Abstract
Background and Purpose
Maternal cigarette smoking increases the risk of neonatal morbidity. We tested the hypothesis that perinatal nicotine exposure causes heightened brain vulnerability to hypoxic-ischemic (HI) injury in neonatal rats via aberrant expression patterns of angiotensin II type 1 (AT1R) and type 2 (AT2R) receptors in the developing brain.
Methods
Nicotine was administered to pregnant rats via subcutaneous osmotic minipumps. HI brain injury was determined in 10-day-old pups. AT1R and AT2R expression patterns were assessed via Western blotting, q-PCR, immunofluorescence and confocal imaging.
Results
Perinatal nicotine exposure significantly increased HI brain infarct size in male, but not female, pups. In fetal brains, nicotine caused a decrease in mRNA and protein abundance of AT2R, but not AT1R. The downregulation of AT2R persisted in brains of male pups, and nicotine treatment resulted in a significant increase in methylation of CpG locus three bases upstream of TATA-box at AT2R gene promoter. In female brains, there was an increase in AT2R, but a decrease in AT1R expression. Both AT1R and AT2R expressed in neurons but not in astrocytes in the cortex and hippocampus. Central application of AT1R antagonist losartan or AT2R antagonist PD123319 increased HI brain infarct size in both male and female pups. In male pups, AT2R agonist CGP42112 abrogated nicotine-induced increase in HI brain infarction. In females, PD123319 uncovered the nicotine’s effect on HI brain infarction.
Conclusion
Perinatal nicotine exposure causes epigenetic repression of AT2R gene in the developing brain resulting in heightened brain vulnerability to HI injury in neonatal male rats in a sex-dependent manner.
Keywords: nicotine, AT1R/AT2R, neonatal rat, hypoxic-ischemic brain injury
Introduction
Hypoxic-ischemic encephalopathy (HIE) occurs in 1 to 6 per 1000 term newborns and causes severe mortality and long-lasting morbidity including cerebral palsy, seizure and cognitive retardation in infants and children.1,2 Although the underlying mechanisms of heightened brain vulnerability to hypoxic-ischemic (HI) injury in newborns remain largely elusive, recent studies suggest a possible cause of aberrant brain development due to fetal insults.3 Maternal cigarette smoking is the single most widespread perinatal insult in the world. As one of the major components in cigarette smoking, nicotine readily crosses the placenta and produces higher nicotine concentrations in the fetal circulation than that experienced by the mother.4 Epidemiological and animal studies have provided evidence linking perinatal nicotine exposure and the increased incidence of neurodevelopmental disorders, neurobehavioral deficits, impaired cognitive performance and increased risk of affective disorders later in life.5,6
However, whether and to what extent perinatal nicotine exposure adversely affects the brain susceptibility to HI injury in newborns remains unknown. The present study tested the hypothesis that maternal nicotine administration during gestation results in the heightened brain vulnerability to HI injury in neonatal rats. Given that brain renin-angiotensin system plays a vital role in the development and progression of cerebrovascular diseases, and both angiotensin II type 1 (AT1R) and type 2 (AT2R) receptors are pivotal players in the pathogenesis of ischemic brain injury,7,8,9 we sought to investigate further the role of AT1R and AT2R in the nicotine-mediated ischemia-sensitive phenotype of neonatal brains. Herein, we present evidence of a novel finding that perinatal nicotine exposure causes epigenetic programming of AT2R gene repression in the developing brain resulting in the increased brain susceptibility to HI injury in neonatal male rats in a sex-dependent manner, and suggest new insights of molecular mechanisms linking maternal cigarette smoking to heightened HIE vulnerability in newborns.
Materials and Methods
Experimental animals
Pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI) and were randomly divided into two groups: 1) saline control and 2) nicotine administration via osmotic minipumps (4 μg/kg/minute) implanted subcutaneously from day 4 of gestation to day 10 after birth.10 On day 21 of pregnancy, some rats were euthanized and fetal (E21) brains were isolated. Other rats were allowed to give birth, and further studies were conducted in 10-day-old neonatal (P10) pups of both sexes. All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Brain HI treatment and intracerebroventricular (ICV) injection
A modified Rice-Vannucci model was conducted in P10 pups.11 Pups were anesthetized with 2% isoflurane and the right common carotid artery was ligated. After recovery for 1 hour, pups were treated with 8% O2 for 1.5 or 2.5 hours. To determine the role of AT1R and AT2R in brain HI injury, AT1R antagonist losartan (Merck), AT2R antagonist PD123319 (Sigma-Aldrich) and AT2R selective agonist CGP42112 (TOCRIS bioscience) were administered intracerebroventricularly, respectively, prior to the HI treatment. Pups were anesthetized and fixed on a stereotaxic apparatus (Stoelting, Wood Dale, IL). An incision was made on the skull surface and bregma was exposed. All agents were injected at a rate of 1 μl/minute with a 10 μl syringe (Stoelting, Wood Dale, IL) on the right hemisphere following the coordinates relative to bregma: 2 mm posterior, 1.5 mm lateral and 3.0 mm below the skull surface.12 Saline was injected as control. The injection lasted 2 minutes and the needle was kept for additional 5 minutes before its removal. The incision was sutured.
Infarct size measurement
Pups were anesthetized and killed 48 hours after the HI treatment. Coronal slices of the brain (2 mm thick) were cut and immersed in a 2% solution of 2,3,5-triphenyltetrazolium chloride monohydrate (TTC; Sigma-Aldrich) for 5 minutes at 37°C and then fixed by 10% formaldehyde overnight. Each slice was weighed, photographed separately and the percentage of infarction area for each slice was analyzed by Image J software (Version 1.40; National Institute of Health, Bethesda, MD), corrected by slice weight, summed for each brain, and expressed as a percentage of whole brain weight.
Western immunoblotting
Brains were homogenized in a lysis buffer containing 150 mM NaCl, 50 mM Tris.HCl, 10 mM EDTA, 0.1% Tween-20, 1% Triton, 0.1% β-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, and 5 μg/ml aprotinin, pH 7.4. Homogenates were centrifuged at 4°C for 10 minutes at 10,000 g, and supernatants collected. Protein concentrations were determined using a protein assay kit (Bio-Rad, Hercules, CA). Samples with equal amounts of protein were loaded onto 10% polyacrylamide gel with 0.1% SDS and separated by electrophoresis at 100 V for 90 minutes. Proteins were then transferred onto nitrocellulose membranes and probed with primary antibodies against AT1R (1:100) and AT2R (1:1000) (Santa Cruze Biotechnology; Santa Cruz, CA), as described previously.13 After washing, membranes were incubated with secondary horseradish peroxidase-conjugated antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to Hyperfilm. The results were analyzed with the Kodak ID image analysis software. Band intensities were normalized to GAPDH.
Real-time RT-PCR
RNA was extracted from brains and abundance of AT1aR, AT1bR and AT2R mRNA was determined by real-time RT-PCR using Icycler Thermal cycler (Bio-Rad, Hercules, CA), as described previously.13 The primers used were: AT1aR, 5′-ggagaggattcgtggcttgag-3′ (forward) and 5′-ctttctgggagggttgtgtgat-3′ (reverse); AT1bR, 5′-atgtctccagtcccctctca-3′ (forward) and 5′-tgacctcccatctccttttg-3′ (reverse); and AT2R, 5′-caatctggctgtggctgactt-3′ (forward) and 5′-tgcacatcacaggtccaaaga-3′ (reverse). Real-time RT-PCR was performed in a final volume of 25 μl. Each PCR reaction mixture consisted of 600 nM of primers, 33 units of M-MLV reverse transcriptase (Promega, Madison, WI), and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) containing 0.625 unit Taq polymerase, 400 μM each of dATP, dCTP, dGTP, and dTTP, 100 mM KCl, 16.6 mM ammonium sulfate, 40 mM Tris-HCl, 6 mM MgSO4, SYBR Green I, 20 nM fluorescing and stabilizers. The following RT-PCR protocol was used: 42°C for 30 minutes, 95°C for 15 minutes, followed by 40 cycles of 95°C for 20 seconds, 56°C for 1 minute, 72°C for 20 seconds. GAPDH was used as an internal reference and serial dilutions of the positive control was performed on each plate to create a standard curve. PCR was performed in triplicate, and threshold cycle numbers were averaged.
Quantitative methylation-specific PCR
CpG methylation at rat AT2R gene promoter was determined as previously described.10,14 Briefly, genomic DNA was isolated from brains of P10 pups using a GenElute Mammalian Genomic DNA Mini-Prep kit (Sigma), denatured with 2 N NaOH at 42°C for 15 minutes, treated with sodium bisulfite at 55°C for 16 hours, and purified by EZ DNA Methylation-Gold Kit™ (Zymo Research), as previously described. Bisulfite-treated DNA was used as a template for real-time fluorogenic methylation-specific PCR (MSP) at CpG−52 locus (forward primer, 5′-ttttttggaaagttggtaagtgttta-3′; reverse primer for C, 5′-ctctaatttccttcttatatattca-3′; reverse primer for Cm, 5′-ctctaatttccttcttatatattcg-3′) and CpG+11 locus (forward primer, 5′-gaaggttttttagtggatag-3′; reverse primer for C, 5′-aaaaaaaactttcaattctatactca-3′; reverse primer for Cm, 5′-aaaaaaaactttcaattctatactcg-3′), respectively. GAPDH was used as an internal reference gene. Real-time MSP was performed using the iQ SYBR Green Supermix with iCycler real-time PCR system (Bio-Rad). Data are presented as the percent of methylation at the region of interest (methylated CpG/methylated CpG + unmethylated CpG ×100), as described previously.10,14
Immunofluorescence staining and confocal imaging
Brains were fixed in formalin and processed to obtain 10 μm tissue slides. Antigens were retrieved with antigen retrieval buffer (Abcam) following heat-induced procedures. The following primary antibodies were employed: mouse anti-NeuN (Millipore); mouse anti-GFAP (Millipore); rabbit anti-AT1R (Santa Cruz); rabbit anti-AT2R (Santa Cruz). After blocking with 1% BSA for 2 hours at room temperature and incubation with the primary antibodies at 4°C overnight, tissue sections were treated with secondary antibodies raised against mouse and rabbit IgG conjugated with FITC and Texas Red (Santa Cruz), respectively, for 2 hours at room temperature. After three washes, sections were stained with Hoechst 33258 (5 μg/ml) (Sigma) for 1 minute. The sections were then covered with Permount reagent (Fisher) and visualized using the Zeiss LSM 710 confocal microscope, as previously described.15
Statistical analysis
Data are expressed as mean ± SEM. Experimental number (n) represents fetuses and neonates from different dams. Statistical significance (P<0.05) was determined by analysis of variance (ANOVA) followed by Neuman-Keuls post hoc testing or Student’s t test, where appropriate.
Results
Nicotine caused asymmetric growth restriction in fetuses and neonates
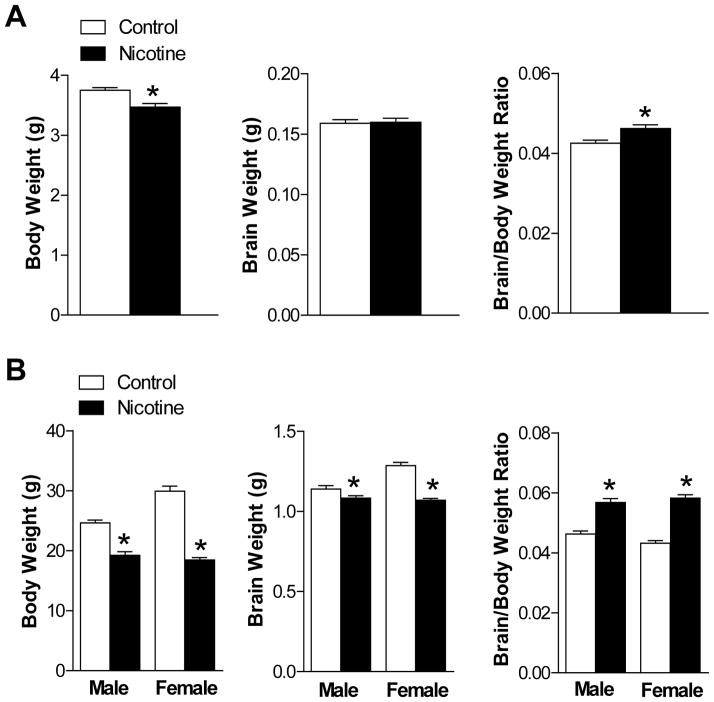
Maternal nicotine administration caused a significant decrease in the body weight, but not the brain weight, in E21 fetuses, resulting in a significant increase in the brain to body weight ratio (Figure 1A). In P10 pups, both body and brain weight was decreased but the brain to body weight ratio remained significantly increased in both sexes (Figure 1B), suggesting asymmetric growth restriction in the fetus and neonate in nicotine-treated animals.
Figure 1. Effect of nicotine on body weight, brain weight and brain to body weight ratio in E21 fetuses (Panel A; n = 27–39) and P10 pups (Panel B; n=9–13).
Data are mean ± SEM. *P < 0.05 versus control group.
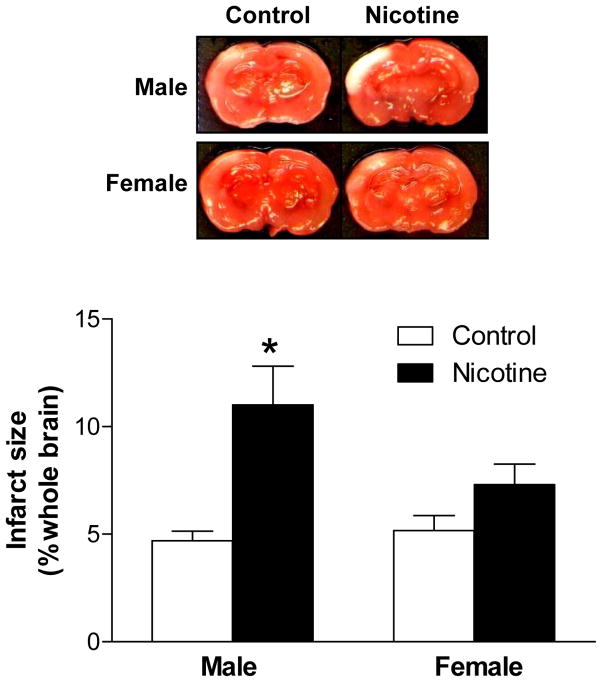
Nicotine increased brain vulnerability to HI injury in male pups
In control animals, there was no significant difference in HI-induced brain infarct size between male and female pups (Figure 2). The nicotine treatment significantly exaggerated HI-induced brain infarct size in male, but not female, pups (Figure 2).
Figure 2. Effect of nicotine on HI-induced brain infarct size in P10 pups.
Data are mean ± SEM, n = 4–6. *P < 0.05 versus control group.
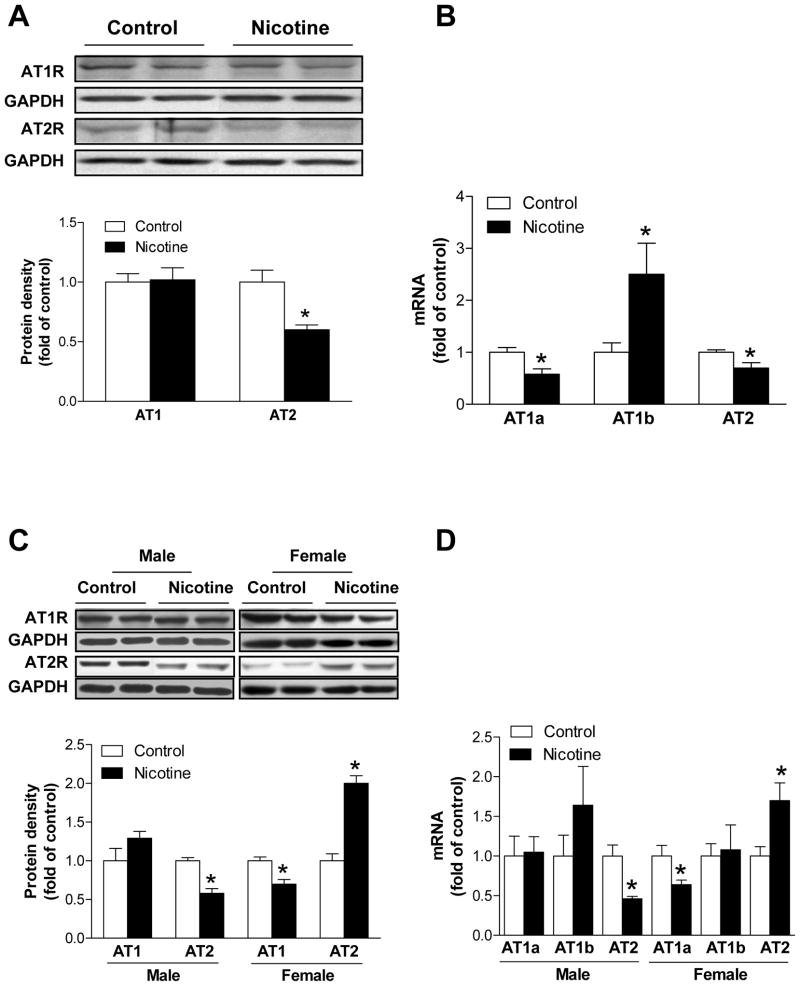
Nicotine altered expression patterns of AT1R and AT2R in fetal and neonatal brains
In E21 fetuses, the nicotine treatment resulted in a significant decrease in brain AT2R protein and mRNA abundance (Figure 3A, 3B). There was no significant effect of nicotine on AT1R protein abundance with a significant decrease in AT1aR mRNA but an increase in AT1bR mRNA abundance in the fetal brain (Figure 3B). In P10 pups, brain AT2R protein and mRNA abundance was significantly decreased in male pups in nicotine-treated animals (Figure 3C, 3D). In contrast, in female pups nicotine caused a significant increase in brain AT2R protein and mRNA abundance (Figure 3C, 3D). There was no significant effect of nicotine on brain AT1R protein, AT1aR and AT1bR mRNA abundance in male pups (Figure 3C, 3D). However, nicotine induced a significant reduction of brain AT1R protein and AT1aR mRNA abundance in female pups (Figure 3C, 3D). Immunofluorecsence and confocal imaging analyses showed that both AT1R and AT2R presented in neurons but not in astrocytes in the cortex (Supplemental Material Figure 1) and hippocampus (Supplemental Material Figure 2) of P10 pups. It appeared that nicotine treatment increased astrocyte numbers in both cortex and hippocampus (Supplemental Material Figure 3).
Figure 3. Effect of nicotine on protein and mRNA abundance of AT1R and AT2R in E21 fetal (Panel A and B) and P10 pup (Panel C and D) brains.
Data are mean ± SEM, n = 4–6. *P < 0.05 versus control group.
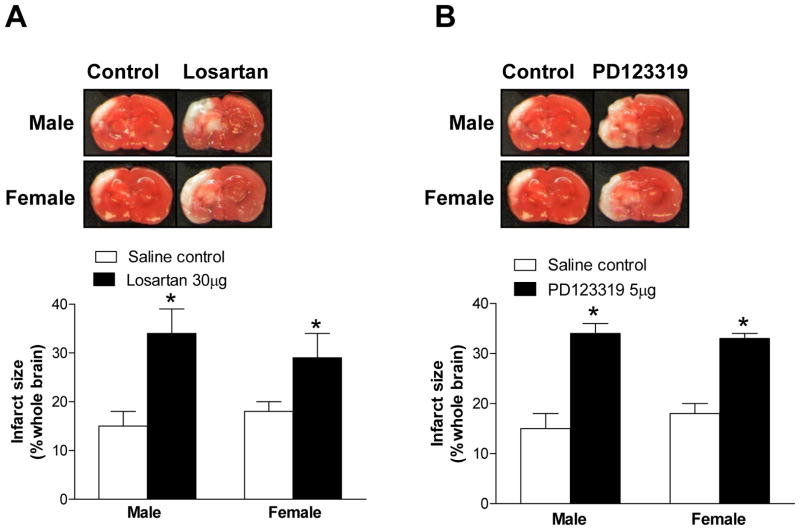
AT1R and AT2R protected neonatal rat brains from HI injury
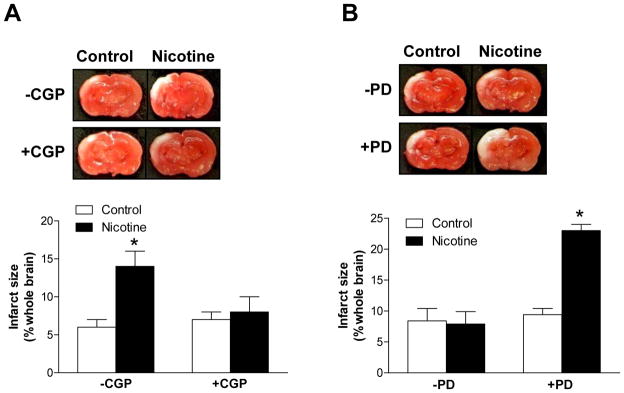
To determine the functional significance of altered AT1R and AT2R expression patterns in nicotine-induced, heightened brain vulnerability to HI injury in neonates, we firstly evaluated the role of AT1R and AT2R in the pathogenesis of HI brain injury in pups via intracerebroventricular (ICV) injection of AT1R or AT2R antagonists. Compared with the saline control, ICV of either losartan (Figure 4A) or PD123319 (Figure 4B) significantly increased brain infarct size in both male and female pups, suggesting that both AT1R and AT2R may be implicated in the pathogenesis of HI brain injury and confer neuroprotective properties in neonatal rat brains.
Figure 4. Effect of losartan (Panel A) and PD123319 (Panel B) on HI-induced brain infarct size in P10 pups.
Data are mean ± SEM, n = 4–6. *P < 0.05 versus control group.
AT2R played a key role in nicotine-induced, heightened brain vulnerability to HI injury in pups
To demonstrate the cause and effect relation between nicotine-induced downregulation of brain AT2R and heightened brain vulnerability to HI injury in male pups, a selective AT2R agonist CGP42112 was administered in male pups that had been treated with nicotine or saline control. As shown in Figure 5A, ICV administration of CGP42112 (3 μg) reversed the effect of nicotine and abrogated the difference in HI-induced brain infarct size between saline control and nicotine-treated male pups. The key role of brain AT2R in nicotine-induced heightened brain vulnerability to HI injury in neonatal rats was further tested in female pups with ICV administration of PD123319. As shown in Figure 5B, in the absence of PD123319, the nicotine treatment had no significant effect on brain HI injury in female pups. However, in the presence of PD123319 (5 μg), the effect of nicotine was uncovered and HI-induced brain infarct size was significantly increased in nicotine-treated, as compared with saline control, female pups (Figure 5B).
Figure 5. Effect of CGP42112 (CGP) in male P10 pups (Panel A) and PD123319 (PD) in female P10 pups (Panel B) on nicotine-induced changes in brain HI injury.
Data are mean ± SEM, n = 4–8. *P < 0.05 versus control group.
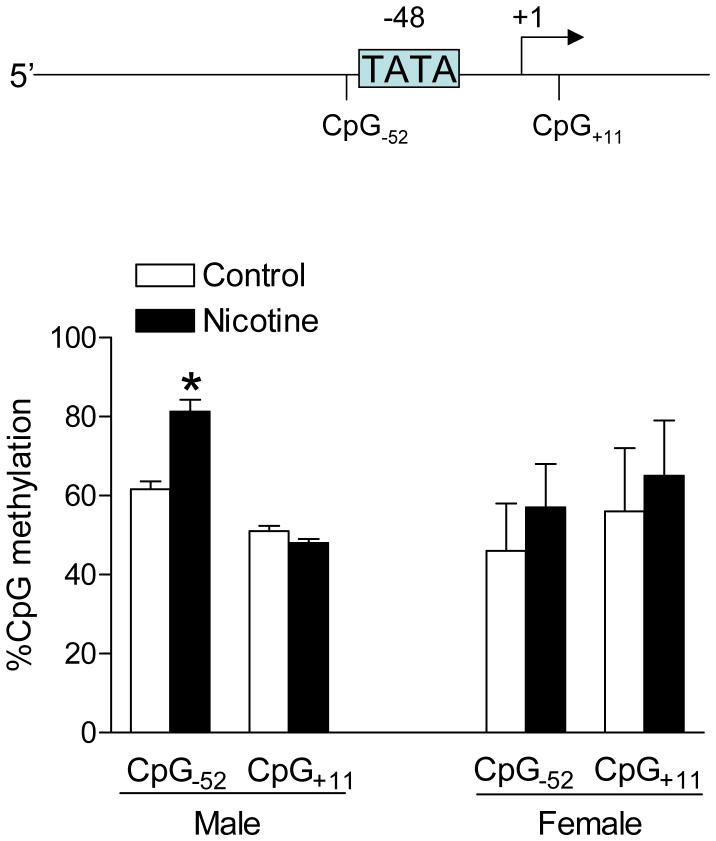
Nicotine treatment increased methylation of CpG−52 locus at AT2R promoter
Recently, we have demonstrated that rat AT2R promoter has a TATA element at −48 from transcription start site, and deletion of the TATA element significantly decreases the promoter activity.13 Two CpG loci were identified at AT2R promoter, one was located three bases upstream of TATA-box (CpG−52) and the other one eleven bases downstream of transcription start site (CpG+11). The previous study showed that increased methylation at CpG locus three bases upstream of TATA-box inhibited the binding of the TATA-box binding protein and decreased promoter activity.16 As shown in Figure 6, nicotine treatment caused a significant increase in methylation of CpG−52 locus in male but not female pup brains, whereas methylation of CpG+11 locus was not significantly affected.
Figure 6. Effect of nicotine on methylation of CpG loci at AT2R promoter in P10 pup brains.
Data are mean ± SEM, n = 5–10. *P < 0.05 versus control group.
Discussion
The new findings of the present study are: 1. perinatal nicotine exposure significantly increases brain vulnerability to HI injury in male rat pups, but not in female pups; 2. this heightened vulnerability is associated with sex-specific reprogramming of AT1R and AT2R expression patterns in the developing brain; 3. both AT1R and AT2R are implicated in the pathogenesis of HI brain injury and exhibit the neuroprotective effect in neonatal brains; 4. downregulation of AT2R in the developing brain plays a causal role in nicotine-induced, heightened brain vulnerability to HI injury in neonatal rats; and 5. increased methylation of CpG locus three bases upstream of TATA-box at AT2R promoter is a mechanism of nicotine-mediated AT2R gene repression.
The present finding that perinatal nicotine exposure increased brain HI injury in neonates is novel and suggests a risk factor of maternal cigarette smoking in heightened brain HIE vulnerability in newborns. The nicotine dose used in the present study resulted in blood nicotine concentrations similar to those found in humans who smoke or use nicotine gum and patch. 4,17 Nicotine readily crosses the placenta into the fetal circulation, resulting in fetal nicotine concentrations being 15% higher than maternal levels.18 It is unclear at present whether observed effects are caused by vascular effects or direct neuronal effects of nicotine. While it may be technically challenging in measuring cerebral blood flow in neonatal rats, possible alterations in cerebral blood flow caused by nicotine treatment deserve further investigation.
The Rice-Vannucci model of unilateral common carotid artery ligation followed by 2.5 to 3 hours 8% oxygen treatment produces extensive brain damage in neonatal rats, and is widely used in studies of potential therapeutic intervention. However, few studies examined the brain susceptibility to mild HI injury in neonates, which may present only subtle differences and require more sophisticated experimental procedures. In the present study, shorter treatment period of pups with 8% oxygen for 1.5 hours produced a mild brain damage of about 10% infarction in the ipsilateral hemisphere. This mild and clinically relevant brain HI injury was significantly increased by more than two-fold in nicotine-treated male pups. However, the longer period of hypoxic treatment with greater brain damage in the model masked the effect of nicotine, suggesting a critical importance of appropriate model in investigating subtle changes of heightened brain vulnerability of HIE in newborns.
The growth restriction found in nicotine-treated animals presents a possible link between perinatal nicotine exposure and enhanced brain HI injury in pups, given that intrauterine growth restriction is a risk factor of neonatal encephalopathy.19 Fetal hypoxia may be another possible factor enhancing the nicotine-mediated effects. Although intermittent injections of nicotine to the mother may produce episodic fetal hypoxia and a decrease in cerebral perfusion with a reduced fetal brain weight,20,21,22 these effects were not observed in continuous low-level infusion of nicotine via a minipump.17
The finding that ICV application of both AT1R and AT2R antagonists enhanced the severity of brain HI injury is intriguing and suggests that both AT1R and AT2R are neuroprotective in the setting of neonatal HI brain injury. Both AT1R and AT2R present in the brain with specific developmental and spatial expression patterns. In adult brains, the AT1R predominates, while fetal brains express high levels of AT2R that decreases during the postnatal development.23 The present study demonstrated that both AT1R and AT2R expressed exclusively in neurons in both cortex and hippocampus in neonatal rat brains, whereas AT1R expressed predominantly in astrocytes in adult brains.24 The neuroprotective effect of AT2R demonstrated in the present study is consistent with previous findings.24,25,26 In contrast, the present finding of neuroprotective effect of AT1R in neonatal brains is somewhat surprising, given that AT1R antagonists have been shown to exhibit neuroprotection in adult rat brains.9,27,28,29 These findings highlight the important differences between immature and mature brains in AT1R-mediated responses. It has been shown that apoptotic cell death is more prominent in immature brains to HI insult, but necrotic cell death is more common in adult brains in response to acute insults such as HI or excitotoxicity.30,31 Although long-term and systemic administration of AT1R antagonists often showed neuroprotective effects of the brain via multiple systemic effects, the acute and local direct effects of AT1R antagonists in modulating brain HI injury are indeed less clear and may be quite different as those seen in the long-term and systemic effects. Indeed, similar to the present finding, the previous studies demonstrated a direct adverse effect of local administration of AT1R antagonists in the setting of acute ischemic injury in the heart,13,32 despite well documented protective effects of long-term and systemic administration of AT1R blockers in preventing the deleterious consequences of ischemia and reperfusion injury and reducing cardiac remodeling.
Of importance, the present study demonstrated that perinatal nicotine exposure-mediated, heightened brain vulnerability to HI injury in male pups was associated with a significant decrease in brain AT2R expression. Additionally, the ICV administration of AT2R agonist CGP42112 abrogated the nicotine’s effect. It has been demonstrated that direct stimulation of AT2R in the brain with CGP42112 confers neuroprotective effects in a conscious rat model of stroke, which is beyond blood pressure regulation.26 These results provide evidence of a causal role of AT2R downregulation in the nicotine-induced increase in brain HI injury in the pups. Our recent study has revealed that rat AT2R promoter has a TATA element at −48 from transcription start site and deletion of the TATA-box significantly decreases the promoter activity.13 The finding that nicotine treatment significantly increased methylation of CpG−52 locus three bases upstream of TATA-box at AT2R promoter in male pup brains is intriguing and suggests an important mechanism of site-specific CpG methylation in epigenetic repression of AT2R gene in the developing brain. It has been demonstrated that increased methylation at CpG locus three bases upstream of TATA-box inhibits the binding of the TATA-box binding protein and decreases receptor activator of nuclear factor-κB ligand gene promoter activity.16 Unlike CpG−52 locus, methylation of CpG+11 locus was not significantly altered, suggesting its minimal role in programming of AT2R gene expression patterns in the brain. Perinatal nicotine-mediated increase in sequence specific CpG methylation has recently been demonstrated in the Egr-1 binding site at PKCε promoter in the developing heart, which causes PKCε gene repression.10 Interestingly, nicotine had no significant effect on methylation of CpG−52 locus in female pup brains, demonstrating a sex-specific effect at a developmental period that sex hormonal influences are minimal. This suggests there are transcriptional distinctions that are wired in males and females long before sex steroids are involved. Similar findings of sex-specific CpG methylation and epigenetic repression of PKCε gene were obtained in male fetal rat hearts in response to hypoxia, in which the greater expression of estrogen receptors in female fetuses may convey a protection in stress-mediated epigenetic modifications.14 In the present study, the mechanism of increased AT2R expression in female pup brains is not clear at present. A possible mechanism is that stress-mediated downregulation of glucocorticoid receptors may contribute to the upregulation of AT2R, as shown recently in fetal rat hearts.13 Additionally, it has been shown that estrogen receptors mediate the downregulation of AT1R, but upregulation of AT2R in rodents.33,34,35 Consistent with the present findings, sex differences in perinatal stress-mediated epigenetic programming of gene expression patterns and subsequent disease development have been well reported previously, with males often being prone to be at higher risk of disease development at an earlier age than females.36,37,38,39
The present investigation provides novel evidence that perinatal nicotine exposure increases brain susceptibility to HI injury via reprogramming of AT1R and AT2R expression patterns in rat pups. Although it may be difficult to translate the present findings directly into humans, the possibility that antenatal stresses may result in programming of specific gene expression patterns in the developing brain resulting in heightened vulnerability of newborn brains to HI injury provides a mechanistic understanding worthy of investigation in humans. The clinical significance of the present study is warranted because maternal cigarette smoking and use of nicotine gum and patch during gestation present a major stress to the developing fetus, and because HIE in newborns causes severe mortality and long-lasting morbidity yet the underlying mechanisms remain largely elusive. Further studies on the epigenetic regulation of AT1R and AT2R gene expression patterns in the developing brain should provide more insights into mechanisms at the molecular level and may suggest new insights of therapeutic strategies that may be beneficial for the treatment of HIE in newborns.
Supplementary Material
Acknowledgments
A portion of this research used the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core, a facility is supported in part by the National Science Foundation through the Major Research Instrumentation program of the Division of Biological Infrastructure Grant No. 0923559 and the Loma Linda University School of Medicine.
Sources of Funding
This work was supported in part by the following grants: National Institutes of Health grants HL082779 (LZ), HL083966 (LZ), HL089012 (LZ), HL110125 (LZ), DA025319 (SY), DA032510 (DX), and California Tobacco-Related Disease Research Program Award 18KT-0024 (DX).
Footnotes
Disclosures
None.
References
- 1.Ferrieo DM. Neonatal brain injury. N Engl J Med. 2004;35:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 2.Verklan MT. The chilling details: hypoxic-ischemic encephalopathy. J Perinat Neonat Nurs. 2009;23:59–68. doi: 10.1097/01.JPN.0000346221.48202.7e. [DOI] [PubMed] [Google Scholar]
- 3.Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–633. doi: 10.1097/MOP.0b013e328010c536. [DOI] [PubMed] [Google Scholar]
- 4.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 5.Wickstrom R. Effects of nicotine during pregnancy: human and experimental evidence. Curr Neuropharmacol. 2007;5:213–222. doi: 10.2174/157015907781695955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr. 2008;97:1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- 7.Sokol SI, Portnay EL, Curtis JP, Nelson MA, Hebert PR, Setaro JF, et al. Modulation of the renin-angiotensin-aldosterone system for the secondary prevention of stroke. Neurology. 2004;63:208–213. doi: 10.1212/01.wnl.0000130360.21618.d0. [DOI] [PubMed] [Google Scholar]
- 8.Schrader J, Luders S, Kulschewski A, Hammersen F, Plate K, Berger J, et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES) Stroke. 2005;36:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- 9.Ando H, Zhou J, Macova M, Imboden H, Saavedra J. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke. 2004;35:1726–1731. doi: 10.1161/01.STR.0000129788.26346.18. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, et al. Foetal nicotine exposure causes PKCε gene repression by promoter methylation in rat hearts. Cardiovasc Res. 2011;89:89–97. doi: 10.1093/cvr/cvq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, et al. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55:158–163. doi: 10.1002/(SICI)1097-4547(19990115)55:2<158::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000;20(15):5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue Q, Dasgupta C, Chen M, Zhang L. Foetal hypoxia increases cardiac AT2R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc Res. 2011;89:300–308. doi: 10.1093/cvr/cvq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson AJ, Chan M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circ Res. 2010;107:365–373. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong F, Xiao D, Zhong L. Norepinephrine causes epigenetic repression of PKCε gene in rodent hearts by activating Nox1-dependent reactive oxygen species production. FASEB J. 2012 Mar 21; doi: 10.1096/fj.11-199422. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitazawa R, Kitazawa S. Methylation status of a single CpG locus 3 bases upstream of TATA-box of receptor activator of nuclear factor-kB ligand (RANKL) gene promoter modulates cell- and tissue-specific RANKL expression and osteoclastogenesis. Mol Endocrinol. 2007;21:148–158. doi: 10.1210/me.2006-0205. [DOI] [PubMed] [Google Scholar]
- 17.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- 18.Koren G. Fetal toxicology of environmental tobacco smoke. Curr Opin Pediatr. 1995;7:128–131. doi: 10.1097/00008480-199504000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009;40:156–167. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Arbeille P, Bosc M, Vaillant MC, Tranquart F. Nicotine-induced changes in the cerebral circulation in ovine fetuses. Am J Perinatol. 1992;9:270–274. doi: 10.1055/s-2007-994787. [DOI] [PubMed] [Google Scholar]
- 21.Önal A, Uysal A, Ülker S, Delen Y, Yurtseven ME, Evinç A. Alterations of brain tissue in fetal rats exposed to nicotine in utero: possible involvement of nitric oxide and catecholamines. Neurotoxicol Teratol. 2004;26:103–112. doi: 10.1016/j.ntt.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Mao C, Yuan X, Cui Y, Li H, Feng X, Liu Y, et al. Prenatal exposure to nicotine with associated in utero hypoxia decreased fetal brain muscarinic mRNA in the rat. Brain Res. 2008;1189:43–50. doi: 10.1016/j.brainres.2007.10.089. [DOI] [PubMed] [Google Scholar]
- 23.Millan MA, Jacobowitz DM, Aguilera G, Catt KJ. Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc Natl Acad Sci U S A. 1991;88:11440–11444. doi: 10.1073/pnas.88.24.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Culman J, Hortnagl H, Zhou Y, Gerova N, Timm M, et al. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 2005;19:617–619. doi: 10.1096/fj.04-2960fje. [DOI] [PubMed] [Google Scholar]
- 25.Iwai M, Liu HW, Chen R, Ide A, Okamoto S, Hata R, et al. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation. 2004;110:843–848. doi: 10.1161/01.CIR.0000138848.58269.80. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy CA, Vinh A, Callaway JK, Widdop RE. Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke. 2009;40:1482–1489. doi: 10.1161/STROKEAHA.108.531509. [DOI] [PubMed] [Google Scholar]
- 27.Dai W, Funk A, Herdegen T, Unger T, Culman J. Blockade of central angiotensin AT1 receptors improves neurological outcome and reduces expression of AP-1 transcription factors after focal brain ischemia in rats. Stroke. 1999;20:2391–2399. doi: 10.1161/01.str.30.11.2391. [DOI] [PubMed] [Google Scholar]
- 28.Lou M, Blume A, Zhao Y, Golhlke P, Deuschl G, Herdegen T, et al. Sustained blockade of brain AT1 receptors before and after focal cerebral ischemia alleviates neurological deficits and reduces neuronal injury, apoptosis and inflammatory responses in the rat. J Cereb Blood Flow Metab. 2004;24:536–547. doi: 10.1097/00004647-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Ando H, Macova M, Dou J, Saavedra J. Angiotensin II AT1 receptor blockade abolishes brain microvacular inflammation and heat shock protein responses in hypertensive rats. J Cereb Blood Flow Metab. 2005;25:878–886. doi: 10.1038/sj.jcbfm.9600082. [DOI] [PubMed] [Google Scholar]
- 30.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 31.Sidhu RS, Tuor UI, Del Bigio MR. Nuclear condensation and fragmentation following cerebral hypoxia-ischemia occurs more frequently in immature than older rats. Neurosci Lett. 1997;223:129–132. doi: 10.1016/s0304-3940(97)13426-7. [DOI] [PubMed] [Google Scholar]
- 32.Ford WR, Clanachan AS, Jugdutt BI. Opposite effects of angiotensin AT1 and AT2 receptor antagonists on recovery of mechanical function after ischemia-reperfusion in isolated working rat hearts. Circulation. 1996;94:3087–3089. doi: 10.1161/01.cir.94.12.3087. [DOI] [PubMed] [Google Scholar]
- 33.Armando I, Jezova M, Juorio A, Terron J, Falcon-Neri A, Semino-Mora C, et al. Estrogen upregulates renal angiotensin II AT2 receptors. Am J Physiol Renal Physiol. 2002;283:F934–F943. doi: 10.1152/ajprenal.00145.2002. [DOI] [PubMed] [Google Scholar]
- 34.Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regulatory peptides. 2005;124:7–17. doi: 10.1016/j.regpep.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Perez AI, Valenzuela R, Villar-Cheda B, Guerra MJ, Labandeira-Garcia JL. Dopaminergic neuroprotection of hormonal replacement therapy in young and aged menopausal rats: role of the brain angiotensin system. Brain. 2012;135:124–138. doi: 10.1093/brain/awr320. [DOI] [PubMed] [Google Scholar]
- 36.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 37.Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 38.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.