Abstract
In the present paper, we report that mitosis is a key step in the cellular response to genotoxic agents in human cells. Cells with damaged DNA recruit γH2AX (phosphorylated histone H2AX), phosphorylate Chk1 (checkpoint kinase 1) and arrest in the G2-phase of the cell cycle. Strikingly, nearly all cells escape the DNA damage checkpoint and become rounded, by a mechanism that correlates with Chk1 dephosphorylation. The rounded cells are alive and in mitosis as measured by low phospho-Tyr15 Cdk1 (cyclin-dependent kinase 1), high Cdk activity, active Plk1 (Polo-like kinase 1) and high phospho-histone H3 signals. This phenomenon is independent of the type of DNA damage, but is dependent on pharmacologically relevant doses of genotoxicity. Entry into mitosis is likely to be caused by checkpoint adaptation, and the HT-29 cell-based model provides a powerful experimental system in which to explore its molecular basis. We propose that mitosis with damaged DNA is a biologically significant event because it may cause genomic rearrangement in cells that survive genotoxic damage.
Keywords: camptothecin, checkpoint adaptation, checkpoint kinase 1 (Chk1), cyclin-dependent kinase 1 (Cdk1), mitosis, mitotic catastrophe
Abbreviations: Cdk, cyclin-dependent kinase; Chk1, checkpoint kinase 1; CPT, camptothecin; DAPI, 4′,6-diamidino-2-phenylindole; GST, glutathione transferase; γH2AX, phosphorylated histone H2AX; IDC, interphasic and DNA-damaged cell; MDC, mitotic and DNA-damaged cell; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; Plk1, Polo-like kinase 1; PP1a, protein phosphatase 1α; TBST, Tris-buffered saline with Tween 20; TDC, total and DNA-damaged cell
INTRODUCTION
To ensure the fidelity of mitosis, cells use biochemical pathways known as checkpoints, which enable them to detect damaged DNA and prevent entry into mitosis [1]. It is widely believed that failure to repair damaged DNA leads to apoptosis and that this protects cells from transmitting damaged DNA to daughter cells [2]. It has also been observed, however, that cells can enter mitosis after being subjected to radiation [3,4] or diverse genotoxic chemicals [5–7] with the possibility of transmitting damaged DNA to daughter cells. The conditions in which cells can enter mitosis with damaged DNA and the biological significance of doing so are poorly understood [8].
Chk1 (checkpoint kinase 1) and Cdk1 (cyclin-dependent kinase 1) (Cdk1–cyclin B complex) are key enzymes in the DNA damage checkpoint and cell cycle pathways [9]. When cells are challenged with damaged DNA, Chk1 is activated by phosphorylation on Ser317 and Ser345 by the upstream kinase ATR (ataxia telangiectasia mutated- and Rad3-related) [10,11]. In addition to phosphorylating Chk1, the PIKK (phosphoinositide 3-kinase-related kinase) family members phosphorylate histone H2AX to convert it into γH2AX (phosphorylated histone H2AX) [12]. This histone can be used as a marker to identify damaged DNA in cells [13]. Chk1 prevents the activation of Cdk1 by promoting the degradation and sequestration of the CDC25 phosphatases [14–16], which are required to dephosphorylate Tyr15 of the catalytic subunit of Cdk1. If Cdk1 remains phosphorylated on Tyr15, the enzyme stays inactive despite high cyclin B1 levels and the cell is blocked in the G2-phase of the cell cycle. Active Cdk1 drives cells into mitosis, which is characterized by major structural and biochemical changes, including cell rounding [17], chromosome condensation and phosphorylation of core histones. By measuring cyclin B1 levels, Cdk1 activity and phosphorylation of histone H3 on Ser10, cells can be followed as they exit G2-phase and enter mitosis [18].
We examined how cells treated with genotoxic compounds exit from a cell cycle arrest and enter mitosis despite containing damaged DNA. We used CPT (camptothecin), a topoisomerase I inhibitor, as a genotoxic agent. The pharmacokinetics of CPT and its clinical derivatives (topotecan and irinotecan) have been investigated extensively in human subjects, providing valuable information to model the genotoxic response in cancer cells [19]. In the present paper, we report that when cells were treated with a cytotoxic and pharmacologically relevant amount of CPT, they became rounded and entered mitosis even though their DNA was still damaged. This morphology change was a convenient measure of mitosis, which we exploited to examine how Chk1 participates in restarting the cell cycle. Our findings suggest that mitosis is a component of the cellular response to cytotoxic anticancer agents. Furthermore, HT-29 cells have features that make them a valuable model to investigate the molecular basis of checkpoint adaptation in human cells.
MATERIALS AND METHODS
Cell culture
The human cell line HT-29 was obtained from the A.T.C.C. (Manassas, VA, U.S.A.). HT-29 cells were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% (v/v) decomplemented fetal bovine serum (PAA Laboratories), 2 mM L-glutamine (Invitrogen) and 10 mM Hepes (pH 7.4). Cells were grown at 37°C in 5% CO2, and the medium was changed every second or third day. Human M059K cells (A.T.C.C.) were grown in DMEM (Dulbecco's modified Eagle medium)/Ham's F12 (Invitrogen) supplemented with 10% decomplemented fetal bovine serum, 2 mM non-essential amino acids and 15 mM Hepes (pH 7.4). The compounds CPT (Sigma), etoposide (Sigma), nocodazole (Sigma), CR8, methyl-CR8 (ManRos Therapeutics), BI2536 (Axon Chemicals) and MG132 (Cedarlane Labs) were dissolved in DMSO to a concentration of 10 mM and stored at −20°C until use.
Mechanical shake-off
HT-29 cells were plated at 400000 cells/25 cm2 flask and cultivated for 48 h before treatment with compounds. At desired times after treatment, culture medium was replaced with a small volume of RPMI 1640 medium (40 μl/cm2), and flasks were tapped on all sides with medium force until rounded cells were released.
Flow cytometry
At desired times after treatment, total cultures or cells in interphase were collected by trypsinization. Rounded cells were collected by mechanical shake-off. Cells were washed in PBS and fixed in 90% ethanol (−20°C) for at least 24 h. Fixed cell suspensions were blocked for 1 h with labelling buffer (PBS, 5% serum, 1% BSA and 0.1% sodium azide) before 1 h of incubation with anti-(Ser10-phosphorylated histone H3) antibody (06-570, Millipore; 1:100 dilution), and 30 min of incubation with FITC-conjugated secondary antibody (sc-2012, Santa Cruz Biotechnology; 1:100 dilution) in labelling buffer, separated by wash/centrifuge steps in wash buffer (PBS, 1% BSA and 0.1% sodium azide). For analysis, samples were incubated for 20 min in wash buffer with 0.02 mg/ml propidium iodide (Invitrogen) and 0.2 mg/ml RNase A (Sigma), and analysed by a FACSCanto™ II flow cytometer (BD Biosciences) using BD FACSDiva™ software. Gating was set using control samples without primary antibody. Experiments were carried out at least twice.
Light microscopy
Images were taken with an Infinity 1.5 camera powered by Infinity Capture (Lumenera Corporation) software. Live cells were detected by dye exclusion with Amresco® Trypan Blue 0.4% solution (VWR). Me-CR8 and CR8 were used at 2.5 μM concentrations with or without 25 nM CPT. Experiments with Me-CR8 or CR8 or MG132 were carried out three times. MG132 was used at 0.3 μM.
Immunofluorescence microscopy
Cells were plated on glass coverslips for 48 h before treatment. Cells collected by mitotic shake-off were attached to glass coverslips coated with poly-L-lysine (Invitrogen). At desired times, cells were fixed in 3% (w/v) formaldehyde for 20 min at room temperature (20°C) and permeabilized for 5 min in 0.2% Triton X-100. Cells were incubated with anti-γH2AX (05-636, Millipore), anti-(Ser10-phosphorylated histone H3) (as above) or anti-cyclin B1 (sc-752, Santa Cruz Biotechnology) for 2 h at room temperature [7]. Texas Red-conjugated anti-rabbit secondary antibodies (Jackson ImmunoResearch/Beckman Coulter) for histone H3 and cyclin B1, or Alexa Fluor® 488-conjugated anti-mouse secondary antibodies (Molecular Probes/Invitrogen) for γH2AX were added for 2 h. Nuclei were stained with 300 nM DAPI (4′,6-diamidino-2-phenylindole) in PBS for 15 min before mounting. Cells were observed using a Zeiss microscope operated by Axiovision 3.1 software. Images were collected with a Zeiss MR camera within the linear dynamic range. Images were prepared for presentation using identical parameters with Adobe Photoshop CS3 10.0 software. Experiments were carried out at least twice. Confocal images were prepared with a Nikon C1+ confocal system equipped with an inverted Eclipse TE 2000U microscope.
Cytotoxicity assays
Cytotoxicity was measured using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay [20]. Results were expressed as IC50 values, the compound concentration that reduced by 50% the absorbance at 590 nm, compared with DMSO-treated cells. All measurements were carried out in triplicate.
Extract preparation
Cells were resuspended in extraction buffer [50 mM Hepes, 50 mM NaF, 10 mM EGTA, 50 mM 2-glycerophosphate, 1 mM ATP, 1 mM DTT (dithiothreitol), 1% Triton X-100, 10 μg/ml RNase A, 0.4 unit/ml DNase I, with Roche protease inhibitor cocktail] at a concentration of 20000 cells/μl, on ice for 30 min. After five passages through a 26-gauge needle at 4°C, the suspension was centrifuged at 12000 g for 10 min at 4°C. Extracts were used either for electrophoresis after being boiled for 3 min in the presence of 2× SDS sample buffer or measurement of Cdk/cyclin activity. Cdk activity was measured by incubating extracts with a GST (glutathione transferase)–Cdk1 substrate or GST alone followed by Western blotting with an anti-(phospho-Thr320 PPP1Ca) (protein phosphatase 1 catalytic subunit) antibody [21]. Details of the Cdk assay are available from R.M.G. on request.
Electrophoresis and Western blotting
Samples (10 μg of total protein per lane) were run on polyacrylamide gels. Protein loading was confirmed by Coomassie Blue staining of gels run in parallel or by anti-actin Western blotting. Molecular-mass markers (Precision Plus) were from Bio-Rad Laboratories. Proteins were transferred on to nitrocellulose membranes with a semi-dry electroblotter system (Bio-Rad Laboratories) for 45 min at 25 V. Subsequently, the membrane was blocked with either 5% (w/v) low-fat milk powder in TBST (Tris-buffered saline with Tween 20: 50 mM Tris/HCl, 150 mM NaCl and 0.1% Tween 20, pH 7.6) or 5% (w/v) BSA in TBST, and incubated overnight with the indicated primary antibody as follows: anti-Chk1 (sc-8408, Santa Cruz Biotechnology; 1:200 dilution); anti-(phospho-Ser345 Chk1) (2341S, Cell Signaling Tehnology; 1:1000 dilution); anti-(cyclin B1) (GNS1, Santa Cruz Biotechnology; 1:200 dilution), anti-Cdk1/Cdc2 (21236-2, Signalway Antibodies; 1:500 dilution), anti-(phospho-Tyr15 Cdk/Cdc2) (11244-2, Signalway Antibodies; 1:500 dilution), anti-(phospho-Thr320 PP1a) (protein phosphatase 1α) (2581S, Cell Signaling Technology; 1:1000 dilution); anti-Plk1 (Polo-like kinase 1) (33-1700, Zymed Laboratories; 1:300 dilution); anti-(phospho-Thr210 Plk1) (558400, BD Biosciences; 1:500 dilution) or anti-actin (SC-58673, Santa Cruz Biotechnology; 1:200 dilution). After washing, the membrane was incubated with horseradish-peroxidase-coupled anti-(mouse IgG) or anti-(rabbit IgG) (GE Healthcare; 1:2000 dilution) for ECL (enhanced chemiluminescence) detection (GE Healthcare) or alkaline-phosphatase-coupled anti-(mouse IgG) or anti-(rabbit IgG) for alkaline phosphatase detection (Bio-Rad Laboratories).
RESULTS
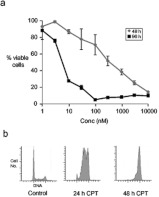
We incubated HT-29 cells with increasing concentrations of CPT and measured their viability at 48 or 96 h by the MTT assay. The IC50 of CPT was 175 nM at 48 h; however, at 96 h the IC50 was 5 nM (Figure 1a). On the basis of these data, we chose 25 nM CPT for subsequent experiments because this concentration is a pharmacologically relevant cytotoxic concentration in human patients [22]. Importantly, at this concentration, more than 80% of the cells were alive at 48 h even though they were destined to die by 96 h. We then treated HT-29 cells with 25 nM CPT and analysed them for cell cycle phase by flow cytometry. Cells accumulated in S-phase and G2/M-phase by 24 h after treatment, and were in the G2/M-phase by 48 h, consistent with the activation of the DNA damage checkpoint (Figure 1b). These data highlighted that HT-29 cells were alive and arrested in the cell cycle at 48 h after treatment with a pharmacologically relevant and cytotoxic concentration of CPT.
Figure 1. HT-29 cells treated with 25 nM CPT are alive at 48 h and arrested in the G2/M-phase of the cell cycle.
(a) Human colon carcinoma cells (HT-29) were treated with graded concentrations of CPT for either 48 h (◆) or 96 h (■) and viability was measured by the MTT assay. Results are mean±S.E.M. percentages of live cells for triplicate experiments. (b) Cells were cultivated with 25 nM CPT and prepared for analysis by flow cytometry at 24 and 48 h after treatments. Control cells (not treated) were also analysed. DNA content was determined by propidium iodide staining.
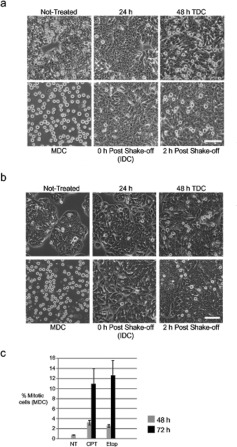
We then investigated the fate of the cells after cell cycle arrest. At 24 h after CPT treatment (Figure 2a), nearly all cells were flat and strongly adherent. At 48 h, when most cells were arrested in G2-phase, relatively large rounded cells appeared, which were weakly adherent. These rounded cells could be collected by mechanical shake-off, leaving behind the flattened cells (Figure 2a). The rounded cells were alive as confirmed by vital dye exclusion (results not shown). We re-cultivated the flattened cells that remained attached after mechanical shake-off and found that new rounded cells appeared by 2 h, as a result of a continuous process. For subsequent experiments, we worked with three types of treated cell populations, which we defined as follows: TDCs (total and DNA-damaged cells), a total cell population with damaged DNA composed of interphase and rounded cells; IDCs (interphasic and DNA-damaged cells), flat interphase cells with damaged DNA that were strongly adherent and could not be collected by mechanical shake-off; and MDCs (mitotic and DNA-damaged cells), rounded cells with damaged DNA, which could be collected by mechanical shake-off.
Figure 2. Cells treated with either CPT or etoposide acquire a rounded shape.
(a) HT-29 cells were either not treated or treated with 25 nM CPT and observed by phase-contrast light microscopy at 24 and 48 h. Rounded cells were visible in non-treated samples, but rarely at 24 h after treatment and commonly by 48 h post-treatment (TDC). Rounded cells were collected after mechanical shake-off (MDC; lower left-hand panel) leaving behind flattened interphase cells (IDC; lower centre panel). New rounded cells appeared within 2 h in the adherent culture (lower right-hand panel). Scale bar, 200 μm. (b) HT-29 cells were either not treated or treated with 3 μM etoposide and analysed as described in (a). (c) The number of live rounded cells (MDC) collected by mechanical shake-off were counted from cultures that were either not treated (NT) or treated with CPT or etoposide at 48 (grey) and 72 (black) h.
To determine whether the MDC morphology was related to genotoxic responses other than CPT, we treated cells with 3 μM etoposide. Like the conditions used for CPT, 3 μM etoposide is a pharmacologically relevant cytotoxic concentration [23]. Etoposide induced the appearance of rounded cells (Figure 2b), indicating that the MDC morphology seen with CPT is probably common to genotoxic agents. The frequency of rounded cells was approximately 2–3% of the total culture at 48 h and 10–12% by 72 h, whereas rounded cells made up approximately 1% of an untreated culture (Figure 2c). This number of MDCs, and the likelihood that cells were continuously entering this population, suggested that this change in cell shape might reflect an important event after cell cycle arrest.
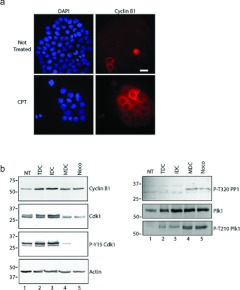
In non-treated cultures, rounded cells that can be collected by mechanical shake-off are typically in mitosis [24]. Therefore we tested whether MDCs induced by CPT treatment were in mitosis. In non-treated cells, immunofluorescence microscopy revealed that only a subpopulation of cells were cyclin B1-positive, which was consistent with cell-cycle-dependent expression of cyclin B1 [25] (Figure 3a). However, in cultures treated for 48 h with 25 nM CPT, nearly all cells were positive for cyclin B1, which was consistent with cells arrested in the G2-phase of the cell cycle. We separated cells into IDC and MDC populations and examined them for cyclin B1 and Cdk1 by Western blotting. Cells without CPT treatment had lower levels of cyclin B1 than those treated with CPT (Figure 3b). Furthermore, the MDCs expressed relatively high levels of cyclin B1 that were comparable with cells arrested in mitosis by nocodazole treatment. We also examined cells for Cdk1 protein levels and Cdk1 phosphorylation on Tyr15. The levels of Cdk1 phosphorylated on Tyr15 were much lower in extracts prepared from MDCs than in non-treated extracts or IDCs. The Cdk1 signal of MDCs was similar to that of nocodazole-treated cells, suggesting that these cells contained the active form of Cdk1. We then compared the amounts of phospho-Thr320 PP1 in each extract. PP1 is phosphorylated on Thr320 by Cdk1 [21] and is used as an indicator of Cdk1 activity [26]. MDCs contained phospho-Thr320 PP1, whereas IDCs did not, confirming our observation that rounded cells have active Cdk1. We examined the levels of Plk1 [27] and activated Plk1 (phospho-Thr210) [28] in these samples. Plk1 levels were relatively high in IDC and MDC samples and low in non-treated samples, consistent with its expression in the G2/M-phase [29]. Phospho-Thr210 Plk1 levels were highest in MDC samples, yet could still be detected in IDC samples.
Figure 3. MDCs express cyclin B1 and the active form of Cdk1.
(a) Cells were grown on coverslips and either not treated (upper row) or treated with CPT for 48 h (lower row), stained with DAPI (blue) and with anti-(cyclin B1) antibodies (red). Cells were analysed by immunofluorescence microscopy. Scale bar, 50 μm. (b) Extracts were prepared from cells either not treated (NT) or treated with CPT. CPT-treated cells were analysed further as total cultures (TDC) or cultures fractionated by mechanical shake-off into adherent interphase cells (IDC) or rounded cells (MDC). Extracts were also prepared from cells treated with nocodazole (Noco). Samples were processed by Western blotting with antibodies against cyclin B1, Cdk1, phospho-Tyr15 Cdk1 (P-Y15 Cdk1), phospho-Thr320 PP1a (P-T320 PP1), Plk1, phospho-Thr210 Plk1 (P-T210 Plk1) or actin. Molecular masses are indicated in kDa.
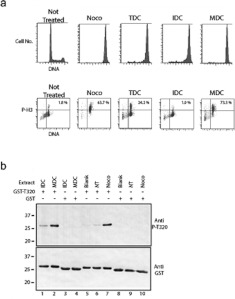
To determine whether cells enter mitosis after CPT treatment, we examined them for phospho-Ser10 histone H3 and DNA content by flow cytometry. A non-treated cell culture displayed a typical DNA distribution with approximately 2% of the cells positive for phospho-Ser10 histone H3, compared with 64% of the cells treated with nocodazole (Figure 4a). At 48 h after CPT treatment, 24% of the TDC population was positive for phospho-Ser10 histone H3. MDCs, which were collected at 48 h, were 73% positive for phospho-Ser10 histone H3, whereas the IDCs had only 1.9% positive cells remaining. We then measured Cdk enzyme activity in extracts prepared from CPT-treated cells. MDCs had much higher Cdk activity than IDCs or non-treated cells as shown by phosphorylation of an artificial Cdk substrate, but similar to that of nocodazole extracts (Figure 4b). The negative control substrate, which did not have a Cdk1 phosphorylation site engineered into it, was not phosphorylated. These results indicate that CPT-treated cells are capable of restarting the cell cycle and entering mitosis.
Figure 4. Rounded cells (MDC) are positive for phospho-Ser10 histone H3 and for Cdk activity.
(a) Cells were either not treated (NT) or treated with nocodazole (Noco) or 25 nM CPT for 48 h (TDC). A second TDC population was fractionated into IDC and MDC populations by mechanical shake-off. All samples were analysed by flow cytometry for DNA content and for phospho-Ser10 histone H3 signals. The percentage of cells positive for phospho-Ser10 histone H3 (P-H3) is listed in the upper right-hand quadrant. (b) Extracts were prepared from IDCs, MDCs, cells not treated (NT) or cells treated with nocodazole (Noco). Extracts were incubated with a GST–Thr320 Cdk1 substrate (lanes 1, 2 and 5–7) or a GST-control substrate (lanes 3, 4 and 8–10). Samples were analysed by Western blotting with an anti-Cdk1 phospho-substrate antibody (upper panel, Anti P-T320). Equal amounts of substrate were used in each sample (lower panel, Anti GST). IDC, MDC, NT and Noco extracts were tested at concentrations representing 500 cells. Blank indicates buffer was used in place of extract. Molecular masses are indicated in kDa.
We then tested whether entry into mitosis after CPT treatment would occur in a cell line other than HT-29. We chose M059K cells, which, in contrast with HT-29, are of brain (glioma) origin, fibroblastic and have a long (48 h) cell cycle. Like HT-29, M059K cells are sensitive to CPT, which is a therapeutic option for glioma. M059K cells were alive after treatment with clinically relevant cytotoxic concentrations of CPT at 48 h yet were destined to die by 96 h (Supplementary Figure S1a at http://www.BiochemJ.org/bj/446/bj4460373add.htm). We observed cell rounding in CPT-treated populations and the appearance of new rounded cells 2 h after mechanical shake-off of a 48 h IDC population (Supplementary Figure S1b). Because M059Ks have a longer cell cycle than HT-29 cells, fewer cells were in mitosis after CPT treatment at any time tested, including those arrested with nocodazole (7%). Importantly, starting at 48 h after CPT treatment, phospho-Ser10 histone H3-positive cells appeared at frequencies that were higher than for non-treated cells (1.5%) or cells under a DNA damage checkpoint at 24 h (0.8%). These data suggest that the phenomenon of entry into mitosis with damaged DNA is common to many cell types.
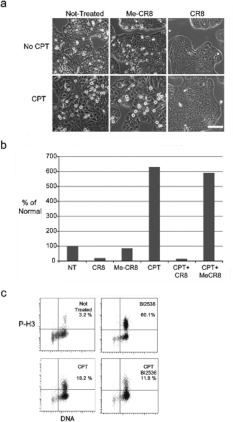
If cells enter mitosis after CPT treatment, we reasoned that a Cdk inhibitor might prevent them from doing so. Cells were either treated or not with CPT for 24 h and then treated further with either CR8, a Cdk inhibitor, or with methyl-CR8, a compound related to CR8, but without Cdk1 inhibitory activity [26]. By 48 h, CR8 reduced the number of rounded cells to near zero in cultures of non-treated cells and in cultures treated with CPT (Figures 5a and 5b). In contrast, methyl-CR8 had little effect upon the number of MDCs. These results confirmed that CPT-treated cells are able to enter mitosis and that this event can be blocked by addition of Cdk1 inhibitors. We then incubated CPT-treated cells with the Plk1 inhibitor BI2536 to determine whether Plk1 activity was required for entry into mitosis with damaged DNA (Figure 5C). First, we demonstrated that 100 nM BI2536 was sufficient to induce a mitotic arrest in HT-29 cells. We then treated cells for 24 h with CPT and then for a further 24 h with CPT and BI2536. Unlike the Cdk1 inhibitor, the Plk1 inhibitor consistently reduced the number of phospho-Ser10 histone H3 cells by approximately 40%.
Figure 5. Cell cycle inhibitors block the entry into mitosis of cells treated with CPT.
(a) Cells were either not treated (upper row) or treated with CPT (lower row). At 24 h after treatment, cells were either treated with methyl-CR8 (Me-CR8) or with CR8 or no further treatment (NT) and cultivated for a further 24 h. Scale bar, 200 μm. (b) The number of MDCs collected by mechanical shake-off were counted at 48 h under each treatment condition and presented as percentages relative to non-treated cells (NT). (c) Cells were either not treated or treated with 100 nM BI2536 for 24 h, 25 nM CPT for 48 h, or co-treated with 25 nM CPT for 48 h with 100 nM BI2536 for the last 24 h. Samples were analysed by flow cytometry for DNA content and for phospho-Ser10 histone H3 signals. The percentage of cells positive for phospho-Ser10 histone H3 (P-H3) is listed in the upper right-hand quadrant.
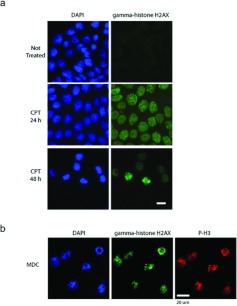
CPT induces a DNA damage checkpoint [30], which arrests cells in the cell cycle, yet we observed that treated cells could enter mitosis. Therefore we wanted to know whether the mitotic cells no longer had damaged DNA, or whether the DNA damage checkpoint was extinguished. We examined cells by immunofluorescence microscopy for γH2AX staining, which is recruited to sites of damaged DNA (Figure 6). Cells were treated with CPT and at various times fixed and stained to detect damaged DNA and mitotic chromosomes. At 24 h after treatment, 97–100% of treated cells displayed γH2AX foci, indicating that this population had damaged DNA (Figure 6a), whereas no signals were observed in cells without CPT treatment. By 48 h, IDCs were still strongly positive; however, cells with condensed chromosomes were also strongly positive, suggesting that cells were entering mitosis even though their DNA was still damaged. It had been reported previously that cells in mitosis might be positive for γH2AX [31], therefore we compared γH2AX signal intensity in mitotic cells after arrest by nocodazole with those treated with CPT. We found that γH2AX staining was much more intense in MDCs, which have damaged DNA, so that they could be readily distinguished from mitotic cells without induced DNA damage (Supplementary Figure S2 at http://www.BiochemJ.org/bj/446/bj4460373add.htm). We then collected MDCs by mechanical shake-off, mounted them on coverslips and observed them by confocal microscopy for DNA organization, phospho-Ser10 histone H3 and γH2AX staining (Figure 6b). The majority of MDCs had poorly formed chromosomes, typical of cells in mitotic catastrophe [32]. They were also phospho-Ser10 histone H3-positive, but, strikingly, they were positive for γH2AX signals. This suggested that these mitotic cells had damaged DNA [33], in addition to atypical chromosome arrangements. We concluded that MDCs had entered mitosis with damaged DNA.
Figure 6. MDCs are in mitosis with damaged DNA.
(a) Cells were either not treated or treated with CPT and stained with DAPI (left-hand panels) and γH2AX antibodies (right-hand panels). Cells were observed after 24 and 48 h of treatment by immunofluorescence microscopy. Scale bar, 10 μm. (b) MDCs were collected, attached to coverslips and stained with DAPI (left) and antibodies against γH2AX (centre) or phospho-Ser10 histone H3 (right, P-H3) and observed by confocal microscopy. Scale bar, 20 μm.
To determine whether mitosis was a frequent event in a population of CPT-treated cells, we counted the number of MDCs at regular intervals after CPT treatment until 96 h, when most, but not all, cells were dead. The time of the greatest number of mitotic cells was 62 h after treatment, although mitotic cells could be detected as early as 40 h. Using video microscopy and cell counting, we found that more than 90% of a cell population entered mitosis, indicating that cells enter mitosis before they die (results not shown).
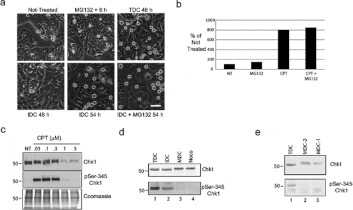
Cells are believed to be able to escape the DNA damage checkpoint by inactivation of Chk1 by either proteolysis [34] or by dephosphorylation [3]. We tested both of these hypotheses using our model. We treated cells with 25 nM CPT and asked whether cells would enter mitosis in the presence of MG132, a proteasome inhibitor (Figure 7a). Cells were cultivated with CPT for 48 h and MDCs were removed by mechanical shake-off. The remaining IDCs were then incubated with MG132 for another 6 h and the number of new MDCs were counted and compared with cells incubated for 6 h without MG132. We detected the same number of MDCs in the presence or absence of the proteasome inhibitor (Figure 7b). We verified that we used an adequate amount of MG132 because we could arrest cells in mitosis by treatment with 300 nM MG132 (see also [35]).
Figure 7. Chk1 is dephosphorylated and not degraded in cells in mitosis with damaged DNA.
Cells were either not treated or treated with MG132 for 6 h or with CPT for 48 h. At 48 h, MDCs were removed by mechanical shake-off from a total culture and the remaining IDCs were either re-incubated with CPT for 6 h (IDC 54 h) or incubated with CPT and MG132 for 6 h (IDC + MG132 54 h). Scale bar, 100 μm. (b) The number of MDCs collected by mechanical shake-off was counted at 48 h under each treatment condition and presented as percentages relative to non-treated cells (NT). (c) Cells were either not treated (NT) or treated with increasing concentrations of CPT (half-log; 0.03–3 μM). Extracts were prepared and analysed by Western blotting with antibodies against either Chk1 or phospho-Ser345 Chk1 or by Coomassie Blue staining. Molecular masses are indicated in kDa. (d) Cells were treated with CPT for 48 h (TDC) and separated into IDC and MDC populations. Mitotic cells without damaged DNA were prepared from nocodazole-treated culture (Noco). Samples were processed by Western blotting with antibodies against either Chk1 or phospho-Ser345 Chk1. Molecular masses are indicated in kDa. (e) Cells were treated with CPT for 48 h (TDC) and separated into IDC and MDC populations (MDC1). The IDC cells were re-cultivated for 2 h and new mitotic cells were collected (MDC2). Samples were processed by Western blotting with antibodies against either Chk1 or phospho-Ser345 Chk1. Molecular masses are indicated in kDa. p, phospho-
We then compared Chk1 protein levels in extracts prepared from cells treated with increasing half-log concentrations of CPT ranging from 0.03 to 3 μM (Figure 7c). We found that Chk1 protein levels were relatively stable up to 0.3 μM CPT, but were much lower when cells were treated with CPT concentrations greater than 0.3 μM. The activated form of Chk1, phospho-Ser345, was present in treated cells, except when Chk1 was low. These data suggested that cells might enter mitosis with damaged DNA by mechanisms other than, but in addition to, Chk1 degradation, depending on the concentration of the genotoxic agent.
We then examined the level of Chk1 in MDCs and compared it with TDCs or IDCs. Chk1 levels were similar in extracts prepared from cells under either condition (Figure 7d). We observed a slight decrease in electrophoretic mobility of Chk1 in MDCs and cells treated with nocodazole, as had been described previously [36]. Strikingly, we found that Chk1 had very low levels of phosphorylation on Ser345 in MDCs. We also observed a loss of phosphorylation on Ser317, a second phosphorylation site on active Chk1 (results not shown). We explored Chk1 dephosphorylation further by examining an IDC population that was re-incubated for 2 h so that new MDCs could be collected (Figure 7e). Under these conditions, we observed again that Chk1 was stable after entry into mitosis and that the dephosphorylation step at Ser345 was closely correlated with entry into mitosis.
DISCUSSION
The events that occur after cell cycle arrest in cells with damaged DNA are poorly understood. To study them, we set up a cell-based assay in which we treated cells with genotoxic agents and observed them at various times. We found that cells became rounded, starting at approximately 40 h after treatment with CPT. The rounded cells are in mitosis, express high levels of cyclin B1, have active Plk1, do not express Cdk1 phosphorylated on Tyr15, have Cdk activity and are positive for phospho-Ser10 histone H3. The relationship between cell rounding and Cdk activity was demonstrated by applying the Cdk inhibitor CR8, which prevented cell rounding. The inactive form of this inhibitor class had no effect upon the transition from IDCs to rounded mitotic cells (MDCs). This cell-based model enables us to study pathways that cells use to exit a DNA damage checkpoint, enter mitosis with damaged DNA and, in some cases, survive.
We set up the assay under conditions that may be relevant for studying the cellular response to cancer drugs in humans. We chose a concentration of CPT that was cytotoxic, as nearly all the cells will die by 96 h. Furthermore, this concentration was within the range of those obtained in the plasma of patients treated with CPT or its derivatives [22]. We confirmed that HT-29 cells responded to CPT in the manner described previously: nearly all cells had multiple γH2AX foci after treatment [7,37], induction of Chk1 by phosphorylation on Ser345 [11] and cell cycle arrest in G2-phase by 48 h.
The rounded cell morphology provided a simple means to examine how cells with damaged DNA enter mitosis. Because the MDCs were rounded and weakly adherent, they could be readily distinguished and isolated from IDCs. Furthermore, by performing sequential mechanical shake-offs, we confirmed that the transition from IDCs to MDCs is a continuous event. At each time tested, starting at approximately 40 h after CPT treatment, cells would enter mitosis. The IDC population appeared to be uniform in their shape and DNA content, yet MDCs appeared asynchronously, hinting that they are following a timing mechanism that we have not yet identified. The lack of synchrony made it difficult to count precisely the proportion of cells that enter mitosis over a period of 48 h. To address this, preliminary observations using video microscopy have revealed that more than 90% of the TDCs enter mitosis before dying, indicating that this is a major cellular event.
We used this model to test the role of Chk1 in exiting the DNA damage-induced cell cycle arrest. Previous studies observed that Chk1 is inactivated by either degradation [34] or dephosphorylation [3]. We found that Chk1 was present in similar amounts in IDCs as in MDCs, although it was no longer phosphorylated on Ser345. A reduced electrophoretic mobility of Chk1 in mitotic cells has been reported to be caused by phosphorylation on Ser286 and Ser301 by Cdk1 [38]. As the MDCs are in mitosis, it is likely that the reduced Chk1 mobility that we observed was due to Cdk1 phosphorylation. Entry into mitosis and Chk1 dephosphorylation on Ser345 were closely linked because we detected Chk1 dephosphorylation in cells that had only entered mitosis after a very short time. We did not detect Chk1 degradation in cells that were treated with pharmacological, yet cytotoxic, concentrations amounts of CPT. Furthermore, treated cells could still enter mitosis even with co-treatment with the proteasome inhibitor, MG132. Our data suggest that when cells are challenged with cytotoxic amounts of damaged DNA, they can use one of two mechanisms to inactivate Chk1. We speculate that the selection of the Chk1 inactivation mechanism (dephosphorylation or degradation) will be linked to the amount of damaged DNA.
The cellular process that we have observed is similar to what has been described in yeast as checkpoint adaptation. Checkpoint adaptation involves three key steps: arrest in the cell cycle due to damaged DNA, overcoming this arrest and resuming the cell cycle even though cells still have damaged DNA [39]. We have detected cell cycle arrest after treatment with CPT and entry into mitosis with poorly formed chromosomes that were γH2AX-positive in human cells. Checkpoint adaptation has been described in human cells that were irradiated [3]. Our data demonstrate that CPT and possibly most genotoxic agents induce checkpoint adaptation.
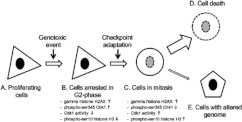
Our observations suggest that checkpoint adaptation is one of the steps that lie between cell cycle arrest and cell death (Figure 8). Interfering with these steps is likely to modify the outcome of the genotoxic response [40,41]. Cells that escape the DNA damage checkpoint with poorly organized chromosomes might activate a mitotic checkpoint [7] and cause the accumulation of MDCs [5]. Furthermore, we confirmed that this phenomenon occurs in osteosarcoma U2OS cells as reported previously [3], and we have detected it in human M059K glioma cells, which indicates that checkpoint adaptation may be a common mechanism in cell death.
Figure 8. A model of the steps required for checkpoint adaptation after treatment with pharmacological concentrations of genotoxic agents.
Cells respond to cytotoxic pharmacological amounts of genotoxic agents through a series of key biochemical events that include: increase in γH2AX levels and increase in phospho-Ser345 Chk1 levels before adaptation. Cells that have undergone checkpoint adaptation still have γH2AX, but a greatly reduced level of phospho-Ser345 Chk1, high Cdk1 activity and phospho-Ser10 histone H3. Although the majority of cells will die, a small number of cells survive, giving rise to cells that have major changes in their genome.
Although cells treated with cytotoxic concentrations of DNA-damaging agents are destined to die, it is important to understand all of the steps that lead to cell death (Figure 8). Entry into mitosis before cell death and mixing of mitotic cells with dying cells may account for previous reports of Cdk activity occurring during apoptosis [42,43]. Knowing that mitosis precedes cell death may help to design strategies to improve the outcome of the majority of current cancer treatments, which use genotoxicity as their mechanism of action. For example, Chk1 inhibitors are being evaluated for use in association with genotoxic agents to improve their clinical activity [44]. Our data suggest that cells already have a mechanism to inactivate Chk1, therefore Chk1 inhibitors might accelerate a process that occurs naturally in cells rather than induce a new means of cell death. We have confirmed that Plk1 may play a role in the entrance into mitosis with damaged DNA. We detected low levels of activated Plk1 in IDCs relative to MDCs, even though the levels of total protein were similar. We also found that the Plk1 inhibitor BI2536 could partially block entry into mitosis in cells with damaged DNA, which is reminiscent of the intermediate effects of Plk1 siRNAs (short interfering RNAs) in cells with damaged DNA forced into mitosis [3]. We are using the HT-29 model to determine whether Plk1 has a precisely timed role during the G2 arrest of the DNA damage response, such as the activation of members of the CDC25 family [45,46].
The mechanisms by which cells die may also have an important effect on the outcome of cancer treatments. During the course of the present study, we collected mitotic cells and re-cultivated them. We observed that a small number of cells actually survive checkpoint adaptation and proliferate. These cells, which were too infrequent to be detected by the MTT assay, appear to have a rearranged genome. Others have predicted that cells that survive genotoxic treatments might be the source of new tumours with major genomic rearrangements [47,48]. In view of the capacity of cells to engage mitosis when confronted with damaged DNA, it is possible that checkpoint adaptation may be not only a major step in cell death, but also a source of major genomic change in surviving cells [49]. The cell model that we describe now gives facile access to this fascinating and medically important problem.
Online data
AUTHOR CONTRIBUTION
Philip Kubara, Brittany Lanser and Sophie Kernéis-Golsteyn performed the experiments. Aurélie Studény contributed preliminary data. Laurent Meijer provided reagents and discussions. Sophie Kernéis-Golsteyn and Roy Golsteyn directed the project. Roy Golsteyn wrote the paper.
ACKNOWLEDGEMENTS
We thank Mr Julian St. Hilaire for help with flow cytometry, and Ms Elfriede Cross, Mr Dominic Mudiayi, Mr Kris Marshall and Mr Bruce McMullin for assistance.
FUNDING
This work was supported by Alberta Innovation-Technology Futures, Alberta Cancer Foundation, Alberta Innovates Sustainability Fund and the University of Lethbridge.
References
- 1.Bartek J., Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007;19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Chen T., Stephens P. A., Middleton F. K., Curtin N. J. Targeting the S and G2 checkpoint to treat cancer. Drug Discovery Today. 2012;17:194–202. doi: 10.1016/j.drudis.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Syljuasen R. G., Jensen S., Bartek J., Lukas J. Adaptation to the ionizing radiation-induced G2 checkpoint occurs in humans cells and depends on checkpoint kinase 1 and polo-like kinase 1. Cancer Res. 2006;66:10253–10257. doi: 10.1158/0008-5472.CAN-06-2144. [DOI] [PubMed] [Google Scholar]
- 4.Hall E. J., Giaccia A. J. Radiobiology for the Radiologist. Philadelphia: Lippincot Williams and Wilkins; 2012. [Google Scholar]
- 5.Clifford B., Beljin M., Stark G. R., Taylor W. R. G2 arrest in response to topoisomerase II inhibitors: the role of p53. Cancer Res. 2003;63:4074–4081. [PubMed] [Google Scholar]
- 6.Demarcq C., Bunch R. T., Creswell D., Eastman A. The role of cell cycle progression in cisplatin-induced apoptosis in Chinese hamster ovary cells. Cell Growth Differ. 1994;5:983–993. [PubMed] [Google Scholar]
- 7.Cahuzac N., Studény A., Marshall K., Versteege I., Wetenhall K., Pfeiffer B., Léonce S., Hickman J. A., Pierré A., Golsteyn R. M. An unusual DNA binding compound, S23906, induces mitotic catastrophe in cultured human cells. Cancer Lett. 2010;289:178–187. doi: 10.1016/j.canlet.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Clemenson C., Marsolier-Kergoat M. C. DNA damage checkpoint inactivation: adaptation and recovery. DNA Repair. 2009;8:1101–1109. doi: 10.1016/j.dnarep.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Smith J., Tho L. M., Xu N., Gillespie D. A. The ATM–Chk2 and ATR–Chk1 pathways in DNA damage signaling and cancer. Adv. Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q., Guntuku S., Cui X., Matsuoka S., Cortez D., Tamai K., Luo G., Carattini-Rivera S., DeMayo F., Bradley A., et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao H., Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 13.Bonner W. M., Redon C. E., Dickey J. S., Nakamura A. J., Sedelnikova O. A., Solier S., Pommier Y. γH2AX and cancer. Nat. Rev. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mailand N., Falck J., Lukas C., Syljuasen R. G., Welcker M., Bartek J., Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 15.Busino L., Donzelli M., Chiesa M., Guardavaccaro D., Ganoth D., Dorrello N. V., Hershko A., Pagano M., Draetta G. F. Degradation of Cdc25A by β-TrCP during S phase and in response to DNA damage. Nature. 2003;426:87–91. doi: 10.1038/nature02082. [DOI] [PubMed] [Google Scholar]
- 16.Sorensen C., Sylijuasen R. G., Falck J., Schroeder T., Rönnstrand L., Khanna K. K., Zhou B. B., Bartek J., Lukas J. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 17.Harris A. K. Location of cellular adhesions to solid substrata. Dev. Biol. 1973;35:97–114. doi: 10.1016/0012-1606(73)90009-2. [DOI] [PubMed] [Google Scholar]
- 18.Juan G., Traganos F., James W. M., Ray J. M., Roberge M., Sauve D. M., Anderson H., Darzynkiewicz Z. Histone H3 phosphorylation and expression of cyclins A and B1 measured in individual cells during their progression through G2 and mitosis. Cytometry. 1998;32:71–77. doi: 10.1002/(sici)1097-0320(19980601)32:2<71::aid-cyto1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Pizzolato J. F., Saltz L. B. The camptothecins. Lancet. 2003;361:2235–2242. doi: 10.1016/S0140-6736(03)13780-4. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Kwon Y. G., Lee S. Y., Choi Y., Greengard P., Nairn A. C. Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2168–2173. doi: 10.1073/pnas.94.6.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivory L. P., Haaz M. C., Canal P., Lokiec F., Armand J. P., Robert J. Pharmacokinetic interrelationships of irinotecan (CPT-11) and its three major plasma metabolites in patients enrolled in phase I/II trials. Clin. Cancer Res. 1997;3:1261–1266. [PubMed] [Google Scholar]
- 23.Slevin M. L., Clark P. I., Joel S. P., Malik S., Osborne R. J., Gregory W. M., Lowe D. G., Reznek R. H., Wrigley P. F. A randomized trial to evaluate the effect of schedule on the activity of etoposide in small-cell lung cancer. J. Clin. Oncol. 1989;7:1333–1340. doi: 10.1200/JCO.1989.7.9.1333. [DOI] [PubMed] [Google Scholar]
- 24.Terasima T., Tolmach L. Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp. Cell Res. 1963;30:344–362. doi: 10.1016/0014-4827(63)90306-9. [DOI] [PubMed] [Google Scholar]
- 25.Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34. Cell. 1989;58:833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- 26.Bettayeb K., Oumata N., Echalier A., Ferandin Y., Endicott J. A., Galons H., Meijer L. CR8, a potent and selective, roscovitine-derived inhibitor of cyclin-dependent kinases. Oncogene. 2008;27:5797–5807. doi: 10.1038/onc.2008.191. [DOI] [PubMed] [Google Scholar]
- 27.Golsteyn R. M., Mundt K. E., Fry A. M., Nigg E. A. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 1995;129:1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang Y. J., Ma S., Terada Y., Erikson R. L. Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J. Biol. Chem. 2002;277:44115–44120. doi: 10.1074/jbc.M202172200. [DOI] [PubMed] [Google Scholar]
- 29.Golsteyn R. M., Schultz S. J., Bartek J., Ziemiecki A., Ried T., Nigg E. A. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J. Cell Sci. 1994;107:1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- 30.Flatten K., Dai N. T., Vroman B. T., Loegering D., Erlichman C., Karnitz L. M., Kaufmann S. H. The role of checkpoint kinase 1 in sensitivity to topoisomerase I poisons. J. Biol. Chem. 2005;280:14349–14355. doi: 10.1074/jbc.M411890200. [DOI] [PubMed] [Google Scholar]
- 31.McManus K. J., Hendzel M. J. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol. Biol. Cell. 2005;16:5013–5025. doi: 10.1091/mbc.E05-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Bruin E. C., Medema J. P. Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat. Rev. 2008;34:737–749. doi: 10.1016/j.ctrv.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Löbrich M., Shibata A., Beucher A., Fisher A., Ensminger M., Goodarzi A. A., Barton O., Jeggo P. A. γH2AX foci analysis for monitoring DNA double-strand break repair: strengths, limitations and optimization. Cell Cycle. 2010;9:662–669. doi: 10.4161/cc.9.4.10764. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y. W., Brognard J., Coughlin C., You Z., Dolled-Filhart M., Aslanian A., Manning G., Abraham R. T., Hunter T. The F box protein Fbx6 regulates Chk1 stability and cellular sensitivity to replication stress. Mol. Cell. 2009;35:442–453. doi: 10.1016/j.molcel.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skoufias D. A., Indorato R. L., Lacroix F., Panopoulos A., Margolis R. L. Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed. J. Cell Biol. 2007;179:671–685. doi: 10.1083/jcb.200704117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng C. P., Lee H. C., Ho C. W., Arooz T., Siu W. Y., Lau A., Poon R. Y. Differential mode of regulation of the checkpoint kinases CHK1 and CHK2 by their regulatory domains. J. Biol. Chem. 2004;279:8808–8819. doi: 10.1074/jbc.M312215200. [DOI] [PubMed] [Google Scholar]
- 37.Goldwasser F., Bae I., Valenti M., Torres K., Pommier Y. Topoisomerase I-related parameters and camptothecin activity in the colon carcinoma cell lines from the National Cancer Institute anticancer screen. Cancer Res. 1995;55:2116–2121. [PubMed] [Google Scholar]
- 38.Shiromizu T., Goto H., Tomono Y., Bartek J., Totsukawa G., Inoko A., Nakanishi M., Matsumura F., Inagaki M. Regulation of mitotic function of Chk1 through phosphorylation at novel sites by cyclin-dependent kinase 1 (Cdk1) Genes Cells. 2006;11:477–485. doi: 10.1111/j.1365-2443.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- 39.Toczyski D. P., Galgoczy D. J., Hartwell L. H. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell. 1997;90:1097–1106. doi: 10.1016/s0092-8674(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 40.Borgne A., Versteege I., Mahe M., Studeny A., Leonce S., Naime I., Rodriguez M., Hickman J. A., Meijer L., Golsteyn R. M. Analysis of cyclin B1 and CDK activity during apoptosis induced by camptothecin treatment. Oncogene. 2006;25:7361–7372. doi: 10.1038/sj.onc.1209718. [DOI] [PubMed] [Google Scholar]
- 41.On K. F., Chen Y., Ma H. T., Chow J. P., Poon R. Y. Determinants of mitotic catastrophe on abrogation of the G2 DNA damage checkpoint by UCN-01. Mol. Cancer Ther. 2011;10:784–794. doi: 10.1158/1535-7163.MCT-10-0809. [DOI] [PubMed] [Google Scholar]
- 42.Golsteyn R. M. Cdk1 and Cdk2 complexes (cyclin dependent kinases) in apoptosis: a role beyond the cell cycle. Cancer Lett. 2005;217:129–138. doi: 10.1016/j.canlet.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Maddika S., Ande S. R., Panigrahi S., Paranjothy T., Weglarczyk K., Zuse A., Eshraghi M., Manda K. D., Wiechec E., Los M. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist. Updates. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Dai Y., Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin. Cancer Res. 2010;16:376–383. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Vugt M. A., Bras A., Medema R. H. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol. Cell. 2004;15:799–811. doi: 10.1016/j.molcel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Lobjois V., Jullien D., Bouche J. P., Ducommun B. The polo-like kinase 1 regulates CDC25B-dependent mitosis entry. Biochim. Biophys. Acta. 2009;1793:462–468. doi: 10.1016/j.bbamcr.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Nakada S., Katsuki Y., Imoto I., Yokoyama T., Nagasawa M., Inazawa J., Mizutani S. Early G2/M checkpoint failure as a molecular mechanism underlying etoposide-induced chromosomal aberrations. J. Clin. Invest. 2006;116:80–89. doi: 10.1172/JCI25716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nitiss J. L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephens P. J., Greenman C. D., Fu B., Yang F., Bignell G. R., Mudie L. J., Pleasance E. D., Lau K. W., Beare D., Stebbings L. A., et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.