Abstract
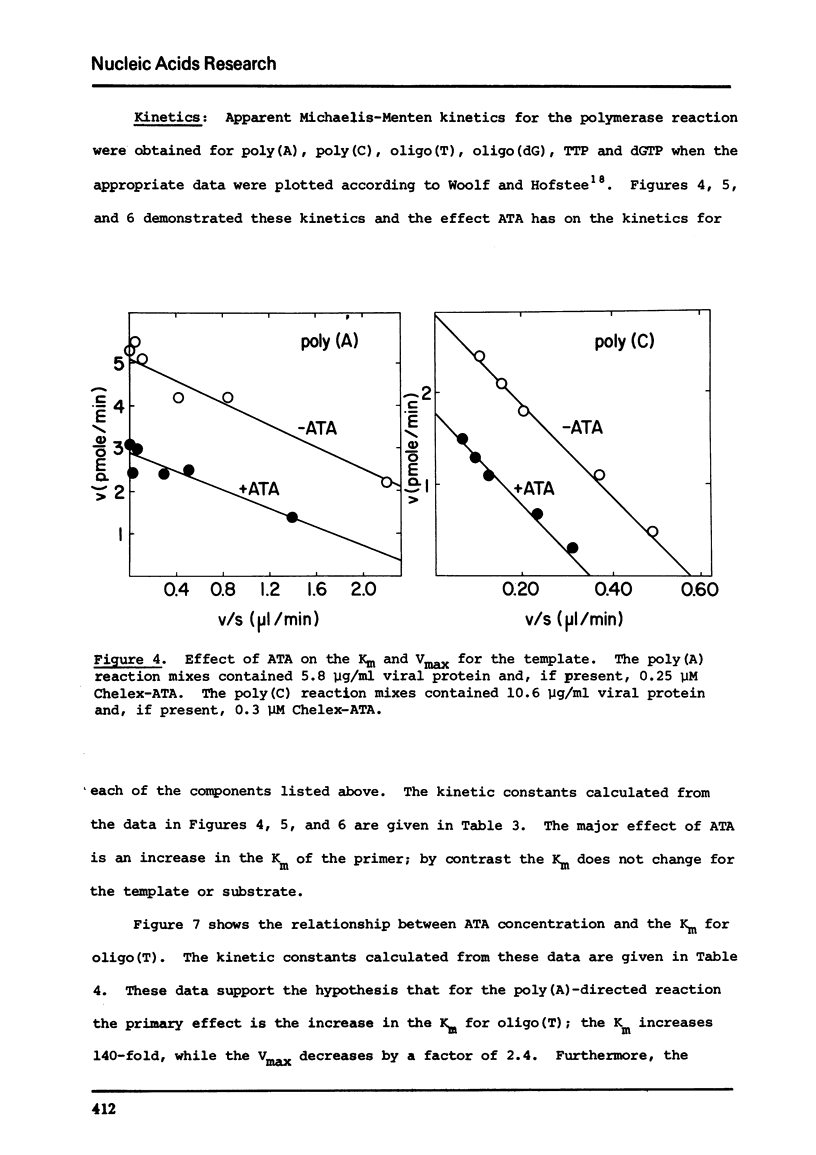
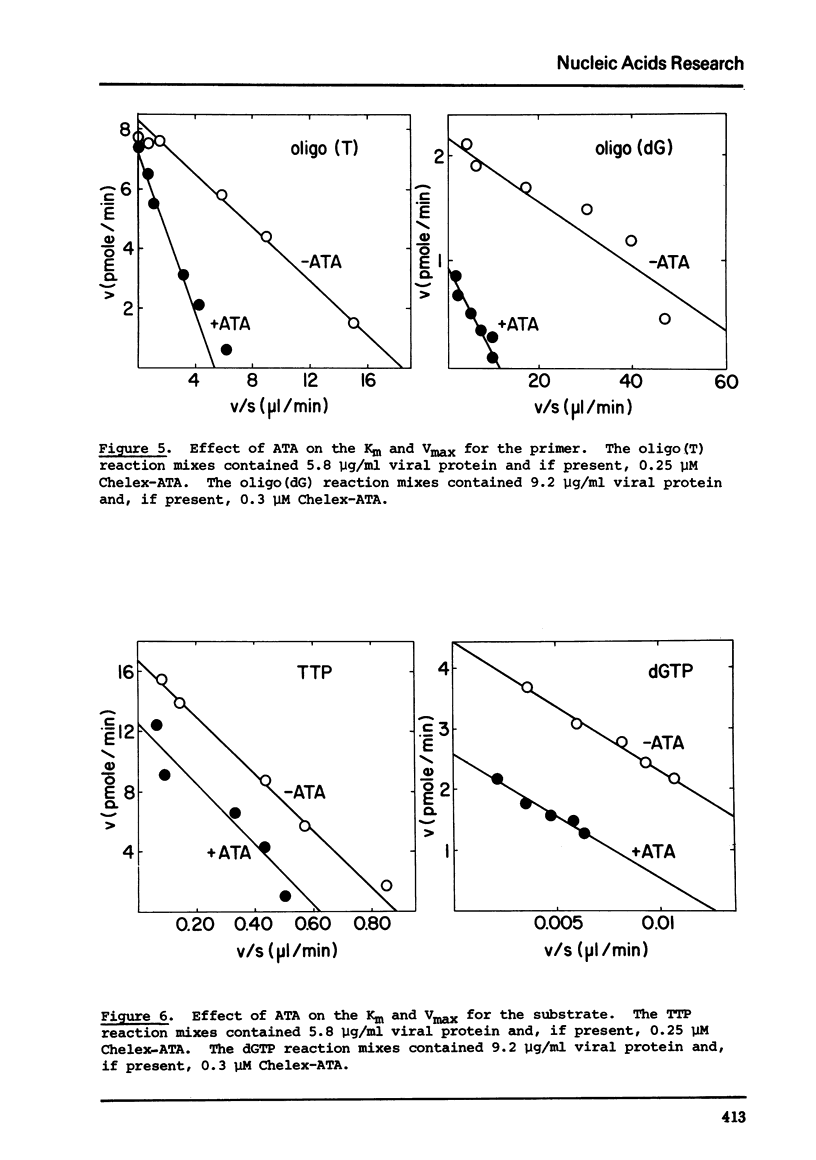
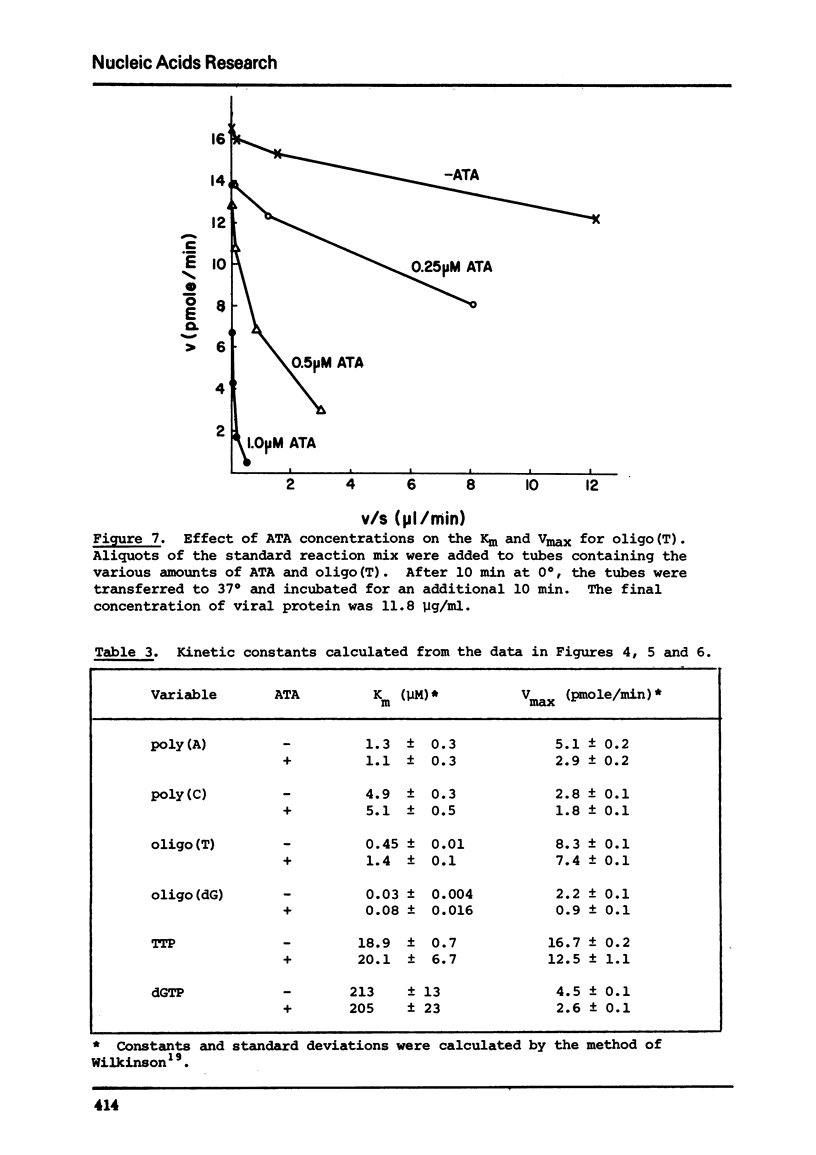
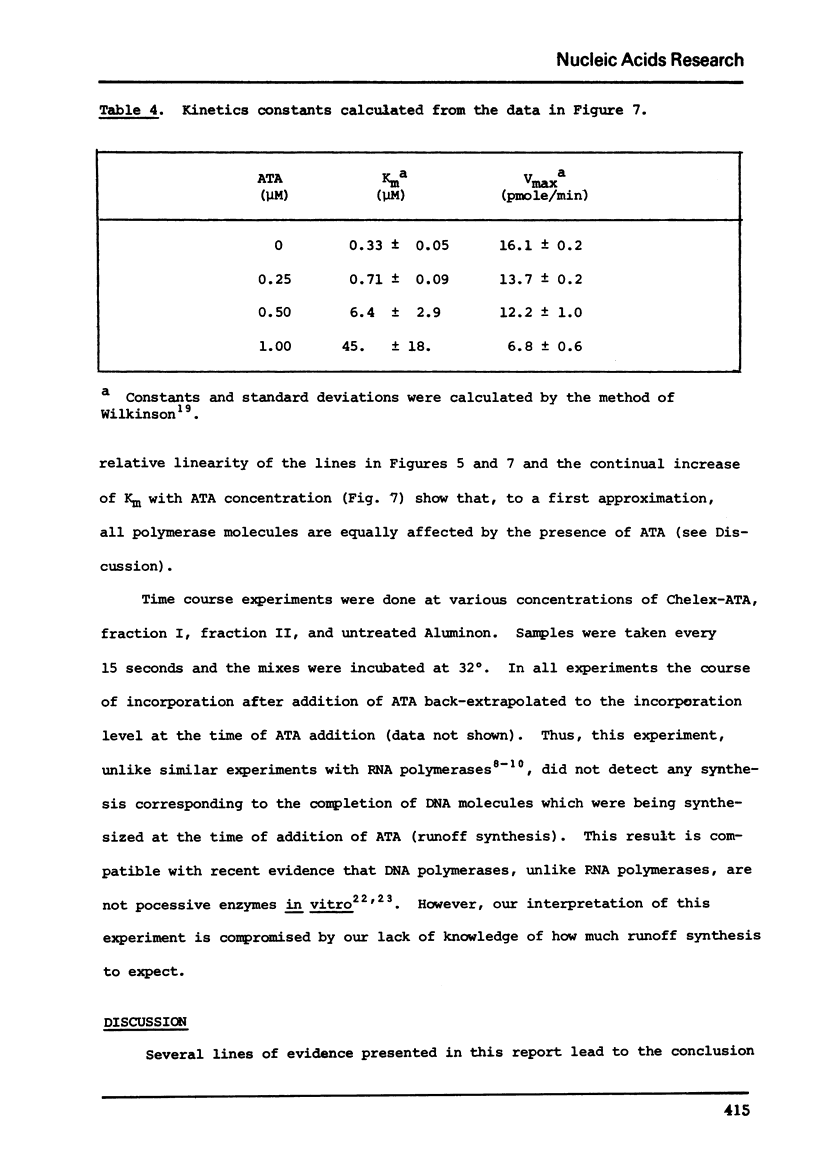
Commercial-grade aurintricarboxylic acid (ATA) inhibits poly(A), poly(C) and viral RNA-directed DNA synthesis by detergent-disrupted virions of Moloney murine leukemia virus. Paper chromatography of crude ATA yields two active components, which appear to behave identically, and at least two inactive components. The concentration of ATA needed to inhibit polymerase activity is proportional to the concentration of viral protein. The inhibition is neither attributable to contaminating heavy metal ions in the ATA preparation nor to chelation by ATA of Mn2+ or Zn2+, the necessary co-factors. Inhibition of the polymerase reaction by ATA greatly increases the Km for the primer [oligo(T)/oligo(dG)], while it only slightly lowers the Vmax and does not affect the Km's for the template [poly(A)/poly(C)] or the substrate (TTP/dGTP). Thus, ATA seems to reduce specifically the affinity of the polymerase for the DNA primer molecule.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Kawaguchi H., Livingston D. M., Vallee B. L. Reverse transcriptase from avian myeloblastosis virus: a zinc metalloenzyme. Biochem Biophys Res Commun. 1974 Apr 23;57(4):967–972. doi: 10.1016/0006-291x(74)90790-6. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A. The inhibition of nucleic acid-binding proteins by aurintricarboxylic acid. Biochem Biophys Res Commun. 1973 Dec 10;55(3):680–688. doi: 10.1016/0006-291x(73)91198-4. [DOI] [PubMed] [Google Scholar]
- Chang L. M. The distributive nature of enzymatic DNA synthesis. J Mol Biol. 1975 Apr 5;93(2):219–235. doi: 10.1016/0022-2836(75)90129-1. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Grollman A. P., Stewart M. L. Inhibition of the attachment of messenger ribonucleic acid to ribosomes. Proc Natl Acad Sci U S A. 1968 Oct;61(2):719–725. doi: 10.1073/pnas.61.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. T., Grollman A. P. Effects of aurintricarboxylic acid on ribosomes and the biosynthesis of globin in rabbit reticulocytes. Mol Pharmacol. 1972 Mar;8(2):111–127. [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Faras A., Woodson B., Jackson J., Bishop J. M. Inhibition of RNA-dependent DNA polymerase of Rous sarcoma virus by thiosemicarbazones and several cations. Proc Natl Acad Sci U S A. 1973 Jan;70(1):164–168. doi: 10.1073/pnas.70.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L. L., Horwitz S. B., Huang M. T., Grollman A. P., Steward D., Martin J. Triphenylmethane dyes as inhibitors of reverse transcriptase, ribonucleic acid polymerase, and protein synthesis. Structure-activity relationships. J Med Chem. 1975 Jan;18(1):117–120. doi: 10.1021/jm00235a029. [DOI] [PubMed] [Google Scholar]
- Manly K. F. A continuous-flow system for the growth of RNA tumor viruses. Anal Biochem. 1975 Feb;63(2):491–500. doi: 10.1016/0003-2697(75)90373-5. [DOI] [PubMed] [Google Scholar]
- Manly K. F. Histones stimulate polyribonucleotide-directed polydeoxyribonucleotide synthesis by murine leukemia virus. J Virol. 1974 Feb;13(2):305–311. doi: 10.1128/jvi.13.2.305-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure W. R., Jovin T. M. The steady state kinetic parameters and non-processivity of Escherichia coli deoxyribonucleic acid polymerase I. J Biol Chem. 1975 Jun 10;250(11):4073–4080. [PubMed] [Google Scholar]
- Poiesz B. J., Battula N., Loeb L. A. Zinc in reverse transcriptase. Biochem Biophys Res Commun. 1974 Feb 27;56(4):959–964. doi: 10.1016/s0006-291x(74)80282-2. [DOI] [PubMed] [Google Scholar]
- Siegelman F., Apirion D. Aurintricarboxylic acid, a preferential inhibitor of initiation of protein synthesis. J Bacteriol. 1971 Mar;105(3):902–907. doi: 10.1128/jb.105.3.902-907.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springgate C. F., Mildvan A. S., Abramson R., Engle J. L., Loeb L. A. Escherichia coli deoxyribonucleic acid polymerase I, a zinc metalloenzyme. Nuclear quadrupolar relaxation studies of the role of bound zinc. J Biol Chem. 1973 Sep 10;248(17):5987–5993. [PubMed] [Google Scholar]
- Stewart M. L., Grollman A. P., Huang M. T. Aurintricarboxylic acid: inhibitor of initiation of protein synthesis. Proc Natl Acad Sci U S A. 1971 Jan;68(1):97–101. doi: 10.1073/pnas.68.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. E., Zinder N. D. Fate of the message-ribosome complex upon translation of termination signals. J Mol Biol. 1969 Jun 28;42(3):425–439. doi: 10.1016/0022-2836(69)90234-4. [DOI] [PubMed] [Google Scholar]
- Wilhelm J. M., Haselkorn R. The chain growth rate of T4 lysozyme in vitro. Proc Natl Acad Sci U S A. 1970 Feb;65(2):388–394. doi: 10.1073/pnas.65.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]


