement of DC function by up-regulation of co-stimulatory molecules and secretion of pro-inflammatory mediators, although most of these studies use chemical inhibitors rather than specific activators. The p38 MAPK is activated in virtually all cell types by cellular stress and TLR signalling [82-84]. The inactivation of MKK3 in mice, one of the upstream kinases of p38, resulted in a complete lack of IL12 production by macrophages and DCs, and in general a decrease in pro-inflammatory responses [85]. Both p38 and NF-κB contribute of up-regulation of co-stimulatory and MHC molecules in monocyte-derived DCs as shown after LPS treatment in the presence of phosphatidyl inositol 3 kinase (PI3K), p38 and NF-κB inhibitors [8]. In fact, p38 activation induces histone phosphorylation, leading to opening of the chromatin structure allowing NF-κB dimmers to bind to promoters controlling transcriptional up-regulation of pro-inflammatory genes [75]. Actually, p38 activation rather than the JNK pathway seems to be critical for human DC maturation after a wide variety of pro-inflammatory stimuli including cytokines, TLRs and other immunostimulatory agents [69]. However, JNK may act in cooperation with the other MAPKs to induce pro-inflammatory responses and immune activation [86-88]. MAPK p38 has been clearly shown to be critical for anti-viral CD8 T cell responses after CD83 up-regulation in human DCs stimulated via CD40-CD40L. CD40 ligation results is strong secretion of pro-inflammatory cytokines such as IL6 and IL12 [80], which could lead to Th1 and Th17 responses. However, there are still some studies using chemical inhibitors in human DCs that may challenge the absolute requirement of p38 over JNK regarding DC maturation [71].
In the case of the MAPK ERK, most of the evidence points out to a regulatory rather than a stimulatory role, which includes inhibition of DC maturation in mouse and human cells [70]. Chemical inhibition of ERK results in up-regulation of co-stimulatory makers and MHC molecules, accompanied by increased expression of pro-inflammatory cytokines such as TNF-α and IL-12 [70]. ERK inhibition also results in p38 activation, which overcomes ERK-mediated immunosuppressive activities, at least in macrophages [89]. However, some evidence suggests that ERK activation is also required for production of some pro-inflammatory cytokines such as TNF-α [90], IL23 and augmentation of natural killer (NK) responses [86, 91]. This could explain why ERK is in most cases phosphorylated after TLR activation. The timing/kinetics of ERK activation in combination with other signal transduction pathways may influence its role either in immune suppression of immune activation [92-95].
Thus, as TLR activation leads to activation of NF-κB, MAPKs and several other signalling pathways, TLR agonists are currently being used in clinical trials for the treatment of infectious diseases and cancer [96-99]. For example, TLR agonists can be combined with inhibitors of immunosuppressive signalling pathways such as PI3K to enhance differentiation of polyfunctional T cells [100]. The advantage of using synthetic TLR agonists for immunotherapy is the control of the exact composition of the adjuvant in the vaccine [101, 102]. This simplifies vaccine design and prediction of therapeutic outcomes. Concluding, all this body of evidence indicates that activation of NF-κB, p38 and possibly JNK, combined with inhibition of suppressive pathways such as PI3K and ERK, would increase anti-tumour capacities possibly by enhancing signals 1, 2 and 3.
Instead of using TLR agonists as vaccine adjuvants, selective activation/inhibition of signalling pathways can also be applied using gene therapy approaches. Thus, DC activation has been achieved by expression of constitutive activators of signalling pathways in human and mouse DCs [103]. Constitutive activation of NF-κB by expression of Kaposi’s sarcoma-associated human herpesvirus (KSHV) vFLIP protein using lentivectors, or by over-expression of the NF-κB inducing kinase (NIK) using adenoviral vectors effectively up-regulates CD80, CD86, CD83, CD40, ICAM I, MHC class I and II [77, 79]. In addition, this is accompanied by strong secretion of the Th1 polarising cytokines IL12, TNFα and other chemokines, ensuring the delivery of a strong signal 3. In fact, constitutive NF-κB activation in mouse DCs was particularly efficient to stimulate Th1 immune responses [79]. As expected, vFLIP expression clearly enhanced anti-tumour immune responses in a mouse lymphoma model and anti-parasitic immunity in a leishmaniasis model [77, 78]. Similarly, activation of p38 and JNK1 pathways in mouse and human DCs by expression of constitutively active mutants of MAPK kinase kinase (MKK)6 (the upstream p38 kinase) or a fusion protein between MKK7-JNK1 produced similar results [24]. These included up-regulation of CD80, CD40 and ICAM-I, although without significant expression of Th1 cytokines IL12 or TNFα. Nevertheless, these DC activators proved to be effective in immunisation, and exhibit anti-tumour activities at least for the p38 activator [22, 24]. In this case, the lentivector preparation could have directly provided adjuvant activities in vivo through TLR3 and TLR7 engagement, at least in mouse models of vaccination [12]. On the other hand, activation of the ERK pathway in mouse and human DCs by expression of a constitutively active MEK1 mutant effectively down-modulated co-stimulatory molecules, particularly CD40 and MHC molecules [23, 24]. This ensures that “positive” co-stimulation does not take place. Moreover, ERK activation in mouse DCs stimulated the production of bioactive TGF-β. This effect was particularly effective in human DCs, for which this third signal drove T cell differentiation towards immunosuppressive Foxp3 Treg cells [23]. Therefore, signals 1, 2 and 3 can also be modulated to achieve immune suppression and tolerance with therapeutic applications towards autoimmune diseases such as inflammatory arthritis [23].
B7 co-stimulatory molecules as targets for immunotherapy
Recently, much attention has been paid to blocking negative co-stimulation during antigen presentation to enhance immunotherapy for cancer and chronic infectious diseases. More specifically, inhibition of the B7 co-stimulatory family with T cell inhibitory properties [104-112]. One of such inhibitory interactions takes place between either PD-L1 or PD-L2 on the surface of DCs with PD-1 on the T cell surface. PD-L1 is a member of the B7 family of co-stimulatory/co-inhibitory molecules widely expressed by many cell types including T cells and DCs [20, 21, 113]. PD-1 is up regulated in activated T cells during antigen presentation and its binding to PD-L1 recruits SHP1 and SHP2 phosphatases to its cytoplasmic domain, which terminate TCR signalling [114-116]. As a matter of fact, PD-L1 expression rather than PD-L2 in peripheral tissues is critical to keep immune tolerance [20, 21].
Therefore, interference of PD-L1/PD-1 using either systemic administration of blocking antibodies or silencing PD-L1 has been applied to enhance positive co-stimulation and improve immunotherapy, both in mouse models or in human DCs [105, 106, 111, 117, 118]. Interestingly, blockade or silencing of PD-L1 significantly enhances recruitment and association between CD8 T cells to DCs in vivo and in vitro in mouse models [22, 119, 120]. This stronger and prolonged association suggests that signal 1 and signal 2 provided to T cells in the immunological synapse are significantly reinforced. Interestingly, PD-L1 silencing in antigen presenting myeloid mouse DCs with a PD-L1-specific short hairpin (sh)RNA decreased TCR down-modulation in activated T cells after antigen presentation [22, 121, 122]. This lack of TCR down-modulation most likely ensured TCR signal transduction to T cells with a concomitant stronger signal 1. In addition, the elimination of negative co-stimulation would in principle increase positive co-stimulation provided by CD80/CD86 and CD40. Therefore, a stronger activatory signal 2. Accordingly, PD-L1 silencing in antigen presenting DCs induced the expansion of a population of hyper activated pro-inflammatory TCRhigh CD8 T cells, with increased levels of interferon (IFN)γ and IL17 [22]. This is particularly interesting for antitumor immunotherapy, considering the key role of cytotoxic CD8 T cells against cancer cells. Cellular vaccination with these PD-L1-silenced DCs inhibited tumour growth in a mouse lymphoma model and prolonged survival. Surprisingly, overall long-term cure rates did not improve in comparison with vaccination using unmodified DCs [22, 121]. This suggested that PD-L1 inhibition accelerated tumour-specific T cell responses, although additional co-stimulatory signals might be needed to enhance T cell cytotoxic activities. We propose that additional manipulation of signal 3 during antigen presentation could improve the results obtained by interference with PD-L1/PD-1 blocking. This strategy could be particularly important to overcome immunosuppressive tumour microenvironments. Removal of PD-L1-based regulation combined with a potent signal 3 provided by cytokine secretion could generate enhanced T cell immunity in a therapeutic setting. While elimination of the PD-L1/PD-1 inhibitory interaction hyperactivates T cells and accelerates their expansion, specific cytokine combinations could potentially modify the type of immune response to be achieved as long as there are provided in cis by antigen presenting DCs.
Expression of other B7 family members by APCs or cancer cells has also been associated to inhibition of T cell responses and poor prognostic in cancer patients. Therefore, their blockade in many instances may be also used to enhance co-stimulation and manipulate T cell differentiation. The B7-H2 co-stimulatory molecule, or inducible T cell co-stimulator (ICOS) ligand (ICOS-L), is expressed in physiological conditions amongst others by immature human DCs [123], human airway epithelial cells [124]. B7-H2/ICOS co-stimulation is required for T cell activation and recall of T and B cell responses [125-127]. However, it has been recently demonstrated that B7-H2 is also a ligand for both CD28 and CTLA-4, involved in activatory and inhibitory signalling in T cells, respectively [128]. Therefore, it could be possible that depending on the context of co-stimulation, B7-H2 may enhance or inhibit immune responses. Thus, B7-H2 expression is up-regulated in a number of cancer cells such as in leukaemia [129] and myeloma cells [130], although possibly linked to increased tumour cell proliferation and survival rather than immune evasion. Finally, the involvement of ICOS-L/ICOS co-stimulation on T cell differentiation is unclear. While some reports suggest that it enhances human Th1/Th17 responses with anti-tumour activities [131], other reports suggest that it is required for Th2 but not Th1 or Th17 differentiation [132, 133].
B7-H3 is preferentially a negative regulator of T cell responses that is expressed by a wide range of cells including DC, monocytes [134-137], T cells [138] and tumour cells [139, 140]. Its up-regulation by cancerous cells confers a mechanism to escape immune responses [139, 141-144]. Therefore, B7-H3 blockade may also enhance tumour immunotherapy by preventing cancer cell escape. However, the role of B7-H3 as an inhibitor of immune responses is still unclear, as many studies also link B7-H3 co-stimulation with increased T cell responses, Th2 differentiation and anti-tumour immunity [145-149]. However, many of these discrepancies might be due to the different experimental systems (most of them murine), as B7-H3 co-stimulation of human T cells seems to be inhibitory, and its expression by tumour cells shows a poor prognosis [150]. Nevertheless, the precise role of B7-H3 in immune activation or inhibition remains highly controversial, and its therapeutic exploitation unclear [147, 151-154].
B7-H4 is another member of the B7 family of co-stimulatory molecules [155] with immunosuppressive activities, similarly to PD-L1. B7-H4 is also expressed by a wide range of cell types including DCs, macrophages, endothelial and mesenchimal cells, and it plays a key homeostatic role by inhibiting T cell functions to maintain immunological tolerance [156-158]. Its expression by tumour cells inhibits T cell cytotoxic activities and provides a means for immune escape [159-163]. Similar strategies as those used for inhibiting PD-L1 co-stimulation could also be applied for B7-H4 to enhance anti-tumour activities, and blocking antibodies are being developed for this purpose [164].
CTLA-4 blockade for tumour immunotherapy
CTLA-4 is a well-known T cell inhibitory B7-receptor that is expressed by activated T cells and particularly by Tregs [165-168]. This receptor constantly traffics between endosomes and the T cell surface [169], and it has a higher affinity than CD28 for their common ligand, CD80 [170]. Several mechanisms of action have been proposed to explain its potent immunosuppressive activities, ranging from competition with CD28 for CD80, delivery of an inhibitory intracellular signal or by CD80/CD86 transendocytosis from the membrane of antigen-presenting cells [171-174]. Nevertheless, independently of its mechanism of action, antibody-mediated CTLA-4 blockade has been largely successful in animal models of cancer. Its blockade clearly enhances T cell cytotoxic responses, inhibits Treg activities and induces differentiation of cytotoxic CD4 T cells [175-177].
Interestingly, CTLA-4 blockade can also be combined with other immunotherapy approaches such as inhibition of inhibitory co-stimulation [178], enhancement of activatory co-stimulation [179, 180], cytokine treatments [181] or superantigens [182]. These approaches turn the balance towards effector T cell activities instead of Tregs [183]. More important, clinical activity is evident and CTLA4 blocking shifts the T cell differentiation balance towards Th1/Th17 in human cancer patients [184]. Clinical efficacy with an approved blocking antibody (ipilimumab) has been reported in a wide range of cancers such as melanoma [185, 186], prostate cancer [187], non-small cell lung cancer [188] amongst others [189]. However, although clinical efficacy might not be apparent after single use, such as in pancreatic adenocarcinoma, significant clinical responses are obtained in combination with other immunotherapeituc strategies, both in mouse models and in humans [190, 191].
This last case on blocking negative co-stimulation, or inhibitory interactions with blocking antibodies is the demonstration and immunotherapy can actually work, with significant clinical efficacy for a wide range of cancers [192]. Immunotherapy will surely be fundamental for the treatment of advanced cancers, due to the capacity of the immune system to detect, reach and eliminate microscopic metastasis.
CONCLUSIONS
The three-signal hypothesis of T cell activation is a convenient mechanistical model that explains the requirements needed for T cell activation and the initiation of immune responses. Thus, T cells require at least three “types” of signals to expand and exert their effector activities. The first one is direct antigen recognition. The second one, co-stimulation, is provided after integration of activatory and inhibitory interactions between receptors and ligands within the immunological synapse. The outcome of these interactions will determine the “activation” status and effector capacities of T cells. However, a third signal is also required to modulate T cell differentiation into different subsets that will control the type of immune responses. This ensures that immune responses are adequate to fight the particular type of pathogen, including cancer. This third signal is provided by a combination of cytokines, chemokines and other inflammatory mediators. Interestingly, there is evidence suggesting that for efficient immune responses, the three signals have to be given to T cells in cis, that is, by the APC directly associated to the T cell. This is a critical observation for designing adequate vaccination regimes, as many adjuvants used for vaccination protocols provide the “third signal” mainly in trans.
Nowadays, it is relatively straightforward to modify DCs to efficiently provide the three signals to T cells, and even modulate co-stimulation to control the strength and type of immune response [3, 103]. It is also possible to induce immune suppression and tolerance [23]. Manipulation of signals 1 and 2 is relatively simple by the co-expression in DCs of antigens, activators/inhibitors of maturation pathways or blocking antibodies that interfere with negative co-stimulation. It is rather more complicated to manipulate signal 3 so that DCs giving the first two signals to T cells can also produce the desired cytokine/chemokine profiles in the immunological synapse. The modulation of the three signals in a directed, targeted way would allow a fine-tuned control of immune responses leading to better immunotherapy treatments.
Blocking antibodies targeted to inhibit negative co-stimulation to T cells are promising for the future of cancer immunotherapy. There are several of these antibodies already approved for human therapy, which are showing clinical efficacy. However, these will probably have to be combined with other immunotherapeutic approaches, most likely because of the necessity of strengthening co-stimulatory signalling and cytokine priming.
ACKNOWLEDGEMENTS
ID is funded by an ERASMUS (EU) scholarship. KB is funded by the Fund for Scientific Research-Flandes. DE is funded by an arthritis research UK career development fellowship (18433).
LIST OF ABBREVIATIONS
- APC
Antigen presenting cell
- CD
Cluster of differentiation
- CTLA4
cytotoxic T-lymphocyte antigen 4
- DC
Dendritic cell
- ERK
extracellularly regulated protein kinase
- ICOS
Inducible T cell co-stimulator
- IFN
Interferon
- IL
Interleukin
- KSHV
Kaposi’s sarcoma-associated human herpesvirus
- MAPK
mitogen activated protein kinases
- MKK
MAPK kinase kinase
- MHC
major histocompatibility complex
- NF
Nuclear factor
- NK
Natural killer
- PD-L1
Programmed cell death 1 ligand 1
- PD-1
Programmed death 1
- PI3K
phosphatidyl inositol 3 kinase
- p-MHC
peptide-MHC complex
- TAA
tumour-associated antigen
- TCR
T cell receptor
- TGF
Transforming growth factor
- Th
T helper cell
- TLR
Toll-like receptor
- TNF
Tumour necrosis factor
Footnotes
Publisher's Disclaimer: This is a pre-print. The copyright belongs to Bentham Publishers, and the final version of the article can be found at: http://www.benthamdirect.org/pages/b_viewarticle.php?articleID=3179321
REFERENCES
- [1].Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- [3].Breckpot K, Escors D. Dendritic Cells for Active Anti-cancer Immunotherapy: Targeting Activation Pathways Through Genetic Modification. Endocrine, metabolic & immune disorders drug targets. 2009;9:328–343. doi: 10.2174/187153009789839156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Olweus J, BitMansour A, Warnke R, Thompson PA, Carballido J, Picker LJ, Lund-Johansen F. Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(23):12551–12556. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Morgado JM, Pratas R, Laranjeira P, Henriques A, Crespo I, Regateiro F, Paiva A. The phenotypical and functional characteristics of cord blood monocytes and CD14(−/low)/CD16(+) dendritic cells can be relevant to the development of cellular immune responses after transplantation. Transpl Immunol. 2008;19(1):55–63. doi: 10.1016/j.trim.2007.11.002. [DOI] [PubMed] [Google Scholar]
- [6].Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- [7].Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188(11):2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96(3):1039–1046. [PubMed] [Google Scholar]
- [9].Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annual review of immunology. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- [10].Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease. Physiol Rev. 2002;82(1):97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- [11].Goold HD, Escors D, Conlan TJ, Chakraverty R, Bennett CL. Conventional dendritic cells are required for the activation of helper-dependent CD8 T cell responses to a model antigen after cutaneous vaccination with lentiviral vectors. J Immunol. 2011;186(8):4565–4572. doi: 10.4049/jimmunol.1002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Breckpot K, Escors D, Arce F, Lopes L, Karwacz K, Van Lint S, Keyaerts M, Collins M. HIV-1 lentiviral vector immunogenicity is mediated by Toll-like receptor 3 (TLR3) and TLR7. J. Virol. 2010;84:5627–5636. doi: 10.1128/JVI.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boisvert J, Edmondson S, Krummel MF. Immunological synapse formation licenses CD40-CD40L accumulations at T-APC contact sites. J Immunol. 2004;173(6):3647–3652. doi: 10.4049/jimmunol.173.6.3647. [DOI] [PubMed] [Google Scholar]
- [14].Rothoeft T, Balkow S, Krummen M, Beissert S, Varga G, Loser K, Oberbanscheidt P, van den Boom F, Grabbe S. Structure and duration of contact between dendritic cells and T cells are controlled by T cell activation state. Eur J Immunol. 2006;36(12):3105–3117. doi: 10.1002/eji.200636145. [DOI] [PubMed] [Google Scholar]
- [15].Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annual review of immunology. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huppa JB, Axmann M, Mortelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, Klein LO, Schutz GJ, Davis MM. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463(7283):963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403(6766):216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- [18].Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, Le J, Ohashi PS, Sarosi I, Nishina H, Lipkowitz S, Penninger JM. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403(6766):211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- [19].Nurieva R, Thomas S, Nguyen T, Martin-Orozco N, Wang Y, Kaja MK, Yu XZ, Dong C. T-cell tolerance or function is determined by combinatorial costimulatory signals. Embo J. 2006;25(11):2623–2633. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101(29):10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liang SC, Greenwald RJ, Latchman YE, Rosas L, Satoskar A, Freeman GJ, Sharpe AH. PD-L1 and PD-L2 have distinct roles in regulating host immunity to cutaneous leishmaniasis. Eur J Immunol. 2006;36(1):58–64. doi: 10.1002/eji.200535458. [DOI] [PubMed] [Google Scholar]
- [22].Karwacz K, Bricogne C, Macdonald D, Arce F, Bennett CL, Collins M, Escors D. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8(+) T cells. EMBO molecular medicine. 2011;3(10):581–592. doi: 10.1002/emmm.201100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arce F, Breckpot K, Stephenson H, Karwacz K, Ehrenstein MR, Collins M, Escors D. Selective ERK activation differentiates mouse and human tolerogenic dendritic cells, expands antigen-specific regulatory T cells, and suppresses experimental inflammatory arthritis. Arthritis and rheumatism. 2011;63:84–95. doi: 10.1002/art.30099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Escors D, Lopes L, Lin R, Hiscott J, Akira S, Davis RJ, Collins MK. Targeting dendritic cell signalling to regulate the response to immunisation. Blood. 2008;111(6):3050–3061. doi: 10.1182/blood-2007-11-122408. [DOI] [PubMed] [Google Scholar]
- [25].Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. The Journal of experimental medicine. 2003;197(9):1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. Journal of immunology. 2003;171(10):5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- [27].Kapsenberg ML, Hilkens CM, Wierenga EA, Kalinski P. The paradigm of type 1 and type 2 antigen-presenting cells. Implications for atopic allergy. Clin Exp Allergy. 1999;29(Suppl 2):33–36. [PubMed] [Google Scholar]
- [28].Ramanathan S, Dubois S, Chen XL, Leblanc C, Ohashi PS, Ilangumaran S. Exposure to IL-15 and IL-21 enables autoreactive CD8 T cells to respond to weak antigens and cause disease in a mouse model of autoimmune diabetes. J Immunol. 2011;186(9):5131–5141. doi: 10.4049/jimmunol.1001221. [DOI] [PubMed] [Google Scholar]
- [29].Curtsinger J, Lins D, Mescher M. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. Journal of experimental medicine. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Curtsinger J, Schmidt C, Mondino A, Lins D, Kedl R, Jenkins M, Mescher M. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. Journal of immunology. 1999;162:3256–3262. [PubMed] [Google Scholar]
- [31].Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8 T cells in vivo. J Immunol. 1999;163:2561–2567. [PubMed] [Google Scholar]
- [32].Schmidt CS, Mescher MF. Peptide Ag priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J. Immunol. 2002;168:5521–5529. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- [33].Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD+ T cells. Nat. Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- [34].Hernandez J,S, Aung KM, Sherman LA. Uncoupling of proliferative potential and gain of effector function by CD8 T cells responding to self-antigens. J Exp Med. 2002;196:323–333. doi: 10.1084/jem.20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154(10):5071–5079. [PubMed] [Google Scholar]
- [36].Maldonado-Lopez R, Smedt TD, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8α+ and CD8α-subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- [38].Mitchell TC, Hildeman D, Kedl RM, Teague TK, Schaefer BC, J. White Y, Zhu JK, Marrack P. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat Immunol. 2001;2:397–402. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]
- [39].McGeachy MJ, Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- [40].Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- [41].Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS ONE. 2008;3(12):e3879. doi: 10.1371/journal.pone.0003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203(7):1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Larsen JM, Bonefeld CM, Poulsen SS, Geisler C, Skov L. IL-23 and T(H)17-mediated inflammation in human allergic contact dermatitis. J Allergy Clin Immunol. 2009;123(2):486–492. doi: 10.1016/j.jaci.2008.09.036. [DOI] [PubMed] [Google Scholar]
- [44].Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, Santamaria M. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol. 2009;86(2):435–443. doi: 10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]
- [45].Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nature reviews. 2010;10(3):170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- [46].O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114(10):1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10(8):801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- [48].Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108(5):1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- [49].Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S, Unutmaz D, Pulendran B. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116(4):916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15(4):401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- [52].Bekeredjian-Ding I, Roth SI, Gilles S, Giese T, Ablasser A, Hornung V, Endres S, Hartmann G. T cell-independent, TLR-induced IL-12p70 production in primary human monocytes. J Immunol. 2006;176(12):7438–7446. doi: 10.4049/jimmunol.176.12.7438. [DOI] [PubMed] [Google Scholar]
- [53].Cisco RM, Abdel-Wahab Z, Dannull J, Nair S, Tyler DS, Gilboa E, Vieweg J, Daaka Y, Pruitt SK. Induction of human dendritic cell maturation using transfection with RNA encoding a dominant positive toll-like receptor 4. J Immunol. 2004;172(11):7162–7168. doi: 10.4049/jimmunol.172.11.7162. [DOI] [PubMed] [Google Scholar]
- [54].Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15(4):410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191(10):1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nature immunology. 2005;6(2):163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- [57].Nolte M, Leibundgut-Landmann S, Joffre O, Sousa C.R.e. Dendritic cell quiescence during systemic inflammation driven by LPS stimulation of radioresistant cells in vivo. Journal of experimental medicine. 2007;204:1487–1501. doi: 10.1084/jem.20070325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hou B, Reizis B, DeFranco A. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29(272-282) doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kratky W, Sousa C.R.e., Oxenius A, Spörri R. Direct activation of antigen-presenting cells is required for CD8+ T-cell priming and tumor vaccination. PNAS. 2011;108:17414–17419. doi: 10.1073/pnas.1108945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- [61].Gallucci SM, Danger P. signals: SOS to the immune system. Curr. Opin. Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- [62].Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194(6):863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schmidt C. Clinical setbacks for toll-like receptor 9 agonists in cancer. Nat Biotechnol. 2007;25:825–826. doi: 10.1038/nbt0807-825. [DOI] [PubMed] [Google Scholar]
- [64].den JH, Lehar S, Bevan M. CD8(+) but not CD8(−) dendritic cells crossprime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Doxsee CL, Riter TR, Reiter MJ, Gibson SJ, Vasilakos JP, Kedl RM. The immune response modifier and Toll-like receptor 7 agonist S-27609 selectively induces IL-12 and TNF-alpha production in CD11c+CD11b+CD8− dendritic cells. J Immunol. 2003;171(3):1156–1163. doi: 10.4049/jimmunol.171.3.1156. [DOI] [PubMed] [Google Scholar]
- [66].Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33(4):827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- [67].Arce F, Kochan G, Breckpot K, Stephenson H, Escors D. Selective Activation of Intracellular Signalling Pathways In Dendritic Cells For Cancer Immunotherapy. Anti-cancer agents in medicinal chemistry. 2012;1:29–39. doi: 10.2174/187152012798764679. [DOI] [PubMed] [Google Scholar]
- [68].Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162(7):3865–3872. [PubMed] [Google Scholar]
- [69].Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166(6):3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- [70].Puig-Kroger A, Relloso M, Fernandez-Capetillo O, Zubiaga A, Silva A, Bernabeu C, Corbi AL. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood. 2001;98(7):2175–2182. doi: 10.1182/blood.v98.7.2175. [DOI] [PubMed] [Google Scholar]
- [71].Nakahara T, Uchi H, Urabe K, Chen Q, Furue M, Moroi Y. Role of c-Jun N-terminal kinase on lipopolysaccharide induced maturation of human monocyte-derived dendritic cells. Int Immunol. 2004;16(12):1701–1709. doi: 10.1093/intimm/dxh171. [DOI] [PubMed] [Google Scholar]
- [72].Yu Q, Kovacs C, Yue FY, Ostrowski MA. The role of the p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and phosphoinositide-3-OH kinase signal transduction pathways in CD40 ligand-induced dendritic cell activation and expansion of virus-specific CD8+ T cell memory responses. J Immunol. 2004;172(10):6047–6056. doi: 10.4049/jimmunol.172.10.6047. [DOI] [PubMed] [Google Scholar]
- [73].Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci. 2006;42(1):1–11. doi: 10.1016/j.jdermsci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- [74].Wang Z, Sokolovska A, Seymour R, Sundberg JP, Hogenesch H. SHARPIN is essential for cytokine production, NF-kappaB signaling, and induction of Th1 differentiation by dendritic cells. PLoS ONE. 2012;7(2):e31809. doi: 10.1371/journal.pone.0031809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3(1):69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- [76].Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, Tanaka K, Nakano H, Iwai K. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471(7340):633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- [77].Rowe HM, Lopes L, Brown N, Efklidou S, Smallie T, Karrar S, Kaye PM, Collins MK. Expression of vFLIP in a lentiviral vaccine vector activates NF-{kappa}B, matures dendritic cells, and increases CD8+ T-cell responses. J Virol. 2009;83(4):1555–1562. doi: 10.1128/JVI.00709-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Karwacz K, Mukherjee S, Apolonia L, Blundell MP, Bouma G, Escors D, Collins MK, Thrasher AJ. Nonintegrating lentivector vaccines stimulate prolonged T-cell and antibody responses and are effective in tumor therapy. J Virol. 2009;83(7):3094–3103. doi: 10.1128/JVI.02519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Andreakos E, Williams RO, Wales J, Foxwell BM, Feldmann M. Activation of NF-kappaB by the intracellular expression of NF-kappaB-inducing kinase acts as a powerful vaccine adjuvant. Proc Natl Acad Sci U S A. 2006;103(39):14459–14464. doi: 10.1073/pnas.0603493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Yanagawa Y, Onoe K. Distinct regulation of CD40-mediated interleukin-6 and interleukin-12 productions via mitogen-activated protein kinase and nuclear factor kappaB-inducing kinase in mature dendritic cells. Immunology. 2006;117(4):526–535. doi: 10.1111/j.1365-2567.2006.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang Z, Potter CS, Sundberg JP, Hogenesch H. SHARPIN is a key regulator of immune and inflammatory responses. Journal of cellular and molecular medicine. 2012 doi: 10.1111/j.1582-4934.2012.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265(5173):808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- [83].Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372(6508):739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- [84].Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell research. 2005;15(1):11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- [85].Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. Embo J. 1999;18(7):1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yang L, Du C, Chen T, Li S, Nie W, Zhu W, Fan F, Zhu J, Yan H. Distinct MAPK pathways are involved in IL-23 production in dendritic cells cocultured with NK cells in the absence or presence of angiotensin II. Molecular immunology. 2012;51(1):51–56. doi: 10.1016/j.molimm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- [87].Trompezinski S, Migdal C, Tailhardat M, Le Varlet B, Courtellemont P, Haftek M, Serres M. Characterization of early events involved in human dendritic cell maturation induced by sensitizers: cross talk between MAPK signalling pathways. Toxicology and applied pharmacology. 2008;230(3):397–406. doi: 10.1016/j.taap.2008.03.012. [DOI] [PubMed] [Google Scholar]
- [88].Hammaker DR, Boyle DL, Inoue T, Firestein GS. Regulation of the JNK pathway by TGF-beta activated kinase 1 in rheumatoid arthritis synoviocytes. Arthritis research & therapy. 2007;9(3):R57. doi: 10.1186/ar2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Xiao YQ, Malcolm K, Worthen GS, Gardai S, Schiemann WP, Fadok VA, Bratton DL, Henson PM. Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-beta. J Biol Chem. 2002;277(17):14884–14893. doi: 10.1074/jbc.M111718200. [DOI] [PubMed] [Google Scholar]
- [90].Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103(7):1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- [91].Qian C, Jiang X, An H, Yu Y, Guo Z, Liu S, Xu H, Cao X. TLR agonists promote ERK-mediated preferential IL-10 production of regulatory dendritic cells (diffDCs), leading to NK-cell activation. Blood. 2006;108(7):2307–2315. doi: 10.1182/blood-2006-03-005595. [DOI] [PubMed] [Google Scholar]
- [92].Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276(40):37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- [93].Luft T, Maraskovsky E, Schnurr M, Knebel K, Kirsch M, Gorner M, Skoda R, Ho AD, Nawroth P, Bierhaus A. Tuning the volume of the immune response: strength and persistence of stimulation determine migration and cytokine secretion of dendritic cells. Blood. 2004;104(4):1066–1074. doi: 10.1182/blood-2003-12-4146. [DOI] [PubMed] [Google Scholar]
- [94].Luft T, Rodionova E, Maraskovsky E, Kirsch M, Hess M, Buchholtz C, Goerner M, Schnurr M, Skoda R, Ho AD. Adaptive functional differentiation of dendritic cells: integrating the network of extra- and intracellular signals. Blood. 2006;107(12):4763–4769. doi: 10.1182/blood-2005-04-1501. [DOI] [PubMed] [Google Scholar]
- [95].Hirata N, Yanagawa Y, Ebihara T, Seya T, Uematsu S, Akira S, Hayashi F, Iwabuchi K, Onoe K. Selective synergy in anti-inflammatory cytokine production upon cooperated signaling via TLR4 and TLR2 in murine conventional dendritic cells. Molecular immunology. 2008;45(10):2734–2742. doi: 10.1016/j.molimm.2008.02.010. [DOI] [PubMed] [Google Scholar]
- [96].Dunne A, Marshall NA, Mills KH. TLR based therapeutics. Current opinion in pharmacology. 2011;11(4):404–411. doi: 10.1016/j.coph.2011.03.004. [DOI] [PubMed] [Google Scholar]
- [97].Goutagny N, Estornes Y, Hasan U, Lebecque S, Caux C. Targeting pattern recognition receptors in cancer immunotherapy. Target Oncol. 2012;7(1):29–54. doi: 10.1007/s11523-012-0213-1. [DOI] [PubMed] [Google Scholar]
- [98].Hedayat M, Netea MG, Rezaei N. Targeting of Toll-like receptors: a decade of progress in combating infectious diseases. Lancet Infect Dis. 2011;11(9):702–712. doi: 10.1016/S1473-3099(11)70099-8. [DOI] [PubMed] [Google Scholar]
- [99].Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1(6):949–964. doi: 10.2217/imt.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Marshall NA, Galvin KC, Corcoran AM, Boon L, Higgs R, Mills KH. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-gamma+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer research. 2012;72(3):581–591. doi: 10.1158/0008-5472.CAN-11-0307. [DOI] [PubMed] [Google Scholar]
- [101].Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS ONE. 2011;6(1):e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunological reviews. 2011;239(1):178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Escors D, Breckpot K. Lentiviral Vectors in Gene Therapy: Their Current Status and Future Potential. Arch Immunol Ther Exp. 2010;58(2):107–119. doi: 10.1007/s00005-010-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Breton G, Yassine-Diab B, Cohn L, Boulassel MR, Routy JP, Sekaly RP, Steinman RM. siRNA knockdown of PD-L1 and PD-L2 in monocyte-derived dendritic cells only modestly improves proliferative responses to Gag by CD8(+) T cells from HIV-1-infected individuals. J Clin Immunol. 2009;29(5):637–645. doi: 10.1007/s10875-009-9313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hobo W, Maas F, Adisty N, de Witte T, Schaap N, van der Voort R, Dolstra H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen-specific CD8+ T cells. Blood. 2010;116(22):4501–4511. doi: 10.1182/blood-2010-04-278739. [DOI] [PubMed] [Google Scholar]
- [107].Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010;184(7):3442–3449. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu LF, Gondek D, Wang Y, Fava RA, Fiser A, Almo S, Noelle RJ. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Butte MJ, Pena-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Molecular immunology. 2008;45(13):3567–3572. doi: 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annual review of immunology. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- [111].Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–1096. [PubMed] [Google Scholar]
- [112].Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- [113].Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- [114].Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- [115].Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, Chaudhary D. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574(1-3):37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- [116].Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhou Q, Xiao H, Liu Y, Peng Y, Hong Y, Yagita H, Chandler P, Munn DH, Mellor A, Fu N, He Y. Blockade of programmed death-1 pathway rescues the effector function of tumor-infiltrating T cells and enhances the antitumor efficacy of lentivector immunization. J Immunol. 2010;185(9):5082–5092. doi: 10.4049/jimmunol.1001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114(8):1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sierro SR, Donda A, Perret R, Guillaume P, Yagita H, Levy F, Romero P. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur J Immunol. 2011;41:2217–2228. doi: 10.1002/eji.201041235. [DOI] [PubMed] [Google Scholar]
- [120].Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10(11):1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Karwacz K, Arce F, Bricogne C, Kochan G, Escors D. PD-L1 co-stimulation, ligand-induced TCR down-modulation and anti-tumor immunotherapy. Oncoimmunology. 2012;1(1):86–88. doi: 10.4161/onci.1.1.17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Escors D, Bricogne C, Arce F, Kochan G, Karwacz K. On the Mechanism of T cell receptor down-modulation and its physiological significance. The journal of bioscience and medicine. 2011;1(1) [PMC free article] [PubMed] [Google Scholar]
- [123].Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni J, Chen L. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood. 2000;96(8):2808–2813. [PubMed] [Google Scholar]
- [124].Kurosawa S, Myers AC, Chen L, Wang S, Ni J, Plitt JR, Heller NM, Bochner BS, Schleimer RP. Expression of the costimulatory molecule B7-H2 (inducible costimulator ligand) by human airway epithelial cells. Am J Respir Cell Mol Biol. 2003;28(5):563–573. doi: 10.1165/rcmb.2002-0199OC. [DOI] [PubMed] [Google Scholar]
- [125].Wong SC, Tan AH, Lam KP. Functional hierarchy and relative contribution of the CD28/B7 and ICOS/B7-H2 costimulatory pathways to T cell-mediated delayed-type hypersensitivity. Cellular immunology. 2009;256(1-2):64–71. doi: 10.1016/j.cellimm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- [126].Wong SC, Oh E, Ng CH, Lam KP. Impaired germinal center formation and recall T-cell-dependent immune responses in mice lacking the costimulatory ligand B7-H2. Blood. 2003;102(4):1381–1388. doi: 10.1182/blood-2002-08-2416. [DOI] [PubMed] [Google Scholar]
- [127].Yong PF, Salzer U, Grimbacher B. The role of costimulation in antibody deficiencies: ICOS and common variable immunodeficiency. Immunol Rev. 2009;229(1):101–113. doi: 10.1111/j.1600-065X.2009.00764.x. [DOI] [PubMed] [Google Scholar]
- [128].Yao S, Zhu Y, Zhu G, Augustine M, Zheng L, Goode DJ, Broadwater M, Ruff W, Flies S, Xu H, Flies D, Luo L, Wang S, Chen L. B7-h2 is a costimulatory ligand for CD28 in human. Immunity. 2011;34(5):729–740. doi: 10.1016/j.immuni.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Tamura H, Dan K, Tamada K, Nakamura K, Shioi Y, Hyodo H, Wang SD, Dong H, Chen L, Ogata K. Expression of functional B7-H2 and B7.2 costimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res. 2005;11(16):5708–5717. doi: 10.1158/1078-0432.CCR-04-2672. [DOI] [PubMed] [Google Scholar]
- [130].Yamashita T, Tamura H, Satoh C, Shinya E, Takahashi H, Chen L, Kondo A, Tsuji T, Dan K, Ogata K. Functional B7.2 and B7-H2 molecules on myeloma cells are associated with a growth advantage. Clin Cancer Res. 2009;15(3):770–777. doi: 10.1158/1078-0432.CCR-08-0501. [DOI] [PubMed] [Google Scholar]
- [131].Paulos CM, Carpenito C, Plesa G, Suhoski MM, Varela-Rohena A, Golovina TN, Carroll RG, Riley JL, June CH. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Science translational medicine. 2010;2(55):55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kadkhoda K, Wang S, Fan Y, Qiu H, Basu S, Halayko AJ, Yang X. ICOS ligand expression is essential for allergic airway hyperresponsiveness. Int Immunol. 2011;23(4):239–249. doi: 10.1093/intimm/dxq476. [DOI] [PubMed] [Google Scholar]
- [133].Frey O, Meisel J, Hutloff A, Bonhagen K, Bruns L, Kroczek RA, Morawietz L, Kamradt T. Inducible costimulator (ICOS) blockade inhibits accumulation of polyfunctional T helper 1/T helper 17 cells and mitigates autoimmune arthritis. Annals of the rheumatic diseases. 2010;69(8):1495–1501. doi: 10.1136/ard.2009.119164. [DOI] [PubMed] [Google Scholar]
- [134].Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- [135].Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, Dong C. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173(4):2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- [136].Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stockl J, Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172(4):2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- [137].Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da Costa J, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, Mak TW. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- [138].Ferlazzo G, Semino C, Meta M, Procopio F, Morandi B, Melioli G. T lymphocytes express B7 family molecules following interaction with dendritic cells and acquire bystander costimulatory properties. Eur J Immunol. 2002;32(11):3092–3101. doi: 10.1002/1521-4141(200211)32:11<3092::AID-IMMU3092>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- [139].Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV, Moretta L, Moretta A, Bottino C. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101(34):12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Chavin G, Sheinin Y, Crispen PL, Boorjian SA, Roth TJ, Rangel L, Blute ML, Sebo TJ, Tindall DJ, Kwon ED, Karnes RJ. Expression of immunosuppresive B7-H3 ligand by hormone-treated prostate cancer tumors and metastases. Clin Cancer Res. 2009;15(6):2174–2180. doi: 10.1158/1078-0432.CCR-08-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung cancer (Amsterdam, Netherlands) 2006;53(2):143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- [142].Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML, Cheville JC, Sebo TJ, Kwon ED. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67(16):7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- [143].Yuan H, Wei X, Zhang G, Li C, Zhang X, Hou J. B7-H3 over expression in prostate cancer promotes tumor cell progression. The Journal of urology. 2011;186(3):1093–1099. doi: 10.1016/j.juro.2011.04.103. [DOI] [PubMed] [Google Scholar]
- [144].Tekle C, Nygren MK, Chen YW, Dybsjord I, Nesland JM, Maelandsmo GM, Fodstad O. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. International journal of cancer. 2012;130(10):2282–2290. doi: 10.1002/ijc.26238. [DOI] [PubMed] [Google Scholar]
- [145].Hofmeyer KA, Ray A, Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci U S A. 2008;105(30):10277–10278. doi: 10.1073/pnas.0805458105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci U S A. 2008;105(30):10495–10500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kobori H, Hashiguchi M, Piao J, Kato M, Ritprajak P, Azuma M. Enhancement of effector CD8+ T-cell function by tumour-associated B7-H3 and modulation of its counter-receptor triggering receptor expressed on myeloid cell-like transcript 2 at tumour sites. Immunology. 2010;130(3):363–373. doi: 10.1111/j.1365-2567.2009.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Nagashima O, Harada N, Usui Y, Yamazaki T, Yagita H, Okumura K, Takahashi K, Akiba H. B7-H3 contributes to the development of pathogenic Th2 cells in a murine model of asthma. J Immunol. 2008;181(6):4062–4071. doi: 10.4049/jimmunol.181.6.4062. [DOI] [PubMed] [Google Scholar]
- [149].Chen C, Zhu YB, Qu QX, Ge Y, Huang JA, Wang Y, Zhang XG. CD40-activated apoptotic tumor cell-pulsed dendritic cell could potentially elicit antitumor immune response: involvement of up-regulation of B7-H3 expression. J Immunother. 2009;32(1):29–35. doi: 10.1097/CJI.0b013e31818c8816. [DOI] [PubMed] [Google Scholar]
- [150].Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, Kreil DP, Dong C, Yamazaki T, Zlabinger G, Pfistershammer K, Steinberger P. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39(7):1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Yamato I, Sho M, Nomi T, Akahori T, Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H, Nakajima Y. Clinical importance of B7-H3 expression in human pancreatic cancer. British journal of cancer. 2009;101(10):1709–1716. doi: 10.1038/sj.bjc.6605375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhang G, Wang J, Kelly J, Gu G, Hou J, Zhou Y, Redmond HP, Wang JH, Zhang X. B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol. 2010;185(6):3677–3684. doi: 10.4049/jimmunol.0904020. [DOI] [PubMed] [Google Scholar]
- [153].Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, Spriggs DR, Dupont J, Allison JP. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23(8):1104–1112. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, Kleeff J, Friess H. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC cancer. 2009;9:463. doi: 10.1186/1471-2407-9-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- [156].Wang X, Hao J, Metzger DL, Ao Z, Chen L, Ou D, Verchere CB, Mui A, Warnock GL. B7-H4 Treatment of T Cells Inhibits ERK, JNK, p38, and AKT Activation. PLoS ONE. 2012;7(1):e28232. doi: 10.1371/journal.pone.0028232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wang X, Hao J, Metzger DL, Mui A, Ao Z, Verchere CB, Chen L, Ou D, Warnock GL. B7-H4 induces donor-specific tolerance in mouse islet allografts. Cell transplantation. 2012;21(1):99–111. doi: 10.3727/096368911X582750. [DOI] [PubMed] [Google Scholar]
- [158].Wang X, Hao J, Metzger DL, Mui A, Ao Z, Akhoundsadegh N, Langermann S, Liu L, Chen L, Ou D, Verchere CB, Warnock GL. Early treatment of NOD mice with B7-H4 reduces the incidence of autoimmune diabetes. Diabetes. 2012;60(12):3246–3255. doi: 10.2337/db11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chen C, Qu QX, Shen Y, Mu CY, Zhu YB, Zhang XG, Huang JA. Induced expression of B7-H4 on the surface of lung cancer cell by the tumor-associated macrophages: a potential mechanism of immune escape. Cancer Lett. 2012;317(1):99–105. doi: 10.1016/j.canlet.2011.11.017. [DOI] [PubMed] [Google Scholar]
- [160].He C, Qiao H, Jiang H, Sun X. The inhibitory role of b7-h4 in antitumor immunity: association with cancer progression and survival. Clinical & developmental immunology. 2012;2011:695834. doi: 10.1155/2011/695834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, Lu BF, Zhang XG. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60(7):1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Qian Y, Shen L, Xu C, Wu Z, Brockmeyer NH, Altmeyer P, Wu N, Yao HP. Development of a novel monoclonal antibody to B7-H4: characterization and biological activity. European journal of medical research. 2011;16(7):295–302. doi: 10.1186/2047-783X-16-7-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Henson SM, Macaulay R, Kiani-Alikhan S, Akbar AN. The use of the inhibitory receptors for modulating the immune responses. Current pharmaceutical design. 2008;14(26):2643–2650. doi: 10.2174/138161208786264124. [DOI] [PubMed] [Google Scholar]
- [166].Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- [168].Jury EC, Flores-Borja F, Kalsi HS, Lazarus M, Isenberg DA, Mauri C, Ehrenstein MR. Abnormal CTLA-4 function in T cells from patients with systemic lupus erythematosus. Eur J Immunol. 2009 doi: 10.1002/eji.200939781. [DOI] [PubMed] [Google Scholar]
- [169].Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2008;105(49):19396–19401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Krummel MF, Allison JP. Pillars article: CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. The journal of experimental medicine. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]; J Immunol. 2011;187(7):3459–3465. [PubMed] [Google Scholar]
- [172].Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nature reviews. 2011;11(12):852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- [173].Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Sansom DM. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology. 2000;101(2):169–177. doi: 10.1046/j.1365-2567.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- [176].Yang YF, Zou JP, Mu J, Wijesuriya R, Ono S, Walunas T, Bluestone J, Fujiwara H, Hamaoka T. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57(18):4036–4041. [PubMed] [Google Scholar]
- [177].Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Yu P, Steel JC, Zhang M, Morris JC, Waitz R, Fasso M, Allison JP, Waldmann TA. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci U S A. 2012;109(16):6187–6192. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Youlin K, Li Z, Xiaodong W, Xiuheng L, Hengchen Z. Combination immunotherapy with 4-1BBL and CTLA-4 blockade for the treatment of prostate cancer. Clinical & developmental immunology. 2012;2012:439235. doi: 10.1155/2012/439235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS ONE. 2011;6(4):e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Sundstedt A, Celander M, Eriksson H, Torngren M, Hedlund G. Monotherapeutically Nonactive CTLA-4 Blockade Results in Greatly Enhanced Antitumor Effects When Combined With Tumor-targeted Superantigens in a B16 Melanoma Model. J Immunother. 2012;35(4):344–353. doi: 10.1097/CJI.0b013e318253ec25. [DOI] [PubMed] [Google Scholar]
- [183].Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Current opinion in immunology. 2006;18(2):206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- [184].Dulos J, Carven GJ, van Boxtel SJ, Evers S, Driessen-Engels LJ, Hobo W, Gorecka MA, de Haan AF, Mulders P, Punt CJ, Jacobs JF, Schalken JA, Oosterwijk E, van Eenennaam H, Boots AM. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35(2):169–178. doi: 10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- [185].Scheier B, Amaria R, Lewis K, Gonzalez R. Novel therapies in melanoma. Immunotherapy. 2011;3(12):1461–1469. doi: 10.2217/imt.11.136. [DOI] [PubMed] [Google Scholar]
- [186].Trinh VA, Hwu WJ. Ipilimumab in the treatment of melanoma. Expert Opin Biol Ther. 2012 doi: 10.1517/14712598.2012.675325. [DOI] [PubMed] [Google Scholar]
- [187].Drake CG, Antonarakis ES. Current status of immunological approaches for the treatment of prostate cancer. Current opinion in urology. 2012;22(3):197–202. doi: 10.1097/MOU.0b013e3283519ad5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Tomasini P, Khobta N, Greillier L, Barlesi F. Ipilimumab: its potential in non-small cell lung cancer. Therapeutic advances in medical oncology. 2012;4(2):43–50. doi: 10.1177/1758834011431718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Dienstmann R, Markman B, Tabernero J. Application of monoclonal antibodies as cancer therapy in solid tumors. Current clinical pharmacology. 2012;7(2):137–145. doi: 10.2174/157488412800228929. [DOI] [PubMed] [Google Scholar]
- [190].Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Totterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. Journal of immunotherapy. 2010;33(3):225–235. doi: 10.1097/CJI.0b013e3181c01fcb. [DOI] [PubMed] [Google Scholar]
- [192].Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]