Abstract
Autophagy is conserved throughout the eukaryotes and for many years, work in Saccharomyces cerevisiae has been at the forefront of autophagy research. However as our knowledge of the autophagic machinery has increased, differences between S. cerevisiae and mammalian cells have become apparent. Recent work in other organisms, such as the amoeba Dictyostelium discoideum, indicate an autophagic pathway much more similar to mammalian cells than S. cerevisiae, despite its earlier evolutionary divergence. S. cerevisiae therefore appear to have significantly specialized, and the autophagic pathway in mammals is much more ancient than previously appreciated, which has implications for how we interpret data from organisms throughout the eukaryotic tree.
Keywords: S. cerevisiae, Dictyostelium, Saccharomyces, autophagy, evolution, omegasomes
Autophagy is fundamental to eukaryotic life, essential for survival following stresses such as starvation as well as cellular homeostasis. These roles are important for all cells and, as such, autophagy is conserved throughout the eukaryotes. From a research point of view, this is has been extremely useful, allowing the use of model organisms such as the yeast Saccharomyces cerevisiae to first identify and subsequently explore the core macroautophagic machinery.1 As autophagy has been described most fully in S. cerevisiae it has become the model to which autophagy in other organisms is compared. However as the number of organisms in which it is studied increases, it is important to reconsider how representative autophagy in S. cerevisiae is of other eukaryotes.
As our knowledge of the autophagic pathway has increased, it has become clear that there are substantial differences between mammalian and S. cerevisiae autophagy. Perhaps the most obvious of these is the presence of a single degradative vacuole in S. cerevisiae, as opposed to a number of acidic lysosomal vesicles in mammalian cells. It has been shown that lysosomal positioning is important to coordinate autophagy, and the reformation of lysosomes from autolysosomes is highly regulated, and therefore these interactions must differ substantially between organisms such as S. cerevisiae with a single lysosome and those with multiple lysosomal compartments.2,3
Another major difference is the location of autophagosome formation. The S. cerevisiae phagophore membrane is both initiated and expands from a single specialized structure termed the phagophore assembly site (PAS) that is disconnected from other cellular organelles.4 In contrast, mammalian autophagosomes form by transiently transforming regions of the endoplasmic reticulum (ER) or mitochondrial membranes into phagophore nucleation sites and can produce many autophagosomes simultaneously.5,6
These contrasting mechanisms of autophagosome formation clearly have different requirements. Therefore, whereas most of the core autophagic machinery is highly conserved, a number of elements are significantly divergent. While the genetic requirements for S. cerevisiae autophagy have largely been identified, the picture is less complete in other organisms and the goal of many groups is to identify new components of the pathway in mammals.
It is frequently assumed that elements of the autophagic pathway present in “higher eukaryotes” (i.e., metazoans) but absent in S. cerevisiae, are more recent adaptations and therefore metazoan specific. While in some cases this is undoubtedly true, during its evolution S. cerevisiae has become exquisitely specialized and undergone genome duplication and drastic gene loss. Therefore in many cases S. cerevisiae is not representative of metazoans, or even other fungi. Recent work, discussed below, indicates that this is also the case for autophagy, where in a number of respects S. cerevisiae is the “odd one out” and the mechanism of autophagosome formation is more universally conserved than previously thought.
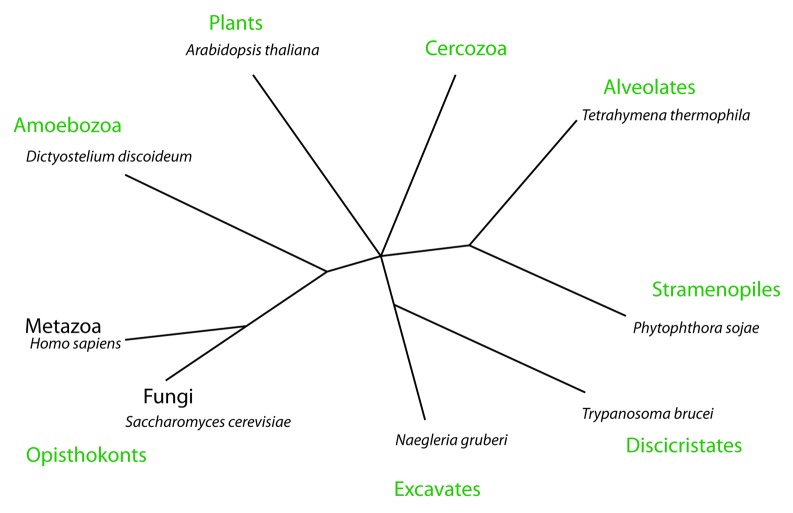
The complete range of eukaryotic organisms is diverse, but most model organisms used to study autophagy in detail are relatively close in evolutionary terms, generally belonging to the opisthokont branch of the phylogenetic tree (including the animal and fungal kingdoms). One of the organisms outside of this group where autophagosome formation has been studied in detail is Dictyostelium discoideum, a representative of the amoebozoa group that diverged from the metazoa at some point after the plants but before fungi (Fig. 1).7,9,10
Figure 1. A simplified eukaryotic tree. This phylogeny is based on references 7 and 8 and nearly all eukaryotes can be placed in one of the eight groups. The organisms named were used as representatives of each clade to search for the presence of autophagy-related genes. The Cercozoa have been excluded from the sequence analysis due the lack of complete genomic data.
Interestingly, studies using these amoeba show that like mammalian cells, the expanding phagophore forms from multiple regions of the ER, highly reminiscent of the omegasome structures observed in mammals.5,11 These regions are transient, and there is no evidence, therefore, of a PAS-equivalent structure. Dictyostelium cells also have a classical lysosomal compartment, consisting of numerous acidified and proteolytic vesicles rather than a single S. cerevisiae-style degradative vacuole.12 As S. cerevisiae diverged from the common eukaryotic ancestor after Dictyostelium, the PAS is likely to be a S. cerevisiae-specific adaptation. It is therefore probable that the generation of phagophores from the ER (and potentially other organelles) is the true ancient method of autophagosome formation, conserved throughout the eukaryotes as far as the fungi, which subsequently diverged.
Although there are no detailed studies of autophagosome formation in organisms covering most of the eukaryotic tree, there is evidence for this conserved mechanism at the genomic level. In recent years there has been much interest in identifying the ‘missing’ proteins required for macroautophagy in metazoans but absent in S. cerevisiae. A screen in Caenorhabditis elegans identified three genes encoding such proteins (epg-3, -4 and -5), two of which reside in the ER. These are vacuolar membrane protein 1 (VMP1) and etoposide-induced protein 24 (EI24) (also named EPG-3 and EPG-4, respectively).13 Interestingly, although absent in S. cerevisiae clear homologs of all three genes can be found in non-metazoans, and VMP1 and EI24 are present in every branch of the eukaryotic tree, along with the core members of the canonical autophagy apparatus (which are comprehensively described elsewhere14,15) (Table 1; Figs. S1 and S2). Interestingly, although they are lost from large parts of the fungal kingdom, including the entire Ascomycota phylum (containing S. cerevisiae) both genes can be found in the Chytrid fungus Batrachochytrium dendrobatidis, and EI24 orthologs are also present across the Basidiomycetes. Both these groups diverged early in the fungal lineage indicating that these genes were lost later, somewhere around the Ascomycota branch point.16
Table 1. Conservation of autophagy-related genes/proteins across the eukaryotes. Each genome was searched using the BLAST algorithm for orthologs of the respective human genes.
| Group | Organism | ATG1 | ATG4 | ATG5 | ATG6 | ATG7 | ATG8 | ATG9 | ATG18 | VMP1 | EI24 | epg-5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opisthokonts |
Saccharomyces cerevisiae |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N† |
N‡ |
N† |
|
Homo sapiens |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
|
Caenorhabditis elegans |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
| Amoebozoa |
Dictyostelium discoideum |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
| Plants |
Arabidopsis Thaliana |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
| Alveolates |
Tetrahymena thermophila |
Y |
Y |
Y |
Y |
Y |
Y |
N§ |
Y |
Y |
Y |
N |
| Stramenopiles |
Phytophthora sojae |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
| Discristates |
Trypanosoma brucei |
Y |
Y |
Y‖ |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
N |
| Excavates |
Naegleria gruberi |
Y | N¶ | Y | Y | Y | Y | Y | Y | N¶ | Y | Y |
Dark green shading indicates highly conserved orthologs with an E-value of < 10−10. Light green shading indicates more distant orthologs with E-values of < 10−5. Red shading indicates where no convincing ortholog could be identified (i.e., E > 10−5). †These genes have no homologs in S. cerevisiae, or any other fungi except Batrachochytrium dendrobatidis. ‡EI24 has no homolog in S. cerevisiae or any other fungi except those belonging to the basidiomycetes subgroup. §Although the Tetrahymena genome contains no obvious ATG9 ortholog, weakly homologous genes are present in other alveolates such as Toxoplasma gondii. ‖Although Trypanosome ATG5 is not identified using the human gene, a homolog can be identified using non-opisthokont ATG5 sequences.14 ¶No ATG4 or VMP1 orthologs appear to be present in any excavates currently sequenced. The accession numbers of sequences used to search were: ATG1 = hsUlk1 (NP_003556), ATG4 = hsAtg4a (AAH41862), ATG5 = hsAtg5 (CAI20314), atg6 = hsBeclin1 (NP_003757), ATG7 = hsAtg7 (AAH00091), ATG8 = hsLC3B (NP_852610), ATG9 = hsAtg9a (EAW70707), ATG18 = hsWIPI2 (Q9Y4P8, VMP1 = hsVMP1 (CAG38552), EI24 = hsEI24 (NP_004870) and epg-5 = mEPG5 (NP_066015).
The presence of VMP1 and EI24 in diverse organisms such as Trypanosoma brucei (a member of one of the earliest groups to diverge from the common eukaryotic ancestor) and plants clearly demonstrates that they were present during early eukaryotic evolution and have been subsequently lost in S. cerevisiae. A conserved function for these genes has also been demonstrated in Dictyostelium where VMP1 is also required for autophagy and both VMP1 and EI24 localize to the ER (see ref. 17; King J, unpublished data). Although functional studies of these genes in other protists is required to confirm a role in autophagy it is likely that the autophagic process observed in metazoans is more highly conserved through evolution than previously thought, and formation from the ER, rather than a PAS, is the true ancient mechanism of autophagosome formation.
The diversity of organisms in which autophagy is being studied is steadily growing, and in particular there is growing interest in autophagy in pathogens such as the trypanosomes, Toxoplasma gondii, Leishmania major and Entamoeba histolytica where it plays important roles in differentiation and pathogenicity (recently reviewed in refs. 14,15). Recent molecular and bioinformatic studies indicate that the function of several core ATG genes are highly conserved18-20 but it is clear that different organisms utilize the autophagic process in different ways. Therefore the upstream signaling and regulation of autophagy in selective organelle removal and development are more divergent than the core machinery and cannot be so easily translated across species.21-23 Currently there are no detailed analyses of autophagosome biogenesis in any of these organisms and the source of the membrane and location of phagophore expansion is unknown. However, as they all retain VMP1 and EI24, I speculate that they also use an omegasome rather than a PAS-type mechanism of autophagosome formation.
When interpreting data from model organisms and extrapolating it to mammalian cells, it is essential to consider the evolutionary background of the process in question. Autophagy is so fundamental to cell health and survival that it is exceptionally well conserved, and studies in S. cerevisiae have been invaluable. However, while some aspects of autophagy such as interactions with the classical apoptotic pathway via BCL2-family proteins clearly are specific to metazoans,24 the yeast are so specialized that the absence of a gene in S. cerevisiae does not necessarily indicate metazoan specificity.
Many other questions about the evolution of autophagy remain. Recently it has been shown that both mitochondria and the plasma membrane can act as sites for mammalian autophagosome biogenesis and it will be interesting to see if this is also conserved.6,25 The eukaryotes are so diverse that there cannot be a generic model for all of them and, despite its limitations, S. cerevisiae remains at the heart of autophagy research. As the number of organisms studied rises, and the level of detail increases we will gain a fuller picture of how autophagy has changed and adapted through evolution. It is already clear that most of autophagy is far more ancient than previously thought.
Supplementary Material
Acknowledgments
I would like to thank Robert Insall and Douwe Veltman for helpful discussions and critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20527
References
- 1.Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–74. doi: 10.1016/0014-5793(93)80398-E. [DOI] [PubMed] [Google Scholar]
- 2.Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13:453–60. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–6. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–81. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–67. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keeling PJ, Burger G, Durnford DG, Lang BF, Lee RW, Pearlman RE, et al. The tree of eukaryotes. Trends Ecol Evol. 2005;20:670–6. doi: 10.1016/j.tree.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Baldauf SL. The deep roots of eukaryotes. Science. 2003;300:1703–6. doi: 10.1126/science.1085544. [DOI] [PubMed] [Google Scholar]
- 9.Calvo-Garrido J, Carilla-Latorre S, Kubohara Y, Santos-Rodrigo N, Mesquita A, Soldati T, et al. Autophagy in Dictyostelium: genes and pathways, cell death and infection. Autophagy. 2010;6:686–701. doi: 10.4161/auto.6.6.12513. [DOI] [PubMed] [Google Scholar]
- 10.Cavalier-Smith T. Kingdoms Protozoa and Chromista and the eozoan root of the eukaryotic tree. Biol Lett. 2010;6:342–5. doi: 10.1098/rsbl.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King JS, Veltman DM, Insall RH. The induction of autophagy by mechanical stress. Autophagy. 2011;7:1490–9. doi: 10.4161/auto.7.12.17924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maniak M. Conserved features of endocytosis in Dictyostelium. Int Rev Cytol. 2002;221:257–87. doi: 10.1016/S0074-7696(02)21014-1. [DOI] [PubMed] [Google Scholar]
- 13.Tian Y, Li Z, Hu W, Ren H, Tian E, Zhao Y, et al. C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell. 2010;141:1042–55. doi: 10.1016/j.cell.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Duszenko M, Ginger ML, Brennand A, Gualdrón-López M, Colombo MI, Coombs GH, et al. Autophagy in protists. Autophagy. 2011;7:127–58. doi: 10.4161/auto.7.2.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiel JAKW. Autophagy in unicellular eukaryotes. Philos Trans R Soc Lond B Biol Sci. 2010;365:819–30. doi: 10.1098/rstb.2009.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature. 2006;443:818–22. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- 17.Calvo-Garrido J, Escalante R. Autophagy dysfunction and ubiquitin-positive protein aggregates in Dictyostelium cells lacking Vmp1. Autophagy. 2010;6:100–9. doi: 10.4161/auto.6.1.10697. [DOI] [PubMed] [Google Scholar]
- 18.Besteiro S, Brooks CF, Striepen B, Dubremetz JF. Autophagy protein Atg3 is essential for maintaining mitochondrial integrity and for normal intracellular development of Toxoplasma gondii tachyzoites. PLoS Pathog. 2011;7:e1002416. doi: 10.1371/journal.ppat.1002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koopmann R, Muhammad K, Perbandt M, Betzel C, Duszenko M. Trypanosoma brucei ATG8: structural insights into autophagic-like mechanisms in protozoa. Autophagy. 2009;5:1085–91. doi: 10.4161/auto.5.8.9611. [DOI] [PubMed] [Google Scholar]
- 20.Williams RA, Woods KL, Juliano L, Mottram JC, Coombs GH. Characterization of unusual families of ATG8-like proteins and ATG12 in the protozoan parasite Leishmania major. Autophagy. 2009;5:159–72. doi: 10.4161/auto.5.2.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan EY, Tooze SA. Evolution of Atg1 function and regulation. Autophagy. 2009;5:758–65. doi: 10.4161/auto.8709. [DOI] [PubMed] [Google Scholar]
- 22.Meijer WH, van der Klei IJ, Veenhuis M, Kiel JAKW. ATG genes involved in non-selective autophagy are conserved from yeast to man, but the selective Cvt and pexophagy pathways also require organism-specific genes. Autophagy. 2007;3:106–16. doi: 10.4161/auto.3595. [DOI] [PubMed] [Google Scholar]
- 23.Rigden DJ, Michels PA, Ginger ML. Autophagy in protists: Examples of secondary loss, lineage-specific innovations, and the conundrum of remodeling a single mitochondrion. Autophagy. 2009;5:784–94. doi: 10.4161/auto.8838. [DOI] [PubMed] [Google Scholar]
- 24.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 25.Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12:747–57. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.