Abstract
Raised blood pressure (BP) is the world’s leading mortality risk factor. Childhood BP substantially predicts adult levels, and although both prenatal and postnatal growth influence it, their relative importance is debated. In a longitudinal study (Avon Longitudinal Study of Parents and Children) of 12 962 healthy children, we aimed to assess the relative contribution of different growth periods and of standardized measures of height versus weight-for-height (an adiposity marker) to BP at age 10 years. Conditional growth modeling was used in the 3230 boys and 3346 girls with BP measurements. Systolic BP was inversely associated with birth weight and weight-for-height but not length (−0.33, −0.27, and −0.12 mm Hg · SD−1; P=0.003, 0.035, and 0.35, respectively). In infancy, weight, weight-for-height, and height gains were all positively associated with systolic BP (0.90, 0.41, and 0.82 mm Hg · SD−1, respectively; all P<0.001). After infancy, all of the growth modalities were positively associated with systolic BP (weight, 1.91; weight-for-height, 1.56; height, 1.20 mm Hg · SD−1; all P<0.001). Similar but weaker associations were found with diastolic BP. Although BP at 10 years was associated with both prenatal and early postnatal growth, their influence was small compared with that of later growth. Because BP ranking relative to the population is substantially determined in the first decade of life, a focus on strategies to reduce the development of adiposity from infancy onward, rather than an emphasis on the nutrition and weight of mothers and infants, should bring greater reductions in population BP.
Keywords: blood pressure, childhood growth, hypertension, obesity, population
Raised blood pressure (BP) is the world’s leading mortality risk factor, responsible for 13% of deaths.1 Effects of BP are continuous across its normal range and not limited to hypertensives.2 Therefore, the greatest population health benefit should come from preventative strategies that reduce population-wide BP rather than targeting individuals with hypertension. BP ranking relative to the population is substantially determined in the first decade, suggesting that effective prevention strategies should begin in early childhood.3
Both genetic and environmental factors contribute to raised BP, and there is considerable interest in the role of growth and habitus. Worldwide prevalence of childhood obesity has increased dramatically over recent decades,4 affecting cardiovascular (CV) risk adversely.5 Because the relative importance of growth in height compared with adiposity for later BP remains unclear, we examined the associations of BP with these types of growth during different phases of childhood.
Controversy remains over how early growth influences later BP. Cross-sectional studies at all ages of childhood and adulthood have shown associations of BP with both birth weight and rapid postnatal “catch-up” growth.6 Two “programming” hypotheses have emerged, with different emphases. One states that offspring BP is determined by maternal factors, which limit fetal growth. As a result, interventions to improve maternal nutrition and to increase infant caloric intake have been proposed.7 The other states that rapid postnatal growth in response to low birth weight is detrimental, so that infant caloric intake should be limited.8
To address this controversy and establish the relative importance of height and adipose growth, we examined the influence of growth patterns on BP at age 10 years in a large, contemporary population of healthy children from the United Kingdom, recruited during gestation and followed up with serial measurements from birth.
Methods
Avon Longitudinal Study of Parents and Children Population
Avon Longitudinal Study of Parents and Children was established in 1990 to investigate the early life and genetic determinants of childhood health, development, and adult disease. The cohort and study design are detailed elsewhere (http://www.alspac.bris.ac.uk).9 Briefly, 14 541 pregnant women, expected to deliver between April 1991 and December 1992, were enrolled. A total of 14 062 live born children were followed up with questionnaires and, since the age of 7 years, at regular clinics until 2006 to establish anthropometric, behavioral, CV, and metabolic phenotypes. The Avon Longitudinal Study of Parents and Children Law and Ethics Committee and the local research ethics committee approved the study. Informed consent and assent were obtained from parents and children, respectively.
Anthropometric Measurements
Trained Avon Longitudinal Study of Parents and Children staff measured weight and crown-heel length (Harpenden Neonatometer; Holtain Ltd, Crymych, United Kingdom) at birth in 62% of subjects. Additional data were sourced from clinical records and birth notification. Weight and height were available from personal child health records until 5 years of age and have been shown to compare favorably with clinic measures from a subgroup.10 Subsequently, height and weight were measured during regular clinics with the children in light clothing, without shoes. Weight was measured to the nearest 100 g using Tanita scales (Wardworth Ltd, Bolton, United Kingdom). Height was measured to the nearest millimeter using a Harpenden stadiometer. Pubertal status was assessed by validated questionnaire.11
BP Measurement
At a median age of 10.6 years, children were asked to rest, without talking, for ≥5 minutes in the seated position before the first oscillometric BP measure was taken from the right arm using a Dinamap 9301 Vital Signs Monitor (Morton Medical, London, United Kingdom). The right arm was supported at midsternum level. Depending on arm circumference, 12- to 19-cm or 17- to 25-cm cuffs were used. The mean of 2 subsequent measures taken at 1-minute intervals was used. If 2 consecutive measures differed by >5 mm Hg, further measures were taken until stable readings were obtained.
Growth Curve Modeling
Age- and sex-dependent growth curves were constructed using data from 12 962 subjects who were born after singleton pregnancies with a normal gestation (37–43 weeks). Growth curves were modeled using Generalized Additive Models for Location, Scale and Shape in R (please see online-only Data Supplement). Models for weight and height were fitted for each sex using smoothly varying functions that describe the age-varying distributions of the data in terms of location (median), scale (coefficient of variation), and shape (skewness and kurtosis). Thus, at any age, measures of height or weight could be transformed to z scores using a population-relevant model of normal growth.
Data Interpolation and Weight-for-Height Z Scores
Individual growth trajectories for height and weight, as a series of z scores, were estimated for subjects with ≥2 temporally separated measures. Data >4 z scores from the median were removed (24 weight estimates [0.03%] and 34 height estimates [0.04%]). For each individual growth trajectory, outliers were determined and removed after statistical comparison with the immediately preceding and following measures (912 weight estimates [1%] and 906 height estimates [1%]).
Weight and height measurements did not always coincide. To calculate weight-for-height z scores, weight and height z scores were interpolated linearly to an arbitrary, fixed-age scale. This was sampled on alternate days from birth to 3 months, then weekly until 1 year, and then every 2.4 months until 10 years. Weight and height at specific ages were estimated by back transformation of z scores to natural units. To assess adiposity trajectories, weight-for-height z scores were used in preference to body mass index (BMI; please see the online-only Data Supplement). Unlike BMI, this adiposity index is statistically independent of height at all ages and can, therefore, be used in multiple regression models to compare the relative strength of associations of height growth and fat accrual with outcomes.
Conditional Growth Modeling
To assess growth over distinct age periods while eliminating some of the problems associated with highly correlated measures, we used conditional growth modeling (please see the online-only Data Supplement).12 For sequential growth intervals, variables were constructed that are statistically independent of each other, allowing inclusion together in multiple regression models. Thus, the influence of growth in specific intervals was assessed in comparison with, and adjusted for, growth in other intervals. We defined the intervals according to the correlation structure of the data so that they had approximately equal variability of growth. This resulted in sequential intervals between birth; 3.4 weeks; 1.9, 4.2, and 7.9 months; and 1.4, 3.2, 6, and 10 years for weight; between birth; 3.7 weeks; 2.0, 4.6, and 8.1 months; and 1.2, 2.2, 5.0, and 10.0 years for height; and between birth; 2.9 weeks; 1.6, 2.7, and 7.4 months; and 1.4, 3.2, 6.0, and 10.0 years for weight for height.
Statistical Analysis
Further analyses were carried out using Stata 11 (StataCorp, College Station, TX). Cross-sectional analyses of the associations of height and weight-for-height with BP were carried out using multiple linear regression, adjusted for sex. Predictors in conditional growth models were initial measure of size, residualized growth variables for all periods up to the specified time point, and sex.
Results
Table 1 describes the cohort. Birth weight and length were greater, and gestational age at birth was 0.09 weeks shorter in boys. Birth measures did not differ by source. Age, parental social class, and systolic BP did not differ by sex but boys were taller than girls, with lower weight, BMI, and diastolic BP at age 10 years. Pubertal status within 6 months of BP measurement was available for 51% of subjects, and although girls were more advanced than boys, only a small minority of boys (0.5%) and girls (7.7%) had advanced beyond Tanner Stage III.
Table 1.
Characteristics of the Study Population
| Boys |
Girls |
||||
|---|---|---|---|---|---|
| Characteristics | N | Mean (SD) | N | Mean (SD) | P Values |
| Birth characteristics | |||||
| Gestational age, wk | 3230 | 39.7 (1.3) | 3346 | 39.8 (1.3) | 0.0016 |
| Birth weight, kg | 3200 | 3.54 (0.49) | 3301 | 3.43 (0.45) | <0.0001 |
| Crown-heel length, cm | 2581 | 51.3 (2.2) | 2691 | 50.5 (2.1) | <0.0001 |
| Current characteristics | |||||
| Age, y* | 3230 | 10.6 (10.5–10.8) | 3346 | 10.6 (10.5–10.8) | 0.64 |
| Weight, kg* | 3226 | 33.4 (29.9–38.2) | 3345 | 34.0 (29.9–39.2) | 0.017 |
| Height, cm | 3222 | 140.4 (6.0) | 3346 | 139.8 (6.2) | 0.0005 |
| BMI, kg/m2* | 3221 | 16.9 (15.7–18.8) | 3345 | 17.4 (15.8–19.5) | <0.0001 |
| Systolic BP, mm Hg | 3230 | 104.0 (9.1) | 3346 | 104.3 (9.2) | 0.19 |
| Diastolic BP, mm Hg | 3230 | 59.6 (7.9) | 3346 | 60.9 (8.1) | <0.0001 |
| Parent-reported Tanner Stage† | |||||
| I | 1068 | 67.5% | 592 | 33.2% | |
| II | 434 | 27.4% | 670 | 37.5% | |
| III | 71 | 4.5% | 385 | 21.6% | <0.0001 |
| IV | 7 | 0.4% | 111 | 6.2% | |
| V | 2 | 0.1% | 27 | 1.5% | |
| Father, manual social class† | 2839 | 50.1% | 3007 | 52.1% | 0.13 |
| Mother, manual social class† | 2683 | 58.2% | 2800 | 58.1% | 0.91 |
P values come from 2-way t test comparisons between the sexes. BP indicates blood pressure; BMI, body mass index.
Values are median (interquartile range) and P values from Wilcoxon rank-sum tests.
Values are percentages and P values from χ2 tests.
At 10 years, 1 SD greater weight (7.1 kg in boys and 7.8 kg in girls) was associated with 1.93 mm Hg (95% CI, 1.72–2.15 mm Hg) greater systolic BP and 0.82 mm Hg (95% CI, 0.63–1.02 mm Hg) greater diastolic BP. In a multiple regression model, systolic BP was similarly associated with weight for height (1.50 mm Hg · SD−1 [95% CI, 1.27–1.72 mm Hg · SD−1]) and height (1.22 mm Hg · SD−1 [95% CI, 1.00–1.44 mm Hg · SD−1]) at 10 years, explaining 4.6% of its variance. Diastolic BP was associated with weight-for-height (1.13 mm Hg · SD−1 [95% CI, 0.93–1.33 mm Hg · SD−1]) but not height (0.01 mm Hg · SD−1 [95% CI, −0.19 to 0.20 mm Hg · SD−1]), explaining 2.5% of its variance. These associations did not differ by sex.
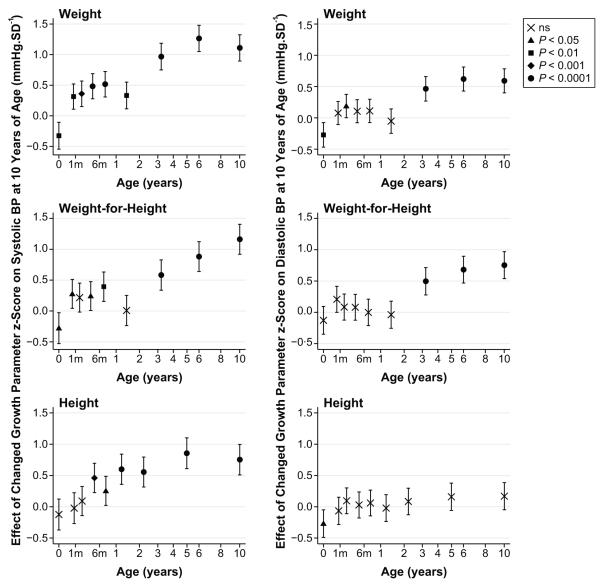
The independent associations of separate growth intervals with BP at 10 are shown in Figure 1. The values represent growth that is greater or less than normal population growth, analogous to the process of “crossing centiles” on growth charts. Using the graph of weight growth versus BP, as an example, highest BP at 10, would be expected for individuals with low birth weight, who cross centiles positively in all periods from birth to age 10 (birth to 3.4 weeks, 3.4 weeks to 1.9 months, etc). The similar modest positive associations with BP for the 5 periods ≤17 months suggest that “catch-up” growth effects are similar at any stage of infancy and less important than centile crossing after infancy.
Figure 1.
Conditional growth models for weight, weight for height, and height in relation to BP at 10 years. Each point shows the strength of association (±95% CI) for the period of growth from the preceding point or throughout gestation for the measure at birth and represents a difference from normal population growth over that period. Symbol shape (key) represents the statistical significance level for the null hypothesis. Age is plotted on a square-root transformed axis. × indicates not significant; ▲, P<0.05; ■, P<0.01;  , P<0.001; ●, P<0.0001.
, P<0.001; ●, P<0.0001.
Figure 1 suggests that a reasonable numeric summary of the results can be obtained using 3 key growth periods to evaluate the influences of growth before birth (prenatal), in the first 17 months of life (infancy), and between 17 months and 10 years (postinfancy growth; Table 2). As in the detailed models, the intervals were defined according to the correlation structure of the data. Although definitions of the term “infancy” vary, we have used it as a convenient description of the 17-month postnatal period defined by our simplified models. Greater systolic BP at 10 years was associated with lower prenatal growth of weight and weight for height but not height and with greater growth in any measure at any stage after birth. Greater diastolic BP at 10 years was associated with lower prenatal growth of weight and length but not weight for height and with postinfancy growth in weight and weight for height but not height. However, infancy growth in any measure had little effect on diastolic BP. In the postinfancy period, an SD change in weight had a greater effect on BP at 10 years than it did prenatally or in infancy.
Table 2.
Associations of Conditional Weight, Weight for Height, and Height Growth With BP at 10 Years for 3 Key Growth Periods (mm Hg · SD−1)
| Variable | Systolic BP (95% CI) | P Value | Diastolic BP (95% CI) | P Value |
|---|---|---|---|---|
| Prenatal growth | ||||
| Weight | −0.33 (−0.55 to −0.11) | 0.003 | −0.28 (−0.47 to −0.08) | 0.006 |
| Weight for height | −0.27 (−0.52 to −0.02) | 0.035 | −0.12 (−0.35 to 0.10) | 0.27 |
| Height | −0.12 (−0.37 to 0.13) | 0.35 | −0.27 (−0.49 to −0.05) | 0.018 |
| Infant growth (0–17 mo) | ||||
| Weight | 0.90 (0.68–1.12) | <0.001 | 0.18 (−0.02 to 0.37) | 0.08 |
| Weight for height | 0.41 (0.16–0.66) | <0.001 | 0.06 (−0.16 to 0.28) | 0.57 |
| Height | 0.82 (0.58–1.07) | <0.001 | 0.08 (−0.14 to 0.30) | 0.48 |
| Postinfancy growth (>17 mo to 10 y) | ||||
| Weight | 1.91 (1.69–2.13) | <0.001 | 0.95 (0.76–1.14) | <0.001 |
| Weight for height | 1.56 (1.32–1.81) | <0.001 | 1.14 (0.92–1.35) | <0.001 |
| Height | 1.20 (0.96–1.45) | <0.001 | 0.22 (−0.002 to 0.43) | 0.052 |
All models were adjusted for sex. BP indicates blood pressure.
To test the possibility that the associations of BP with prenatal and infant growth might be explained by a group of low birth weight subjects catching up on growth after birth, analyses were repeated excluding subjects with less than median birth weight. This gave similar results: 1-SD lower weight at birth was associated with 0.56-mm Hg higher systolic BP (P=0.028) and 0.49-mm Hg higher diastolic BP (P=0.031), and infancy weight gain was positively associated with later systolic BP (0.82 mm Hg · SD−1; P<0.001) but not diastolic BP (0.02 mm Hg · SD−1; P=0.9). Formal interaction testing showed no significant difference in the associations between growth in infancy and BP at 10 years, according to birth weight. Thus, the independent associations of reduced birth weight and of increased postnatal growth with raised BP at 10 are not confined to low birth weight subjects.
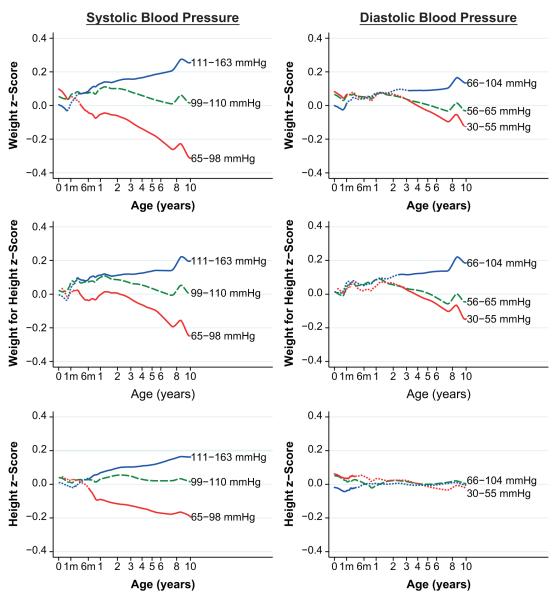
Average growth patterns in the 3 size parameters, according to categories of systolic and diastolic BPs, are shown in Figure 2. Children with higher systolic BP at age 10 years started life with, on average, lower weight, no difference in length, and no difference in weight for height from children with a lower BP. They steadily gained height, weight, and weight for height at a greater than average rate in their first 10 years, whereas those with lower BP grew at a lower rate than average. Most of the divergence in these parameters occurred after infancy, supporting the findings of the conditional growth models. All of the parameters showed a similar pattern, with weight having the strongest association. Patterns of associations of weight and weight for height with diastolic BP were very similar, although the effects were generally smaller, but associations of height with diastolic BP were markedly different. Children with higher diastolic BP at age 10 years started life shorter, whereas those with lower diastolic BP at age 10 years were longer, but there was little difference in height growth after this between children in the different diastolic BP groups.
Figure 2.
Mean growth trajectories for the upper (thin blue line) and lower (thick red line) quartiles of BP at 10 years. The middle half of the population (dashed green line) is shown for comparison. Where the other lines are dotted, P>0.05 for a 2-way t test of the null hypothesis that the growth parameters are the same. Age is plotted on a square-root transformed axis.
Discussion
In the largest reported contemporary cohort of healthy, prepubertal children, we found that the strongest anthropometric association with BP at 10 years was weight gain after infancy, with contributions from both height and weight for height (a marker of adiposity). There were effects of size at birth and of early postnatal growth, as reported previously,6 but their magnitude was substantially smaller. Because BP at age 10 years is related substantially to adult BP,3 our findings support public health policy to reduce adiposity in children.
There is increasing interest in the effects of early life on future CV risk. Considerable emphasis has been put on prenatal and early postnatal influences. These were confirmed in our study but found to be independent of each other and relatively small. Our findings support previous associations between catch-up growth in low birth weight individuals and later BP. However, the influence of early postnatal growth did not depend solely on a subpopulation catching up from low weight, because similar associations were found when subjects with less than median birth weight were excluded. Growth after infancy was more strongly associated with later BP than growth in infancy or prenatal growth, supporting evidence that growth in early life has less influence on adult BP than growth in later childhood.13 This is consistent with evidence that growth after 11 years has a greater effect on adult BP than previous growth14 and with recent findings from this cohort that later adiposity increments have a greater association with BP at 16 years than earlier ones.15
It is interesting that height and adipose growth had almost equal associations with systolic BP. This suggests that, in postnatal life, acquisition of mass, regardless of tissue type, increases systolic BP. However, this was not the case for diastolic BP, which was only associated with postinfancy growth. Lowest BP was associated, not with average growth, but with growth that was lower than predicted by early size. This supports the concept that mismatch between early life specification of systems and eventual demands placed on them may determine BP status. Animal data16 suggest that demands on renal capacity made by rapid childhood growth may not be met completely by renal development, resulting in compensatory BP increases. Other systems may also be affected. Although nephron numbers are largely determined by birth, so too are cardiac myocyte populations, arteriolar wall structures, and vessel densities.17
Our data, at first sight, might suggest that limiting growth after birth in any form should be beneficial. Although this might lower BP, negative consequences are likely. For example, final height is inversely related to coronary heart disease risk.18 Thus, strategies to reduce adiposity alone are more likely to be beneficial, given known associations between obesity and CV risk.5,19
Body mass explained ≈5% of systolic BP variance at age 10 years. However, we have not fully accounted for the considerable momentary and daily BP variability in individuals or the ±5% to 10% error of BP devices. Thus, we may have underestimated the true association of growth with long-term BP status. Although other factors undoubtedly influence BP, meaningful reductions in CV risk could be achieved by BP reductions resulting from decreased adiposity.5,19
BP in childhood is less likely to be influenced by the effects of aging and chronic disease on the CV system. Thus, effects of growth should be more apparent. We chose to study BP at age 10 years to minimize the effects of puberty on BP. Although our questionnaire assessment of pubertal status was limited, it was sufficient to determine that the majority of subjects had not achieved advanced puberty. Deviations from average growth in this cohort were assessed using relevant data from the same population rather than a reference population. Many longitudinal growth studies have examined the stages of childhood growth using only a few divisions of the age range. In contrast, we chose intervals defined by the correlation structure of our data so that each had an approximately equal change in rank ordering of individuals for a given measure. This yielded periods of differing chronological time but of arguably similar biological importance. The amount of data available in our study, particularly in early life, enabled unusually short periods of growth to be studied while maintaining good levels of precision. This allowed for a detailed assessment of the influence of different growth periods on later BP. It also enabled a simpler model, limited to 3 growth periods of prenatal life, infancy, and postinfancy to be constructed. Unfortunately, the timing and influence of adiposity rebound could not be examined because of sparse data at age 6 years.
We used weight-for-height z score to assess adiposity. Unlike BMI or ponderal index, this measure removes the age-varying correlation between height and weight entirely and therefore allows for meaningful comparisons between age groups and estimation of relative contributions of height versus adipose growth. In common with other indices, this measure estimates adiposity indirectly and may, therefore, be influenced by changes in lean mass that are not related to height growth. Direct measures using technologies such as dual-energy x-ray absorptiometry are possible but not practical in studies like ours. However, such measures would be necessary to estimate the portion of overall mass attributed to the expected increase in bone mineral density that results from increased adiposity.20 We showed recently in this cohort that weight-for-height indices such as BMI are comparable to x-ray absorptiometry fat mass in their associations with CV risk factors.5,19
Perspectives
Raised BP is associated with more global mortality than any other risk factor. Many adults require lifelong treatment, often with multiple drugs, to achieve target BP levels. Nevertheless, their risk remains much higher than for people with the same BP who were never hypertensive.21 Furthermore, such therapies do little to alter the risk profile of the whole population, where the number of deaths from BP-related conditions exceeds that for the hypertensive subpopulation.22 Because BP ranking relative to the population is substantially determined in the first decade of life,3 BP reduction strategies aimed at children should have lifelong benefit in terms of CV risk. Our study suggests that a focus on strategies to reduce the development of adiposity from infancy onward, rather than an emphasis on the nutrition and weight of mothers and infants, should bring greater reductions in population BP.
Supplementary Material
Acknowledgments
We thank the families, midwives, the team from the Avon Longitudinal Study of Parents and Children, Dr Vivek Muthurangu, and Prof Clive Osmond for help with the article.
Sources of Funding This work was supported by an National Institute for Health Research BMC grant (AJ [09CC04]); the Medical Research Council MRC, Wellcome Trust, United Kingdom Department of Health, Department of the Environment, Department for Education and Employment, National Institutes of Health, and others (Avon Longitudinal Study of Parents and Children); and a British Heart Foundation grant (to J.E.D. and A.D.H. [FS/05/125]).
Footnotes
Disclosures None.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.111.187716/-/DC1.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization . Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization; Geneva, Switzerland: [Accessed January 13, 2011]. 2009. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. [Google Scholar]
- 2.van den Hoogen PC, Feskens EJ, Nagelkerke NJ, Menotti A, Nissinen A, Kromhout D. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world: Seven Countries Study Research Group. N Engl J Med. 2000;342:1–8. doi: 10.1056/NEJM200001063420101. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falaschetti E, Hingorani AD, Jones A, Charakida M, Finer N, Whincup P, Lawlor DA, Davey Smith G, Sattar N, Deanfield JE. Adiposity and cardiovascular risk factors in a large contemporary population of prepubertal children. Eur Heart J. 2010;31:3063–3072. doi: 10.1093/eurheartj/ehq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJP, Eriksson JG, Forsén TJ, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 8.Singhal A, Cole TJ, Fewtrell M, Kennedy K, Stephenson T, Elias-Jones A, Lucas A. Promotion of faster weight gain in infants born small for gestational age: is there an adverse effect on later blood pressure? Circulation. 2007;115:213–220. doi: 10.1161/CIRCULATIONAHA.106.617811. [DOI] [PubMed] [Google Scholar]
- 9.Golding J, Pembrey M, Jones R. ALSPAC-the Avon Longitudinal Study of Parents and Children: I–study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 10.Howe LD, Tilling K, Lawlor DA. Accuracy of height and weight data from child health records. Arch Dis Child. 2009;94:950–954. doi: 10.1136/adc.2009.162552. [DOI] [PubMed] [Google Scholar]
- 11.Rubin C, Maisonet M, Kieszak S, Monteilh C, Holmes A, Flanders D, Heron J, Golding J, McGeehin M, Marcus M. Timing of maturation and predictors of menarche in girls enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2009;23:492–504. doi: 10.1111/j.1365-3016.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson JG, Osmond C, Kajantie E, Forsen TJ, Barker DJ. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia. 2006;49:2853–2858. doi: 10.1007/s00125-006-0459-1. [DOI] [PubMed] [Google Scholar]
- 13.Adair LS, Martorell R, Stein AD, Hallal PC, Sachdev HS, Prabhakaran D, Wills AK, Norris SA, Dahly DL, Lee NR, Victora CG. Size at birth, weight gain in infancy and childhood, and adult blood pressure in 5 low- and middle-income-country cohorts: when does weight gain matter? Am J Clin Nutr. 2009;89:1383–1392. doi: 10.3945/ajcn.2008.27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamborg M, Andersen PK, Baker JL, Budtz-Jorgensen E, Jorgensen T, Jensen G, Sorensen TI. Life course path analysis of birth weight, childhood growth, and adult systolic blood pressure. Am J Epidemiol. 2009;169:1167–1178. doi: 10.1093/aje/kwp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howe LD, Tilling K, Benfield L, Logue J, Sattar N, Ness AR, Davey Smith G, Lawlor DA. Changes in ponderal index and body mass index across childhood and their associations with fat mass and cardiovascular risk factors at age 15. PLoS One. 2010;5:e15186. doi: 10.1371/journal.pone.0015186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weder AB, Schork NJ. Adaptation, allometry, and hypertension. Hypertension. 1994;24:145–156. doi: 10.1161/01.hyp.24.2.145. [DOI] [PubMed] [Google Scholar]
- 17.Dilley RJ, Schwartz SM. Vascular remodeling in the growth hormone transgenic mouse. Circ Res. 1989;65:1233–1240. doi: 10.1161/01.res.65.5.1233. [DOI] [PubMed] [Google Scholar]
- 18.Paajanen TA, Oksala NK, Kuukasjarvi P, Karhunen PJ. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur Heart J. 2010;31:1802–1809. doi: 10.1093/eurheartj/ehq155. [DOI] [PubMed] [Google Scholar]
- 19.Lawlor DA, Benfield L, Logue J, Tilling K, Howe LD, Fraser A, Cherry L, Watt P, Ness AR, Davey Smith G, Sattar N. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ. 2010;341:c6224. doi: 10.1136/bmj.c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timpson NJ, Sayers A, Davey-Smith G, Tobias JH. How does body fat influence bone mass in childhood? A Mendelian randomization approach. J Bone Miner Res. 2009;24:522–533. doi: 10.1359/jbmr.081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor DA, Kim L, Morris R, Amuzu A, Whincup P, Ebrahim S. Survival with treated and well-controlled blood pressure: findings from a prospective cohort study. PLoS One. 2011;6:e17792. doi: 10.1371/journal.pone.0017792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.