Abstract
Background & Aims
Radiofrequency ablation (RFA) is a safe alternative to esophagectomy for patients with dysplastic Barrett’s esophagus (BE). Although some studies have indicated that RFA is effective at eradicating dysplasia, most have found that RFA is not as effective in eradicating intestinal metaplasia. We investigated whether uncontrolled reflux is associated with persistent intestinal metaplasia after RFA.
Methods
Thirty-seven patients with BE underwent RFA, high resolution manometry, and 24 hour impedance-pH testing; they received proton pump inhibitors twice daily. Patients returned every 2 months for repeat treatment or standard surveillance. Patients were classified as complete responders (CRs) if all intestinal metaplasia was eradicated in fewer than 3 ablation sessions. We analyzed clinical parameters to identify factors associated with a CR or incomplete response (ICR).
Results
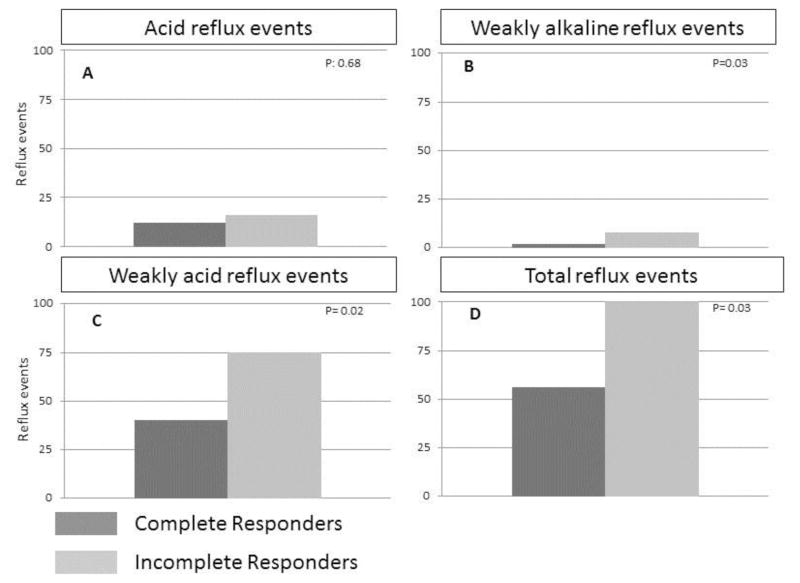
Among the 37 patients, 22 had a CR and 15 had an ICR. Mann-Whitney U tests revealed that length of BE, size of hiatal hernia, and frequency of reflux, but not acid reflux, differed between CRs and ICRs. CRs had fewer weakly acidic than ICRs (29.5 vs 52; P<.05) and total reflux events (33.5 vs 60; P<.05), and a trend towards fewer weakly alkaline events (1.0 vs 5.0; P=.06). No other clinical or manometric features differed between groups.
Conclusion
Uncontrolled, predominantly weakly acidic reflux despite twice daily proton pump inhibitor therapy before RFA increases the incidence of persistent intestinal metaplasia after ablation in patients with BE. Length of BE and size of a hiatal hernia were also associated with persistent intestinal metaplasia after RFA.
Keywords: esophagus, esophageal cancer, risk factor, prognosis
Introduction
Barrett’s esophagus (BE) carries an increased risk of adenocarcinoma compared to the general population1. This observation has led to considerable interest in ablative therapies for dysplastic BE, with the goal of decreasing the risk of adenocarcinoma. Recent investigations have focused on radiofrequency ablation (RFA) using the HALO (BARRX Medical Inc., Sunnyvale Ca) ablative system. A randomized controlled trial demonstrated that the rate of complete eradication of low grade dysplasia (LGD) and high grade dysplasia (HGD) was 90% and 81% respectively.2 However, the efficacy of RFA for eradication of intestinal metaplasia (IM) varies widely in the reported literature with complete response for IM (CR-IM) rates ranging between 46 and 98%2–12. The largest randomized controlled trial to date2 reported only a 70% eradication rate for IM and eradication rates for dysplasia are consistently reported to be greater than eradication rates for IM. The recent 5-year ablation durability study reported that when CR-IM was achieved, the response seemed to be durable for 3 years after ablation13. However, that study also revealed that many patients required at least 4 ablations in the first year to achieve this response and 25% of patients had recurrent IM during follow-up. A recent investigation by Vacarro et al reported that 25% of patients had recurrent IM within one year of ‘successful’ ablation 14. It is unclear why some patients have a durable, complete eradication of IM while others have recurrent or persistent IM after RFA. The presence of IM after ablative therapy is concerning as it implies ongoing malignant potential.
In vitro studies have shown that both acid and other gastric constituents (i.e. bile salts) are toxic to the esophagus, and lead to early changes in the pathogenesis of BE 15,16 . Patients with BE have been shown to carry the highest reflux exposure of any patient population.17 Previously, the clinical relevance of this reflux exposure was dismissed, as most patients are asymptomatic with once daily PPI therapy. However, given the role of reflux in the pathogenesis of BE, it is conceivable that differences in ongoing reflux exposure may be critical in the persistence of IM after RFA therapy. Indeed, some studies suggest that aggressive reflux management may decrease the recurrence of IM after ablation 18. Furthermore, a recent pilot investigation reported that when RFA was combined with antireflux surgery, 80% of patients achieved complete eradication of IM with only one ablation.19 These data suggest that ongoing reflux may contribute to the persistence of IM after ablative therapy. As such, we sought to investigate whether ongoing reflux exposure (both acidic and non-acidic) was associated with persistent IM after RFA.
Methods
Patient selection
Thirty-seven consecutive patients were recruited from our outpatient GI faculty practice. All patients underwent high resolution esophageal pressure topography (EPT) and 24 hour impedance-pH monitoring prior to ablation therapy. The diagnosis of BE and dysplasia was confirmed by an expert gastrointestinal pathologist at our institution. Barrett’s esophagus was defined by the presence of specialized columnar mucosa with goblet cells. Patients with non-dysplastic BE were included if they were deemed high risk (long segment BE with a family history of esophageal adenocarcinoma). Patients with raised or nodular lesions were excluded, as were patients with prior endoscopic therapy for BE. This study was approved by the Northwestern University Institutional Review Board.
High resolution manometry
A solid-state manometric assembly with 36 circumferential sensors spaced at 1 cm intervals was used (Given imaging, Los Angeles, CA). Studies were done after at least a 6-hour fast. The patients underwent transnasal placement of the manometric catheter and positioned to record from the hypopharynx to the stomach. Once in a correct position, the catheter was taped to the nose to maintain it throughout the study. Measurements were collected in both supine and sitting positions to assess esophageal and EGJ function. The manometric protocol included at least ten 5-ml swallows in each posture as well as a 5-minute period to assess basal sphincter pressure.
Impedance-pH testing
The impedance-pH catheter (Medical Measurement Systems Inc., Denmark) was positioned transnasally into the esophagus so that the pH electrode was 5 cm proximal to the EGJ based on landmarks provided by manometry, performed earlier on the same day. Recordings lasted for 24 hours, during which time the impedance-pH data were collected and stored in an external receiver attached to the catheter. Patients were encouraged to engage in their usual daily activities. All patients were taking twice-daily proton pump inhibitors (omeprazole 20 mg bid, esomeprazole 40 mg bid, lansoprazole 30 mg bid, rabeprazole 20 mg bid, or pantoprazole 40 mg bid) for at least two weeks prior to and during impedence-pH testing.
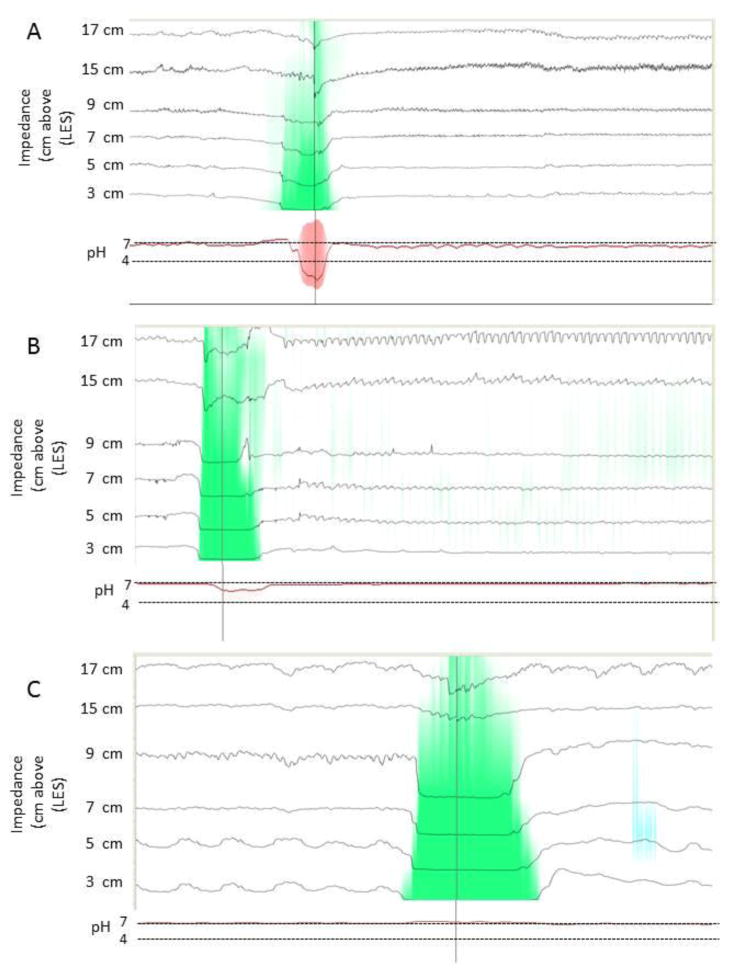
Reflux events were automatically identified and counted. Acid exposure time (AET) was reported as the percentage of the 24-hour study that the pH was less than four. . Each reflux event was correlated with the associated pH of that event and labeled either acidic (AR, pH<4), weakly acidic (WAR, pH 4–7), or weakly alkaline (WalkR, pH≥7) as per consensus guidelines 20. Impedence-pH data were independently reviewed in a blinded manner to verify the automated findings (Figure 1).
Figure 1.
Impedance-pH recordings demonstrating acid reflux (A), WAR (B), and WAlkR (C). The green colorization on the impedance tracings illustrates the retrograde flow of refluxate to the most proximal recording site (17 cm). The corresponding pH tracing in red at the bottom demonstrates the nadir pH to be 2.3 in A, 5.8 in B, and 7.2 in C.
Radiofrequency ablation protocol
The ablation protocol was standardized for all patients and all endoscopy was performed by a single highly experienced endoscopist blinded to the results of the impedance-pH and manometry studies. Initial circumferential ablation was performed with the HALO360 catheter (BARRX, Sunnyvale Ca). Patients returned after 2 months, at which time residual BE was focally ablated using the HALO90 ablation catheter. All patients received circumferential ablation during the first treatment session and focal ablation with the Halo 90 device on all subsequent ablations. Patients with dysplasia underwent at least two ablations (one circumferential and one focal). In that cohort, we included at least one focal ablation to the newly formed squamocolumnar junction. However, if this was not achieved until the 3rd endoscopy, it was not our protocol to routinely ablate what endoscopically appeared to be a complete response.
Patients then returned in two months for surveillance endoscopy. Surveillance endoscopy was done using high definition endoscopes (Olympus GIF-H180, Olympus corp., Tokyo Japan) with white light and narrow band imaging. At that point, repeat RF treatment was performed if columnar mucosa was noted endoscopically; otherwise surveillance was performed with a standard biopsy protocol (see below). Patients with complete eradication of all dysplasia and IM at the third endoscopy were considered complete responders (CR) and those with residual dysplasia or IM were considered incomplete responders (ICR). Complete responders underwent surveillance again at one year from the initiation of therapy. If IM was found on subsequent biopsy in a patient labeled CR, they were re-categorized as ICR. Incomplete responders underwent repeat treatment every two months until eradication of IM/dysplasia was achieved. Patients were followed in this protocol for one year after the initial ablation therapy. All patients were placed on twice-daily proton pump inhibitor therapy consistent with their prior usage and were provided viscous lidocaine, sucralfate, and acetaminophen with codeine for short-term symptom relief.
Biopsy Protocol
After ablative therapy patients underwent surveillance. Biopsies were taken using jumbo forceps (RJ4, Boston Scientific, Natick, MA) in four quadrants, starting in the gastric cardia and continuing proximally at 2 cm increments until the proximal margin of the BE segment was sampled. Biopsies were evaluated for the presence of dysplasia and IM. Biopsies were interpreted by one of two expert gastrointestinal pathologists at our institution who were blinded to patient status.
Outcome
The primary aim of the study was to determine whether the severity of ongoing reflux exposure prior to treatment was associated with persistent IM after RFA therapy. Patients with durable, complete eradication of all dysplasia and IM after two ablations were labeled CR, whereas those patients with persistent IM after two ablations were labeled ICR. While reflux exposure was our primary variable of interest between CR and ICR, we also considered the potential impact of manometric and clinical factors on persistent IM after RFA. The manometric properties evaluated included esophagogastric junction (EGJ) pressure, percent failed peristalsis and the distal contractile integral (length × amplitude × duration of the distal esophageal contraction in mmHg-s-cm 21). The clinical factors analyzed were hiatal hernia size, baseline histology, length of BE, age, body mass index (BMI) and smoking history.
Statistical Analysis
Data were entered and analyzed in SPSS v.20. Measurement of central tendency revealed that the data were not normally distributed and violated the assumption of homogeneity of variances. Medians are reported in place of means. To estimate effect sizes for future studies powered to examine predictors of response with logistic regression, we performed independent sample non-parametric tests to determine differences between ICR and CR with respect to several factors likely to influence response. Alpha was set at .05. Actual statistical power for these analyses was 82%. Reflux parameters (WAlkR, WAR, TR, percent acid exposure) clinical and manometric factors including hiatal hernia length, maximum length of BE as determined by the Prague classification, baseline histology, smoking history (current, past, never), age and BMI were compared between groups using Mann Whitney U and Fisher’s Exact tests.
Results
Pre-treatment, clinical and manometric variables by responder status
Thirty-seven consecutive patients (median age 64.0; range 30–79) underwent ablative therapy; all but 3 had a hiatal hernia. Among the 37 patients entered, 22 patients achieved eradication of all IM in less than three ablation sessions (CR- complete responders) while 15 patients required three or more ablations due to persistent IM or dysplasia (ICR- incomplete responders). After a third ablation 24 patients were CR and 13 ICR. In total 35 of 37 patients achieved CR-IM at the end of the 12-month study follow up period, with two patients being persistent non responders and subsequently referred for cryoablation. The median number of ablations in the total cohort was 2 (range 2–6). and in the ICR group, 4 (range 3–6). All of the CR group underwent two ablations. The median number of endoscopies in the CR group was 4 (range 3–4) and in the ICR group, 5 (range 4–7.) Only one patient who was initially labeled CR was re-categorized as ICR on follow-up; this did not change the analysis.
There were no manometric features distinguishing CRs from ICRs. One patient in the CR group had distal esophageal spasm. Median basal EGJ pressure in the CRs was 7.5 mmHg compared to 6.0 mmHg in the ICRs (ns). The median DCI was 648 mmHg-s-cm in the CRs compared to 566 mmHg-s-cm in the ICRs (ns). Weak and failed peristalsis was equally common in the CRs and ICRs (CR 47% weak, 10% failed; ICR 53% weak, 9% failed; ns).
There were significant differences between CR and ICR with respect to length of BE (4.0 vs. 6.0 cm, z = 3.1, p < .01, [95% CI = −5.0,0.1] and size of hiatal hernia (2.3 vs. 3.0 cm, z = 3.2, p < .01 [95% CI = −3.0, −0.5). There were no significant differences between groups with respect to the other clinical parameters—age, BMI, smoking history or histology (Table 1).
Table 1.
Variables by responder group CR vs. ICR (Mann Whitney U test). Four factors were significantly different between groups: BE length, hiatal hernia length, WAR, and TR. While there was a trend for WAlkR, it was not statistically significant.
| Variable | CR (N = 22) | ICR (N =15 ) | Test Statistic | ||||
|---|---|---|---|---|---|---|---|
| Median | Variance | Median | Variance | z | p | 95% CI | |
| Baseline Characteristics | |||||||
| Age | 63.0 | 147 | 68.0 | 101 | 1.5 | 0.14 | |
| BE (cm) | 4.0 | 3.9 | 6.0 | 10.4 | 3.1 | <.01 | −5.0,0.1 |
| Histology NDBE/LGD/HGD | 7/10/5 | 4/7/4 | X2 = 0.14, p =0 .93 | ||||
| HH (cm) | 2.3 | 1.5 | 3.0 | 9.2 | 3.2 | <.01 | −3,−0.5 |
| BMI | 28.0 | 16.0 | 30.0 | 21.1 | 1.3 | 0.19 | |
| Never Smoked | 15 (68%) | 12 (80%) | X2 = 1.0, p = 0.59 | ||||
| High Resolution Manometry | |||||||
| EGJP (mmHg) | 7.5 | 62.8 | 6.0 | 28.9 | −0.82 | 0.42 | |
| DCI (mmHg-s-cm) | 648 | 1501343 | 566 | 732623 | −0.43 | 0.68 | |
| Peristalsis Weak/ Absent (%) | 35.0 | 1140 | 30.0 | 1174 | −0.03 | 0.99 | |
| Peristalsis present (%) | 50.0 | 1023 | 60.0 | 1167 | 0.05 | 0.53 | |
| pH-Impedance Monitoring | |||||||
| Acid exposure time (%) | 0.20 | 26.6 | 0.20 | 119 | 0.26 | 0.57 | |
| AR | 2.0 | 360 | 3.0 | 1076 | 0.48 | 0.64 | |
| WAlkR | 1.0 | 25.4 | 5.0 | 82.1 | 1.9 | 0.06 | −8.0,0 |
| WAR | 29.5 | 695 | 52.0 | 2762 | 2.2 | 0.03 | −65.0,− 2.0 |
| TR | 33.5 | 999 | 60.0 | 2779 | 2.1 | 0.03 | −67.0,−4.0 |
Abbreviations: CR, complete response; ICR, incomplete response; BE, Barrett’s Esophagus; NDBE, non-dysplastic Barrett’s esophagus; LGD, low grade dysplasia; HGD, high grade dysplasia; HH, hiatal hernia; BMI, body mass index, EGJP, esophagogastric junction pressure; DCI, distal contractile integral; AR, acid reflux; WAlkR, weakly alkaline reflux; WAR, weakly acidic reflux; TR, total reflux
Comparison of reflux exposure between CR and ICR
As hypothesized,reflux exposure was most strongly asso ciated with response status. The AET varied greatly, but was on average less than 5%, the upper limit of normal in both groups (median 2.0% range 0–22%). Seven of the 37 patients (20%) with BE had abnormal distal esophageal acid exposure despite twice daily PPI therapy. Weakly acid reflux events were significantly more common in ICR vs. CR (52 vs. 29.5, z = 2.2, p = .03 [95%CI = −65.0,2.0), and TR (60.0 vs. 35.5, z = 2.1, p = .03 [95%CI = −67.0,−4.0). Weakly alkaline reflux events were rare but there was a trend for them to be more common in ICR (5.0 vs. 1.0, z = 1.9, p = .06 [95%CI = −8.0,0). Acid reflux events did not differ between groups.
In order to evaluate whether our findings were the result of insufficient ablation treatments, we re-evaluated the data classifying patients as CR or ICR on the basis of at least 3 ablation sessions. This resulted in two patients in the ICR group being re-categorized to CR but it did not change the overall analysis as we continued to find significantly more WAR, WAlkR and TR events in ICR compared to CR. These results are illustrated in Figure 2.
Figure 2.
Reflux exposure was compared between ICR and CR after recalculating the data based on at least 3 ablations. (A) AR varied considerably, but there was no significant difference between ICR and CR. (B) Weakly alkaline reflux events (pH>7) were uncommon, however, they were significantly more frequent in ICR compared to CR. Weakly acidic reflux (C and D) events accounted for the bulk of total reflux events and were significantly more frequent in ICR compared to CR.
Finally, we performed Pearson correlations to determine the extent of overlap between variables.) Length of BE was positively correlated with size of hiatal hernia (r = 0.54, p < .01). Length of BE and hiatal hernia were also positively correlated with age (r = 0.34, p < .05; r = 0.54, p < .01, respectively) and negatively correlated with WAlkR (r = −0.59, p < .05).
Body mass index was positively correlated with WAlkR (r = 0.39, p < .05) and length of BE (0.34, p < .05). Acid reflux was positively correlated with EGJ pressure (.37, p < .05) and BMI (.34, p < .05). Weakly acidic reflux and TR were positively correlated with size of hiatal hernia (r = 0.35, p < .05; r = 0.40, p < .05).
Discussion
The major finding from this study was that the severity of ongoing reflux exposure despite twice daily PPI therapy was associated with persistent IM in BE patients after RFA. In particular, WAR events, which comprise a majority of reflux events in patients taking PPIs were most strongly associated with persistent IM after RFA. Additional factors associated with an incomplete response to RFA were hiatal hernia size and length of BE. Baseline histology, manometric variables, age, smoking history and BMI were not associated with persistent. These data suggest that uncontrolled reflux, irrespective of acidity, predisposes BE patients to an incomplete RFA response.
A multitude of studies have reported on the efficacy of RFA for BE ablation and it is clear that RFA is a safe and effective therapy for BE-associated dysplasia. 2,3,5–10,22–24 The current report suggests that RFA is less effective at eradicating IM. A plausible explanation for this is that RFA does not address the underlying pathophysiology of BE, which is abnormal reflux. 25,26 In fact, patients with BE are known to have more reflux than patients with peptic strictures and severe erosive esophagitis, regardless of concomitant symptoms. 27 Although there are favorable mucosal changes after RFA therapy, these patients likely continue to have uncontrolled reflux as demonstrated with impedance-pH reflux monitoring prior to treatment. Proton pump inhibitors are usually effective at decreasing the acidity of this refluxate, but several studies have shown that even this is not uniformly achieved. 28–30 In our dataset, 20% of patients had continued abnormal esophageal acid exposure despite twice daily PPI therapy. Furthermore, considerable in-vitro and ex-vivo data suggest that bile salts are equally caustic to the esophageal epithelium and are by themselves sufficient to cause BE and BE related cancer. 16,26,31–34 Consistent with these observations, we found that patients with a greater number of WAR and WAlkR events were less likely to achieve eradication of IM. We also noted an association between size of hiatal hernia and response to RFA. This is potentially attributable to the anatomic deformity imparted by the hernia itself or to its pathophysiological significance in both facilitating reflux events and impairing refluxate clearance. 35–38 With respect to anatomy, the hernia may compromise the ability to achieve adequate tissue apposition with the ablation catheter. This limitation was addressed in a study of intraoperative RFA at the time of fundoplication in patients with large hiatal hernias. Laparoscopy assisted RFA was performed to facilitate increased mucosa contact time and CR-IM was achieved in 80% of patients after only one ablation.19 However, it is uncertain as to whether that excellent response rate was the result of the intraoperative ablation or the correction of reflux with fundoplication.
The largest existing follow-up study for RFA (AIM dysplasia) reported that in patients who achieved CR-IM at two years, 25 percent had recurrent IM at year five. Furthermore, many patients required more than four ablation sessions to achieve a complete response. 24 These findings highlight two important issues regarding RFA: 1) that patients often require multiple ablation sessions over a period of a year to eradicate all dysplasia and IM and 2) that despite complete eradication of all dysplasia and IM, recurrence of IM is frequently observed during subsequent surveillance. The current investigation identified ongoing reflux as a potential risk factor for persistent IM after two ablation sessions. Although our follow-up period was insufficient to address the second phenomenon, it is possible that ongoing reflux may also be contributing to recurrent IM as well.
In this investigation, we categorized patients as CRs if they achieved complete eradication of all dysplasia and IM after two ablations sessions. Those with persistent IM/dysplasia at that time point were labeled ICR. Other investigations have reported that the median number of ablations to achieve CR-IM was three. However, there is currently no consensus on the number of treatments needed to define a non-responder. A common investigational approach is of ablation every two months until visible IM is gone. We have found that approximately 60% of patients achieve CR-IM after two ablation sessions, whereas the remainder required additional treatments. That observation led us to adopt the two treatment criterion for defining CRs. In fact, in our patient population, the median number of ablations needed for CR was two. To explore the importance of the threshold selected, we also evaluated differences in WAR among patients with at least three treatments. Using that definition, an additional two patients achieved CR-IM, but that did not change the overall results of our analysis. Two patients were complete non-responders (persistent IM or dysplasia) at the end of the study follow-up. As such, 95% of patients in our cohort eventually achieved CR with RFA therapy.
There are a few limitations of this study. Our sample consisted of only 37 subjects, unequal sample sizes, and a non-equal distribution of means and variance. An analysis using logistic regression would have required a minimum sample size of 138 (69 per group) based on the preliminary data we acquired in this study. Hence, the study was underpowered to address the question of which factors predict response to RFA in BE, and instead can only report on factors that differ between CR and ICR. The study was, however, sufficiently powered for the analysis performed (β = .82), thereby providing the most comprehensive data yet available on reflux and esophageal physiology in patients undergoing RFA therapy. Lastly, as we only had two persistent non-responders, we cannot comment on the role of reflux exposure on that outcome. In addition, this was a single center study and assumes the inherent bias of that.
In conclusion, we found that the severity of ongoing reflux exposure, hiatal hernia size, and length of BE were associated with persistent IM after RFA therapy. While we are unable to speak to the unique contribution of reflux exposure on response to RFA because of significant autocorrelation between hiatal hernia and reflux, that correlation was quite modest.. These data suggest that post-ablation reflux exposure, specifically WAR, in BE patients is an important determinant of persistent IM and hence, may imply a persistent risk of adenocarcinoma after RFA. Further investigation with larger cohorts and long term follow-up will be needed to determine whether physiologic testing and operative management of reflux should be considered in patients who undergo RFA.
Acknowledgments
Grant Support: Supported by R01 DK56033 (PJK) and R01 DK079902 (JEP) from the Public Health Service
Footnotes
Author Disclosures:
Kumar Krishnan: None
John Pandolfino: Consultant: Given
Peter Kahrilas: Consultant: AstraZeneca, Eisai, EndoGastric Solutions, Ironwood, Torax, Reckitt Benckiser
Laurie Keefer: None
Lubomyr Boris: None
Srinadh Komanduri: Consultant: Boston Scientific, BARRX
Speaker: Novartis
Author participation
Study concept and design: KK, JP, SK
Acquisition of data: KK, LB
Analysis and interpretation of data: KK, LK. SK
Drafting of the manuscript: KK, SK, PK
Critical revision of the manuscript for important intellectual content: JP, SK, PK
Statistical analysis: KK, LK
Study supervision: JP, SK
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O'Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol. 1999 Aug;94(8):2037–2042. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 2.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009 May 28;360(22):2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 3.Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010 Oct;42(10):781–789. doi: 10.1055/s-0030-1255779. [DOI] [PubMed] [Google Scholar]
- 4.Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett's esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008 Jul;68(1):35–40. doi: 10.1016/j.gie.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Stepwise circumferential and focal ablation of Barrett's esophagus with high-grade dysplasia: results of the first prospective series of 11 patients. Endoscopy. 2008 May;40(5):359–369. doi: 10.1055/s-2007-995567. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez JC, Reicher S, Chung D, et al. Pilot series of radiofrequency ablation of Barrett's esophagus with or without neoplasia. Endoscopy. 2008 May;40(5):388–392. doi: 10.1055/s-2007-995747. [DOI] [PubMed] [Google Scholar]
- 7.Pouw RE, Gondrie JJ, Sondermeijer CM, et al. Eradication of Barrett esophagus with early neoplasia by radiofrequency ablation, with or without endoscopic resection. J Gastrointest Surg. 2008 Oct;12(10):1627–1636. doi: 10.1007/s11605-008-0629-1. discussion 1636–1627. [DOI] [PubMed] [Google Scholar]
- 8.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010 Jan;8(1):23–29. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Roorda AK, Marcus SN, Triadafilopoulos G. Early experience with radiofrequency energy ablation therapy for Barrett's esophagus with and without dysplasia. Dis Esophagus. 2007;20(6):516–522. doi: 10.1111/j.1442-2050.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007 Feb;65(2):185–195. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 11.Vassiliou MC, von Renteln D, Wiener DC, Gordon SR, Rothstein RI. Treatment of ultralong-segment Barrett's using focal and balloon-based radiofrequency ablation. Surg Endosc. 2010 Apr;24(4):786–791. doi: 10.1007/s00464-009-0639-4. [DOI] [PubMed] [Google Scholar]
- 12.Velanovich V. Endoscopic endoluminal radiofrequency ablation of Barrett's esophagus: initial results and lessons learned. Surg Endosc. 2009 Oct;23(10):2175–2180. doi: 10.1007/s00464-009-0364-z. [DOI] [PubMed] [Google Scholar]
- 13.Shaheen NJ, Peery AF, Overholt BF, et al. Biopsy depth after radiofrequency ablation of dysplastic Barrett's esophagus. Gastrointest Endosc. 2010 Sep;72(3):490–496. e491. doi: 10.1016/j.gie.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaccaro BJ, Gonzalez S, Poneros JM, et al. Detection of Intestinal Metaplasia After Successful Eradication of Barrett's Esophagus with Radiofrequency Ablation. Dig Dis Sci. 2011 Jul;56(7):1996–2000. doi: 10.1007/s10620-011-1680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huo X, Zhang HY, Zhang X, et al. Acid and Bile Salt Induced CDX2 Expression Differs in Squamous Cells from Patients with and without Barrett's Esophagus. Gastroenterology. 2010 Mar 17; doi: 10.1053/j.gastro.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett's metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008 Aug;295(2):G211–218. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 17.Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology. 1996 Nov;111(5):1192–1199. doi: 10.1053/gast.1996.v111.pm8898632. [DOI] [PubMed] [Google Scholar]
- 18.Ferraris R, Fracchia M, Foti M, et al. Barrett's oesophagus: long-term follow-up after complete ablation with argon plasma coagulation and the factors that determine its recurrence. Aliment Pharmacol Ther. 2007 Apr 1;25(7):835–840. doi: 10.1111/j.1365-2036.2007.03251.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Connell K, Velanovich V. Effects of Nissen fundoplication on endoscopic endoluminal radiofrequency ablation of Barrett's esophagus. Surg Endosc. 2011 Mar;25(3):830–834. doi: 10.1007/s00464-010-1270-0. [DOI] [PubMed] [Google Scholar]
- 20.Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004 Jul;53(7):1024–1031. doi: 10.1136/gut.2003.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z, Roman S, Pandolfino JE, Kahrilas PJ. Automated calculation of the distal contractile integral in esophageal pressure topography with a region-growing algorithm. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012 Jan;24(1):e4–e10. doi: 10.1111/j.1365-2982.2011.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett's neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy. 2008 May;40(5):370–379. doi: 10.1055/s-2007-995589. [DOI] [PubMed] [Google Scholar]
- 23.Pouw RE, Gondrie JJ, Curvers WL, Sondermeijer CM, ten Kate FJ, Bergman JJ. Successful balloon-based radiofrequency ablation of a widespread early squamous cell carcinoma and high-grade dysplasia of the esophagus: a case report. Gastrointestinal Endoscopy. 2008 Sep;68(3):537–541. doi: 10.1016/j.gie.2008.03.1086. [DOI] [PubMed] [Google Scholar]
- 24.Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology. 2011 Aug;141(2):460–468. doi: 10.1053/j.gastro.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spechler SJ, Goyal RK. The columnar-lined esophagus, intestinal metaplasia, and Norman Barrett. Gastroenterology. 1996 Feb;110(2):614–621. doi: 10.1053/gast.1996.v110.agast960614. [DOI] [PubMed] [Google Scholar]
- 26.Triadafilopoulos G. Acid and bile reflux in Barrett's esophagus: a tale of two evils. Gastroenterology. 2001 Dec;121(6):1502–1506. doi: 10.1053/gast.2001.30090. [DOI] [PubMed] [Google Scholar]
- 27.Stein HJ, Barlow AP, DeMeester TR, Hinder RA. Complications of gastroesophageal reflux disease. Role of the lower esophageal sphincter, esophageal acid and acid/alkaline exposure, and duodenogastric reflux. Annals of surgery. 1992 Jul;216(1):35–43. doi: 10.1097/00000658-199207000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spechler SJ, Sharma P, Traxler B, Levine D, Falk GW. Gastric and esophageal pH in patients with Barrett's esophagus treated with three esomeprazole dosages: a randomized, double-blind, crossover trial. Am J Gastroenterol. 2006 Sep;101(9):1964–1971. doi: 10.1111/j.1572-0241.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 29.Katzka DA, Castell DO. Successful elimination of reflux symptoms does not insure adequate control of acid reflux in patients with Barrett's esophagus. Am J Gastroenterol. 1994 Jul;89(7):989–991. [PubMed] [Google Scholar]
- 30.Gerson LB, Boparai V, Ullah N, Triadafilopoulos G. Oesophageal and gastric pH profiles in patients with gastro-oesophageal reflux disease and Barrett's oesophagus treated with proton pump inhibitors. Aliment Pharmacol Ther. 2004 Sep 15;20(6):637–643. doi: 10.1111/j.1365-2036.2004.02127.x. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald RC. Molecular basis of Barrett's oesophagus and oesophageal adenocarcinoma. Gut. 2006 Dec;55(12):1810–1820. doi: 10.1136/gut.2005.089144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong J, Behar J, Wands J, et al. Role of a novel bile acid receptor TGR5 in the development of oesophageal adenocarcinoma. Gut. 2010 Feb;59(2):170–180. doi: 10.1136/gut.2009.188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaiswal K, Lopez-Guzman C, Souza RF, Spechler SJ, Sarosi GA., Jr Bile salt exposure increases proliferation through p38 and ERK MAPK pathways in a non-neoplastic Barrett's cell line. Am J Physiol Gastrointest Liver Physiol. 2006 Feb;290(2):G335–342. doi: 10.1152/ajpgi.00167.2005. [DOI] [PubMed] [Google Scholar]
- 34.Dvorak K, Payne CM, Chavarria M, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett's oesophagus. Gut. 2007 Jun;56(6):763–771. doi: 10.1136/gut.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sloan S, Rademaker AW, Kahrilas PJ. Determinants of gastroesophageal junction incompetence: hiatal hernia, lower esophageal sphincter, or both? Annals of internal medicine. 1992 Dec 15;117(12):977–982. doi: 10.7326/0003-4819-117-12-977. [DOI] [PubMed] [Google Scholar]
- 36.Sloan S, Kahrilas PJ. Impairment of esophageal emptying with hiatal hernia. Gastroenterology. 1991 Mar;100(3):596–605. doi: 10.1016/0016-5085(91)80003-r. [DOI] [PubMed] [Google Scholar]
- 37.Parrilla P, Ortiz A, Martinez de Haro LF, Aguayo JL, Ramirez P. Evaluation of the magnitude of gastro-oesophageal reflux in Barrett's oesophagus. Gut. 1990 Sep;31(9):964–967. doi: 10.1136/gut.31.9.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coenraad M, Masclee AA, Straathof JW, Ganesh S, Griffioen G, Lamers CB. Is Barrett's esophagus characterized by more pronounced acid reflux than severe esophagitis? Am J Gastroenterol. 1998 Jul;93(7):1068–1072. doi: 10.1111/j.1572-0241.1998.00331.x. [DOI] [PubMed] [Google Scholar]