Abstract
The facilitated diffusion of glucose, galactose, fructose, urate, myoinositol and dehydroascorbic acid in mammals is catalyzed by a family of 14 monosaccharide transport proteins called GLUTs. These transporters may be divided into 3 classes according to sequence similarity and function/substrate specificity. GLUT1 appears to be highly expressed in glycolytically active cells and has been co-opted in vitamin C auxotrophs to maintain the redox state of the blood through transport of dehydroascorbate. Several GLUTs are definitive glucose/galactose transporters, GLUT2 and GLUT5 are physiologically important fructose transporters, GLUT9 appears to be a urate transporter while GLUT13 (HMIT1) is a proton/myoinositol co-transporter. The physiologic substrates of some GLUTs remain to be established. The GLUTs are expressed in a tissue specific manner where affinity, specificity and capacity for substrate transport are paramount for tissue function. Although great strides have been made in characterizing GLUT-catalyzed monosaccharide transport and mapping GLUT membrane topography and determinants of substrate specificity, a unifying model for GLUT structure and function remains elusive. The GLUTs play a major role in carbohydrate homeostasis and the redistribution of sugar-derived carbons among the various organ systems. This is accomplished through a multiplicity of GLUT-dependent glucose sensing and effector mechanisms that regulate monosaccharide ingestion, absorption, distribution, cellular transport and metabolism and recovery/retention. Glucose transport and metabolism have co-evolved in mammals to support cerebral glucose utilization.
INTRODUCTION
The mammalian monosaccharide transporters or Glucose Transport proteins (GLUTs) belong to a family of integral membrane proteins that catalyzes the facilitated diffusion (transport down a concentration gradient) of hexose and pentose sugars into and out of cells. Some GLUTs also mediate transport of dehydroascorbate, urate or myoinositol. The physiologic substrates for some GLUTs are not known.
The GLUTs are expressed in nearly all mammalian cells although most cells typically express one GLUT isoform as the major monosaccharide transport protein and lower levels of one or more of the remaining 13 isoforms. GLUT1 has been described as a ubiquitously expressed transporter. This may be true or may be a consequence of tissue vascularization because GLUT1 is highly expressed in vasculature smooth muscle and endothelial cells.
The cell membrane is a very effective barrier to the transmembrane flow of monosaccharides in the absence of specific sugar transport proteins. For example, inhibition of GLUT1-mediated sugar transport in human red cells by use of specific inhibitors reduces the glucosepermeability of red cells by 5 orders of magnitude(446).The GLUTs enhance membrane permeability to pentose and hexose monosaccharides that adopt pyranose and furanose chair conformations(446). Unlike the active glucose transporters (SGLTs) of the absorptive and reabsorptive epithelia (633), the GLUTs are not coupled to the co-transport of Na+. GLUT-mediated glucose transport proceeds via facilitated diffusion. When sugars are present both inside and outside of the cell, these transporters catalyze unidirectional sugar uptake and unidirectional sugar exit. The direction of net sugar transport is always in the direction of the sugar gradient (617). As a consequence, the highest concentration of cytoplasmic sugar that a cell may attain is the prevailing extracellular sugar level. Even this requires the absence of intracellular sugar metabolism.
The GLUTs therefore provide a pathway for cellular sugar import and export. In most cells, import is the most important function because it provides a source of metabolic fuel. In some cells (e.g. liver, kidney and gastrointestinal tissues), export of sugars imported from luminal contents or formed in the cytoplasm by gluconeogenesis is important. In yet other cells (e.g. endothelial cells forming blood-tissue barriers), trans-cellular sugar transport is vital for the delivery of metabolic fuel to the protected tissue.
The importance of GLUT function is illustrated in studies of transgenic animals and through analysis of GLUT1 mutations. GLUT1 homozygous knockouts are embryonic lethal(607). GLUT2 and GLUT4 knockouts are not lethal but are nevertheless associated with mild to severe perturbations of carbohydrate homeostasis which may be partially compensated by upregulated expression of other GLUT isoforms(550). GLUT1 deficiency syndrome affected individuals have haplo-insufficiency for GLUT1because of missense, nonsense, splice site, insertional, or deletional mutations in one GLUT1 gene (465). Several GLUT1 mutations have been observed in humans where the phenotype can range from mild to severe developmental and metabolic perturbation. The resulting transporter may be absent, dysfunctional or have compromised transport potential. These observations, which we shall expand upon in later sections, point to a central role for the GLUTs in organismal carbohydrate homeostasis. The physiologic substrates of the GLUTs are only partially resolved. GLUT1, GLUT3 and GLUT4 are glucose/galactose and dehydroascorbic acid transporters, GLUT2 and GLUT5 catalyze fructose transport, GLUT9 appears to be a urate transporter and GLUT13 (HMIT1) is a proton/myoinositol co-transporter. The preferred substrates of the remaining GLUTs remain to be established.
This chapter examines our current understanding of the monosaccharide transport proteins, their structure, the mechanism of monosaccharide transport, where these proteins are expressed, how they contribute directly to mammalian glucose metabolism, and how their activities and expression are regulated to coordinate the distribution, uptake and metabolism of monosaccharides. We also consider the roles played by these proteins in the transport of other small molecules.
MEMBRANE PROTEINS
Membrane proteins may be broadly categorized into two groups. Peripheral membrane proteins are associated with the cell membrane through covalent, ionic or hydrophobic interactions with lipids or other membrane-associated proteins. These proteins are typically displaced from the cell membrane by high salt or by high pH media and are stable in aqueous suspension following displacement (546). Integral membrane proteins are embedded in the membrane. These proteins are stably associated with the cell membrane and require the use of lipid bilayer-disrupting detergents in order to escape the cell membrane (643). Following their release, integral membrane proteins are stabilized in aqueous media by a surrounding annulus of detergent molecules. The most common form of integral membrane protein is the transmembrane protein or TM, which fully spans the cell membrane. Single pass membrane proteins span the membrane only once exposing the N- and C- termini to opposite sides of the membrane. Multi-pass membrane proteins cross the cell membrane more than once. If the number of membrane spanning domains is odd, the amino and carboxy terminal domains are exposed at opposite sides of the cell membrane. If the number of membrane spanning domains is even, amino and carboxyl-terminal domains are exposed at the same surface. Transporters are typically multi-pass TMs with as many as 14 membrane spanning domains (504).
TRANSPORTERS
Membrane transport may be defined as the movement of molecules across a membrane barrier. Transport can describe solute flow into a cell (import), out of a cell (export) and across a cell (transcellular transport) as in epithelia. Transport may be passive or active. Passive transport describes the movement of molecules down an electrochemical or chemical gradient. Passive transport is bi-directional and proceeds until an equilibrium is achieved in which import is exactly balanced by export and intra- and extracellular concentrations of transported substrate are constant. This is precisely the result that would be obtained if transport occurred by simple diffusion although, when mediated by a transport protein, equilibrium is achieved many times more rapidly. This explains why protein-mediated passive transport is termed "facilitated diffusion."
Active transport, in contrast, is defined as the net movement of a molecule against an electrochemical gradient. This requires energy expenditure either by coupling transport to ATP-hydrolysis (primary active transport; e.g. the Na+,K+ATPase (335)) or to the movement of a second transported species down an electrochemical or chemical gradient (secondary active transport) (547). Active transport is conservative in that it stores the free energy released upon ATP-hydrolysis or substrate flow down a concentration gradient in the form of a new concentration gradient. In secondary active transport, the driving, transported species is typically Na+ or H+ and favorable electrochemical Na+ or H+ gradients are established by primary active transport of these cations. Secondary active transporters may be divided into two sub-groups: antiporters and symporters. Antiporters catalyze active transport by transporting one molecule down its concentration gradient in exchange for transport of another molecule against its concentration gradient and in the opposite direction. The NCX Na/Ca eXchangers, which import three sodium ions for every calcium ion exported out of the cell, are antiporters (6) and can only perform useful work (Ca2+ export) because the Na+,K+ATPase establishes and maintains a Na+ gradient directed into the cell. Symporters catalyze the co-transport of two different molecular species in the same direction by using the free energy available in the electrochemical gradient of one molecule to drive the transport of the second species against its concentration gradient. An example of a symporter is the SGLT family of glucose transporters, which mediate glucose reabsorption or absorption in the kidney and gastrointestinal system by coupling net uphill or concentrative glucose uptake to sodium transport down its electrochemical gradient (348). Again, the conservative co-transporter or symporter can only perform useful work (glucose uptake from the lumen) because the Na+,K+ATPase establishes and maintains an inwardly directed Na gradient.
Facilitated diffusion can be mediated by pores, channels and carriers. The pores and channels permit extremely high flows of transported species down an electrochemical or chemical gradient whereas the carriers transport substrates at least 100- to 1,000-fold more slowly. Recent studies of the ClC Cl− channel have suggested that channels and carriers may be more closely related than previously recognized (4, 427). Unlike pores and channels, carriers, it has been proposed, present a single substrate binding site that can exist only alternately at either side of the membrane (288, 616) but see (427). Substrate binding sites in channels are though to be simultaneously accessible from both sides of the membrane. Carriers undergo a conformational change upon substrate binding, which results in translocation of bound substrate through the protein and across the plasma membrane. Translocation through pores and channels does not require as extensive a conformational change (93). Finally, the carrier mechanisms can be adapted to passive or active transport, while pores and channels are strictly passive transport proteins. The transporters of the GLUT family are carriers that catalyze "passive transport" or "facilitated diffusion" of sugars and other small molecules (547). GLUT13 (HMIT) may be an exception in that it catalyzes proton-myoinositol co-transport or symport (secondary active transport)(589
MAJOR FACILITATOR SUPERFAMILY
The mammalian facilitative glucose transport (GLUT) family of proteins is a member of the Major Facilitator (MFS) Superfamily of transporter proteins, which is one of two membrane protein families found ubiquitously in living organisms. The ATP-binding Cassette (ABC) superfamily is the second such family (293, 406, 460). The MFS superfamily currently consists of 29 established families of transport proteins, including sugar, ion, and drug transporters, hexose proton symporters, and sugar ion symporters found ubiquitously in all species including bacteria, plants, and mammals (505). Unlike the ABC transporters, which can exist as multiprotein complexes and transport both large and small molecules via ATP hydrolysis, MFS superfamily proteins are characterized as single polypeptides that transport small molecules without the use of energy (460). Though the superfamily is extremely diverse, there are two signature structural elements that define MFS proteins. First, MFS proteins contain 12 (or rarely 14) TM domains divided into two symmetrical halves connected by an intracellular loop. The symmetry between both halves of these proteins is thought to have occurred through a gene duplication event (257, 460). Second, the cytoplasmic loop between TM 2 and TM 3 in MFS proteins contains the following sequence: G-[X1]-L-[G/A/S]-[D/N]-[R/K]-[F/Y]-G-R-[R/K]- [R/K/P]-[X2]-[L/I/M], where X1 is R, K, P, A, T, or Y, and X2 is L, I, V, G, S, or T. In many MFS members, this sequence is also roughly duplicated in some form in the cytoplasmic loop connecting TM 8 and TM 9 (460). While sequence analysis of the 29 families has been extensive, visualizing the three-dimensional structure of these proteins has proven more difficult, since membrane protein hydrophobicity and carrier conformational flexibility can makes many carriers refractory to crystallography (483). To date, only four of the over 5,000 MFS superfamily members have been crystallized: the glycerol-3-phosphate transporter GlpT (353), the lactose permease LacY (3), the multi-drug transporter EmrD (641) and the fucose-proton symporter FucP (144) all from Escherichia coli. In addition, the structure of the oxalate-formate exchange protein OxlT from Oxalobacter formigenes has been visualized by cryo-electron microscopy, but not by X-ray diffraction (262). The resolved structures of these proteins provide general structural insights into the organization of MFS superfamily proteins. LacY and GlpT structures are oriented in the inward or so-called e1 conformation in which a deep, amphipathic cavity containing a bound substrate is exposed to cytoplasm (3). FucP is oriented in the outward or so-called e2 conformation in which a deep amphipathic cavity is exposed to the periplasm (144). While the lack of sequence identity between family members makes specific conclusions about the structural basis of substrate specificity challenging and subject to uncertainty, the simple carrier hypothesis suggesting that MFS proteins alternate between inward and outward conformations (288) is strongly reinforced by these observations.
SUGAR PORTERS
The first family in the MFS superfamily, the sugar porter family, is the largest with as many as 133 members identified to date (506). Sugar porters are expressed in a range of organisms from bacteria to mammals, with protein sizes ranging from 404 to 818 amino acids (506). Sugar porters are characterized by 12 transmembrane spanning domains as well as hydrophilic, intracellular N- and C-termini. Most members of the sugar porter family transport sugars, though some also transport compounds such as cations, inositols, and quinates through uniport, solute-solute antiport, or cation-solute symport mechanisms (460).
A subset of the sugar porter family called the facilitative sugar transporter family is responsible for the majority of organism-wide sugar transport in mammals. The mammalian facilitative sugar transporter (or GLUT) family of proteins, contains 14 identified members, which are members of the SLC2A (Solute Linked Carrier 2A) gene family. The proteins are named GLUT1-GLUT12, GLUT14, and HMIT1 (GLUT13) based on the order they were discovered and cloned (294, 590, 635). The GLUT proteins contain approximately 500 amino acids and share between 25–68% amino acid sequence identity with one another (578). To date, none of the GLUT proteins has been crystallized. However, using the crystal structures of the MFS protein GlpT (353), as well as the ion channel MscL from M. tuberculosis103)and the water channel aquaporin (109, 606), GLUT1 and GLUT3 virtual structures have been computed by homology modeling (168, 508). While these homology models may be generally useful for understanding overall GLUT architecture, three independent experimental approaches suggest that readers should exercise caution when inferring specific roles to specific amino acid residues in the homology-modeled structures. 1) Using the crystal structure of LacY as a template, Lemieux (354) compared the homology modeled structure of GlpT to the crystal structure of GlpT and found that although the overall architecture of the modeled structure was correct, key residues in the active site were modeled incorrectly. 2) Mueckler and colleagues have undertaken a systematic analysis of GLUT1 topology by scanning cysteine mutagenesis and find several inconsistencies with the homology modeled GLUT1 structure (440). 3) Chemical footprinting of exposed GLUT1 lysine side chains and analysis of side chain exposure to proteolytic enzymes by using mass spectrometry reveal significant differences between modeled and experimental exposures (62).
All GLUT proteins are predicted to contain 12 hydrophobic, membrane spanning, α-helical domains (TMs) connected by hydrophilic loops of varying length, with a large intracellular loop between TM domains 6 and 7 of the protein (295, 593). There is also a highly conserved 5 amino acid motif, RXGRR/K, which is located in the loop between TM domains 2 and 3 and duplicated in the loop between TM’s 8 and 9 (295, 593). This sequence is a variation of the motif found in all MFS superfamily proteins mentioned previously (255, 256), and may aid in determining proper topology during plasma membrane insertion (512). In addition, there are certain sugar transport signatures that are commonly found in all GLUTs that are thought to be essential for substrate and inhibitor selectivity and sensitivity. Among them are a PMY domain in TM4, a PESPRY/FLL domain in the large intracellular loop 6, a QQLSGIN domain in TM7 thought to aid in glucose binding, a GXXXXP motif in TM10which potentially determines inhibitor and substrate binding, a single W in TM11 which has been shown to be critical for transport function in GLUT1, and a VPETKG in the C-terminus of the protein (645). GLUT proteins contain intracellular N- and C-termini and a single glycosylation site on the exofacial side of the protein, either in the loop between TMs 1 and 2, or 9 and 10 (294, 578, 593). Sequence analysis suggests that the GLUT family may be sub-divided into three classes based on structural similarities. Class 1 GLUTs comprise GLUT1–4 and GLUT14; Class 2 GLUTs comprise GLUT5, 7, 9, and 11; and Class 3 GLUTs comprise GLUT 6, 8, 10, 12, and HMIT1. These subdivisions are based on sequence similarities between each class of proteins, and are not indicative of substrate transport capability (294). However, all of the GLUT proteins, with the exception of HMIT1 have demonstrated the capacity to transport glucose, fructose, or both sugars even if glucose and fructose are not the primary substrate for the GLUT in question (578). When expressed on Xenopus oocytes, the Class 2 and 3 GLUTs catalyze very low rates of glucose transport in comparison to their Class 1 counterparts (578). HMIT1 transports myoinositol when coupled with a proton, but does not transport glucose or fructose (232).
CLASS 1 GLUCOSE TRANSPORTERS
The class 1 transporters, GLUT1–4 and GLUT14, are the best-characterized of the group, since (with the exception of GLUT14) they were discovered relatively early and have been studied extensively. Loop 1–2 of class 1 GLUTs is longer than the other five extracellular loops, and contains a single N-linked glycosylation site. These transporters also share a QL motif in TM5, and a STSIF motif found in extracellular loop 7–8 (296). STSIF may be important in GLUT conformational changes associated with the transport cycle (159). The QL sequence is thought to specify sugar recognition motifs (520). In addition, in the cytoplasmic loop following TM10 of class 1 GLUTs, there is a tryptophan after the GXXXXP motif that is thought to confer substrate specificity as well as sensitivity to the competitive inhibitor cytochalasin B (227, 254, 296, 334). Class 1 GLUTs are typically glucose transporters, though GLUT1, GLUT3, and GLUT4 are also able to transport dehydroascorbic acid (DHA), an oxidized form of vitamin C (52, 422, 502, 601). With the exception of GLUT1, GLUT proteins demonstrate highly tissue-specific expression patterns. GLUT1, the only GLUT to be biochemically purified ex vivo (307, 651), and the first of the GLUTs to be cloned was originally cloned in liver HepG2 cells (439), but has since been shown to be expressed throughout the body, with highest expression levels in erythrocytes, cardiac muscle cells, smooth muscle, blood-tissue barrier cells, astrocytes, and in developing embryos (238, 396, 563). GLUT1 has been termed a high-affinity glucose transporter and serves to maintain basal glucose uptake throughout the body, but is critical for glucose transport across the blood-brain barrier (140, 163, 377, 461). GLUT2 expression is localized to pancreatic β-cells, the liver, the hepatic portal vein, intestine, and kidney (579). Of the class 1 GLUTs, GLUT2 has the lowest reported affinity for glucose and cytochalasin B; catalyzes fructose transport, but not transport of dehydroascorbic acid (254, 334). Originally, the low affinity displayed by GLUT2 for glucose was thought to allow it to function as a high capacity transporter, but we now know that high expression and catalytic turnover are more important factors (247). In the context of where it is expressed, GLUT2 has been proposed to act either as a glucose sensor protein or an integral component of a glucose sensing system in the intestine, liver and pancreas (334). GLUT3 is a glucose transporter primarily found in neurons, making it the major transporter responsible for neuronal glucose uptake, but is also expressed in thrombocytes, white blood cells, pre-implantation embryos, the testes, spermatozoa, and some carcinomas (227, 540). Like GLUT1, GLUT3 has been shown to transport dehydroascorbate, thus allowing neurons to potentially take up and metabolize vitamin C (493) in addition to mannose, xylose, and galactose (540). The presence of both GLUT3 and GLUT1 in the brain are critical for cerebral glucose homeostasis. GLUT4, the insulin-sensitive glucose transporter, is expressed most highly in cardiac and skeletal muscle, and adipocytes (578). Unlike the other class 1 GLUT proteins, GLUT4 is targeted to specialized intracellular pools under non-insulin stimulated conditions due to the presence of an FQQI motif in the N-terminus, a dileucine motif in the C-terminus and an endosomal targeting TELEYLGP motif in the C-terminus (530). Exposure to insulin causes a rapid, 3- to 12-fold increase in plasma membrane GLUT4 levels in muscle and fat, thereby increasing glucose uptake from the blood and lowering blood glucose. GLUT4 can also transport DHA in addition to glucose (493) and works in concert with GLUT2 to affect organismal glucose homeostasis. GLUT14, the most recently cloned class 1 transporter, is proposed to be a gene duplication (a duplicon) of GLUT3, since it shares 95% sequence identity to GLUT3 (635). Unlike GLUT3, GLUT14 is only expressed in the testes. Although the characterization of GLUT14 is not complete, it is assumed to be a glucose and DHA transporter like GLUT3.
CLASS 2 GLUCOSE TRANSPORTERS
Unlike class 1 GLUTs, the class 2 transporters (GLUT5, GLUT7, GLUT9, and GLUT11) are primarily fructose transporters, although they have been shown to transport glucose as well as other substrates, such as uric acid (28, 73, 106, 160, 362). Another notable difference that distinguishes class 2 GLUTs is the absence of the tryptophan residue the GXXXXP motif in TM10 (296). This lack of tryptophan may explain why class 2 GLUTs are insensitive to cytochalasin B and may contribute to their selectivity for fructose as opposed to glucose (296). Like their class 1 counterparts, tissue-specific expression is a hallmark of class 2 GLUT proteins. GLUT5 was the first of the class 2 GLUTs to be discovered, and is the major fructose transporter in the intestines, kidney, and spermatozoa, though it has also been shown to be expressed at the blood-brain barrier, erythrocytes, in skeletal muscle, and in fat (73, 145, 519). GLUT7 is expressed mainly in the colon and small intestine, but may also be found in the testes and prostate (106). It is possible that GLUT7 transports substrates other than sugars, but as of yet, such a substrate has not been found. GLUT7 is also hypothesized to aid in the uptake of fructose and glucose in the gut when sugar levels are low (519). GLUT9 is expressed primarily in liver, kidney, and developing embryo, but is also found to be expressed in the heart, lung, and leukocytes (28, 88). GLUT9 contains a dileucine motif in the N-terminus, but the motif does not sequester GLUT9 to intracellular pools, unlike most other GLUT family members (28). GLUT9 exists as two splice variants, dubbed GLUT9a and GLUT9b, that demonstrate differential localization in the proximal tubules of the kidney, with GLUT9a localizing to the basolateral membrane, and GLUT9b localizing to the apical membrane (28, 156). Studies show that GLUT9 plays an important role in mediating glucose uptake in the very early stages of embryonic development (88). However, the main role for GLUT9 appears to be urate transport as evidenced by genetic and functional studies (156, 413, 477). Kinetic analysis indicates that GLUT9- mediated urate transport is stimulated by the presence of glucose and fructose on the opposite side of the membrane, suggesting that GLUT9 plays a role in sugar reabsorption from the urine as well as urate clearance from the bloodstream (102). The final member of the class 2 glucose transporters, GLUT11, exists as three known splice variants, two of which have different expression patterns. The full length form (GLUT11L) is expressed in the brain, lung, leukocytes, small intestine, placenta, and liver (636), while the shorter form GLUT11S is expressed almost exclusively in the heart and muscle (160). The third splice variant encodes a severely truncated version of the protein that has yet to be characterized (636). Both the long and short forms of GLUT11 transport glucose and fructose; and unlike the other class 2 GLUTs, GLUT11 shows a low affinity for cytochalasin B (160, 636).
CLASS 3 GLUCOSE TRANSPORTERS
The class 3 GLUTs comprise GLUT6, GLUT8, GLUT10, GLUT12, and HMIT and are the least well-characterized of the GLUT proteins to date. This class of proteins differs structurally from the other two classes. Most strikingly, class 3 GLUT proteins contain a single glycosylation site on exofacial loop 9–10, as opposed to exofacial 1oop 1–2 for classes 1 and 2. Also, all class 3 GLUTs contain N- or C-terminal sequence motifs, which direct the protein to intracellular compartments (296). However, despite these differences, class 3 GLUT proteins, like class 1 GLUTs, contain the conserved post GXXXXP motif tryptophan. Class 3 GLUTs demonstrate tissue-specific expression like the other GLUT proteins. GLUT6 is prevalent in the brain, spleen and leukocytes; and preliminary analysis shows transport activity for glucose and sensitivity to cytochalasin B, with low affinity for both (157). GLUT6 also contains an N-terminal dileucine motif, which traffics the transporter to intracellular pools. Cell stimulation by a number of factors such as insulin, phorbol esters, and osmotic shock does not recruit GLUT6 to the plasma membrane (369). The physiological role of GLUT6 has yet to be determined. GLUT8, which was the first of the class 3 transporters to be cloned, is expressed at highest levels in the brain, testes, liver, spleen, adipose, and lung, and may also be expressed in muscle, heart, and kidney (276). GLUT8 is a high-affinity glucose transporter that is inhibited by cytochalasin B, fructose and galactose, which indicates both sugars as potential substrates for the transporter (158, 276). Like GLUT6, GLUT8 contains an N-terminal dileucine motif that targets the transporter to intracellular stores. In blastocysts, GLUT8 translocates to the plasma membrane in response to insulin, since GLUT4 is absent at this stage of development (87). GLUT8 recruitment to the plasma membrane has not been seen in fully developed mammals (369, 515, 618). While the function of GLUT8 requires further study, it is thought to act as an intracellular glucose transporter responsible for moving glucose between organelles (515). GLUT10 expression is localized to the heart, lung, brain, placenta, liver, kidney, pancreas, and skeletal muscle (421). Although initial characterization shows GLUT10 to be a high-affinity glucose transporter that is inhibited by cytochalasin B, but not fructose (149), the exact role of GLUT10 in mammals is unclear. Recent studies suggest that GLUT10 plays a role in vascular changes occurring in Type 2 diabetes, since mutations in the GLUT10 gene alter angiogenesis and cause arterial tortuosity syndrome (133). However, this requires more study. GLUT12 is localized to the heart, muscle, brain, placenta, pancreas, kidney, and adipose tissue (494, 495) where, having similar targeting motifs to the other class 3 GLUTs, as well as GLUT4, it localizes to intracellular stores. GLUT12 is responsive to insulin, and translocates to the plasma membrane upon insulin stimulation and hyperglycemia (549, 624). GLUT12 has been characterized as a glucose, fructose, and galactose transporter that is inhibited by cytochalasin B (494), and it is hypothesized to compensate for insulin-sensitive glucose transport when GLUT4 is knocked out in mice (308). The final member of the class 3 GLUTs, HMIT or GLUT13, is a H+-myoinositol symporter, a secondary active carrier that shows no transport affinity for hexoses even though it retains many signatures of glucose transport in its structure. While it is able to transport myoinositol in the absence of a transmembrane proton gradient, the presence of such a gradient increases the rate of myoinositol transport. HMIT is expressed in the brain, predominantly in astrocytes, but may also be expressed in neurons, adipose and kidney. HMIT is localized to intracellular stores, but translocates to the plasma membrane upon membrane depolarization and may play an important role in brain myoinositol metabolism (589, 592).
BIOCHEMISTRY AND STRUCTURE OF GLUCOSE TRANSPORT PROTEINS
CLASS 1 TRANSPORTERS
GLUT1 is a 492 amino acid protein with a calculated molecular weight of 54,117 Daltons. The sequence NQT at amino acids 45–47 in exofacial loop 1 comprises the single N-linked glycosylation sequence found in GLUT1. Glycosylation of GLUT1 is heterogeneous, causing the protein to run as a smeared band from 45–65 kDa when visualized by SDS-PAGE or Western blot (213). This smeared band collapses to a single band of approximately 38 kDa upon deglycosylation (26). GLUT1 deglycosylation causes a 50% decrease in sugar uptake and a 2.5-fold decrease in affinity for glucose, though targeting to the plasma membrane remains unaffected (26). These data suggest that the glycan plays a role in maintaining GLUT1 structure, and its affinity for substrate. The sequence GRRTLHLIGLAG, which corresponds to amino acids 332–343 in loop 8 and TM9 of GLUT1, is a Walker B nucleotide-binding domain (191, 357, 359). GLUT1 is an ATP binding protein, and nucleotide binding plays an important role in transport regulation (95, 244, 253). Truncation of the C-terminal 37 amino acids of GLUT1 is without effect on GLUT1 trafficking to the plasma membrane but ablates transport activity by eliminating the GLUT1 exofacial sugar binding site (456).
GLUT1 Secondary Structure
Approximately 60% of GLUT1’s primary structure comprises hydrophobic amino acids, and hydropathy analysis predicts twelve membrane spanning α-helical domains (442). Fourier transform infrared spectroscopy of GLUT1 proteoliposomes confirms the α-helical nature of GLUT1 (15); and circular dichroism spectroscopy analysis indicates that GLUT1 structure is approximately 82% α-helical, 10% β-turn, and 8% randomcoil structure(111). GLUT1 α-helices are perpendicular to the plasma membrane, confirming the hypothesized membrane-spanning structure of the protein (112). D-glucose increases the ordered secondary structure of purified GLUT1 as measured by circular dichroism, while cytochalasin B has no effect on structure (467). Mueckler and colleagues used the technique of scanning glycosylation mutagenesis to determine which of the hydrophilic loops that connect putative membrane-spanning domains are exposed to the lumen of the endoplasmic reticulum and are thus accessible to glycosylation by the oligosaccharyltransferase complex (270). Their findings fundamentally confirm the predicted topology of the transport. Transporter topology has also been examined by extensive chemical footprinting using mass spectrometric analysis of GLUT1 accessibility to membrane-impermeant NHS-biotin and trypsin (62). These findings also support the general topology proposed for GLUT1 which is summarized in Figure 1. Two striking findings emerged from mass spectrometry analysis of reconstituted, purified human GLUT1. TMs 1 and 8 are released from the membrane following GLUT1 trypsinization indicating that each TM is poised at the limits of membrane solubility and is constrained only by the intact polypeptide backbone. TM1 is released in the absence of substrate. The sugar transport inhibitor cytochalasin B (but not the transport substrate D-glucose) promotes TM8 release from trypsinized GLUT1 indicating that TM8 is unstable only in the GLUT1-cytochalasin B complex. GLUT1 behavior is strikingly similar to that of the α-subunit of the primary active carrier Na,K-ATPase. The Na,K-ATPase TM5-TM6 hairpin is released following trypsinolysis, but release is prevented by the pump inhibitor ouabain or by Rb occlusion (379). P1 ATPase crystal structures reveal that the TM5-TM6 hairpin forms the major cation binding site of this family of primary active carriers (436). Thus an amphipathic region of a primary active carrier undergoes conformational change upon ligand binding and is released from the carrier scaffold only in the absence of substrate.
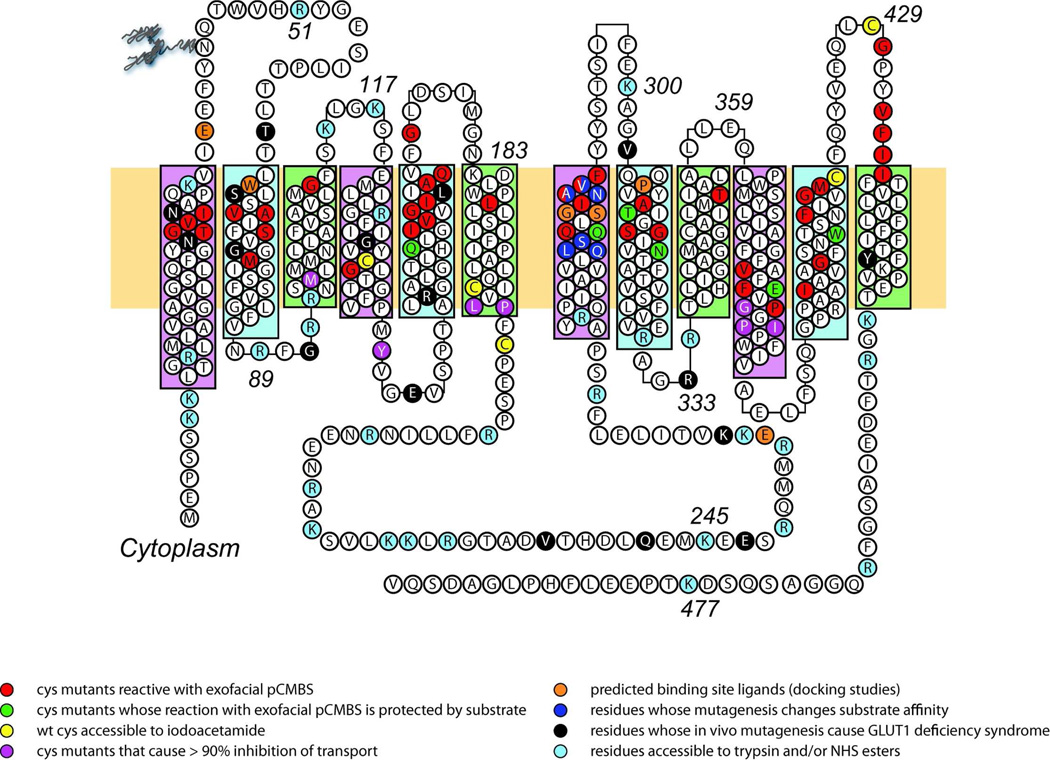
FIGURE 1. Putative GLUT1 topology and helix packing.
GLUT1 topology adapted from the GlpT homology model (508). Group 1 TMs are highlighted in pink. Group 2 and Group 3 TMs are highlighted in blue and green respectively. Some TMs extend beyond the bilayer boundaries (indicated by horizontal yellow rectangle). The bilayer-embedded region of TMs 1–12 comprise amino acids 17–39, 64 – 86, 93–112, 120 –141, 157–178, 187–207, 267–291, 305–325, 335–356, 362–385, 401–421, and 431–452, respectively. GLUT1 is glycosylated at Asn45. TMs 6 and 7 are linked by the large cytoplasmic loop (L6 –7). Amino acid residues are show using 1 letter code. The key indicates residues that are accessible to a variety of agents. The red, green and purple residues indicate residues which when mutagenized to cysteine are reactive with exofacial pCMBS, whose labeling by pCMBS is protected by substrate or whose substitution causes transport inhibition respectively (441). The yellow residues indicate those native cysteine that are accessible to alkylation by iodoacetamide (62). The orange residues are predicted to be important in ligand binding based on docking studies (141, 508). The dark blue residues are important in substrate binding as judged by mutagenesis studies (399). The black residues are known to be modified in GLUT1 deficiency syndrome (haploinsufficiency) (319). The light blue residues are accessible in native GLUT1 to trypsin and NHS-esters (62).
GLUT1 Tertiary Structure
Eight of the GLUT1 putative membrane spanning α-helices are amphipathic and have been proposed to form a water-accessible, translocation pathway that is alternately accessible to extra-and intracellular sugar (442). The accessibility of the GLUT1 translocation pathway to small water-soluble covalent probes has been examined in several ways. Mueckler's group has undertaken a painstakingly systematic cysteine scanning mutagenesis approach in which they individually substituted each residue of each putative membrane spanning domain with cysteine then asked whether transport is affected by extracellular or intracellular application of sulfhydryl-reactive molecules such as pCMBS. Mueckler and Makepeace (see (440) for a comprehensive summary of this extensive body of work) show that GLUT1 membrane spanning domains vary in accessibility to interstitial polar molecules but, for the most, part are amphipathic with a periodicity consistent with that expected of α-helices. Except for TMs 4 and 12, the examined TMs show a solvent accessibility consistent with the major facilitator superfamily helix-packing model that will be described below.
The solved crystal structures of the Major Facilitator Superfamily members GlpT and LacY (3, 353) have been used as a scaffold to predict GLUT1 tertiary structure based on homology of helical packing and secondary structure (267, 508). These models suggest that each GLUT1 exists as two symmetrical halves connected by a long cytoplasmic loop between TMs 6 and 7. TMs 1, 2, 4, 5, 7, 8, 10 and 11 form a funnel-like translocation channel or catalytic center which is oriented by an external framework comprising TMs 3, 6, 9, and 12. The long, intracellular loop between TMs 6 and 7 and the N- and C-termini of GLUT1 appear as disordered random coil structures (Figure 2). The symmetry of the three-dimensional model, combined with some of the conserved sequence symmetry in the first and second halves of the protein (i.e. the GRR/K motif in TM2 and 8), support the hypothesis that GLUT1, and indeed the GLUT family of transporters, arose as a result of a gene duplication event (392). However, it should be noted, that while the two halves of GLUT1 can associate and form a glucose-sensitive cytochalasin B binding unit when co-expressed in cells, each half of the protein is unable to accomplish this when expressed individually (130) indicating that neither half is sufficient for transporter function.
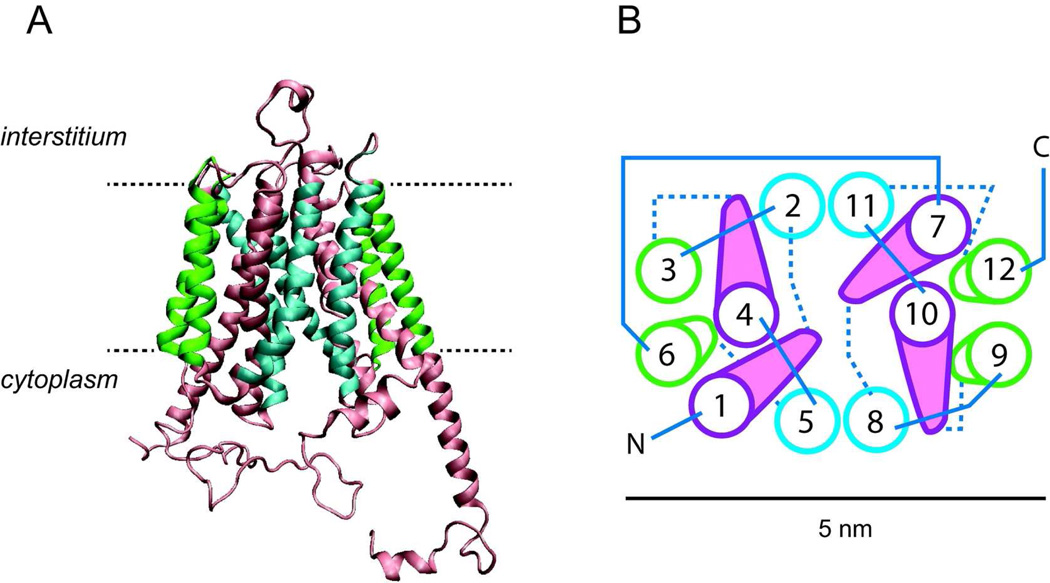
FIGURE 2. Putative GLUT1 homology modeled structure.
adapted from the GlpT homology model (508) and analyzed using the software program VMD 1.8.5 (© University of Illinois 2006). GLUT1 coordinates were obtained from the RCSB Protein Data Bank (entry No. 1SUK). A. GLUT1 viewed as a membrane spanning protein along the bilayer plane. The limits of the bilayer are indicated by the dashed lines. Membrane spanning helices (TMs) are color coded as in Figure 1. B, putative helix packing arrangement viewed from the cytoplasmic surface. TMs are numbered and colored as in 2A. Cytoplasmic and exofacial loops are indicated by solid and dashed linesrespectively. A scale bar (5 nm) is indicated.
The structures of LacY, GlpT and GlpT-homology modeled GLUT1 suggest an overall trapezoidal shape of dimensions 60 Å (intracellular domain along the membrane) by 60Å (along the membrane normal - cytoplasm to interstitium). Normal to the membrane, the transporter is oval shaped with approximate dimensions of 30Å by 60Å. The molecule contains a large, hydrophilic cavity open at the cytoplasmic side with dimensions of 25Å by 15Å suggesting that the crystal structure of LacY and GlpT captures the endofacial orientation of the transporter that presents a substrate binding site to the cytoplasm (3, 353). The recent FucP crystal structure presents an MFS transporter oriented in the outward or so-called e2 conformation exposing a deep amphipathic cavity to the interstitium/periplasm (144). This orientation substantially confirms the simple carrier hypothesis suggesting that MFS proteins alternate between inward and outward conformations (288).
GLUT1 Quaternary Structure
GLUT1 resolves as a monomer upon reducing and nonreducing SDS PAGE. Non denaturing size exclusion chromatography of detergent-solubilized, purified GLUT1 suggests that the transporter is either a monomer (378) or dimer or tetramer (245, 246, 654). Freeze fracture electron microscopy of reduced GLUT1 (transporter purified in the presence of reductant) reveals membrane particles consistent with the size of a GLUT1 dimer (215, 261). Non reduced transporter produces particle sizes consistent with a GLUT1 tetramer (215). Dynamic light scattering analysis of detergent-solubilized reduced GLUT1 suggests that some detergents (octyl glucoside, cholic acid, Triton X100) stabilize GLUT1 tetramers while others (dodecyl maltoside, CHAPS) cause tetramer dissociation (215). Chemical crosslinking studies support the hypothesis that GLUT1 forms dimers and tetramers (245) and radiation inactivation studies support the idea that human erythrocyte glucose transporter is a tetramer (298). Studies using GLUT1-GLUT4 chimerae expressed in CHO cells also suggest oligomer formation, since immunoprecipitation of GLUT1-GLUT4 chimerae with GLUT4 C-terminal antibody also pulls down parental (CHO cell-resident) GLUT1 (470). Co-expression studies in Xenopus oocytes suggest that if GLUT1 and GLUT3 form hetero-oligomers, each subunit is functionally independent of its partnering non-identical subunit (74). GLUT1/GLUT3 chimera studies have illuminated the determinants of GLUT1 oligomerization. Replacement of GLUT1 TM9 sequence with GLUT3 TM9 sequence prevents GLUT1 oligomerization with the GLUT1/GLUT3 chimera1 and conversely, replacement of GLUT3 TM9 sequence with GLUT1 TM9 sequence permits GLUT1 oligomerization with the GLUT3/GLUT1 chimera1. Only TM9 is able to function in this way in GLUT1 - GLUT3 association experiments1. Heterologously expressed GLUT1 mutants influence the activity of parental GLUT1 in HEK or Cos cells (358, 360) suggesting that GLUT1 oligomeric complexes comprise functionally interacting subunits.
Class 2 and class 3 transporters have not yet been subjected to extensive biochemical analysis.
TRANSPORT STEREOSPECIFICITY
While the class 2 and 3 GLUTs have not been subjected to biochemical analysis, the substrate preferences and pharmacologic sensitivity of the class 1 and 2 transporters have revealed insights into the determinants of GLUT specificity (399).
Functional characterization ofGLUTs 1–5 reveals that only GLUT2 and 5 transport glucose and fructose (73, 122)but thatGLUT5 has only a limited capacity for glucose (309). GLUT2 transports glucosamine and 2-deoxy-D-glucose with equal facility suggestingthat the C2 position is unimportant in hydrogen bonding(591). The other class II GLUTs (73, 309, 362, 398)also transport glucose as well as fructose, and do so with high affinity (398). None of these transportersrecognize 2-deoxy-D-glucose as a substrate, which may explain why earlier expression cloning strategies employing 2-deoxy-D-glucose failed to identify these transporters. Naturally occurring and engineered GLUT1 point mutations suggestthathelix 7 containsseveral residues that are important for transport function. GLUTs 1, 3, and 4, which transport glucose but not fructose, have the QLS sequence in helix 7, whereas GLUTs 2 and 5, which both transport fructose, have HVA or MGG, respectively(399). GLUT2/GLUT3 chimeras, which contain GLUT2 sequence from the beginning of helix 7 to the COOH terminus and N-terminal GLUT3 sequence behave like a glucose/fructose transporter with GLUT2 kinetics (high capacity/low affinity)(23). GLUT2 mutations in which HVA is replaced with QLS and GLUT3 mutations carrying the reverse QLS to HVA insertion are characterized by partially reversed kinetics and substrate selectivities (520).
Comparison of the ability of hexose analogs to act as class 1 GLUT transport substrates or inhibitors indicates that substrate binding involves hydrogen bonding between protein and hexose. In GLUTs 1, 3, and 4, the hydroxyls on C1 and C2 of the hexose ring are critical for binding and transport, whereas binding to C4 is possible but not essential (123). Analysis of GLUTs 1–4 suggests that when substrate enters the exofacial binding site, hydrophobic interactions between the methylene group on C6 of the substrate and part of the pore lining contribute to the affinity of substrate binding (40, 123).
GLUT2 and GLUT5 bind and transportfructose, which isomerizes between furanose (30%) and pyranose (70%) forms in aqueous solution(123). GLUT2 recognizes the furanose form of fructose, allowing alignment with the same residues within the binding pocket as for the pyranose structures. Thus C2 and C3 of the furanose ring form the hydrogen bonds, whereas C6 provides the hydrophobic interaction. GLUT2 does not form the conventional hydrogen bond in which the protein serves as the proton donor to the hydroxyl on C3 (glucose) or C2 (fructose). Rather, the proton is donated by the sugar (122). GLUT5recognizes fructose in both furanose and pyranose conformations and binding involves interactions with C1, C2, C3, and C4 positions of the hexose (305). In all cases, the hexose enters the exofacial binding site leading with C1.
The stereospecificity of the endofacial binding site is somewhat different with interactions between protein and hexose C6 becoming more important. Substitution studies (exclusively undertaken using GLUT1) indicate that the C1 position of any hexose complexed with the endofacial binding site, faces the cytoplasm (39). Thus, the orientation of the hexose appears to remains unaltered during the transport.
TRANSPORT KINETICS
The glucose transporters are termed uniporters because they catalyze sugar uptake in the absence of intracellular sugar and catalyze sugar export in the absence of extracellular sugar. Transporters that only able to import one substrate in exchange for export of a second substrate are called antiporters or exchange-only transporters. The GLUTs are capable of both uniport and antiport. Symport describes simultaneous uptake (or exit) of two different molecular species in which the net downhill flow of one substrate drives the net uphill flow of a second substrate. HMIT (a Class 3 transporter) is a H+myoinositol symporter.
CLASS 1 TRANSPORTERS
Sugar transport catalyzed by GLUTs 1, 2 and 4 has been extensively characterized in isolated erythrocytes, hepatocytes and adipocytes. GLUT3 and GLUT5-mediated transport has been examined in rather less detail using heterologous expression systems or in primary cell cultures. This section summarizes our current understanding of the kinetics of sugar transport and transporter interactions with inhibitors.
GLUT1
GLUT1-mediated sugar transport has been most extensively studied in human erythrocytes where uniform cell size has permitted a level of analysis rarely possible in other cell types (547). As described in the preceding section, GLUT1 prefers hexose and pentose sugars that adopt the pyranose form (39, 40) and while the impact of substitutions at C1 on sugar uptake may vary depending on whether they adopt the α-(the substituent lies below C1) or β-configurations (the substituent is co-axial with C1) (40), GLUT1 recognizes α-and β-D-glucose equally (97, 351). L-Glucose is neither transported nor bound by GLUT1 and while the disaccharide maltose is bound by GLUT1 at exofacial and endofacial sugar binding sites, its size precludes transport (251, 547).
2-Deoxy-D-glucose (2–DOG) is transported by GLUT1 and, upon entering the cell, is phosphorylated by hexokinase to form 2-deoxy-D-glucose-6-phosphate. Phosphoglucose isomerase is unable to utilize 2-deoxy-D-glucose-6-phosphate (30) and, since 2-deoxy-D-glucose-6-phosphate is not transported by GLUT1, it becomes trapped and accumulates within the cell. Thus, when transport rate-limiting for 2-DOG uptake and metabolism, measurement of 2-deoxy-D-glucose-6-phosphate accumulation is an accurate reflection of the rate of 2-DOG transport. In contrast, 3-O-methyl-D-glucose is a sugar analog that is transported by GLUT1 but is not phosphorylated by hexokinase (289). In human red cells where metabolic rates are some 3 orders of magnitude slower than transport rates (283), it is possible to measure the kinetics of D-glucose, 2-deoxy-D-glucose and 3-O-methylglucose transport without fear of secondary complications arising from the metabolism of the sugars. In other cells, D-glucose and 2-deoxy- D-glucose are more rapidly metabolized thus unambiguous transport determinations require the use of 3-O-methylglucose.
GLUT1 transport activity is inhibited by a number of molecules such as cytochalasin B, phloretin, forskolin, and maltose. Cytochalasin B and forskolin appear to inhibit transport by binding with high affinity to the endofacial sugar binding site (or to a site whose occupancy is mutually exclusive with the endofacial sugar binding site), while maltose binds to the exofacial sugar binding site with 100 - 1,000-fold lower affinity (43, 58, 524). Phloretin may act at both endo- and exofacial substrate binding sites (43).
A series of ingenious transport measurements have been developed in order to eliminate ambiguities in data interpretation. These are summarized in three important reviews (446, 547, 617) and are:
Zero Trans experiments in which sugar transport is measured in the direction cis to trans under conditions where the concentration of starting cis sugar is varied and the starting concentration of sugar at the opposite or trans-side of the membrane is zero. In a zero-trans uptake experiment, the cis-side is the interstitium, the trans-side is the cytoplasm and the procedure measures Km and Vmax for sugar uptake. Zero-trans exit measures Km and Vmax for sugar efflux.
Equilibrium Exchange experiments in which intracellular [sugar] = extracellular [sugar] and unidirectional sugar movements are measured using radiolabeled sugars. These experiments therefore measure Vmax and Km for equilibrium exchange sugar uptake and efflux.
Infinite-cis experiments in which sugar flux is measured in the direction cis to trans, the starting concentration of cis sugar is saturating and the starting concentration of trans sugar is varied from zero to saturating. Thus infinite cis exit measures Vmax for zero-trans exit and Km for uptake into cells containing saturating sugar levels. Infinite cis entry measures Vmax for zero-trans uptake and Km for exit into medium containing saturating sugar levels.
Infinite-trans experiments in which sugar flux is measured in the direction cis to trans, the starting concentration of cis sugar is varied and the starting concentration of trans sugar is saturating. Thus infinite trans exit measures Vmax for equilibrium-exchange exit and Km for exit into medium containing saturating sugar levels. Infinite trans entry measures Vmax for equilibrium exchange uptake and Km for uptake into cells containing saturating sugar levels.
The combination of these assays has been used to define the kinetics of GLUT-mediated transport in a variety of systems, with the most detailed analysis of GLUT1 kinetics having been performed in the red blood cell.
Kinetics of GLUT1 Transport: Transport mechanism
Two fundamentally different models have been proposed for the facilitated diffusion of sugars. The simple carrier describes a transport mechanism, which alternately presents an endofacial or an exofacial sugar binding site (288, 331, 363, 616). In the absence of bound sugar, the transporter undergoes reversible conformational changes between endofacial and exofacial orientations, which in the absence of bound sugar are termed "relaxation". When sugar binds to endofacial or exofacial orientations, the carrier reversibly reorients between endo- and exofacial conformations but this may now proceed at a different rate (more rapidly or more slowly) and is now called "translocation" because the bound sugar is carried along as cargo. Assuming that this transport model is correct, it is not entirely clear whether translocation and relaxation represent physically similar conformational changes. With GLUT1, the Gibbs free energy of activation for relaxation at ice-temperature is some 3 to 6-fold greater than for translocation indicating that relaxation follows a very different reaction pathway than translocation (605). These differences become less marked at physiologic temperature. With GLUT4, relaxation and translocation proceed at similar rates at all temperatures studied (572, 583) indicating that substrate interaction with GLUT4 may not introduce alternative reaction pathways.
The two-site or fixed site carrier (31, 33, 446, 447) describes a transporter that simultaneously presents exofacial and endofacial sugar binding sites. In order for exchange transport to proceed, the transporter must permit sugars that are initially bound at exofacial and endofacial sites to simultaneously translocate in opposite directions. This could occur through a central, water-filled cavity or, as we shall see below, via individual transport pathways.
Lieb and Stein (365) developed a very useful mathematical description of simple carrier kinetics which contains 4 experimentally determinable constants. This model can also be adapted to fixed-site carriers although interpretation of the constants is model-specific (94). This means that when a carrier's steady-state behavior is compatible with the simple carrier model, it must also be compatible with the fixed-site carrier model. However, while the fixed site transporter predicts steady-state transport behavior that is indistinguishable from the simple carrier, it also allows for more complex behaviors (94). Thus compatibility with the simple carrier does not exclude an alterative explanation. Regardless of these subtleties, experimental analysis confirms that the steady-state sugar transport behavior of human red blood cells is incompatible with both models.
Analysis of zero-trans and equilibrium exchange transport data permits computation of all 4 independent transport parameters (94, 365). This has been accomplished for D-glucose transport at 4°C to 37°C and for 3-O-methylglucose transport at 4°C (32, 93, 98, 117, 232, 306, 356, 376, 428–430, 617). Human red cell sugar transport is asymmetric. Vmax and Km for sugar export at 20°C are 4 to 5-fold greater than Vmax and Km for zero-trans sugar uptake (32, 376). Human red cell sugar transport also shows trans-acceleration in which Vmax and Km for equilibrium exchange import and export are identical but greater than the equivalent parameters for zerotrans exit and entry (32, 376). Asymmetry and trans-acceleration are amplified as temperature falls and diminish (but persist) as temperature increases towards 37°C (376, 603).
The equilibrium relationship required of a passive transport system is not contravened by asymmetry (617) because the ratio Vmax/Km is identical for zero-trans uptake, zero-trans exit and equilibrium exchange (94, 376). However, the use of the resultant transport constants to compute Km for infinite-cis uptake invariably predicts a value that is 5 to 10-fold greater than the Km that is experimentally determined (32, 94, 117, 205, 364). This is observed for glucose, galactose and 3-O-methylglucose transport thus the result is independent of the transported substrate. Only two interpretations are possible: transport determinations are either technically flawed or their results are incompatible with both models.
It should be emphasized that kinetic analysis does not inform about physical mechanism. However, if a putative physical model predicts a specific kinetic behavior, which is not observed experimentally, the putative model must be wrong. Indeed, a putative physical model for transport is only useful if it predicts both biochemical and kinetic behavior.
While the critical infinite-cis experiment describes transport under non-physiological conditions (saturating sugar levels at one side of the membrane with varying levels at the other side), it is a well-conceived experimental test, which through its very design, prevents data misinterpretation resulting from poor experimental design(547). Rather like voltage clamp experiments that take membrane potential and channels to non-physiologicpotentials, the infinite-cis experiment reveals important insights into transporter function. The infinite-cis test yields experimental results that support the simple carrier hypothesis for uridine transport in red cells (83) and for GLUT4-mediated glucose transport in adipose(572). This establishes its utility and argues against specific technical problems in its application to red cell transport systems or to glucose transport systems in general. However, the same test refutes the simple and fixed-site carrier hypotheses for choline transport in red cells (547) and its failure to produce findings that conform to expectations of a simple carrier or a fixed-site carrier for GLUT1-mediated sugar transport demonstrates that red cell GLUT1 does not function as these models describe.
If steady-state transport data are unrevealing with respect simultaneous or mutually exclusive endo- and exofacial sugar binding sites, the use of sided inhibitors has been more successful. Krupka and Devés (331) developed an insightful strategy based on measurements of sugar transport in the presence of transport inhibitor pairs where one inhibitor is a competitive inhibitor of sugar uptake and the other is a competitive inhibitor of sugar efflux. This approach can be used to determine whether such inhibitors bind to the transporter simultaneously or whether their binding is mutually exclusive. The first experiments performed using this approach are consistent with mutually exclusive endo- and exofacial sugar binding sites (331) but with one proviso - the inhibitor pair phloretin and cytochalasin B do not show negative cooperativity if binding is mediated by a fixed site carrier. These experiments were later repeated using maltose and cytochalasin B as the inhibitor pair (phloretin behavior is more complex than is expected of a simple exofacial inhibitor (43)) and the results are more in keeping with the predictions of the fixed site carrier (96). Other experiments also reveal that cytochalasin B and phloretin binding to GLUT1 display a type of negative cooperativity that might be expected of a two-site carrier (251).
There is a problem, however. Earlier studies with purified human erythrocyte GLUT1 clearly demonstrate that GLUT1 ligand binding is consistent with the simple carrier - endofacial and exofacial ligand binding sites are mutually exclusive (211, 212, 261). Each transport protein can bind 1 molecule of cytochalasin B (an endofacial site ligand) and when saturated with cytochalasin B, the carrier does not expose an exofacial binding site. These preparations of purified GLUT1 were isolated in the presence of reductant. GLUT1 isolated in the absence of reductant exposes only 1 cytochalasin B binding site per 2 molecules of GLUT1 and behaves like a fixed-site carrier (92, 120, 245, 246, 654). Both observations were reconciled when it was discovered that nonreduced GLUT1 is a cooperative homotetramer in which two subunits must present cytochalasin B (endofacial) sites and two subunits must present maltose (exofacial) binding sites at any instant (98). This was reinforced by the observation that red cell resident GLUT1 forms a complex that presents at least two exofacial maltose binding sites and two endofacial cytochalasin B binding sites (118, 229) and that cooperative behavior and tetrameric structure are lost by treatment with reductant, by mutagenesis of GLUT1 cysteine residues 347 and 421 to serine (229, 654) or by exofacial trypsinization (120). Dimeric GLUT1 behaves as if it comprises two structurally associated but functionally independent GLUT1 subunits (654). As with aquaporin (172), dimeric and tetrameric anion exchange transporter (99), and the Na+K+ATPase (339) the membrane complex is a multimer of subunits in which each subunit provides an individual transport pathway. Studies from this laboratory (361) indicate that the oligomerization interface between GLUT1 subunits is provided by TM9 in a manner similar to the dimerization of glycophorin A (503).
Interpretation of ligand binding studies has frequently made the simplifying assumption that exofacial and endofacial sugar binding sites correspond to exofacial and endofacial inhibitor binding sites. This may not be true (although they may be mutually exclusive) and final resolution of this interesting problem must await GLUT1 crystallization in the presence and absence of reductant and trans inhibitor pairs.
One further aspect of erythrocyte sugar transport deserves mention. Human erythrocyte sugar transport is a multiphasic process. At all concentrations of 3-O-methylglucose, transport appears to be biphasic with fast and slow components of uptake (59, 60, 119, 243, 350, 351). Quench-flow analysis at very short time points reveals the presence of a third, rapid phase of transport, which corresponds to glucose binding to GLUT1 (59) and this sugar can be occluded within the transporter. All three phases are protein-mediated, though the physical explanation for complexity (initially and incorrectly thought to represent sugar binding at intracellular sites (244) or differential transport of α- and β-sugar (350)) is probably intrinsic to transport kinetics (351, 447). Similar behavior is observed with GLUT1-mediated sugar transport in pre-erythroid K562 cells (119).
GLUT2 and GLUT4
3-O-Methylglucose transport in rat hepatocytes is mediated by GLUT2 (579) and displays transport symmetry and no trans-acceleration (135). Km for 3-O-methylglucose zero-trans uptake at 20 °C is some 4 - 5-fold greater than for zero-trans uptake by erythrocytes but Km for zero-trans exit and equilibrium exchange are similar to those observed with GLUT1. The capacity for 3-O-methyglucose transport in hepatocytes (Vmax) approaches that of human red cells. These behaviors are compatible with simple- and fixed site carrier models for sugar transport (94).
GLUT4-mediated sugar transport has been examined in rat adipocytes before and after insulin stimulation of transport. Zero-trans sugar transport is symmetric (571) and equilibrium exchange transport does not show trans-acceleration (583). While Km for uptake and exit are unaffected by insulin, Vmax for zero-trans and equilibrium exchange transport are increased 17- fold (583). These behaviors are compatible with simple- and fixed site carrier models for sugar transport (94).
GLUT3
Studies of GLUT3 in rat cerebellar granule neurons (389) demonstrate that GLUT3 kcat (the number of transport cycles catalyzed by a single GLUT3 molecule) is 6,500/s at 37°C. This is some 5 - 6-fold greater than, kcat for GLUT1 in human erythrocytes, 3T3-L1 adipocytes or Xenopus oocytes (1,200/s), or GLUT4 in 3T3-L1 adipocytes or oocytes (1,300/s) (376, 453, 459). GLUT3 displays higher affinity for glucose than does GLUT1 or GLUT4 (399). Beyond these descriptions of high affinity, high capacity transport, insufficient data exist to characterize the kinetic mechanism of GLUT3-mediated sugar transport.
CLASS 2 AND 3 TRANSPORTERS
Insufficient experimental data are available to draw conclusions regarding transport mechanisms for Class 2 and 3 transporters.
TRANSPORT REGULATION
Glucose uptake is rate-limiting for utilization in cells where glucose transport capacity is low relative to rates of sugar metabolism (e.g. smooth, striated and cardiac muscle, adipose, nucleated erythrocytes and certain cultured cell lines). Net sugar uptake in these cells is rapidly (seconds to minutes) stimulated when glucose utilization is increased (129, 242, 264, 265, 529). GLUT1-mediated sugar transport in nucleated erythrocytes and cultured macrophages and GLUT4-mediated transport in muscle are stimulated 3- to 50-fold by cellular metabolic depletion (153, 242, 265, 529) while insulin produces an 8- to 40-fold stimulation of sugar transport in muscle and fat (537, 571, 583, 627). Some cells respond acutely to stimuli by increasing cell surface sugar transport content (537) while others respond with increased GLUT intrinsic activity (153, 529).
Glucose transport in cardiomyocytes, smooth muscle and astrocytes is rate-limiting for utilization. Transport regulation in these cells is necessary for metabolic homeostasis. Erythrocytes and endothelial cells, however, transport glucose some 50- to 500-fold more rapidly than they utilize sugar (203, 284). In spite of this, red cells respond to ATP depletion with 4- to 10-fold increased glucose uptake (153), endothelial cells respond to acute hypoglycemia with increased Vmax for net sugar uptake (142) and respond to chronic hypoglycemia or hypoxia with increased GLUT1 expression (194, 373, 396) resulting in enhanced (1.5 to 2-fold) glucose uptake (558). Why regulate transporter activity or content in cells where glucose supply exceeds demand? One reason may be that transport in these cells subserves glucose transfer to other tissues where demand for glucose is greater. In the brain, for example, glucose utilization by astrocytes and neurons requires glucose transport across the blood brain barrier. This barrier comprises endothelial cells that constitute only 0.1% of the mass of the brain (235) but which nevertheless transport sugar to the much greater cell mass.
FACTORS INFLUENCING GLUT ACTIVITY
Endothelial glucose transport in vivo responds acutely to neuronal stimulation with increased Vmax for uptake (132) and GLUT1-mediated transport in hippocampal astrocytes is instantly stimulated upon exposure to glutamate – an agent released by some neurons during stimulation (372). These observations suggest that local humoral factors acutely influence GLUT1-mediated sugar transport in endothelial cells and astrocytes.
Endothelial cell, astrocyte and human red cell GLUT1 are phenotypically homologous (558). Human GLUT1 (red cell-resident or heterologously expressed in CHO cells and HEK cells) responds acutely to cellular ATP-depletion with enhanced glucose import capacity (98, 244, 283, 299, 350, 358, 360, 569, 610). ATP reduces Km and Vmax for net sugar import, Vmax for net sugar exit and Km for exchange transport in human red cells (116, 253). This transport modulation reflects a direct action of ATP on GLUT1 (95), is competitively inhibited by H+AMP and ADP but does not involve ATP hydrolysis (244). At ice temperature (4°C) where transport in human red cells is easier to measure, transport asymmetry is 10-fold (376). ATP depletion results in the loss of transport asymmetry and stimulates Vmax for sugar uptake by 10- fold (253). At physiological temperature, transport asymmetry is 1.4 to 2-fold (376) thus loss of transport asymmetry would result in a 1.4 to 2-fold stimulation of transport.
ATP-modulation of GLUT1 structure has been examined by analysis of GLUT1 susceptibility to proteolysis; GLUT1 susceptibility to covalent modification by lysine-reactive water-soluble molecules and GLUT1 accessibility to peptide directed IgGs. All 3 approaches show that GLUT1 is a dynamic structure that is acutely modulated by ATP (Figure 3).
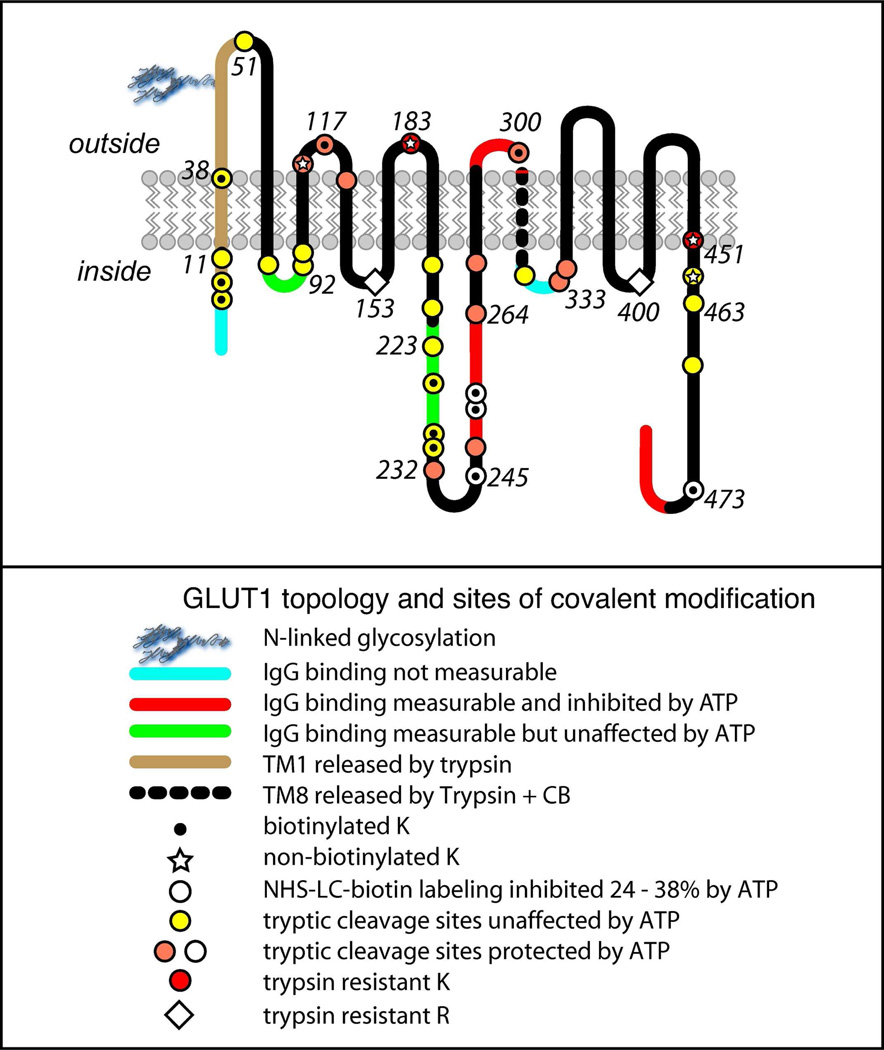
FIGURE 3. Biochemical analysis of GLUT1 topography.
Membrane-resident GLUT1 was digested with trypsin or, following labeling with NHS-LC-biotin by α-chymotrypsin and then analyzed by reverse phase HPLC-ESI-MS/MS(62). Peptides containing the indicated cleavage sites were positively identified by MS/MS. The 12 TMs are indicated in schematic form relative to the lipid bilayer. The key indicates accessible and inaccessible residues and how accessibility is modified when GLUT1 is complexed with ATP (61). Trypsin cuts GLUT1 at the C-terminal side of accessible lysine (K) and arginine (R) residues. The figure also shows whether peptide directed IgGs interact with bilayer resident GLUT1 and how that interaction is modified in the presence of ATP (61).
Purified GLUT1 proteoliposomes are unsealed and expose both membrane surfaces to ligands, IgGs and proteases (22, 552). GLUT1 proteolysis by trypsin proceeds rapidly at 37°C, is protected by ATP (but not GTP) in a dose-dependent manner (61)and involves protection of GLUT1 N-terminal, loop 6 and C-terminal domains. ATP addition to GLUT1 proteoliposomes inhibits the extent of NHS-biotin incorporation into GLUT1 by 50%. Fourteen out of a total of 16 GLUT1 lysine residues are biotinylated and ATP reduces biotinylation at lysine residues 245, 255, 256 and 477 (61). Residues 245, 255 and 256 fall within the C-terminal half of loop 6 while lysine 477 is located in the GLUT1 Carboxy terminus. ATP inhibits binding of C-terminus directed IgGs to GLUT1 (61, 95) but does not affect binding of loop 2-directed IgGs or IgGs directed against an N-terminal region of loop 6. Lysine-biotinylation in this region of loop 6 is not protected by ATP. Binding of loop 7- and C-terminus -directed IgGs is inhibited by ATP. IgG binding to loop 8 (a putative ATP binding domain(357, 358)) is undetectable in GLUT1 proteoliposomes (61). GLUT1 domains involved in ATP regulation of GLUT1 and a potential mechanism for GLUT1 regulation are summarized in Figure 4.
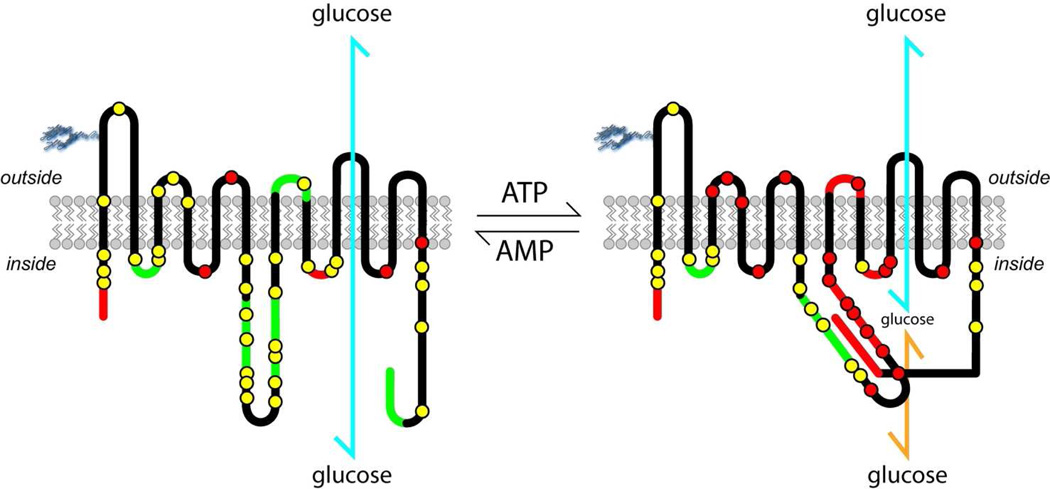
FIGURE 4. Model for ATP regulation of GLUT1.
GLUT1 experimentally-determined membrane-spanning topography (61, 62) is illustrated. The leftmost topography summarizes observations in the presence of AMP. Accessible tryptic cleavage sites (lysine or arginine residues) or sites of biotinylation (lysine residues) are shown as yellow circles (please refer to Figure 3 for specific details). Inaccessible tryptic cleavage sites (lysine or arginine residues) and inaccessible biotinylation (lysine) sites are shown as red circles. GLUT1 sequence that is inaccessible to peptide-directed antibodies is shown in red while sequence that is accessible to peptide-directed antibodies is shown in green. When ATP binds to GLUT1 (rightmost topography), a significant GLUT1 conformational change takes place rendering more sequence inaccessible to peptide-directed antibodies and making specific lysine and arginine residues less accessible to trypsin and lysine residues less accessible to biotinylating probes. This conformational change is proposed to restrict glucose release (yellow arrow) from the translocation pathway (blue arrow).
Thus lysine-biotinylation and IgG binding data show that exofacial loop 7, the cytoplasmic Carboxy terminus and a C-terminal portion of cytoplasmic loop 6 undergo significant but localized conformational changes in the presence of ATP. Alanine scanning mutagenesis of a GLUT1 putative nucleotide binding domain (cytoplasmic loop 8, which connects TM8 and TM9; (357–359)) reveals 2 critical residues - Glu329 and Arg333 (or the adjacent Arg334), which when substituted with alanine result in the loss of ATP-modulation of transport. When expressed in Cos-7 cells, the GLUT1E329A mutant exerts a dominant-negative effect on Cos cell endogenous GLUT1 which, together with the mutant, loses the ability to respond to reduced cytoplasmic ATP levels with low affinity sugar transport (358). Thus specific residues within cytoplasmic loop 8 contribute to ATP-binding or to transduction of the effects of ATP-binding.
FACTORS ACUTELY INFLUENCING CELL SURFACE GLUT EXPRESSION
GLUT4 recycling between plasma membrane and intracellular membrane compartments in adipose and skeletal muscle has been reviewed extensively in (69, 104, 304, 328, 424, 458, 537, 553). When muscle or adipose are acutely stimulated by insulin, metabolic stress or by osmotic stress, cellular glucose transport is rapidly stimulated by reversible recruitment of intracellular GLUT4 proteins to the cell membrane.
The cellular location of GLUT4 is governed by a tightly controlled recycling mechanism, whereby GLUT4 endocytosis, sorting into specialized vesicles, exocytosis, tethering, docking, and fusion are highly regulated. When insulin is absent, adipocyte GLUT4may be actively sequestered away from the general pool of recycling endosomes into GLUT4-specialized compartments, thereby reducing the amount of GLUT4 at the plasma membrane(297). Targeting to this specialized compartment may involve GLUT4 ubiquitination (337). In muscle, cell surface GLUT4 in the basal state appears to be very rapidly internalized and internalization is inhibited upon exposure to insulin (180). Insulin stimulates the net translocation of a portion of intracellular GLUT4 to the plasma membrane. This involves the microtubule network and actin cytoskeleton, which act either to coordinate regulatory signaling components or to direct vesicle trafficking from the perinuclear region of the cell to the plasma membrane (500). Once at the plasma membrane, GLUT4 vesicles dock and fuse with the cell membrane, allowing for GLUT4 membrane insertion (104).
The insulin response is mediated by specialized insulin-responsive vesicleswhose protein composition consists primarily of GLUT4, IRAP, sortilin, LRP1 and v-SNAREs(304).Insulin receptor activation involves receptor autophosphorylation which leads to tyrosine phosphorylation of intracellular substrates. Two signaling pathways are required for insulin dependent GLUT4 translocation in fat and muscle cells. Tyrosine phosphorylation of the IRS proteins after insulin stimulation leads to an interaction with and activation of wortmannin-inhibitable phosphatidylinositol 3-kinase. These kinases then initiate a cascade of phosphorylation events, resulting inGLUT4 translocation. A separate pool of the insulin receptor may also phosphorylate substrates, which interact with the lipid raft protein flotillin. This interaction recruits phosphorylated substrate into the lipid raft which in turn initiates a cascade of phosphorylation events, resulting inGLUT4 translocation.
Metabolic stress also stimulates sugar transport in cardiac and skeletal muscle and in adipose by recruitment of intracellular GLUT4 to the cell membrane but exploits a different signaling pathway. The insulin dependent pathway involves the wortmannin-sensitive phosphatidylinositol 3-kinase pathway. The metabolic response pathway is the wortmannin-insensitive AMP-activated protein kinase (AMPK) pathway(56, 437, 638). In muscle, AMPK phosphorylation is increased by contraction and by oxidative metabolic stress (e.g. hypoxia or treatment with oligomycin) and is associated with stimulated glucose transport activity. The rate of GLUT4 exocytosis is rapidly stimulated by insulin, but insulin does not alter the rate of endocytosis (638). Like insulin, muscle contraction stimulates GLUT4 exocytosis but does not affect endocytosis. By contrast, metabolic stress is without effect on GLUT4 exocytosis but reduces GLUT4 endocytosis(638).
The sugar transport capacity of other cell types is also regulated by the reversible recruitment of intracellular sugar transporters to the plasma membrane. Cerebral microvasculature endothelial cell sugar transport is stimulated during metabolic stress by AMPK-dependent , reversible recruitment of intracellular GLUT1 to the cell membrane (142). Thrombin causes a rapid and pronounced platelet shape change, secretion of most α-granules and a concomitant 3-fold increase in glucose transport and cell surface GLUT3 expression by mobilizing intracellular GLUT3 from α-granules to the cell surface (248).
GLUCOSEPHYSIOLOGY
While glucose is not an essential nutrient (some individuals subsist on a high-fat and protein diets because they cannot tolerate dietary glucose and galactose), glucose does assume a central role in mammalian energy metabolism serving as a preferred metabolic substrate in brain and exercising skeletal muscle. It is unsurprising, therefore, that mammalia maintain blood glucose within narrow limits (4–12 mM) in spite of continuously variable carbohydrate ingestion and elimination (metabolism and excretion; (633
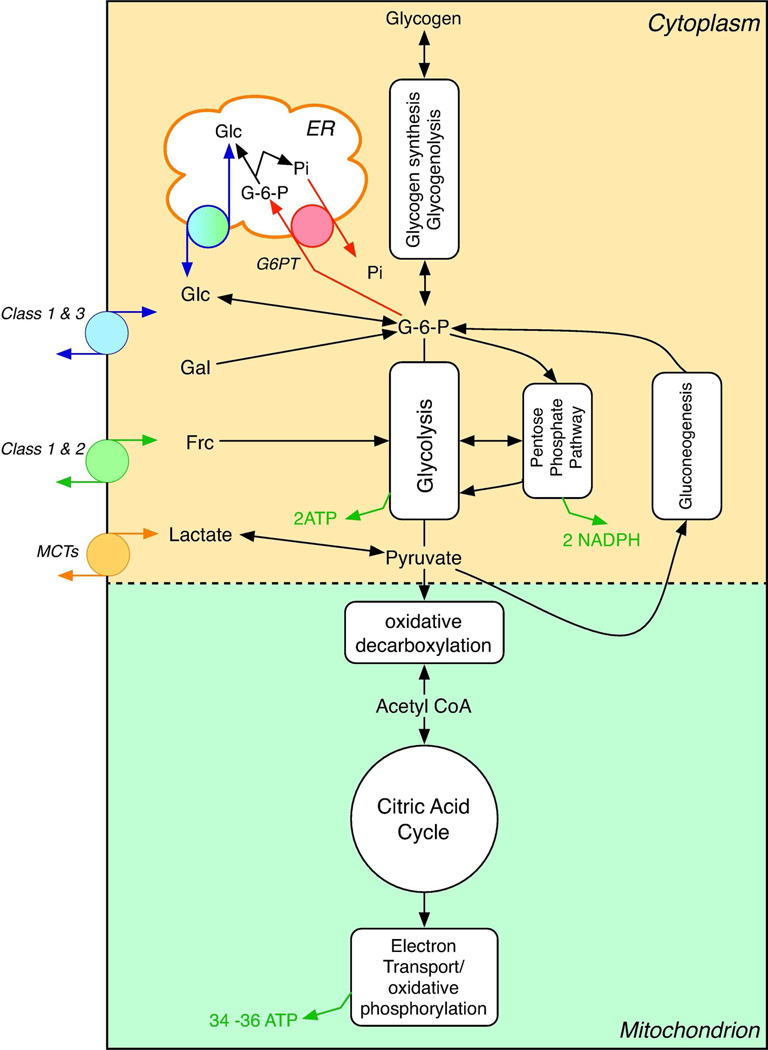
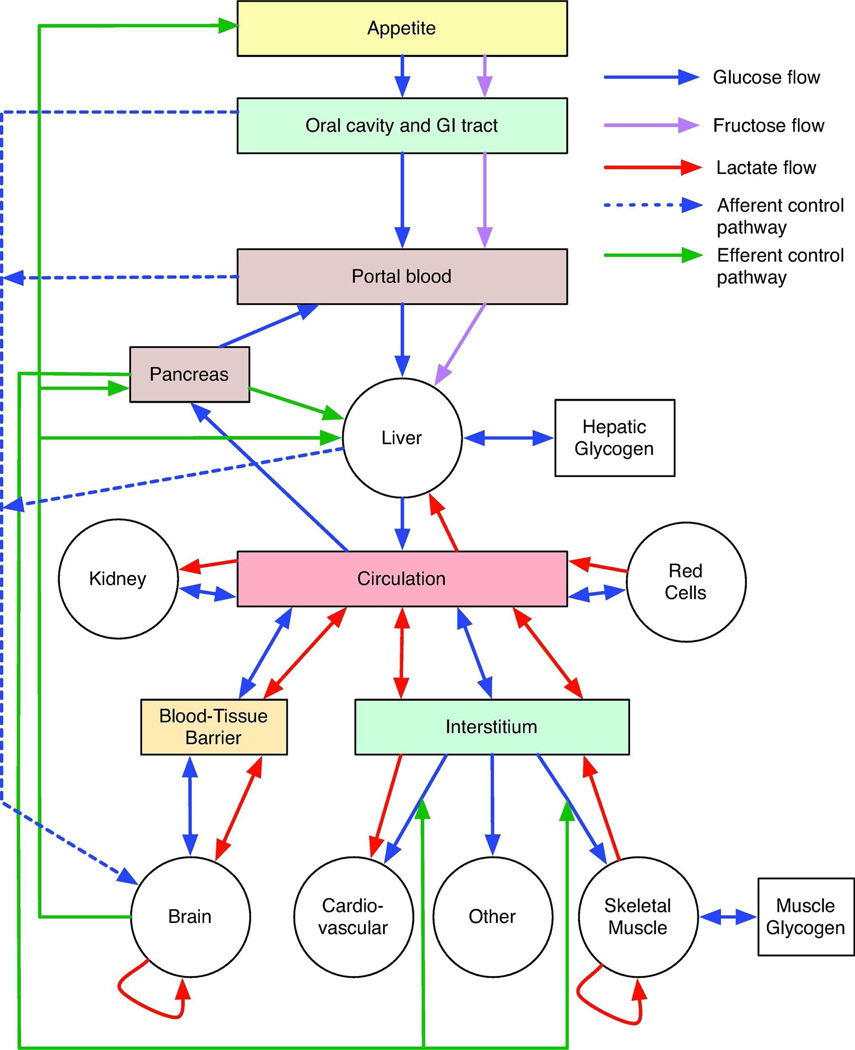
The daily glucose requirement of a typical adult human being depends on activity levels and ranges from 160–260 g (489). Of this, 120 g is used to fuel brain metabolism, which accounts for 50 to 75% of total glucose consumption. The amount of glucose present in body fluids is about 20 g, and that readily available from glycogen, a storage form of glucose, is approximately 190 g. Thus, available glucose reserves are sufficient to meet glucose needs for about a day. Glucose stores (glycogen) are typically replenished by carbohydrate ingestion (as much as 300 grams/day) from which the average adult is able to absorb 125 grams of glucose daily from the gut. Acute and chronic shortfalls between glucose absorption and glucose utilization must be bridged and this is accomplished through the breakdown of glycogen stores (glycogenolysis) and by de novo glucose synthesis (gluconeogenesis).
Glycogen synthesis, glycogenolysis and gluconeogensis are active hepatic functions. Following a meal when serum glucose and insulin levels are elevated, the liver imports glucose where it is converted to glycogen and stored for later use. When serum glucose and insulin fall to normal levels, glucose is produced by the liver through glycogenolysis or by gluconeogenesis (conversion of lactate to glucose). While glycogen synthesis and breakdown occur in many tissues, only glucose released via hepatic glycogenolysis can be made accessible to other tissues. During the 8–12 hours following restoration of serum glucose to pre-prandial levels (4–6 mM), liver glycogen-derived glucose (40–80 g/day; (472)) becomes a primary source of blood glucose.
Gluconeogenesis is fueled by lactate produced during glucose metabolism in erythrocytes and skeletal muscle but may also be fueled by amino acids and glycerol. Adult human gluconeogenesis averages 180 g/day of which 73% occurs in the liver and 27 % in the renal cortex (202). Significant redundancy/adaptability is built into human gluconeogenic capacity since the kidney can compensate almost completely for lost hepatic glucose output for example during hepatic failure (202).
The kidney also plays a major role in glucose recovery from the glomerular filtrate. Each day the kidney filters 180 liters of plasma (approximately 72 serum volumes), which, in the absence of glucose reabsorption, would result in the loss of 180 grams of glucose to the urine. Virtually all (99%) of the filtered glucose is reabsorbed in the proximal tubule and less than 0.5 grams are lost (633).
There exists, therefore, a great cycle of glucose ingestion, absorption, metabolism, synthesis, redistribution and recovery between organ systems. Glucose transporters play a central role in delivering glucose to or secreting glucose by each of these organ systems. Glucose transporter expression levels, sites of expression, and interplay with cellular glucose metabolism determine precisely how each organ system contributes to organismal, carbohydrate and energy homeostasis. Since lactate is an end product of anaerobic glucose metabolism and feeds into the oxidative pathway there is also an interesting interplay between cellular glucose and lactate import/export and metabolism. This section of the review describes our current understanding of these transport and metabolic cycles.
MONOSACCHARIDE UTILIZATION
The flow of monosaccharide-derived carbons from interstitium to cytoplasm, their exchange between metabolic pathways and the roles played by the GLUTs and MonoCarboxylate Transporters (MCTs) in this cycle are summarized schematically in Figure 5. We focus on three GLUT substrates (D-glucose, D-galactose and D-fructose), two key intermediate metabolites that are also substrates for important transporters (glucose-6-phosphate and lactate) and the cycle of monosaccharide-derived carbons between tissues in the forms of glucose and lactate. The GLUTs play a central role in this carbon cycle. They are also pivotal in the central control and pre-conditioning of nutrient consumption, digestion, absorption and metabolism by serving as key partners in peripheral and central glucose sensing mechanisms that acutely modulate food intake and nutrient metabolism. In order to appreciate carbon cycling, we must first undertake a rudimentary review of monosaccharide metabolism and examine how glucose, galactose and fructose enter these metabolic pathways.
FIGURE 5. Pathways for monosaccharide metabolism in mammals.
The major pathways for monosaccharide metabolism in cells and their intracellular locations are shown in schematic. The 3 compartments are cytoplasm, mitochondrion and endoplasmic reticulum (ER). Glucose (Glc), Galactose (Gal) and Fructose (Frc) enter and exit the cell on Class 1, 2 and 3 GLUTs. Lactate (produce by glycolysis) can enter or leave the cell on monocarboxylate transporters (MCTs). Galactose and glucose enter glycolysis as glucose-6-phosphate (G-6-P). Fructose enters at later steps in the glycolytic pathway as either dihydroxyacetone phosphate or glyceraldehyde-3-phosphate. Glycolysis produces 2 ATP molecules per entering glucose molecule. The Pentose phosphate pathway produces to NADPH molecules and mitochondrial oxidative phosphorylation produces 34–36 ATP molecules per glucose. Gluconeogenesis, which can be fed by pyruvate, amino acids or glycerol, produces G-6-P which is transported into the ER by a G-6-P/Pi antiporter (G6PT), dephosphorylated to Glc, which is then exported to the cytoplasm by ER GLUTs. G-6-P is converted reversibly to a useful glucose storage form (glycogen) by glycogen synthesis. Anabolic use of Glc, Gal or Frc in membrane protein and lipid glycosylation is not shown.
Glycolysis
Glycolysis is the cytoplasmic metabolic pathway that converts glucose into pyruvate. The free energy released during glycolysis is exploited to form ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide). The overall stoichiometry of glycolysis is:
glucose + 2 NAD+ + 2 ADP + 2 Pi→ 2 pyruvate + 2 NADH + 2 H+ + 2 ATP + 2 H2O
Glycolysis is a sequence of ten reactions involving ten intermediates, with each intermediate providing alternative entry points to glycolysis. Glucose entry into the glycolytic pathway is mediated by hexokinase which combines with intracellular glucose and ATP to transfer the terminal phosphate of ATP to carbon 6 of glucose forming glucose-6-phosphate (G-6-P).
Hexokinase
Hexokinases I, II, and III have high affinity for glucose (Km(app)< 0.1 mM) and are strongly inhibited by their product, glucose-6-phosphate. Hexokinase I is found in all mammalian tissues, predominating in brain and kidney, (420), and is considered a "housekeeping enzyme," because its expression unaffected by most physiological, hormonal, and metabolic changes. Hexokinase II is the principal regulated isoform in many cell types (especially in adipose, muscle and heart) and its expression is upregulated in many cancers. Hexokinase III is inhibited by glucose at physiologic concentrations and is expressed in cell bodies of mature neurons and brain white matter (myelinated axons) as well as in kidney, heart, skeletal muscle and spleen (121).
Mammalian hexokinase IV or glucokinase, differs from the other hexokinases in kinetics and function. Glucokinase Km for glucose is 100 times higher than that of hexokinases I, II, and III. Glucokinase, displays positive cooperativity with glucose, and is not allosterically inhibited by glucose-6-phosphate. It is present in the liver, pancreas, hypothalamus, small intestine, and perhaps certain other neuroendocrine cells, and plays an important regulatory role in carbohydrate metabolism. The activity of hepatic glucokinase is regulated by a 68-kDa inhibitory protein, glucokinase regulatory protein (GKRP). Glucokinase is bound to GKRP within the nucleus when the cell is metabolically quiescent and translocates to a free state in the cytoplasm in response to a rise in extracellular glucose (24, 281).
Two kinetic properties distinguish glucokinase from the other hexokinases and are suggested to permit glucokinase to function in a special role as glucose sensor: 1) Glucokinase has a lower affinity for glucose (Km(app) = 8 mM) than the other hexokinases (Km(app)< 0.1 mM); 2) Glucokinase is not inhibited by its product, glucose-6-phosphate. This allows continued product formation in the presence of significant amounts of product and thus provides a continuous "read out" of cellular glucose in the form of glucose-6-phosphate.
Lactic acid fermentation
If glycolysis were to continue indefinitely, cellular NAD+ would be consumed, and glycolysis would arrest. In order to maintain glycolysis, NADH must be oxidized back to NAD+. This is accomplished by a process called lactic acid fermentation in which pyruvate is converted to lactate by lactate dehydrogenase:
pyruvate + NADH + H+ → lactate + NAD+
This process occurs in bacterial yogurt cultures (lactic acid causes the milk to curdle) and in animals experiencing hypoxia such as in overworked muscles starved of oxygen, or infarcted heart muscle cells. Most animal tissues cannot maintain anaerobic respiration for an extended length of time. The end product, lactate, is a substrate for MCT1 and MCT2 and, as we shall see below, is exported by muscle, red cells, astrocytes and neurons during increased glycolysis.
Oxidative Metabolism
Oxidative metabolism occurs in four stages. First, pyruvate is converted to acetyl-CoA and CO2 within the mitochondria in a process called pyruvate decarboxylation. One molecule of NADH is formed per pyruvate oxidized. This step links glycolysis and the Krebs cycle.
Second, Acetyl-CoA enters the citric acid cycle, or Krebs Cycle, within the mitochondrial matrix where it is fully oxidized to carbon dioxide and water. Three pathways converge on the citric acid cycle: Glycolysis through pyruvate, Gluconeogenesis through malate and fatty acid degradation through acetyl CoA. Each turn of the cycle generates 1 GTP, 3 NADH and 1 FADH2.
Third, NADH is oxidized to NAD+ by the electron transport chain, using oxygen as the final electron acceptor. During oxidative phosphorylation, electrons are transferred from NADH and FADH2 to oxygen in a coordinated series of redox reactions catalyzed by mitochondrial protein complexes. These complexes are called electron transport chains. The energy released by electron flow through the electron transport chain drives H+ transport across the inner mitochondrial membrane, in a process called chemiosmosis. This creates a protongradient across the inner membrane of the mitochondria.
This proton gradient is key to ATP synthesis. and is used by ATP synthase - a large, membrane-spanning enzyme complex - which couples the downhill transport of protons into the mitochondrial matrix to ADP phosphorylation thereby synthesizing ATP from ADP and Pi. The net result of glycolysis and oxidative phosphorylation of one molecule of glucose is the production of 36 ATP molecules.
Glycogen synthesis/glycogenolysis
G-6-P is central in glycogen synthesis and glycogenolysis. Cytoplasmic G-6-P is reversibly converted to glucose-1-phosphate by phosphoglucomutase. Uridine triphosphate (UTP) then reacts with glucose-1-phosphate to form UDP-glucose in a reaction catalysed by UDP-glucose pyrophosphorylase. Glycogen is synthesized from monomers of UDP-glucose by glycogen synthase, which progressively lengthens the glycogen chain with (α1→4) bonded glucose.
Glycogenolysis, in contrast, occurs when glycogen is cleaved from the nonreducing ends of the chain by glycogen phosphorylase to produce monomers of glucose-1-phosphate, which is then converted to glucose 6-phosphate by phosphoglucosemutase. The resulting glucose 6-phosphate monomers have three possible fates: glucose 6-phosphate enters the glycolytic pathway and is used as fuel; glucose 6-phosphate enters the pentose phosphate pathway via glucose-6-phosphate dehydrogenase to produce NADPH and 5-carbon sugars; or in the liver and kidney, glucose 6-phosphate is dephosphorylated by glucose 6-phosphatase to form glucose. This is the final step in gluconeogenesis and takes place in the endoplasmic reticulum.
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that generates glucose from non-carbohydrate, carbon substrates. All citric acid cycle intermediates, through conversion to oxaloacetate, amino acids other than lysine or leucine, and glycerol are also substrates for gluconeogenesis. Gluconeogenesis occurs during fasting, starvation, low-carbohydrate diets, or intense exercise and is highly endergonic: the pathway from phosphoenolpyruvate to glucose-6-phosphate requires 6 molecules of ATP. Transamination or deamination of amino acids facilitates their entry into the cycle directly (as pyruvate or oxaloacetate), or indirectly via the citric acid cycle.
In humans, gluconeogenesis is restricted to the liver and the kidney. Oxaloacetate production from pyruvate and citric acid cycle intermediates occurs in the mitochondrion, and phosphoenolpyruvate conversion to glucose in the cytosol. Gluconeogenesis begins in the mitochondria or cytoplasm, depending on the starting substrate. Several of the reactions are essentially glycolysis in reverse and lead to fructose 6-phosphate. Glucose-6-phosphate is formed from fructose 6-phosphate by phosphoglucoisomerase and can be used in other metabolic pathways or dephosphorylated to free glucose.
Whereas free glucose is readily exported by the cell, glucose-6-phosphate is nontransportable and is, therefore, trapped within the cell. Glucose formation occurs in the lumen of the endoplasmic reticulum, where glucose-6-phosphate is converted to glucose by glucose-6-phosphatase. Cytoplasmic G-6-P is transported into the endoplasmic reticulum by G6PT - an exchange transporter that imports G6P in exchange for endoplasmic reticulum Pi (108). Glucose is then shuttled into the cytosol by GLUTs expressed in the endoplasmic reticulum membrane or, in hepatocytes, may be secreted by a vesicle-mediated mechanism (76, 226).
Pentose Phosphate Pathway
The pentose phosphate pathway generates NADPH and pentoses (5–carbon sugars) from glucose-6-phosphate (a hexose) and comprises oxidative and non-oxidative phases. The oxidative phase generates NADPH and the non-oxidative phase synthesizes 5-carbon sugars. The pentose phosphate pathway is an alternative to glycolysis and while it involves glucose oxidation, its primary purpose is anabolic rather than catabolic.
The overall reaction is:
Glucose-6-phosphate + 2 NADP+ + H2O → ribose-5-phosphate + 2 NADPH + 2 H+ + CO2
NADPH is used in reductive biosynthetic cellular reactions (e.g. fatty acid synthesis); ribose-5-phosphate is used in the synthesis of nucleotides and nucleic acids and erythrose-4-phosphate (E4P) is used in the synthesis of aromatic amino acids.
METABOLIC ENTRY POINTS
Glucose
Glucose enters the glycolytic cycle directly and is phosphorylated by hexokinase or glucokinase to form glucose-6-phosphate. Cytoplasmic glucose derives either from extracellular glucose which requires the action of cell surface glucose transport proteins (Class I, II and III GLUTs - although not all GLUTs are known to be glucose transporters) to mediate sugar import or from endoplasmic reticulum glucose (formed as the last step of gluconeogenesis) which requires the activity of ER GLUTs to mediate export into the cytoplasm.
Each GLUT protein undergoing expression in a cell may contribute to endoplasmic reticulum glucose transport while en route to the cell surface (559). However, a specific GLUT may mediate glucose transport across the endoplasmic reticulum membrane (515). GLUT8 contains an N-terminal late endosomal/lysosomal-targeting motif [DE]XXXL[LI] (29). Mutagenesis of these leucine residues causes GLUT8 to accumulate at the surface of heterologously expressing cells (516) suggesting that GLUT8 is a constitutively intracellular transporter. Intracellular GLUT8 is distributed throughout the secretory system (515) suggesting that intracellular sugar transport scavenges monosaccharides (glucose, galactose) throughout the pathway.
Galactose
Galactose enters cells via the Class 1 and Class III sugar transporters (578). However, there are no catabolic pathways for galactose metabolism, so intracellular galactose must first be converted into a glycolytic intermediate. This is achieved in 4 steps resulting in the formation of glucose 6-phosphate. The first step in the pathway is galactose phosphorylation to galactose-1- phosphate by galactokinase. The final stepcatalyzes glucose-1-phosphate isomerization to glucose-6-phosphate - a glycolytic intermediate.
Fructose
Cellular import of fructose is catalyzed by GLUT2 and by GLUT5 (578). Fructose is an increasingly important monosaccharide in European and North American diets (344, 568) where consumption of fructose has risen from 16–24 g daily (obtained from fruits and honey) to as high as 100 g daily (162). Most of this is derived from refined or processed fructose (221).Fructose is largely metabolized in the liver, using the fructose 1-phosphate pathway. The initial step, following GLUT2-mediated fructose uptake, is catalyzed by fructokinase, which phosphorylates intracellular fructose to form fructose 1-phosphate. Fructose 1-phosphate is then split by fructose 1-phosphate aldolase into glyceraldehyde and dihydroxyacetone phosphate (DHAP) - aglycolytic intermediate. Glyceraldehydephosphorylation produces glyceraldehyde 3-phosphate (G-3-P) - another glycolytic intermediate. Both intermediates then feed directly into glycolysis. Fructose can also be directly phosphorylated to fructose-6-phosphate by hexokinase. However, the affinity of hexokinase for glucose is 20-fold greater than its affinity for fructose hence very little fructose-6-phosphate is formed in the liver where normally high levels of glucose competitively inhibit fructose phosphorylation. The largest part of fructose metabolism occurs in the liver where it is converted to glycogen, enters gluconeogenesis or is converted into triose-phosphate and can therefore be oxidized fully. About 12% of fructose is metabolized in a similar way by the absorptive cells (enterocytes) of the small intestine (567).
TISSUE SPECIFIC TRANSPORT AND METABOLISM
All tissues are capable of using glucose to produce ATP but the studies of (411) illustrate the major sites of glucose uptake and utilization in the human body. Using 18F 2-fluoro-2-deoxyglucose and three-dimensional positron emission tomography (PET) to monitor glucose utilization in healthy volunteers, Masud et al. demonstrate that skeletal muscle, liver, heart and brain are the major organs of glucose consumption in humans. At rest, the rate of glucose utilization by brain is six-fold greater than the rate of glucose utilization by heart or liver and is some 20-fold greater than the rate of utilization in skeletal muscle. During light exercise, glucose utilization in brain is reduced slightly, is unchanged in heart, falls slightly in liver but increases four-fold in skeletal muscle. Moderate exercise further reduces brain glucose utilization, leaves glucose utilization in the heart and liver unchanged and increases glucose consumption by thigh muscle by some sixfold over resting conditions.
Cardiac & smooth muscle
Cardiac muscle and smooth muscle utilize several substrates for energy metabolism including glucose. GLUT1 is the major monosaccharide transporter expressed in vascular smooth muscle (396) where glycolysis is essential for maintaining normal Na, KATPase activity while glycogen-derived pyruvate and fatty acid metabolism are essential to support contractile activity (380). Both GLUT1 and GLUT4 are expressed in cardiac muscle, which undergoes a gradual shift from dependence on carbohydrate metabolism to use of fatty acid metabolism as the animal matures from neonatal to adult (374, 375). The failing or hypertrophied heart, however, undergoes an interesting reversion to glycolytic metabolism (38, 509).
Skeletal Muscle
Basal metabolism in resting skeletal muscle is fueled by oxidation of free fatty acids released by adipose (290). As muscle work increases, fatty acid oxidation increases several-fold and glucose metabolism (aerobic and anaerobic) becomes important (496). Exercise increases the rate of lipolysis and fatty acid release from adipose tissue. During moderate-intensity exercise, increased ß-adrenergic stimulation increases lipolysis approximately threefold. In addition, exercise doubles the blood flow to adipose tissue and halves reesterification which, when coupled to increased blood flow to skeletal muscle, increases the delivery of fatty acids to muscle several-fold.
Plasma glucose and especially muscle glycogen become more important as exercise intensity increases (496). Exercise increases muscle glucose transport by promoting GLUT4 translocation to the sarcolemma (193, 210). GLUT4 is the major skeletal muscle glucose transport protein and only a small fraction of total cellular GLUT4 is expressed at the sarcolemma of resting muscle. Glucose is stored in skeletal muscle as glycogen and this store is a major source of energy during most forms of muscle activity. There is a direct correlation between muscle glycogen concentration and time to fatigue during moderately intense exercise (60–80% of maximal oxygen uptake) (49, 260). Fast muscle consumes ATP, producing ADP and Pi, much faster than it can regenerate ATP. Previously considered to be a consequence of oxygen lack in contracting skeletal muscle, we now understand that lactate is formed and utilized continuously under fully aerobic conditions (68). During exercise, lactate oxidation accounts for 70–75 % of lactate utilization, with gluconeogenesis accounting for the remainder. Working skeletal muscle both produces and uses lactate as a fuel, with much of the lactate formed in glycolytic fibers being exported via MCTs and taken up and oxidized in adjacent oxidative fibers. This is a cell-cell lactate shuttle (68). Glycolysis in combination with lactate oxidation permits high flux rates and the maintenance of redox balance in cytosolic and mitochondrial compartments.
CNS
Glucose is a major source of metabolic fuel to the brain. In humans, 100 − 120 g glucose (0.5 mol/min or 50 to 75% of total glucose consumption by the body) is used daily to fuel brain metabolism (165). In resting individuals, the brain undertakes a small net lactate secretion into the blood catalyzed by endothelial cell MCT1 (482). During heavy exercise, excess lactate produced during skeletal muscle contraction raises blood lactate from resting levels of 0.9 mM to as great as 7 mM (482). Under these conditions, lactate uptake by the brain reaches a maximal rate of 1 mmol/min then declines upon cessation of exercise and recovery (482). GLUT1- mediated non-oxidative glucose uptake by the brain may double during recovery from intense exercise (482) suggesting a mechanism for acute regulation of glucose transport across the blood brain barrier. The brain, therefore, imports lactate at a rate that equals or exceeds basal glucose uptake (0.5 mmol/min). The conventional view of the liver as the organ that clears the blood of lactate via the Cori cycle (see below) should, therefore, be extended to include lactate uptake by brain. This favors distribution of carbohydrate energy in the body and economy in glucose utilization (482).
Adipose
Increased insulin-dependent, GLUT4-mediated glucose uptake by adipose during hyperglycemia results in increased triglyceride synthesis and storage. The glucose-fatty acid cycle describes the interplay between carbohydrate and fat metabolism (484). Elevated blood glucose stimulates insulin secretion, which suppresses non-esterified fatty acid release from adipose. This eliminates competition for substrate utilization in muscle allowing insulin-stimulation of glucose utilization unimpeded by high concentrations of fatty acids. When plasma non-esterified fatty acid concentrations are high (normally because glucose and insulin concentrations are low), fatty acids are the major fuel for skeletal muscle.
Liver
The liver plays a major role in glucose homeostasis by releasing precisely that amount of glucose into the systemic circulation that is necessary to meet rates of extra-hepatic glucose utilization and thus maintain normal limits of plasma glucose concentrations (566). The liver accomplishes this by mobilizing glucose stored within hepatocytes as glycogen and/or by converting lactate, glycerol, and amino acids into glucose (gluconeogenesis). Net glucose release is catalyzed by GLUT2 (579) and results from these two parallel processes, which must be precisely regulated. Plasma glucose levels reflect the balance between hepatic glucose production and extra-hepatic glucose utilization and, if utilization exceeds production, plasma glucose concentrations fall leading to compromised CNS function. After an overnight fast, the liver is the major site of glucose output into the circulation. Glycogenolysis and gluconeogenesis each contribute ~50% of hepatic glucose output. The liver is also the major site of fructose metabolism (see above and (567)).
Erythrocytes
Red blood cells use only glucose as a fuel and, lacking the machinery for oxidative metabolism, release the product of glucose metabolism as lactate. Thus, carbon flow (largely in the forms of glucose, fructose, galactose and lactate) within and between cells of the same and different organ systems is highly interconnected. Human red cells express GLUT1 and MCT1 at very high concentrations (67, 307, 651).
CARBON CYCLING BETWEEN TISSUES
The Cori Cycle
Lactate is produced by active skeletal muscle and erythrocytes and is exported (via MCTs) into the intertsitium where it becomes an important source of energy for other organs (68). Erythrocytes lack mitochondria and thus can never fully oxidize glucose. Contracting skeletal muscle produces glycolytic pyruvate at a rate that exceeds the rate at which it can be oxidized by the citric acid cycle. Furthermore, under these conditions the rate of NADH formation by glycolysis is greater than the rate of its oxidation by aerobic metabolism.
This accumulation of NADH is reversed by lactate dehydrogenase, which oxidizes NADH to NAD+ as it reduces pyruvate to lactate and thereby eliminates a major roadblock to continued glycolysis (NAD+ loss). Lactate formation buys time and shifts part of the metabolic burden from muscle to other organs or to other cells within the same organ. For example, lactate produced and exported by glycolytic skeletal muscle fibers may enter the circulation or may be imported by adjacent oxidative muscle fibers where it is converted to pyruvate and oxidized in a cell-cell lactate shuttle (68). Exported lactate that enters the peripheral circulation is taken up by heart, brain, liver and kidney (482).
Cardiomyocytes and hepatocytes contain cell membrane transporters (MCTs) that greatly enhance their permeability to lactate and pyruvate. Both substances are transported out of active skeletal muscle into the blood and then into heart and liver. Once inside well-oxygenated cardiomyocytes, lactate is converted back to pyruvate and metabolized through the citric acid cycle and by oxidative phosphorylation to generate ATP. The use of lactate in place of glucose by these cells makes more circulating glucose available to active muscle cells.
Excess lactate also enters the liver and is converted first into pyruvate and then into glucose by gluconeogenesis. Thus, the liver restores the level of glucose necessary for active, glycolytic muscle cells, which derive ATP from the glycolytic conversion of imported glucose into lactate. Contracting skeletal muscle supplies lactate to the liver, which uses it to synthesize glucose. These reactions constitute the Cori cycle. The renal cortex is also capable of gluconeogenesis using circulating lactate to produce and secrete glucose into the circulation. Under normal circumstances 10–30% of gluconeogenic glucose is produced in the kidneys (202) with the remainder coming from the liver.
Brain - ANLS/NALS
The brain has also been suggested to be a site of cell-cell glucose/lactate cycling (386, 468) but the direction and extent of lactate cycling between cerebral astrocytes and neurons continues to stimulate significant debate (291, 394).
Under normal circumstances (the well-fed state), glucose is the obligate energetic fuel for the mammalian brain. Most studies of cerebral energy metabolism assume that the majority of cerebral glucose utilization fuels neuronal activity via oxidative metabolism, both in the basal and activated state. Glucose transporter (GLUT) proteins deliver glucose from the circulation to the brain: GLUT1 in the microvascular endothelial cells of the blood-brain barrier and glia; GLUT3 in neurons. Lactate, a glycolytic product of glucose metabolism, is transported into and out of neurons by the monocarboxylate transporters (MCT): MCT1 in the blood-brain barrier and astrocytes and MCT2 in neurons (539).
Magistretti (386, 468) proposed the astrocyte-neuron lactate shuttle hypothesis (ANLS) in which it is hypothesized that astrocytes play the primary role in cerebral glucose utilization and generate lactate for oxidative, neuronal energetics, especially during activation. The reasons for this are several-fold: 1) Neurons are thought not to respond to activation with increased glycolysis (291); 2) The burden of Na+-dependent neurotransmitter (glutamate) re-uptake by peri-synaptic astrocytes is thought to stimulate astrocytic Na export via the Na+,K+ATPase and thus increase the demand for glycolytic ATP; 3) Neurons appear to be the major site of O2 consumption in brain (291); 4) Studies of primary cultures of neonatal astrocytes and neurons suggest that astrocytic and neuronal glucose transport and utilization rates are similar (466, 476); 5) In situ studies using the fluorescent amino sugars (6NBDG and 2NBDG) indicate that astrocyte import and metabolize sugars faster than neurons (42).
In contrast, Simpson et al (394, 395, 539) have proposed the neuron-astrocyte lactate shuttle hypothesis (NALS) in which neurons play the primary role in cerebral glucose utilization and generate lactate for oxidative, astrocytic energetics at rest and during activation. The reasons for this are several-fold: 1) Numerous studies report that neurons do respond to activation with increased glycoslysis (394); 2) The major thermodynamic burdens of neurotransmission are neuronal and result from activation of neuronal Na+,K+ATPase and Ca2+ATPase in order to restore pre- and post-synaptic intracellular Na+K+ and Ca2+ levels (this burden is significantly greater than that experienced by surrounding astrocytes in clearing Na+ uptake from Na+- Glutamate co-transport). Moreover, proton-dependent glutamate transport into presynaptic vesicles is critically dependent on glycolytic activity not oxidative metabolism (277); 3) Neurons have 12-fold greater glucose transport capacity than do astrocytes based on cytochalasin B binding studies of astrocytic and neuronal plasma membranes isolated from rat brain homogenates and immunoblot and immuno-histochemical analyses of rat brain (539). 4) Since the identification of the GLUTs and MCTs in brain, much has been learned about their transport behavior (i.e. capacity and affinity for substrate) and cellular expression, which must be included in models of cerebral glucose uptake and utilization. Using concentrations and kinetic parameters of GLUT1 and GLUT3 in cerebral microvasculature endothelial cells, astrocytes, and neurons, along with the corresponding kinetic properties of the MCTs, Simpson et al (539) successfully modeled brain glucose and lactate levels as well as lactate transients in response to neuronal stimulation. Simulations based on these parameters suggest that glucose readily diffuses through the basal lamina and interstitium to neurons, which are primarily responsible for glucose uptake, metabolism, and the generation of the lactate transients observed on neuronal activation.
More quantitative analysis suggests that the amount of lactate that shuttles between neurons and astrocytes (in either direction with each model) accounts for less than 10% of total cellular energy metabolism (394). Thus while interesting as yet another example of cell-cell glucose/lactate cycling, the overall contribution to cellular metabolism may be rather less than originally considered.
GLUCOSE SENSING
Glucose is the primary metabolic fuel in the brain and sustained brain activity requires a continuous glucose supply at serum levels of 5 mM or greater. Because high serum glucose can exert long-term deleterious effects on cellular function, it is also important that blood glucose does not exceed 10 mM or greater. In order to control blood glucose, critical glucose-sensing systems are located in the systemic and hepatoportal circulations and in the autonomic nervous system. These sensors regulate glucose homeostasis, feeding behavior, and energy balance. Glucose is, therefore, an important regulatory metabolic signal that controls hormonal secretion by endocrine cells, the activity of specific peripheral and central nervous system neurons and the metabolic activity of specific tissues that utilize glucose. The monosaccharide transport proteins play key roles in glucose sensing mechanisms.
Marty et al (410) describe 6 elements of carbohydrate homeostasis that become activated upon glucose ingestion. These are:
The Cepahlic phase of insulin secretion. This is initiated by oral and olfactory taste receptors and oral/pharyngeal mechanoreceptors, occurs before serum glucose levels rise and is essential for normal glucose tolerance (the rate at which blood glucose levels return to fasting levels following a specified oral dose of glucose).
Potentiation of insulin secretion. Cells of the intestinal mucosa secrete hormones (gluco-incretins) in response to food intake that acutely potentiate glucose-stimulated insulin secretion and chronically increase beta cell mass.
Activation of the hepatic portal vein glucose sensors. This activation increases glucose utilization by peripheral tissues, inhibits the phenomenon of counterregulation (see below) and terminates food intake.
Allosteric control of metabolic pathways by substrate. Intracellular glucose allosterically modulates hepatocyte function in several ways. First, increased intracellular glucose and glucose-6-phosphate inhibit glycogen phosphorylase and stimulate glycogen synthase respectively leading to increased glycogen storage. Second, increased glucose flow through the pentose phosphate pathway stimulates fatty acid synthesis.
Direct actions on pancreatic islets. Elevated blood glucose directly promotes insulin secretion by pancreatic beta cells, which stimulates glucose uptake and glycogen synthesis in muscle, glycogen synthesis in liver and glucose uptake and lipogenesis in adipose. Glucose induced pancreatic insulin secretion is primarily responsible for suppressing hepatic gluconeogenesis but imposition of a simultaneous hyperglycemia induces an additional suppression of glucose production.
A fall in blood glucose below 5 mM induces a rapid counter regulatory response to restore normoglycemia. This involves glucagon secretion by pancreatic alpha cells and catecholamine secretion by adrenal glands which act in a concerted manner to stimulate hepatic gluconeogenesis and glucose output.
Central control. Changes in CNS glucose levels regulate hypothalamic and brain stem glucose-sensitive neurons, which control counter regulation, feeding, and energy expenditure via the hypothalamic melanocortin pathway.
Although the cephalic phase of insulin secretion amounts to only 1–3% of total insulin secretion, rats with transplanted (denervated) islets are glucose intolerant after a meal intake. Bypassing the oral cavity by direct administration of glucose to the GI tract also promotes glucose intolerance. Treatment of denervated rats with a dose of insulin that matches the cephalic response restores tolerance (reviewed in (7)).
Enteral nutrition provides a more potent insulinotropic stimulus than an intravenous isoglycemic challenge (164). The incretin hypothesis posits that gastrointestinal hormones cause increased pancreatic beta cell insulin secretion after eating, but before blood glucose levels become elevated. The first incretin identified, glucose-dependent insulinotropic polypeptide (GIP), is a 42-amino acid peptide synthesized in duodenal and jejunal entero-endocrine K cells in the proximal small bowel (464). GIP has weak effects on gastric acid secretion but is a more potent insulinotropic agent. Glucagon-like peptide-1 (GLP-1) is made in enteroendocrine L cells of the distal ileum and colon, but plasma levels of GLP-1, like GIP, also increase within minutes of eating (487). Hence endocrine and neural signals are thought to promote the rapid stimulation of GLP-1 secretion long before digested food transits the gut to activate the L cells of the small bowel and colon. GIP and GLP-1 act via G-protein-coupled receptors (GPCRs) on the surface of pancreatic β cells, and less abundantly in adipose and the CNS. The GLP-1 receptor (GLP-1R) is expressed in pancreatic α and β cells, the central and peripheral nervous systems, heart, kidney, lung, and the gastrointestinal tract. β cell incretin receptor activation enhances glucose-dependent increases in intracellular cAMP and calcium and thus promotes insulin secretion.
The hepatoportal vein glucose sensor response was first characterized by Thorens and coworkers (77, 78) who demonstrated that glucose infusion through the portal vein induces insulin-independent hypoglycemia by a somatostatin-inhibitable mechanism that increases glucose utilization in skeletal muscle, heart and brown fat. Hypoglycemia and increased glucose turnover do not develop after portal glucose infusion in GLUT2 knockout mice (RIPGLUT1:GLUT2−/− mice in which GLUT1 is specifically re-expressed in the pancreatic β-cells). This suggests that GLUT2 is involved in the function of the hepatoportal glucose sensor and that somatostatin either acts on GLUT2-expressing cells or on tissues where glucose utilization is activated. Increased muscle glucose utilization is suppressed in mice lacking skeletal muscle GLUT4 but is normal in mice with muscle-specific insulin receptor gene knockdown (75). This confirms that the hepatoportal vein glucose sensor response is independent of insulin-signaling pathways. Moreover, the hepatoportal vein glucose sensor response in muscle is completely dependent on the activity of skeletal muscle AMPK, because enhanced hexose disposal is eliminated in mice expressing a muscle-specific dominant negative AMPK.
The hepatoportal vein glucose sensor is connected to the lateral hypothalamus (517, 532) and the nucleus of the tractus solitarius (5) via afferent hepatic branches of the vagus nerve (408). The firing rate of these glucose-sensitive nerves is inversely proportional to the concentration of glucose infused into the portal vein (451).
Hypergylcemia stimulates pancreatic beta cell insulin secretion resulting in elevated serum insulin levels and stimulation of glucose uptake and glycogen synthesis in muscle, glycogen synthesis in liver and glucose uptake and lipogenesis in adipose. Glucose-dependent insulin secretion is observed with isolated islets (577) and requires the import of metabolizable glucose analogs (37). GLUT2 is the pancreatic glucose transporter but can be substituted by GLUT1 in transgenic mice without impacting insulin release (78) indicating that the glucose signaling system is not GLUT2-specific but, rather, simply requires sugar import into the cytoplasm.
In a comprehensive series of studies in dogs, Madison and co-workers (124, 384) assessed the roles of insulin and glucose concentrations in determining hepatic glucose conservation (suppression of production, uptake and storage). Glucose loads, increase glycogen synthesis and reduce glucose production. Overall glucose uptake by the liver (when corrected for suppression of gluconeogenesis) amounts to about 25% of the load. Further analysis demonstrates that insulin is primarily responsible for suppressing glucose production, although simultaneous hyperglycemia plus insulinsuppress gluconeogenesis further resulting in greater net glucose uptake by the liver (41).
A fall in blood glucose below 4 mM induces a rapid counter regulatory response, which restores normoglycemia. This involves direct hypoglycemia-induced glucagon secretion by pancreatic alpha cells (481) and indirect catecholamine secretion by adrenal glands (336) which act to stimulate hepatic gluconeogenesis and glucose output and to reduce glucose uptake by peripheral tissues. The catecholamine response requires the involvement of the autonomic nervous system (336). Plasma glucagon levels in RIPGLUT1:GLUT2−/− mice in the fed state are twice as high as in control mice but are normalized by the ganglionic blockers hexamethonium or chlorisondamine (79) suggesting an increased autonomic tone to pancreatic alpha cells in the absence of GLUT2 . Plasma glucagon levels are reduced or increased by hyper- and hypoglycemic clamps respectively. These responses are absent in RIPGLUT1:GLUT2−/− mice. Cellular glucoprivation by intraperitoneal or intracerebro ventricular administration of 2-deoxy- D-glucose (a glucose analog that does not undergo glycolysis) fails to stimulate glucagon secretion in RIPGLUT1:GLUT2−/− mice. Thus central GLUT2-dependent glucose sensors are also involved in the counter regulatory response (409).
Restoration of GLUT2 expression in glial cells but not in neurons of RIPGLUT1:GLUT2−/− mice also restores glucagon secretion in response to intraperitoneal 2-deoxy-D-glucose. This suggests a class of central glucose sensors that require GLUT2 expression in glial cells and functional coupling between glial cells and neurons (409). Metabolic coupling between astrocytes and neurons is reminiscent of Magistretti's proposal (386) that astrocytic glucose uptake (in this instance via GLUT2) is coupled to neuronal function by lactate production and export.
GLUCOSE SENSING MECHANISMS
Oral Cavity/Cephalic response
The oral cavity/cephalic response is initiated by oral and olfactory taste receptors and oral/pharyngeal mechanoreceptors and glucose transport is not a necessary component of the sensing pathway (7).
Mucosa (incretins)
RIPGLUT1:GLUT2−/− mice show normal GIP secretion but reduced GLP-1 secretion in response to oral glucose ingestion (86). Glucose dependent incretin secretion also requires incretin receptors. GLP-1 secretion is reduced in GLUT2 and GIP receptor-knockout mice but not in GLP-1 receptor knockout mice. GIP secretion is independent of GLUT2 expression but does require the presence of GIP- and GLP-1 receptors.
This observation is confounded by the demonstration that upper intestinal tract administration of nonmetabolizable substrates (3-O-methylglucose and α-methylglucoside) of the sodium dependent glucose transporter SGLT1 stimulate incretin secretion as effectively as glucose and that secretion promoted by each of the 3 sugars is inhibited by the SGLT inhibitor phloridzin (435, 487). Intravenous application of these sugars is without effect on incretin secretion. SGLT3 is also found in the intestinal mucosa where it is suggested to act as an intestinal glucose sensor that regulates gastrointestinal secretion and motility (286). However, 3MG, is not transported by SGLT2 or SGLT3 (634) but is a potent stimulant of incretin secretion thereby arguing against the involvement of SGLT3 in glucose-induced incretin secretion in mice.
SGLT1 is expressed in the apical membrane of murine K-cells, and murine duodenal cultures secrete GIP in response to α-methylglucoside (464). This response is sensitive to phloridizin but is also abolished by the KATP channel agonist diazoxide suggesting that secretion requires membrane depolarization resulting from the coupled inflow of sugar and sodium ions. Tolbutamide (a K channel blocker) does, however, stimulate incretin secretion suggesting 2 mechanisms that produce glucose dependent secretion (464). Both mechanisms involve membrane depolarization-induced elevated cytoplasmic Ca2+ which in turn promotes GLP-1 and GIP secretion. One mechanism involves intracellular metabolism of imported sugar leading, as in pancreatic beta cells, to elevated cytoplasmic ATP:ADP ratios which close tolbutamide-inhibitable and diazoxide-activatable KATP channels. This leads to membrane depolarization, which in turn opens voltage sensitive Na+ and Ca2+ channels leading to full blown membrane depolarization and secretion. The second mechanism involves direct membrane depolarization resulting from glucose: Na+ cotransport, which is sufficient to activate voltage sensitive Na+ channels and Ca2+ channels (487). Thus, while GLUT2 may play a role in GLP-1 secretion, SGLT1 appears to be the critical glucose sensor in GIP and GLP-1 secretion.
Hepatoportal sensing
A hepatoportal arterial glucose gradient is necessary for hepatoportal glucose sensing (551). GLUT2 knockout mice that express GLUT1 in the pancreas (and thus secrete insulin in a glucose dependent manner) or GLUT2 in hepatocytes fail to respond to hepatoportal glucose (79, 576). Thus GLUT2 appears to be essential for the response. The specific cells mediating this response are not yet identified.
Pancreas
Insulin secretion by pancreatic beta-cells is regulated by blood glucose (412). The first step in glucose dependent insulin secretion involves GLUT2-catalyzed sugar uptake followed by glucokinase-mediated glucose phosphorylation to glucose-6-phosphate. Glucose-6-phosphate then enters the glycolytic pathway whence pyruvate enters the oxidative pathways (see sugar metabolism above) to generate secretion coupling factors. The best understood secretagogue is ATP and the increased cytoplasmic ATP/ADP ratio resulting from glucose metabolism. Glucose metabolism causes reduced membrane K+ conductance, which results in membrane depolarization.
Decreased K+ conductance results from the closure of ATP-sensitive K+ channels (KATP channels) (126). These channels govern resting membrane potential and are tetramers of a complex of two proteins: the sulfonylurea receptor (SUR1) and an inwardly rectifying K+ channel (Kir 6.2 (522)). Sulfonylureas and diazoxide act directly on SUR1 to close and open the channel respectively. SUR1 contains the Mg2+.ADP binding site which opens the channel, whereas ATP acts on Kir 6.2 to close the channel. Membrane depolarization resulting from elevated ATP/ADP causes voltage-sensitive Ca2+ channels to open and the resulting Ca2+ entry triggers insulin granule exocytosis.
Glucose also stimulates insulin secretion when cells are depolarized either by KATP channel block using sulfonylureas or, by opening the channels with diazoxide and then raising extracellular K+ to depolarize the membrane (258). This amplification also requires glucose metabolism but does not act via the KATP channel-dependent pathway and, while insulin secretion remains Ca2+-dependent, does not increase cytoplasmic Ca2+ levels. Rather, greater secretion is effected at similar cytoplasmic Ca2+ levels. The glucose metabolism-dependent signals that produce greater sensitivity of the exocytotic pathway to Ca2+ are not known.
The rate-limiting step in glucose stimulated insulin secretion is not glucose uptake but glucose phosphorylation. This is catalyzed by glucokinase (250), which displays a non Michaelis-Menten, sigmoidal dose dependence on glucose levels. The concentration dependencies of glucose phosphorylation, utilization, oxidation and glucose induced insulin secretion are superimposable (412) further supporting the view that glucokinase is the rate limiting event in secretion. GLUT2 knockout mice produce islets with severely compromised glucose dependent insulin secretion (576). This can be corrected with pancreas specific GLUT2 or GLUT1 expression indicating that the defect is glucose transport specific but not glucose transporter specific (576). This is consistent with the hypothesis that glucose phosphorylation, not transport, is rate-limiting for insulin secretion.
Central
Electrophysiologic analysis of hypothalamic slices reveals that the firing activity of some neurons is modulated in response to changes in extracellular glucose levels (17, 81, 492). These are glucose-excited (GE) neurons, which increase their firing rate with elevation in extracellular glucose concentrations, or glucose-inhibited (GI) neurons, which are activated by a decrease in extracellular glucose concentration or by cellular glucoprivation (499, 640). Both types of neurons are widely distributed in the brain but highly represented in hypothalamic nuclei and the brain stem, regions involved in the control of energy homeostasis and food intake.
Evidence for glucose-regulated neurons was initially obtained by intravenous or intracerebroventricular application of glucose anti-metabolites 2-deoxy-D-glucose or 5-thioglucose. The glucoprivic signal generated by these compounds induces metabolic or behavioral responses. The roles of hypothalamic nuclei, in particular the ventromedial nucleus of the hypothalamus (VMH), have examined by lesion studies and by pharmacological or genetic interference with glucose detection systems (189). Glucagon secretion is induced by direct injection of 2-deoxy-D-glucose into the VMH (66) whereas hypoglycemia-induced glucagon secretion is suppressed by direct VMH injection of glucose (65).
Glucose sensing by CNS cells may involve several different mechanisms (639). One mechanism may be analogous to pancreatic beta-cell glucose signaling (see above). Glucose signaling in these cells requires GLUT2-mediated glucose uptake, glucose phosphorylation by glucokinase, and its further glycolytic and oxidative metabolism to increase the intracellular ATP:ADP ratio. Thus GE neurons in the VMH respond to glucose, galactose, glyceraldehyde, glycerol and lactate with increased firing rate. Pyruvate does not alter GE firing. Activation is inhibited by glucosamine (a glucokinase inhibitor), phloridzin (a weak inhibitor of the GLUTs), iodoacetamide (an inhibitor of glycolysis) or 2-deoxy-D-glucose (a glucose analog that is phosphorylated by hexokinase but which cannot be metabolized further). Glucose activated firing is stimulated by tolbutamide (a KATP channel blocker) whereas diazoxide (a KATP channel agonist) inhibits glucose stimulation of about 50% of GE neurons tested. Thus the VMH GE neurons respond to glucose in a manner analgous to pancreatic beta cells (639).
Tolbutamide activates glucose-stimulated neurons but fails to inhibit or activate glucose inhibited neurons of the ventromedial hypothalamus (640). Inhibitors of glucose transport and glycolysis, e.g. the glucokinase inhibitor glucosamine, block the effect of glucose on glucose-inhibited neurons. GI neurons are activated by 2-deoxy-D-glucose. Conversely, glucose-inhibited neurons are inhibited by glycolytic metabolites, including lactate, but not pyruvate. These findings suggest that hypoglycemia induces electrical activity in glucose-inhibited neurons by attenuating glycolysis. There are striking parallels in the sensitivity to metabolites in both GE and GI neurons and the behavior of pancreatic beta cells. However, there are also very interesting differences. For example, beta cells do not respond to 2-deoxy-D-glucose, which is thought not to inhibit glucokinase. Nevertheless, sensitivity to glucosamine (a glucokinase inhibitor) suggests that glucokinase may be involved. Glucosamine is not a GLUT1 or GLUT4 substrate but is a GLUT2 substrate (547, 591) suggesting that it can only enter GLUT2- expressing cells to inhibit glucokinase2. Thus, some observed effects of 2-deoxy-D-glucose may be indirect through hexokinase-expressing neurons or astrocytes in close proximity to the recorded neurons.
Glucose inhibition of orexin/hypocretin neurons in the lateral hypothalamus suggests a fundamentally different sensor mechanism (80). Orexin/hypocretin neurons promote wakefulness and regulate metabolism and reward. Their inhibition by glucose appears to be mediated by TASK (TWIK-related acid-sensitive potassium, K2P) channels that respond to physiologic variations in glucose levels observed between meals. Moreover, glucose appears to act at an extracellular site and signaling to the channels does not involve ATP, Ca2+or glucose. These findings suggest a novel energy-sensing pathway in neurons that regulate states of consciousness and energy balance. Orexin/hypocretin neurons in TASK1 and TASK3 channel knockout mice, however, are still inhibited by glucose (209). Thus, TASK channels may enhance neuronal excitability and high-frequency firing but are not essential for orexin cell responses to glucose and pH. GLUT2-dependent central glucose sensors are associated with counter-regulation and are found in brain stem structures known to contain glucose-sensitive neurons (410).
KINETICS OF GLUCOSE DISTRIBUTION AND METABOLISM
The flow of carbohydrate beginning as undigested, ingested carbohydrate through absorbed glucose and fructose to cellular metabolites is illustrated in Figure 6.
FIGURE 6. Glucose distribution pathways in mammals.
The major distribution routes for monosaccharides and monosaccharide sensing/effector pathways are summarized schematically. The key illustrates glucose (blue arrows), fructose (pink arrows) and lactate flows (red arrows) between compartments; afferent input to the hypothalamus and brain stem (blue dashed arrows) from glucose sensors and effector output from the brain (autonomic) or pancreas (endocrine) to target organs.
GI SYSTEM
Carbohydrates are absorbed in the small intestine as monosaccharides. Oral and gastric carbohydrates are digested by salivary and pancreatic amylase, gastric acid and, upon release into the small intestine, by intestinal brush border disaccharidases (maltase, isomaltase, lactase, saccharase) to monosaccharides (glucose, fructose and galactose) (162).
Glucose and galactose absorption is mediated by the secondary active transport protein SGLT1 - an intestinal Na+-glucose cotransporter that uses the inwardly directed electrochemical Na+ gradient from small intestinal lumen to enterocytic cytoplasm to drive sugar and water against their concentration gradient across the apical membrane of the enterocyte into the cell (632). Glucose and galactose are both transported by SGLT1, whereas fructose is transported across the brush border by the facilitated fructose transporter GLUT5 (147). All three monosaccharides exit the enterocyte via GLUT2 which is normallyexpressed only at the basolateral membrane of enterocytes (575). Interstitial monosaccharides then diffuse into the portal blood. GLUT2 may also be transiently expressed at the apical membrane of jejunum enterocytes in response to monosaccharide ingestion and is chronically expressed at the apical membrane in experimental models of diabetes and in high fructose- or fat-induced insulinresistant states (312).
GLUT7 expression appears to be limited to the ileum - a distal region of small intestine (362). This contrasts with SGLT1, GLUT5, and GLUT2, which are expressed predominantly in the proximal regions of the small intestine. GLUT7 catalyzes relatively high affinity glucose (Km(app) =300 µM) and fructose (Km(app) = 60 µM) transport but is unable to transport galactose (362). These observations suggest that GLUT7 transports sugars under low abundance conditions and may, therefore, be important in scavenging luminal glucose and fructose in the ileum.
Hepatoportal Vein
The splanchnic circulation includes the blood flow through the stomach, small and large intestine, pancreas, spleen and liver. Seventy-five percent of the flow to the liver occurs via the hepatic portal vein, which carries the venous blood draining from each of these organs except the liver. The hepatic arteries form the second arm of the liver’s dual blood supply and provide 25% of hepatic blood flow. The hepatic portal vein does not drain into the heart. Rather, it delivers venous blood into the hepatic sinusoids of the liver. In carrying venous blood from the gastrointestinal tract to the liver, the hepatic portal vein accomplishes two tasks: 1) It supplies the liver with metabolic substrates and, 2) It ensures that ingested substances are first processed by the liver before reaching the systemic circulation. Upon transiting the liver sinusoids, blood from the liver is drained by the hepatic vein into the general circulation.
Liver
The hepatic sinusoids carrying portal and arterial blood are lined with fenestrated endothelial cells that permit the free passage of nutrients and proteins but not red blood cells. Monosaccharides transported from the small intestine via the portal vein are freely accessible to the basolateral (sinusoidal) membranes of hepatocytes. While GLUT1 is expressed in the endothelium, GLUT2 is the major glucose transporter isoform present in sinusoidal membrane of hepatocytes (578). GLUT2 expression is greater in periportal than in perivenous hepatocytes (575). GLUTl is present in the basolateral membranes of perivenous hepatocytes, which surround the terminal hepatic venules and also express GLUT2 (565). It is not known why these cells express cell surface GLUTl but the explanation may lie in the roles played by specific hepatocytes along the periportal-perivenous axis. Periportal hepatocytes are strongly gluconeogenic while perivenous hepatocytes are more glycolytic (300). As in kidney, cellular GLUT1 expression and glycolytic activity are correlated while GLUT2 expression and gluconeogenic capacity are linked. GLUT2 expression may also permit hepatocyte fructose uptake and metabolism since liver does not express GLUT5 but does metabolize fructose efficiently.
PERIPHERAL TISSUES
Upon exiting the liver, blood borne carbohydrates are returned to the heart where they are distributed throughout the circulation to the organ systems of the body. The subsequent metabolism of glucose, galactose and fructose and the cycling of their metabolites between the various organ systems to facilitate whole body carbohydrate homeostasis (see above) are crucially dependent upon the presence of specific monosaccharide and metabolite (specifically monocarboxylate) transporters that catalyze tissue metabolite import and export.
In fasted humans, resting serum [fructose] is 1.9 mM but peaks at 17 mM within 30 min of an oral load (273). Interestingly, fructose absorption is stimulated by inclusion of glucose in the oral load. In fasting rats given a large meal of fructose by gastric intubation, the maximum concentration of fructose found in portal vein blood is in the range 1.1 – 2.2 mM (582). The corresponding range in the systemic circulation is 0.1 to 0.33 mM. Fractional hepatic fructose uptake is 54.9% and 71.5% in fed and fasting rats, respectively with little extra-hepatic fructose removal. Fructose absorption raises blood lactate concentrations in both fed and fasting animals but does not increase lactate production by the intestine. No evidence was found for intestinal conversion of fructose to glucose (582).
Resting serum [Galactose] in humans is 0.1 mM and rises within 30 minutes to 1–2 mM after an oral dose of 0.5g/Kg body weight. Co administration of oral glucose competitively inhibits galactose absorption (621). Studies with preruminant calf indicate that 100% of galactose (70g/L) infused into duodenum is absorbed and serum [galactose] rises to 2.8 mM within 1 hour. One-half of the absorbed galactose is converted to glucose (127).
Pancreas
The pancreas is one of the 6 major glucose sensor systems of the body. Beta cells import glucose via GLUT2 resulting in insulin secretion (577). The counter regulatory response is mediated in part by pancreatic α-cell glucagon secretion. The question as to whether glucose inhibits α-cells directly or by paracrine mechanisms has been difficult to resolve (481). Paracrine signaling appears to be critical for glucose inhibition of glucagon secretion in rats (188, 611), but glucose acts directly in mice and humans (27, 381). The direct effect of glucose is demonstrable in isolated murine and human cells where paracrine effects are negligible, and in intact islets treated with inhibitors of paracrine signaling (381, 534). Glucose inhibits glucagon release at concentrations below the threshold for beta-cell activation and insulin release (381, 602). The use of transgenic mice supports the role of glucose-modulated KATP channels in α-cell function (219, 381). In humans, the Glu23Lys polymorphism in the KCNJ11 channel subunit is associated with diminished suppression of glucagon release during hyperglycaemia (585).
Αlpha-cells express GLUT1, whereas beta cells express GLUT2. Nevertheless, glucose transport is not a limiting factor in α-cell glucose metabolism (214, 249). In addition, it appears that alpha-cells rely on anaerobic glycolysis (480, 518) whereas beta-cells are more efficient at mitochondrial oxidation of glucose (523, 644). Again we observe that GLUT1 expression and glycolytic activity are well correlated. GLUT9 is also expressed in pancreatic beta cells (175). GLUT9 knockdown by RNAi reduces ATP levels and glucose induced insulin secretion in MIN6 cells and rat INS cells (a rat insulinoma cell line) suggesting (in the absence of off-target effects of the siRNA) a crucial role for GLUT9 in control of glucose-induced insulin secretion.
Muscle
Skeletal muscle accounts for 36 – 42% of a normal adult’s body mass. This simultaneously explains the blood glucose lowering capacity of muscle upon insulin stimulation of muscle glucose transport and during contractile activity when blood flow is redistributed to muscle (367) and muscle glucose utilization increases significantly (see above). During moderate to heavy exercise, the large amounts of lactate released by contracting skeletal muscle fibers are either metabolized by adjacent oxidative fibers, utilized by the liver in gluconeogenesis or oxidatively metabolized by the brain to make up any shortfall resulting from glucose redistribution to skeletal muscle. Skeletal muscle is the primary site of insulin-dependent glucose disposal. Skeletal muscle resistance to insulin-dependent glucose uptake and phosphorylation is an early step in the development of type 2 diabetes.
GLUT4 is the major glucose transporter in skeletal muscle fibers. When muscle is exposed to insulin or stimulated to contract, GLUT4 is translocated from intracellular, membrane-bound compartments to the sarcolemma and transverse t-tubules (343, 405, 475, 627). This response results in greatly increased glucose uptake, increased glycogen synthesis (when insulin is the agonist) or increased glycolysis and glycogenolysis (when contractile activity or hypoxia are the stimulant) (233). The net effect is reduced blood glucose.
High resolution, confocal microscopy techniques in combination with the expression of fluorescent glucose transport proteins in transgenic mice have facilitated a level of analysis of GLUT4 translocation in muscle that has not been previously feasible (340, 342). These developments allow detailed analysis of the spatial and temporal kinetics of contraction- and insulin-stimulated GLUT4 translocation, steady-state recycling, and subsequent reinternalization in live, anesthetized mice. Insulin promotes translocation of GLUT4-EGFP (a GLUT4 fused to enhanced green fluorescent protein) to the sarcolemma more rapidly than GLUT4-EGFP translocation to the t-tubules. The delay appears to result from differences in the kinetics of insulin signaling to each pathway. Following insulin removal, GLUT4-EGFP reinternalization from the sarcolemma is more rapid than recovery from the t-tubules. In contrast, the kinetics of contraction-stimulated GLUT4 translocation and re-internalization to and from the sarcolemma and t-tubules are similar for each compartment (341, 342). These behaviors reinforce the biochemical evidence indicating that insulin- and contraction-stimulated GLUT4translocation signaling mechanisms are distinct.
Muscle-specific GLUT4 knockout mice are insulin resistant, mildly diabetic and show increased muscle glycogen content in the fasted state (315). Reduced skeletal muscle glucose transport increases glycogen synthase activity as a result of increased levels of hexokinase II, glucose-6-phosphate, and regulatory and catalytic components of the glycogen synthesis pathway (315). Cardiomegaly is observed in GLUT4-null mice and muscle-specific (650) GLUT4 knockout mice.
The absence of severe diabetes in the muscle-specific GLUT4 null mouse and the persistence of a modest, insulin-stimulation of glucose uptake into soleus muscle (548) were quite surprising. Seven human skeletal muscle-specific GLUT isoforms have since been identified. These are (in decreasing order of mRNA expression) GLUT4, GLUT5, GLUT12, GLUT8, GLUT11, GLUT3, and GLUT1 but of these, GLUT4, GLUT5, and GLUT12 account for 98% of the mRNA (550). GLUT4, GLUT5, and GLUT12 proteins are expressed in normal human muscle and immunofluorescence studies reveal that GLUT4 and GLUT12 are predominantly expressed in type I oxidative (red) fibers, while GLUT5 is expressed in type II (white) fibers (550). The ratio of muscle GLUT4:GLUT12 mRNA is 12:1 whereas the ratio of protein levels is closer to 8:1 (550).
GLUT4 and GLUT12 have carboxyl termini that contain a dileucine motif, which in other proteins targets intracellular expression (296). GLUT8 also contains the amino terminal dileucine motif, but is expressed at a much lower level than GLUT12 (550). GLUT4 and GLUT12 are translocated to the cell surface upon insulin stimulation of skeletal muscle where 12% of the insulin-translocatable GLUTs are accounted for by GLUT12 (549).
Fat
Adipose tissue is responsible for only a small percentage (20%) of total glucose uptake of an oral glucose load in humans (207, 407) and 3–5% of glucose uptake during euglycemic hyperinsulinemic clamps in rats (498). Insulin dependent glucose uptake promotes increased adipocyte lipid synthesis and inhibition of lipolysis (497).
Adipocyte glucose transport is mediated by GLUT1 and GLUT4 under basal (fasting) conditions and, following glucose-induced insulin release, is mediated by a large excess of GLUT4 which is mobilized from intracellular vesicles to the plasma membrane by a mechanism entirely analogous to that utilized in skeletal muscle (69, 328, 371, 507, 537, 608).
Adipocyte-specific GLUT4knockout mice develop normally and adipocyte number and size are unaffected (2). This has been interpreted to suggest that GLUT1-mediated glucose transported in adipocytes is sufficient to generate adequate levels of glycerol 3-phosphate for triglyceride synthesis (431). Heart weight is normal in adipose-GLUT4 knockout mice in contrast to the cardiomegaly observed in GLUT4-null mice, cardiac-specific GLUT4 knockout mice and (1) and muscle-specific (650) GLUT4 knockout mice.
Although white adipose tissue accounts for less than 20% of whole-body glucose uptake (285), adipose-specific GLUT4 knockout mice are insulin resistant and glucose intolerant (431). As expected, insulin-stimulated (GLUT4-dependent) 2-deoxy-D-glucose uptake into white and brown adipose in vivo is markedly reduced. Although glucose uptake into skeletal muscle in vitro is unaffected, muscle glucose uptake in vivo is impaired despite normal GLUT4 expression in muscle. In addition, insulin-induced suppression of hepatic glucose production is impaired in adipose-specific GLUT4 knockout mice. This may result from impairment of insulin-dependent activation of hepatic phosphoinositide-3-kinase in adipose-specific GLUT4 knockout mice (2) and may contribute to defective insulin responses in these tissues. Reduced insulin-stimulated glucose transport in adipocytes therefore secondarily induces insulin resistance in other insulin target tissues and may be caused by altered secretion of an as yet unidentified adipocyte derived molecule that affects insulin action in other tissues (431). GLUT10 and GLUT12 mRNAs are also expressed in adipose (630, 631) although the functional significance of these transcripts is unknown.
Cardiac Muscle
Cardiac muscle expresses two major glucose transporter isoforms - GLUT1 and GLUT4 (330). GLUT1 is expressed at the cardiomyocyte surface and in intracellular vesicles whereas GLUT4 is restricted to intracellular locations under basal conditions. The ratio of GLUT1:GLUT4 in cardiomyocytes is approximately 1:4. Insulin and exercise increase cardiac glucose uptake by translocating GLUT4 to the cell surface (330, 543). Thus GLUT1 is thought to mediate glucose transport under basal (fasted and resting) conditions and GLUT4 mediates the increase in glucose transport observed during insulin exposure and exercise.
In cardiac-specific GLUT4 knockout mice, cardiac hypertrophy is more modest than observed in GLUT4 null mice (1). Plasma glucose and insulin levels and glucose tolerance curves are normal although basal glucose transport is increased two-fold and insulin stimulation of glucose uptake by the heart is eliminated (1).
Using the CreLoxP system to delete GLUT4 in muscle tissue including heart, Kaczmarczyk et al. (302) demonstrate reduced GLUT4 expression in all muscle tissues (including heart but not brown adipose) to levels 15–30% of control mice. In mice expressing Cre recombinase, there is a further reduction in cardiac tissue GLUT4 to almost undetectable levels. Cardiac sugar uptake under basal and insulin-stimulated conditions is normal in hearts expressing 15% of normal GLUT4 levels but is markedly reduced in mice with the more profound reduction in GLUT4. Cardiac enlargement occurs only when GLUT4 levels are less than 5% of normal values. It therefore seems that there is a threshold level of GLUT4 in heart above which insulin-stimulated glucose uptake is maintained. As little as 5% of normal GLUT4 levels expressed in heart is sufficient to prevent the development of cardiac hypertrophy.
Reproductive system
Placenta
The fetal circulation is presented to maternal blood in the placenta via a network of placental villous trees, which bathe in maternal blood in the placental intervillous space (47). Since the fetal villous capillary endothelium is fenestrated (345), maternal and fetal blood are separated only by the syncytiotrophoblast which forms the surface of the villous trees and serves as an epithelium separating the two fluid compartments. The apical plasma membrane of the syncytium faces the maternal intervillous space and the basal plasma membrane faces the fetal capillary endothelium. Transport of solutes from maternal to fetal circulation requires transcellular transport across both apical and basal membranes. This transport is catalyzed by facilitated diffusion (carriers and and ion channels) and by secondary and primary active transport (292, 491).
Glucose transport across the placenta occurs by facilitated diffusion (292). As a consequence, net transport of glucose across the placenta (always from high to low glucose concentrations) is determined by maternal and fetal blood glucose concentrations. The earliest measurements of glucose transport across the sheep placenta carry the distinction of giving rise to the modern era of carrier-mediated transport. Widdas’s measurements of hexose transfer across the sheep placenta enabled him to develop the first model for facilitated diffusion that qualitatively and in some instances quantitatively accounts for carrier-mediated nonelectrolyte transport in cells (616). Although later biochemical studies (211, 612) refuted the physical mechanism he initially proposed for transport (a mobile particle that carried glucose across the membrane), it remains a caprice of kinetic analysis that the mobile carrier model is mathematically indistinguishable from the simple or alternating carrier hypothesis, the fixed-site carrier hypothesis (94) or from a gated channel model for transport (266). While compatibility of a transporter's kinetic behavior with the predictions of a putative physical mechanism for transport does not prove that the proposed mechanism is correct, the utility of kinetic analysis is that incompatibility remains sufficient grounds to reject a hypothesis.
Messenger RNAs encoding six members of the GLUT family are expressed in human placenta (44, 225, 278). In normal human pregnancy, syncytiotrophoblast expression of GLUT isoforms changes as pregnancy progresses. GLUT1 is highly expressed in the placental barrier throughout pregnancy (287). GLUT 3 and the insulin-sensitive isoforms GLUT4 and 12 are expressed in the syncytium in early pregnancy (173, 225). At term, GLUT3 and GLUT4 expression is limited to endothelial (241) and stromal cells (637) respectively. GLUT1 expression and glucose transport activity at the basal plasma membrane are lower than at the microvillous (apical) membrane of the syncytiotrophoblast (287). It seems likely, therefore, that transport across the basal membrane is rate-limiting for trans-placental glucose transport (287). Studies using polarized BeWo cell monolayers directly support this hypothesis (598). The BeWo choriocarcinoma cell line is derived from trophoblast cells and forms a polarized monolayer on cell culture membrane inserts (370).
Transfer of glucose across the placental barrier may proceed as follows: intervillous glucose is transported by GLUT3 and GLUT1 in the apical syncytiotrophoblast membrane into the cytoplasm. Glucose then leaves the syncytiotrophoblast via GLUT1, and enters the fetal circulation by crossing the endothelial cell through numerous fenestrations (533). In the rat, the synctiotrophoblast surrounding the fetal circulation comprises two cell layers connected by gap junctions or connexins (533) and GLUT3 is also expressed at the plasma membrane of the inner syncytiotrophoblast layer (layer II) that faces the outermost syncytiotrophoblast (layer I in contact with maternal blood). This may serve to recover glucose that somehow crosses the syncytiotrophoblast I cell layer (533).
In rodents and sheep (and unlike humans), placental GLUT 3 mRNA and protein levels increase as gestation advances whereas GLUT 1 abundance is unchanged or may decrease towards term (45, 649). In the second half of gestation, placental GLUT abundance is altered in an isoform-specific manner byglucocorticoid administration and by variations in nutrient availability (187). Increases and decreases in GLUT protein abundance are dependent on the timing and duration of glucocorticoid and nutritional manipulations (187) indicating that placental GLUT protein abundance is responsive to environmental conditions and/or the concomitant changes in fetal growth that these conditions induce.
Ovary
The ovary body comprises large cortical and smaller, inner medullary regions that are composed of fibroblast-like cells and smooth-muscle cells. The medullary region contains the arterial and venous blood vessels and the cortical region nurses thefollicles containing individual oocytes (450). Primordial follicles mature into Graafian follicles which comprise (from outer to innermost layers): the Theca cell layer, the basal lamina then the Granulosa cell layer that surrounds the fluid filled Antrum. The Theca cell layer is vascularized by capillaries. The primary oocyte is enveloped by a single layer of Cumulus cells, is associated with the innermost cells of the granulosa cell layer and extends into the follicular fluid of the Antrum. During ovulation, the ovarian surface is ruptured and the oocyte is released. Thereafter, follicleremnants transform into the corpus luteum, which secretes female sex hormones (450).
mRNAs encoding GLUT1 and 3 are present in the bovine follicle and corpus luteum and are expressed at levels comparable to those in seen in brain and heart (452). Much lower levels of GLUT4 are also present in these tissues. Isoform-specific expression is tissue- and stage-specific. Similar levels of GLUT1, 3 and 4 mRNAs are expressed in subordinate follicles and dominant, estradiol-active follicles during luteal and follicular phases. GLUT expression is significantly reduced in dominant estradiol-inactive follicles undergoing atresia. Follicular fluid glucose concentrations and granulosa cell GLUT1 and GLUT3 mRNA levels are negatively correlated in atretic follicles, suggesting that transporter expression is affected by substrate availability (452).
Metabolic shift is a peri-ovulatory, gonadotropin-driven event characterized by enhanced ovarian glucose uptake (25) and may serve to meet the increased energy needs of the growing follicle and the meiotically active oocyte. GLUT1 and GLUT3 proteins are present in cultured whole ovarian dispersates from rat and Interleukin 1b increases expression levels and glucose transport by 3 − 4-fold (324). Other members of the GLUT family (GLUTs 2, 4, and 5) are undetectable. Ovarian GLUT3 (but not GLUT1) expression surges at the time of ovulation (324). GLUT1 and GLUT3 are expressed most highly in follicle granulosa cells. GLUT1 and GLUT3 mRNAs are expressed throughout the ovary but at levels much lower than in follicles. GLUT3 is characterized by the highest intrinsic activity of all the GLUTs studied thus far (389) and is a high affinity (low Km(app)) glucose transporter. GLUT3 expression may, therefore, permit granulosa cells to catalyze high capacity glucose transport at low glucose concentrations.
In sheep, follicle granulosa cell GLUT1 levels are 7–18 fold greater than theca cell levels (623). GLUT4 is expressed at similar concentrations in bothcell types. GLUT1 and GLUT4 expression levels and ovulation rate are unchanged by substrate availability.
Mammary GLUTs
Lactose is the major carbohydrate and the major osmotic constituent of human milk. Lactose synthesis is therefore the major determinant of milk volume in the lactating human mammary gland. Lactose is synthesized from glucose and UDP-galactose. Glucose transport is therefore not only required at the plasma membrane but also at intracellular membranes that define the intracellular compartment of lactose synthesis (231).
The fundamental unit of a lactating mammary gland is the alveolus or acinus, which is a hollow cavity, a few millimeters large, lined with milk-secreting mammary epithelial cells, surrounded by myoepithelial cells and enveloped by a basement membrane. Individual acini connect, via a duct system, to form lobules, which drain into openings in the nipple via a lactiferous duct (514). Mammary epithelial cells are the milk-elaborating cells of the mammary gland. These cells are connected via tight junctions near the apical (lumen-facing) membrane and the individual constituents that comprise milk can only enter the acinar lumen by a limited number of pathways: 1) Milk protein, lactose, and other aqueous components undergo exocytosis in Golgi-derived secretory vesicles; 2) Milk fat is secreted via the milk fat globule; 3) Monovalent ions, water, and glucose are transported across the apical membrane of the cell via protein-mediated transport systems; 4) Components of the interstitial space may enter the ascinar lumen via transcytosis; 5) Plasma components may enter the lumen via the paracellular pathway but this route is open only during pregnancy, involution, and in inflammatory states such as mastitis (528).
Lactating mammary tissue transports D-glucose via facilitative diffusion. Guinea pig mammary tissue slices and both rat and murine mammary acini transport 2-deoxy-D-glucose and 3-O-methylglucose by a temperature-sensitive, saturable, cytochalasin B and phloretin inhibitable process (16, 479, 580). Guinea pig mammary tissue has more than one type of saturable transport system for D-glucose (16) in which cytochalasin B and phloretin do not completely inhibit 2-deoxy-D-glucose uptake.
Rat and bovine mammary tissue expresses GLUT1 mRNA and GLUT1 protein (82, 385, 646) and GLUT1 expression is greatest at the basolateral aspect of the mammary epithelium (84). This is consistent with the observation that 2-deoxy-D-glucose transport occurs from the blood-side of the mammary gland (581). GLUT4 has not been detected in lactating rat mammary epithelial cells (82) but is expressed in mammary adipocytes (82). Lactating rat mammary epithelial cells do not appear to express GLUT2 or GLUT5 (82). Lactating bovine mammary tissue does not express GLUT2 mRNA although low levels of GLUT3 and GLUT5 mRNA are present (647).
Na-dependent glucose transporter (SGLT1) is present in lactating rat mammary tissue (527, 531) but the expression locus and functional significance of this are unknown.
D-Glucose Transport at the Apical Membrane
The levels of D-glucose and other monosaccharides in the milk of most species are much lower than those found in plasma (178). D-Glucose and galactose are rapidly lost from the milk space following introduction into the lactating goat mammary gland via the teat canal (177). Fructose, however, is not removed suggesting that: 1) D-glucose and galactose cross the mammary epithelium via a transcellular, not a paracellular pathway; 2) The mammary epithelium apical membrane does not express fructose transporters (GLUT2, GLUT5 or GLUT7). This is consistent with the observation that radiolabeled 3-O-methyl-D-glucose introduced into the milk space of the goat mammary gland via the teat enters venous blood more rapidly than radiolabeled sorbitol (178).
GLUT1 protein is not detected on the apical membrane (84) but GLUT12 targets the apical membrane (382). It should be noted, however, that immunoblotting techniques using a GLUT1- antibody directed against a GLUT1-C-terminal peptide reveal different levels of GLUT1 at luminal and transluminal membranes of rat brain microvasculature endothelial cells. Related measurements using antibodies directed against the GLUT1 middle loop or the purified GLUT1 protein reveal similar amounts of GLUT1 in both membranes (536). Thus it is possible that the GLUT1 C-terminus can become masked at one membrane within a cell but not at another and the use of GLUT C-terminal peptide-directed antibodies may falsely report expression levels.
Transport of D-Glucose Across the Golgi Membrane
D-Glucose must cross the Golgi membrane to reach the site of lactose synthesis. Golgi vesicle fractions from lactating rat mammary tissue transport D-glucose (614) and contain a GLUT1-like protein. It is possible that GLUT1 en route to cell surface expression also transports D-glucose to the site of lactose synthesis (385). Madon et al. (385) used quantitative Western blotting and cytochalasin-B binding studies to demonstrate that GLUT1 is the major glucose transporter species in plasma membranes but constitutes only half of the glucose transporters in the Golgi membranes of lactating rat mammary epithelial cells. GLUT8 is also highly expressed in mammary gland (648) and this protein may function as an intracellular glucose transporter in other cells (515).
High capacity, intracellular glucose transport is almost unique to mammary epithelial cells although all cells undertaking gluconeogenesis (e.g. hepatocytes and kidney cells) must export glucose from the Golgi or ER into cytoplasm in order to release glucose into the blood. Note, however, that Thorens and co-workers (76, 226) have described normal hepatic glucose output in GLUT2-knockout mice suggesting that hepatocytes may secrete glucose via a vesicle-mediated process rather than by GLUT2-mediated facilitated diffusion of glucose from Golgi/ER to cytoplasm then from cytoplasm to interstitium. It is not known whether GLUT1-expressing perivenous hepatocytes (565) can compensate by upregulating gluconeogenesis in GLUT2 knockout mice.
Subcellular fractionation studies show that lactose synthesis occurs in the Golgi (310) where GLUT1 and GLUT8 are co-expressed (385, 515). GLUT1 is targeted to an intracellular Brefeldin A-sensitive compartment of Golgi-related vesicles in mammary epithelial cells in culture (231, 385). Brefeldin A inhibits protein transport from ER to Golgi (317).
Control of D-Glucose Transport
Bovine mammary GLUT1, GLUT8, GLUT12, SGLT1, and SGLT2 mRNAs increase from 4- to several hundred-fold in an isoform specific manner from day −40 to +7 relative to calving. GLUT1 and GLUT8 have the highest levels of expression (648). Intracellular GLUT1 concentrations in mouse mammary epithelial cells increase approximately 15-fold in response to prolactin and hydrocortisone (230). Mammary gland lactose synthesis and glucose uptake increase abruptly at the time of parturition and decline rapidly as the gland stops secretion before involution (148, 148). The expression of GLUT1 mRNA and protein fall within 24 h of litter removal in the rat (85).
Milk secretion in the rat is controlled by both prolactin and growth hormone, which act synergistically to maintain GLUT1 transporter expression in rat mammary gland plasma membranes (179). Prolactin may upregulate GLUT1 expression in cultured mouse mammary epithelial cells (272) and stimulates 2-deoxy-D-glucose uptake by mouse mammary gland explants (471).
Overnight starvation reversibly reduces 2-deoxy-D-glucose and 3-O-methylglucose uptake by rat mammary gland by 90% (581). Starvation decreases cytochalasin B-sensitive 3-O-methyl- D-glucose uptake in lactating mouse mammary epithelial cells and is reflected as decreased Vmax for uptake and decreased numbers of cytochalasin B binding sites on mammary cell plasma membranes (478). Rat mammary GLUT1 content is unchanged by starvation, suggesting that the reduced D-glucose transport results from translocation of GLUT1 carriers from the plasma membrane to an intracellular site in response to reduced interstitial glucose (84).
Testes
GLUT3 is expressed in the human testis in Sertoli cells, peritubular myoid cells, early spermatocytes, macrophage-like interstitial cells and cells in the small vessels walls (322). GLUT1, GLUT2, and GLUT3 are strongly expressed in the Sertoli cells, early spermatocytes, peritubular myoid cells, macrophage-like interstitial cells, and testicular endothelial cells (323). The newest member of the human GLUT family is GLUT14 (635) which exists as two alternatively spliced forms and is a duplicon of GLUT3. Variation from GLUT3 and differences between alternative splice forms are localized to the extreme N-terminus of the protein (635). GLUT14 is expressed almost uniquely in the testis.
Sperm
Spermatozoa express several hexose transporter isoforms that allow for the efficient uptake of glucose, fructose, and dehydroascorbic acid (19). GLUT3 and GLUT5 were the first GLUTs to be detected in sperm (73, 227). GLUT1, GLUT2, GLUT3, GLUT5, and low levels of GLUT4 are present in human, rat, and bull spermatozoa. Each transporter isoform has a typical subcellular localization in the sperm head and tail. GLUT3 and GLUT5 are expressed in the middle tail piece, GLUT1 is present in the principal tail piece, and the localization of GLUT2 differs according of the species examined. Bovine spermatozoa transport 2-deoxy-D-glucose, fructose, and the oxidized form of vitamin C, dehydroascorbic acid. Transport of 2-deoxyglucose and dehydroascorbic acid is inhibited by cytochalasin B indicating that facilitative transporters transfer both substrates. Transport of fructose is not affected by cytochalasin B, which is consistent for an important role for GLUT5 in fructosetransport in these cells. GLUT8 is expressed in mouse testis during spermatogenesis (208) but is limited to spermatids and spermatozoa. Expression begins when round spermatids are formed at postnatal day 24, persists throughout spermiogenesis, and is detected in spermatozoa, but not in immature germ cells, Sertoli cells and interstitial tissue. GLUT8 localization is restricted to the acrosome membrane and is also found inside the acrosomic lumen suggesting that this transporter plays some role in the fuel supply of spermatozoa and in the traffic of sugars during the capacitation and fertilization processes.
Oxidative metabolism may take place in the spermatozoan midpiece where a mitochondrial sheath is located, whereas glycolytic enzymes are concentrated in the principal piece of the tail, connected to the fibrous sheath (170, 186, 425, 426). Once again, although within a single cell in this instance, GLUT1 is associated with glycolytic metabolism.
Capacitation (the change in mammalian sperm that occurs after exposure to the female genital tract making the sperm competent to undergo the acrosome reaction) is associated with changes in the level of expression and/or location of several GLUTs; these changes seem to be species-specific (71). Capacitation causes spermatozoan GLUTs 1, 2, 3 and 5 to undergo cellular relocalization in dog sperm but not in other species (Bucci et al, 2010a). Dog sperm is able to achieve capacitation in medium lacking sugars and generates ATP through the mobilization of intracellular glycogen stores ((9, 10). Capacitation in other species requires the presence of extracellular sugars (70, 71).
Travis and colleagues (584) describe a positive correlation between increasing glucose concentrations during capacitation and fertilization, and increasing fertilization of zona pellucida (ZP)-intact eggs. Thus glucose is required in the fertilization medium in a post-sperm capacitation manner. While some binding and fusion between the plasma membrane of the sperm and egg occurs at glucose concentrations from 0 to 1 mM, glucose concentrations of 1 mM or higher greatly facilitate binding and fusion. These observations suggest that 1 mM glucose represents a threshold level that facilitates binding and fusion and that glucose is required during capacitation and fertilization under normal physiologic conditions.
IMMUNE SYSTEM
The immune system is crucial for the defense against infectious organisms and toxic products. A defect in any of its components can cause impaired immunity leading to systemic infections, cancer, autoimmune disorders and metabolic impairments (89). White blood cells (leukocytes) are the cells of the immune system and comprise five diverse types - neutrophils, eosinophils, basophils, lymphocytes and monocytes (monocytes differentiate into macrophages upon migration from the blood into organ systems) (72, 653).
Lymphocytes divide rapidly, use glucose as a primary fuel source and maintenance of immune homeostasis requires strict regulation of glucose utilization (89). GLUT1, GLUT3, GLUT4, GLUT5 and GLUT6 are expressed in lymphocytes (157, 174, 192, 402). Lymphocytes mobilize intracellular glucose transporters to the cell surface in a cell survival response - especially GLUT1 - the major glucose transporter in these cells (48, 282, 619, 629).
Control and insulin-stimulated glucose uptake are lower in splenocytes (leukocytes isolated from the spleen) than in thymocytes (leukocytes or T-lymphocytes derived from the thymus) (89). Uptake in thymocytes declines with age, while transport by splenocytes remains responsive to insulin. Thymocyte glucose uptake is blocked by antibodies directed against extracellular domains of GLUT1 and GLUT4 while the insulin response is also blocked by an anti-GLUT3 antibody. Splenocyte transport is blocked only by GLUT1 and GLUT4 antibodies (89).
Maratou and co-workers (402) have examined cell surface expression of GLUT1, GLUT3 and GLUT4 in resting and activated T-lymphocytes, B-lymphocytes, monocytes, polymorphonuclear leukocytes (PMLs) and natural-killer (NK) cells in the absence or presence of insulin. GLUT1 does not respond to insulin in either resting or activated cells. Insulin increases the cell surface abundance of GLUT3 and GLUT4 in resting monocytes and Blymphocytes; in contrast, T-lymphocytes and PMLs are unresponsive to insulin. Activated monocytes, B- and T- lymphocytes increase cell surface expression of all three GLUT isoforms, whereas only GLUT1 and GLUT3 are increased in PMLs. Insulin increases GLUT4 and GLUT3 total expression levels in all leukocytes (402). During infection, these mechanisms may redistribute glucose to the cells that mediate the immune response and which are crucial to survival.
In a separate study, Fu et al (192) demonstrated that lymphocytes express GLUT1 and GLUT3 proteins, and that cellular levels of both are increased 3–6-fold upon lymphocyte activation. Monocytes express 8.4-fold more GLUT3 protein and 88% less GLUT1 than lymphocytes, and activation increases GLUT1 levels 2-fold. Differentiation of monocytes into macrophages is associated with marked induction of GLUT3 and GLUT5 protein expression and high levels of GLUT1, GLUT3, and GLUT5 are maintained after macrophage transformation to foam cells. High GLUT1 and GLUT3 expression may provide fuel for the immune response, and high levels of high-affinity GLUT3 in macrophages might allow the cell to compete with pathogens for hexoses, even in the presence of low interstitial glucose concentrations. Foam cell GLUT1 and GLUT3 may provide hexose substrates and promote lipid loading. The role for the fructose transporter GLUT5 in macrophages and foam cells is unknown for while serum fructose levels range from 0.1 - 2 mM (273), the capacity of these cells to metabolize fructose has not been systematically studied.
SKELETAL SYSTEM
Bone contains several cell types (54).Osteoblasts, or immature bone cells, are mononucleate bone-forming cells derived from osteoprogenitor cells. They are found on the surface of osteoid seams (narrow regions of non-mineralized, newly formed organic matrix on the surface of a bone) and secrete osteoid (a protein mixture largely comprising Type I collagen), which mineralizes to become bone. Osteoblasts secrete prostaglandins, which modify bone, they produce alkaline phosphatase, which plays a role in bone mineralization and they produce matrix proteins. Osteoblasts become entrapped in the bone matrix where they develop into osteocytes - the mature bone cell. Osteoclasts breakdown and resorb bone allowing new bone to be deposited by osteoblasts and, therefore, play a major role in bone remodeling.(573).
Osteoblasts mediate phloridzin-inhibitable, insulin and parathyroid hormone stimulated, saturable sugar transport and express GLUT1 and GLUT3 mRNAs and proteins but not GLUT4 (228, 574, 652). As with primary rat osteoblasts (146, 280, 556), sugar transport and glycogen synthesis are increased by parathyroid hormone. IGF I and supra-physiological insulin concentrations also stimulate sugar uptake and [3H]-thymidine incorporation into DNA. The effects of IGF I on transport are acute whereas those of PTH are chronic. Thus IGF I, rather than insulin, may be a physiological regulator of sugar transport and glycogen synthesis in osteoblasts (652).
Osteoclasts express GLUT1 (560) and GLUT3 but not GLUT2 or GLUT4 (314). Neither GLUT1 nor GLUT3 expression are increased by RANKL (osteoclast differentiation factor) suggesting that transporter levels are constitutively maintained during osteoclastogenesis. Glucose metabolism is accelerated during osteoclast differentiation and includes a metabolic shift towards mitochondrial respiration (314).
Osteoclasts degrade bone by pumping molar quantities of HCl to dissolve the calcium salts of bone and by secreting enzymes that digest the organic components of the remaining matrix. This is an energy-intensive process supported by abundant mitochondria. Glucose, and to a lesser extent lactate, support osteoclastic bone degradation. Fatty acids (palmitate, myristate and stearate), essential amino acids plus 20 mM alanine, or ketone bodies (acetoacetate, betahydroxybutyrate and alpha-ketoglutarate) are unable to support bone degradation. Resorption is glucose concentration dependent (K1/2 = 3 mM) and glucose transport is stereoselective and inhibited by cytochalasin B (622). Osteoclasts cultured on bone, transport glucose twice as rapidly as cells cultured in the absence of bone and medium acid accumulation parallels glucose uptake. Glucose is, therefore, the principal energy source required for bone degradation and fluctuations in serum glucose concentration are an important component in regulation of osteoclastic bone degradation (622). Larsen et al. have proposed that osteoclasts utilize a glucose-sensing mechanism similar to that of β-cells whereby changes in the ATP/ADP ratio result in the mobilization of intracellular Ca2+ and activation of calmodulin dependent protein kinase II (338). While various osteoblast membrane channels are sensitive to ATP, these channels are sensitive to extracellular not intracellular purine nucleotides (35) and involve P2Y and P2X purinoceptors (444, 609). High D-glucose levels, (10–25 mM) but not L-glucose inhibit osteoblast differentiation into osteoclasts via a reactive oxygen species-dependent mechanism (628) and inhibit bone mineralization by inhibiting Ca uptake by osteoblasts (35).
Mice lacking the osteoblast-secreted molecule osteocalcin are characterized by decreased pancreatic islet β-cell proliferation, glucose intolerance and insulin resistance. Osteocalcin stimulates insulin expression in β-cells and adiponectin secretion, an insulin-sensitizing adipokine, in adipocytes and improves glucose tolerance (346). Hence the skeleton exerts an endocrine regulation of carbohydrate homeostasis.
Transcripts (mRNAs) for 9 monosaccharide transporter isoforms (GLUT1, 3, 5, 6, 8, 9, 10, 11 and 12) are expressed in human articular cartilage (488). Immunohistochemistry confirms that GLUT1, GLUT3 and GLUT9 proteins are expressed in normal human articular cartilage. Chondrocyte (cartilage cells) 2-deoxy-D-glucose transport is temperature sensitive, inhibited by cytochalasin B and phloretin, and is accelerated by IGF-I. Sugar transport is unaffected by insulin. Secretion of MMP-2 (a type IV collagenase) is increased in the absence of glucose. Thus glucose transport and metabolism assume a central role in the synthesis and degradation of cartilage (488).
BLOOD SYSTEM AND BLOOD TISSUE BARRIERS
Bone marrow
Bone marrow contains three types of stem cells: 1) Hematopoietic stem cells which give rise to the blood cells that are found in the circulation: red cells (erythrocytes), leukocytes, and platelets (thrombocytes)(271, 625, 626); 2) Mesenchymal stem cells are found arrayed around the central sinus in the bone marrow and can differentiate into osteoblasts, chondrocytes, myocytes, and other types of cells (469, 474, 510); 3) Endothelial stem cells (513).
Erythropoiesis
Erythropoiesis involves the differentiation of proerythroblasts into erythroblasts, which are transformed into reticulocytes then finally mature into erythrocytes. Immature reticulocytes are generated through the process of enucleation, which occurs within bone marrow erythroid niches called erythroblastic islands. Reticulocyte maturation requires approximately 72 hours for completion, and for two-thirds of this time occurs in the marrow - the final third occurring in the circulation (57, 105). Maturation involves the complete loss of intracellular organelles, including mitochondria, endoplasmic reticulum, Golgi apparatus, and endocytic vesicles (220). Equally important is the extensive remodeling of the plasma membrane which results in the progressive loss of specific membrane proteins. These include the transferrin receptor (iron for heme synthesis is no longer required) and adhesion receptors such as β1-integrin whose presence would otherwise cause mature circulating red cells to adhere to vascular endothelial cells (490).
Canine reticulocytes remodel membrane protein content by shedding exosomes or small membrane vesicles. These vesicles contain heat shock protein cognate 70 (Hsc70), transferrin receptors (TfR), the Na+,K+ATPase α-subunit, GLUT1 and stomatin (a lipid raft-associated protein) (325). Extrusion of these proteins leads to their depletion in erythrocytes, while the major protein constituents of erythrocyte membranes, spectrin and the anion transporter are retained in reticulocytes (325).
Fetal and newborn mice express GLUT1 as a major erythrocyte glucose transporter as judged by surface binding of Human T Cell Leukemia Virus 1 and 2 (HTLV-1 and −2) envelope glycoprotein binding to cells and GLUT4 as judged by immunoblot analysis (433, 434). HTLV-1 and 2 inhibit glucose transport by interacting with GLUT1. Receptor binding and HTLV envelope-driven infection are selectively inhibited when glucose transport or GLUT1 expression are blocked by cytochalasin B or siRNAs, respectively (393). The amount of GLUT1 and GLUT4 falls rapidly (t1/2 = 9 −15 days) until only GLUT4 is detected in adult murine erythrocytes. Dolznig et al (161) report very high levels of GLUT1 mRNA expression in fetal murine erythroblasts which increases 7-fold within 48 hours of initiation of differentiation.
In a classic study, Taylor and co-workers (433) demonstrate that erythropoietin-stimulated erythropoiesis of human CD34+ progenitor cells results in the appearance of erythroid progenitors and, using cell surface expression of glycophorin A, the transferrin receptor and GLUT1 to track the progression of erythropoiesis, they observe that GLUT1 is not detected on immature progenitors but is induced at the basophilic erythroblast stage and increases further in acidophilic erythroblasts. Glycophorin A expression precedes GLUT1 expression but from the acidophilic erythroblast stage both markers remain elevated throughout the differentiation process. GLUT1 protein is not present in CD34+ cells expanded in the absence of erythropoietin where GLUT1 mRNA is barely detectable. GLUT1 mRNA levels increase by 3-orders of magnitude following erythropoietin-induced differentiation. The electrophoretic mobility of GLUT1 increases during erythropoiesis, consistent with the previously reported loss of glycosylation (438).
Although avian erythrocytes are nucleated and retain some mitochondria, avian erythroid progenitor cells also undergo significant membrane and organelle remodeling during erythropoiesis (329). Grdisa and White (216–218) have demonstrated that pre-erythroid avian HD3 cells are characterized by high glucose transport activity which is lost upon differentiation to the red cell phenotype. This maturation is accompanied by significant loss of GLUT1 and GLUT3 mRNAs.
Hematopoietic cytokines stimulate erythroid precursor cell proliferation and promote the survival and function of mature cells. These processes require energy, and cytokines such as IL- 3, lL-1, and GM-CSF enhance glucose transport in hematopoietic cells (152).
Red Cells
Human red blood cells contain 250,000 − 500,000 copies of GLUT1 (91, 366, 525, 544, 570, 651) and significantly fewer copies of GLUT5 (125). GLUT5 catalyzes fructose transport whereas GLUT1 catalyzes glucose and galactose transport.
Dog (455) and mouse (161, 433, 434) erythrocytes express GLUT4 as the major glucose transporter whereas higher primates, guinea pigs and fruit bats (mammals unable to synthesize ascorbic acid from glucose) express GLUT1 as the major sugar transporter (433, 434). In keeping with this association, cetacean erythrocytes express very high levels of GLUT1 (134, 138, 143) and cetaceans may also require dietary vitamin C (204). It should be noted that rat erythrocytes catalyze protein-mediated sugar transport characterized by accelerated exchange suggesting that they do not express GLUT4 (252, 445)
What is the link between the need for dietary vitamin C and erythrocyte GLUT1 content? The first clues arose when Mann and Newton (397) demonstrated that human red cells import dehydroascorbate via a facilitated diffusion mechanism and that this transport is strongly inhibited by physiologic glucose levels. This observation was expanded further by the demonstration that placental dehydroascorbate uptake is inhibited by cytochalasin B and by 3-O-methylglucose (279). In an important series of studies, Vera, Golde and colleagues (599–601) demonstrate that GLUT1 expressed in Xenopus oocytes catalyzes dehydroascorbate transport inhibitable by 3-O-methylglucose and by cytochalasin B and that these functional properties are recapitulated in human HL-60 myeloid leukemia cells. Rumsey and co-workers later added GLUT3 and GLUT4 but eliminated GLUT2 and GLUT5 from the cast of glucose transporters that transport dehydroascorbate in a 3-O-methylglucose or cytochalasin B inhibitable manner (501, 502). Thus dehydroascorbate transport is mediated by GLUT1, GLUT3 and GLUT4 in a glucose and cytochalasin B inhibitable manner.
May and co-workers have shown that in human red cells, intracellular dehydroascorbate is reduced to ascorbate by glutathione thereby allowing the cell to accumulate ascorbate in excess of extracellular levels (414). Intracellular ascorbate donates electrons to a transmembrane oxidoreductase which reduces extracellular oxidants and reoxidizes intracellular ascorbate to dehydroascorbate which is then either transported down its concentration gradient out of the cell or is reduced by glutathione to reform ascorbate (414). In the presence of 100 µM dehydroascorbate, erythrocytes are able to regenerate 35 µM ascorbate every 3 min and this absolutely requires intracellular glutathione. Recycled ascorbate is released from cells into plasma at a rate less than one tenth that of dehydroascorbate uptake and conversion to ascorbate, and protects human LDL α-tocopherol from oxidation by free radicals. Thus recycling of ascorbate in erythrocytes helps to maintain the antioxidant reserve of blood (423) and dehydroascorbate transport by erythrocytes in vitamin C auxotrophs allows the blood to maintain antioxidant reserves. Guinea pig red cells use glucose to regenerate glutathione whereas rabbit red cells (which possess much lower glucose transport capacity and, based on their lack of accelerated exchange glucose transport possibly utilize GLUT4 (486)) metabolize adenine and inosine (554, 555). Since GLUT1, GLUT3 and GLUT4 can mediate dehydroascorbate transport, the question remains - why do erythrocytes of vitamin C auxotrophs express GLUT1 vs GLUT4?
Rumsey and co-workers answer this question by demonstrating that GLUT1 and GLUT3 transport sugars and dehydroascorbate at similar rates while GLUT4 transports sugars at rates quite similar to GLUT1 but transports dehydroascorbate some 6–12-fold more slowly (501, 502). Thus GLUT1 expression may provide greater dehydroascorbate transport capacity for vitamin C auxotroph erythrocytes and thereby permit ascorbate recycling rates compatible with the rate of production of extracellular oxidants. This becomes even more important when one considers that GLUT1 and GLUT4-mediated dehydroascorbate transport are inhibited by some 66–70% by physiologic glucose levels (279, 414, 501, 502, 599–601).
Fruit bat and dolphin erythrocytes express GLUT1 at levels quite similar (30 − 50%) to their human counterparts (136, 138). Newborn guinea pig erythrocytes are also highly glucose permeable. However, there is a progressive loss of transport so that by the time a guinea pig is 7 months old, glucose permeability and the presence of GLUT1 can no longer be demonstrated (327). This raises an interesting question. How old were the guinea pigs used in studies demonstrating high levels of cell surface GLUT1 using eGFP-tagged HTLV receptor-binding domain (HRBD) fusion protein (432, 433)? If ascorbate recycling is required to maintain blood redox equilibrium, how do adult guinea pigs achieve this when their red cells have lost GLUT1 and the capacity to transport sugars (327)? Do they also express a GLUT1-independent dehydroascorbate transporter as suggested by Bianchi and Rose (52)? Perhaps expression of this transporter and GLUT1 are developmentally linked in neonatal vitamin C auxotrophs. Or, perhaps as suggested by Montel-Hagen and colleagues, GLUT1 develops the capacity to transport dehydroascorbic acid when cellular stomatin levels increase. Stomatin expression is proposed to decrease glucose transport capacity and result in the loss of glucose-inhibition of GLUT1-mediated dehydroascorbate transport (433). This is an interesting hypothesis that requires further investigation. Previous findings demonstrate strong inhibition of human erythrocyte dehydroascorbate uptake by physiologic glucose levels (397); it has also been suggested that the reported differential effects of glucose on sugar and dehydroascorbate uptake (433) may reflect an impact on substrate metabolism rather than transport (90).
A second question arising from the high level of GLUT1 expression in higher primate and cetacean erythrocytes is what possible advantage does this very high glucose transport capacity afford the organism? Human red cell sugar transport capacity is almost 3-orders of magnitude greater than erythrocyte metabolic capacity (284, 615). Dolphin red blood cells catabolize D-glucose even more slowly than human red blood cells (239) suggesting that overall ratio of transport : metabolic capacities are similar in both species. Craik and colleagues have suggested that high glucose transport capacity maximizes glucose delivery to specific regions of the brain under conditions of physiological stress (e.g. hypoxia in aquatic mammals) (138). For example, human erythrocytes loaded with 5 mM glucose can export more than 66% of cellular glucose in 500 msec at 37°C (376). This means that the effective glucose space of the blood is serum plus cytosolic water (i.e. 90% of blood space). Since the mean transit time for a red blood corpuscle in the cerebral microvasculature is approximately 500 msec (155), extracellular glucose and 66% of intracellular glucose may be transferred to the brain if transport across the blood brain barrier is not limiting. Measurements of glucose consumption by the brain and rates of glucose delivery to the central nervous system support this conjecture both in odentocetes (100) and humans (539). Primates and odontocetes are notable for large, complex central nervous systems and high brain mass/body mass ratios (259).
Platelets
Platelets play a crucial role in hemostasis and thrombosis. They originate from megakaryocytes in the bone marrow and adhere to sites of injury. In their resting state, platelets are nonadhesive, have a discoid shape, and contain a large number of α-granules. α-Granules contain adhesive proteins such as fibrinogen, fibronectin, von Willebrand factor (542) and growth factors such as TGFβ and PDGF (368). Platelets become activated at sites of injury in a process that promotes the formation of a platelet thrombus and wound healing (248). Extracellular glucose is an important source of energy for human platelets where much of the metabolic flux is directed toward lactate production (8).
Human platelets catalyze rapid, stereoselective, saturable, cytochalasin B inhibitable 2-deoxy-D-glucose uptake (355). Because sugar uptake is not inhibited by fructose, it is probable that platelets do not express GLUT2 or GLUT5. Platelets do express GLUT3 but not GLUT1 (137). Thrombin causes a rapid and pronounced platelet shape change, secretion of most α- granules and a concomitant 3-fold increase in glucose transport and cell surface GLUT3 expression. Intracellular GLUT3 is mobilized from α-granules to the cell surface (248).
BLOOD TISSUE BARRIERS
Some tissues are protected from serum-borne nutrients and electrolytes by blood-tissue barriers. These tissues include the brain, the retina, the olfactory epithelium, the inner ear, peripheral nerve and the myocardium (396, 561). These barriers are formed by endothelial cells, which line the capillaries and are sealed by tight junctions (561). GLUT1 is expressed at high levels in endothelial cells and constitutes the major pathway for transcellular glucose movement across these barriers. Glucose transport into brain, peripheral nerve and retina is especially important because glucose stores in these tissues are small relative to glucose demand (332).
Blood Brain Barrier
The cells of the mammalian brain do not contain large stores of glycogen. It is important, therefore, that glucose uptake by the brain matches or exceeds glucose utilization in order to support brain function. To enter the brain, serum glucose must cross the blood brain barrier - an epithelium comprising endothelial cells connected by tight junctions that prevent paracellular diffusion of glucose and other nutrients. Thus, glucose transport into the brain requires trans-endothelial cell transport. This process is catalyzed by the glucose transport protein GLUT1, which is expressed at both luminal and abluminal membranes of the endothelium (185, 201, 303, 462, 561, 562). Developmental expression of GLUT1 in rat brain microvessels increases progressively from birth through at least day 30 postnatal when 80% of adult GLUT1 expression levels are attained (594).
GLUT3 and GLUT5 are also expressed in blood tissue barriers (400) but it appears the level of expression is lower than GLUT1 expression. GLUT1 appears to be the major glucose transporter found in endothelial cells of the rat blood brain barrier (349); whereas dog and bovine blood brain barriers express GLUT1 and GLUT3 (36, 199, 401). Proteomic analysis of membrane proteins isolated from human and mouse brain microvessels indicates that in humans, GLUT1 levels are some 32-fold greater than GLUT3 expression levels. GLUT3 levels in humans are at least 7-fold greater than in mouse microvessels but GLUT1 levels are almost identical in both species (587) and, since the rate of glucose uptake by human and rodent brain is similar (223, 463), glucose supply into the brain must be largely mediated by GLUT1.
Brain glucose import and metabolism are finely balanced. At 5 mM serum glucose the rate of glucose uptake is 0.34 µmol/g/min (51, 132, 150, 206, 224). The rate of glucose consumption is 0.25 to 0.35 µmol/g per minute in rodent and human cortex under basal conditions (115, 150, 222, 240, 386, 588). Glucose transport activity is, therefore, just sufficient to keep up with basal glucose utilization in brain.
Endothelial cells of the blood-brain barrier (bEND) differ from those of the peripheral circulatory system (pEND) in several important ways. 1) bEND cells contain 2–5-fold more mitochondria than pEND cells (457). 2) Brain capillary walls are 40% thinner than capillary walls of the peripheral circulation (128). 3) pEND cells present significantly fewer tight junctions than bEND cells (151). 4) bEND cell tight junction complexes result in polarized cell surface protein expression that is less marked or absent in pEND cells (151). The resulting bEND cell architecture may give rise to behaviors that differ from those of pEND cells but that resemble those of other metabolically active cells and thereby optimally support blood brain barrier physiology.
The importance of endothelial cell GLUT1 to brain glucose uptake is illustrated by three behaviors: 1) adaptations to altered substrate availability; 2) The impact of GLUT1 mutations on brain function; 3) Correlations between GLUT1 expression and glucose utilization.
1) Sugar transport into the brain only narrowly exceeds brain glucose utilization under normal conditions (539). Under conditions of metabolic stress, such as hypoxia (236), hypoglycemia (333, 416, 538), and seizures (132, 463), the glucose import capacity (Vmax)of the brain is up-regulated. Endothelial cell affinity for transported sugars appears to be unchanged (132) suggesting: a) increased GLUT1 at the plasma membrane, either through increased protein expression or recruitment of intracellular stores; b) enhanced intrinsic activity of GLUT1; or c) a combination of both effects. Chronic stress induces transcriptional up-regulation of endothelial GLUT1 levels in vitro (63) and in vivo (64). Endothelial cell glucose transport activity is also acutely unregulated in vitro by AMPK-dependent translocation of intracellular GLUT1 to the plasma membrane (142).
2) Naturally occurring GLUT1 mutations can reduce GLUT1 expression or transport activity and thereby reduce glucose transport across the blood-brain-barrier. Such mutations reduce cerebrospinal glucose levels and cause seizures and delayed development (263, 313, 318, 521).
3) A positive correlation between regional GLUT1 and GLUT3 expression levels and 3-Omethylglucose transport and glucose utilization is demonstrable in rat brain (166, 167).
Blood-Peripheral nerve barrier
Each nerve comprises a collection of nerve fascicles each surrounded by a perineurial sheath and containing a parallel series of axons and endoneurial capillaries (114). The blood-nerve barrier consists of endoneurial capillaries and the perineurial sheath. Endothelial cells of the endoneurial capillaries are connected by tight junctions and thus do not form a fenestrated endothelium. The perineurial sheath restricts fluid movements between the extracellular spaces surrounding nerve fascicles and the endoneurium (604). Blood-neural barriers are functionally and dynamically regulated by factors recruited from or released by adjacent cells (114).
GLUT1 and the tight junction protein occludin are present in the cells of the perineurium and endoneurial capillaries (586) where they constitute a pathway for the selective transfer of glucose across the barrier while preventing the nonspecific flow of blood constituents respectively. In rats, GLUT1 is expressed in the plasma membrane and cytoplasm of myelinating Schwann cells around the nodes of Ranvier and in the Schmidt-Lanterman incisures, making them potential routes for transcellular glucose transport. GLUT1 is not detected in axonal membranes. GLUT3 mRNA and polypeptide are barely detected in peripheral nerve from young adult rats. However, in 13-month-old rats, GLUT3 polypeptide is present in myelinated fibers, endoneurial capillaries, and perineurium. GLUT3 may be preferentially expressed in the paranodal regions of Schwann cells and nodal axons of myelinated fibers, but is also present in internodal regions. These findings suggest that Schwann cell GLUT1 and axonal and Schwann cell GLUT3 are involved in the transport of glucose into the metabolically active regions of peripheral axons (387).
Blood-Olfactory nerve barrier
The sensory cells of the olfactory system, olfactory receptor neurons, are embedded in the nasal olfactory epithelium and project directly to the olfactory bulb of the brain. The apical domain of the olfactory receptor neuron serves as the olfaction sensor and, accordingly, is exposed to the lumen. Immunohistochemical analysis shows that the junctions of the olfactory epithelium contain the tight junction protein occludin (274). Endothelial cells in the blood vessels in the lamina propria of the olfactory mucosa also express occludin. These observations suggest that the olfactory system is guarded from both the external environment and the blood. GLUT1 is highly expressed in occludin-positive endothelial cells (274) suggesting that GLUT1- mediated glucose transport across the capillary endothelium is an important source of metabolic fuel for the cells of the olfactory system.
Blood Retina Barrier
The blood-retina barrier comprises two key membranes - the retinal capillary endothelium of the inner layer of the retina (forming the inner blood-retinal barrier) and the retinal pigment epithelium formed by retinal pigment epithelial cells of the outer layer of the retina (forming the outer blood-retinal barrier) and supplied by the choriocapillaries (268). Retinal capillary endothelial cells of the inner layer are connected by tight junctions as are the retinal pigment epithelial cells of the outer layer (196, 234, 268, 269).
Current understanding of blood-retina barrier function is based on two types of measurement. The retinal uptake index method uses a rapid intracarotid injection of test substrates followed by retinal sampling to assess the permeability properties of the rat barrier (13). For example, transport of D-glucose and L-lactate from the blood to the retina is catalyzed by carrier-mediated processes (12, 13). Studies with cultures of retinal endothelial cells permit analysis of the sugar transport properties of isolated cells (50) or, when tight junctions are formed in monolayers of cells, studies of trans-endothelial sugar transport become possible(20, 443, 526). The permeability of rat retinal endothelial cell monolayers to 3-O-methylglucose is significantly greater than monolayer permeability to other test compounds (526). GLUT1 and GLUT3 are expressed in human and bovine retinal endothelial cells (36, 320, 557).
Inner ear-blood barrier
The cochlea is the auditory portion of the inner ear containing the Organ of Corti - the sensory organ of hearing. The cochlea comprises two connected, fluid or perilymph-filled chambers (vestibular and tympanic) sandwiching an inner fluid chamber or scala media, which is filled with endolymph. The scala media houses the Organ of Corti, which sits on the basilar membrane between the scala media and scala tympani.
The upper portion of the outer wall of the cochlea (the periosteum) contains numerous capillary loops and small blood vessels, is termed the stria vascularis and produces endolymph for the scala media. The stria is a stratified epithelium containing three cell types (marginal, intermediate, and basal cells) and intraepithelial capillaries. The marginal cells face the endolymphatic space. These cells have highly folded basolateral membranes, are replete with mitochondria and are connected by tight junction proteins. The middle of region of the stria vascularis contains capillaries and intermediate cells. Multiple layers of flat, interleaved basal cells connected by tight junctions face the spiral ligament (the perilymphatic side). Basal and intermediate cells are connected by gap junctions, suggesting that these cells are coupled as a syncitium, allowing exchange of intracellular contents.
The stria vascularis of the cochlea generates the endocochlear potential and secretes K+ into the endolymph each being essential for the sensory transduction that leads to hearing. To maintain these characteristics, the stria vascularis consumes a large amount of energy (403), has a high respiratory quotient (404) suggesting that the primary energy source is carbohydrates, and has relatively small glycogen stores (564). Glucose uptake mechanisms are, therefore, indispensable for strial function.
GLUT1 is expressed in the basal and marginal cells of guinea pig stria vascularis (642), while other studies suggest that GLUT1 is expressed only in basal cells, not in marginal cells (18). GLUT1 is observed in the basal side of strial tissue and in capillaries in both rats and guinea pigs (169). Dissociated guinea pig strial basal and capillary endothelial cells, but not marginal cells, stain positively for GLUT1 (169). These results suggest that GLUT1 is not expressed in the marginal cells where another GLUT isoform must be expressed. Conventional RT-PCR and quantitative real-time PCR analysis indicates that GLUTs 1, 3, 4, 5, 8, 10, 12 and HMIT are present in the stria vascularis, whereas no SGLT isoforms are detected (169). GLUT1, GLUT5, and HMIT messages are 2-, 3- and 12-fold greater respectively in the stria vascularis versus spiral ligament. These findings strongly suggest that a number of GLUT isoforms participate in glucose transport in the stria vascularis and the spiral ligament.
The auditory hair cells are located within the organ of Corti and derive their name from the tufts of stereocilia or hair bundles that protrude from the apical surface of the cell into the scala media. Mammalian cochlear hair cells come in two types: the outer and inner hair cells. Outer hair cells do not send neural signals to the brain. Rather, they mechanically amplify low-level sound that enters the cochlea (184). Amplification may be powered by movement of hair bundles, or by an electrically driven motility of their cell bodies. Inner hair cells transform the sound vibrations in the fluids of the cochlea into electrical signals that are relayed to the auditory brainstem and to the auditory cortex via the auditory nerve (184).
GLUT5 is expressed in the lateral walls of outer hair cells (46, 449) but is not detected until 15 days post birth after electromotility of the outer hair cells is fully developed (46). Electromotility is known to be present only in outer hair cells (301), but GLUT5 is also expressed in inner hair cells (197) and supporting cells (Nakazawa et al., 1995). These observations appear to eliminate the possibility (197) that GLUT5 is the motor protein of outer hair cells.
CENTRAL NERVOUS SYSTEM
The GLUT content of the CNS has been discussed in two recent reviews (101, 539) and can be summarized as follows:
A 55 kDa form of GLUT1 is observed in the endothelial cells of the blood brain barrier.
A 45 kDa form of GLUT1 is found in epithelial cells from choroid plexus, ependymal cells, glial cells, and astrocytic end feet abutting endothelial cells.
GLUT2 is expressed in the embryonic granule layer of cerebellum and in ependymal hypothalamic cells and may play a role in glucose sensing (195, 454)
GLUT3 is the major glucose transporter expressed in neurons (see below).
GLUT4 is expressed in hippocampus and cerebellum (see below)
GLUT5 is expressed in the embryonic cerebellum (454)
GLUT8 is expressed in some neurons of hippocampus and other brain areas (275).
Two different molecular weight forms of GLUT1 (45 and 55 kDa based on electrophoretic mobility) have been detected in mammalian brain. The differences in apparent molecular weight are catalytically silent and result from differential glycosylation (55). The higher molecular weight 55 kDa form, which resembles that form found human erythrocytes, is expressed in the luminal and abluminal membranes of microvascular endothelial cells that comprise the blood brain barrier (154, 176, 237, 390, 541). Early studies of rat cerebral microvasculature endothelial cells suggested an asymmetric distribution of GLUT1 among these compartments: 11% luminal membranes, 44% abluminal membranes, and the remaining 45% residing in the intracellular pool (131, 176). Recent fractionation, kinetic studies, and electron microscopy studies indicate that GLUT1 is evenly distributed between luminal and abluminal membranes (201, 536, 539). While early studies maintained that GLUT1 was expressed only in the blood brain barrier, Western blot analysis of brain membranes free of vascular cell contamination demonstrates a 45 kDa GLUT1 peptide (390, 391, 541). This form of GLUT1 is found in all glial cells, as well as the basolateral and apical membranes of the choroid plexus, and ependyma. GLUT1 is expressed at only low levels in the astrocytic endfeet adjacent to the endothelial cell (539). There is limited in vivo expression of 45kDa GLUT1 in neurons, although expression is increased in response to environmental stressors or when neurons are placed in culture (200, 347, 388, 613).
GLUT3 in brain is localized almost exclusively to neurons (198, 390, 418, 448). During cerebral maturation the increase in GLUT3 expression precedes the expression of the glial 45 kDa GLUT1 and parallels neuronal maturation, synaptogenesis, functional activity, and increased rates of cerebral glucose utilization (595, 597).
Insulin-mobilized, intracellular vesicles that contain GLUT4 in skeletal muscle and adipose have been termed GLUT4 storage vesicles. Recent studies show that the known component proteins of GLUT4 storage vesicles (e.g. IRAP, sortilin and GLUT4), are expressed in the brain at significant levels (14, 21, 113, 171, 181–183, 321, 326, 352, 415, 417, 485, 596). Multiple neurons in forebrain, cerebral cortex and hippocampus express GLUT4. However, areas involved in the regulation of metabolism (hypothalamic nuclei) and motor activity (sensorimotor cortex, cerebellum, motor nuclei of cranial nerves and motor neurons of the ventral horn of the spinal cord) express GLUT4 most highly (419, 499).
GLUT4 storage vesicles are present in cerebellar neurons and these vesicles are distinct from small synaptic vesicles that characteristically mediate “kiss and run” or flickering neurosecretion (34, 545, 620). Neuronal GLUT4 vesicles are translocated to the plasma membrane in response to insulin stimulation and exercise (34).
SUGAR REABSORPTION
The kidneys play two crucial roles in glucose homeostasis - glucose recovery from the glomerular filtrate and gluconeogenesis. The glomerulus does not impede glucose transfer from serum to glomerular filtrate owing to the highly fenestrated nature of the glomerular endothelium (511). Hence, the glucose concentration in the glomerular filtrate is close to that in plasma. Assuming a glomerular filtration rate of 180 liters/day (approximately 72 volumes of serum) and a plasma glucose concentration of 4 mM, the filtered glucose load is 180 grams or 1 mole/day. However, only 0.1–0.3 grams glucose (0.06 − 0.2% of the filtered load) are excreted daily in the urine (311, 383). This remarkable retention process is brought about by the interplay of glucose transport proteins in the apical and basal membranes of the nephron (633).
Glucose reabsorption from the luminal fluid is a saturable process. Significant amounts of glucose are excreted by the kidney when plasma glucose exceeds 9 mM and the maximum reabsorption capacity is reached when plasma glucose reaches 15 mM. Above 15 mM, the rate of glucose excretion is directly proportional to the filtered load (633).
The proximal tubule of cortical nephrons ranges from 12 − 24 mm in length yet 90% or more of the filtered glucose is reabsorbed within the first 2–3 mm of the tubule (190). Since the glucose concentration within the luminal contents falls precipitously over the first 3 mm, this means that net glucose reabsorption must proceed against a concentration gradient. Studies of glucose transport in isolated brush border and basolateral membrane vesicles (316) demonstrate that glucose uptake across the brush border membrane is concentrative, sodium-dependent, and phlorizin-sensitive (i.e. occurs via Na+/glucose cotransport) whereas transport across the basolateral membrane is neither concentrative, sodium-dependent, nor phlorizin-sensitive and, therefore, proceeds via facilitated diffusion. Na+-dependent glucose transporters SGLT1 and SGLT2 are expressed in the kidney where SGLT2 is the major concentrative species in brush border membranes of the proximal tubule (633). GLUT2 is expressed in the basolateral membranes of the proximal tubule while SGLT1 is expressed in brush border membranes (139). In situ hybridization studies indicate that RNAs for GLUTs 1, 2, 4 and 5 are expressed in rat kidneys (110). GLUT1 is expressed in all parts of the nephron excluding the proximal convoluted tubule. GLUT2 expression is restricted to the proximal tubule. GLUT4 is restricted to the Thick Ascending Limb of Henlé and GLUT5 is expressed in the proximal straight tubule. Only GLUT1 is found in the collecting duct. A recent study in mice suggests that GLUT4 is expressed in the proximal and distal tubules in addition to the thick ascending limb of Henlé (11).
CONCLUDING REMARKS
Glucose transport and metabolism have co-evolved in mammalia to support cerebral glucose utilization. This necessitates controlling blood glucose within narrow limits and recycling and redistributing carbons between the various organ systems as efficiently as possible. This is accomplished through a complex series of glucose sensing and effector mechanisms that regulate monosaccharide ingestion, absorption, distribution, cellular transport and metabolism and recovery/retention. In almost all instances, sensing and effect involve the direct participation of monosaccharide transport proteins. GLUT1 appears to be highly expressed in glycolytically active cells and has been co-opted in vitamin C auxotrophs to maintain the redox state of the blood through transport of dehydroascorbate. The other 13 transporter isoforms are expressed in a tissue specific manner where affinity, specificity and capacity for substrate transport are paramount. The mechanism of GLUT-mediated glucose transport is ill-defined at both phenomenological and structural levels.
Table 1.
| GLUT Group |
Isoform | Symmetry | Trans- acceleration |
Preferred Substrate |
Catalyzes 2DOG and galactose transport |
High or low affinity |
High or low capacity |
Affinity for cytochalasin B |
Homo- oligomers |
|---|---|---|---|---|---|---|---|---|---|
| 1 | GLUT1 | No | Yes | Glc/Dehydr oascorbic acid |
Yes | high | high | high | Yes |
| 1 | GLUT2 | Yes | No | Glc/Frc | Yes | low | high | low | ?1 |
| 1 | GLUT3 | ? | Yes | Glc | Yes | high | very high | high | ?1 |
| 1 | GLUT4 | Yes | No | Glc | Yes | high | high | high | ? |
| 1 | GLUT142 | ? | ? | Glc | Yes | high | high | high | ? |
| 2 | GLUT5 | ? | ? | Frc | No | low | high | very low | ? |
| 2 | GLUT7 | ? | ? | Frc/GIc | No | high | ? | very low | ? |
| 2 | GLUT9 | ? | ? | Urate | No | ? | very low | ? | |
| 2 | GLUT11 | ? | ? | Glc | No | low | ? | very low | ? |
| 3 | GLUT6 | ? | ? | Glc | ? | low | ? | ? | ? |
| 3 | GLUT8 | ? | ? | Glc/Frc | Yes | high | ? | ? | ? |
| 3 | GLUT10 | ? | ? | Glc | Yes | high | ? | ? | ? |
| 3 | GLUT12 | ? | ? | Glc/fruc | ? | high | ? | ? | ? |
| 3 | HMIT | ? | ? | myo- oinositol |
? | high | ? | ? | ? |
The mammalian GLUTs and their characteristics. Each GLUT isoform is listed with its GLUT Class. Symmetry refers to kinetic behavior in which Vmax for zero trans entry : Vmax for zero trans entry = 1. Trans-acceleration refers to a kinetic behavior in which Vmax for zero trans entry or exit < Vmax for equilibrium exchange. The preferred substrates are glucose (Glc), fructose (Frc), dehydroascorbate, urate or myo-inositol. Catalyzes 2-deoxy-D-glucose and galactose transport indicates whether these are substrates for the transport. High or low affinity refers to low or high Km transport respectively. Affinity for cytochalasin B indicates whether cytochalasin B inhibits with high affinity (low Ki(app) or very low affinity (high Ki(app)). Homooligomers indicates whether the isoform has been shown to form homo-multimeric complexes.
? indicates that the answer is unknown.
GLUT2 and GLUT3 do not form functional hetero-oligomers based on co-expression in Xenopus oocytes.
The functional properties of GLUT14 are inferred from GLUT14 sequence homology with GLUT3.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Julie DeZutter and Sabrina Vollers for helpful comments on the manuscript. This work was supported by NIH grants DK44888 and DK36081.
Footnotes
Levine, K., DeZutter, J and Carruthers, A unpublished.
Some reports suggest that oral glucosamine adversely affects glucose metabolism in subjects with impaired glucose tolerance (53, 473) possibly by inhibiting insulin-dependent GLUT4 recruitment to the cell surface (107). While a comprehensive study suggests that this is not observed in humans (535), this action, if real, must be independent of any effects glucosamine on GLUT4-mediated sugar transport.
REFERENCES
- 1.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 3.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 4.Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- 5.Adachi A. Electrophysiological study of hepatic vagal projection to the medulla. Neurosci Lett. 1981;24:19–23. doi: 10.1016/0304-3940(81)90352-9. [DOI] [PubMed] [Google Scholar]
- 6.Ahern CA, Kobertz WR. Chemical Tools for K+ Channel Biology†. Biochemistry. 2008;48:517–526. doi: 10.1021/bi8018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 8.Akkerman JW. Regulation of carbohydrate metabolism in platelets. A review. Thromb Haemost. 1978;39:712–724. [PubMed] [Google Scholar]
- 9.Albarracin JL, Fernandez-Novell JM, Ballester J, Rauch MC, Quintero-Moreno A, Pena A, Mogas T, Rigau T, Yanez A, Guinovart JJ, Slebe JC, Concha II, Rodriguez-Gil JE. Gluconeogenesis-linked glycogen metabolism is important in the achievement of in vitro capacitation of dog spermatozoa in a medium without glucose. Biol Reprod. 2004;71:1437–1445. doi: 10.1095/biolreprod.104.029041. [DOI] [PubMed] [Google Scholar]
- 10.Albarracin JL, Mogas T, Palomo MJ, Pena A, Rigau T, Rodriguez-Gil JE. In vitro capacitation and acrosome reaction of dog spermatozoa can be feasibly attained in a defined medium without glucose. Reprod Domest Anim. 2004;39:129–135. doi: 10.1111/j.1439-0531.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 11.Albiston AL, Yeatman HR, Pham V, Fuller SJ, Diwakarla S, Fernando RN, Chai SY. Distinct distribution of GLUT4 and insulin regulated aminopeptidase in the mouse kidney. Regul Pept. 2011;166:83–89. doi: 10.1016/j.regpep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Alm A, Tornquist P. Lactate transport through the blood-retinal and the blood-brain barrier in rats. Ophthalmic Res. 1985;17:181–184. doi: 10.1159/000265371. [DOI] [PubMed] [Google Scholar]
- 13.Alm A, Tornquist P, Maepea O. The uptake index method applied to studies on the blood-retinal barrier. II. Transport of several hexoses by a common carrier. Acta Physiol Scand. 1981;113:81–84. doi: 10.1111/j.1748-1716.1981.tb06865.x. [DOI] [PubMed] [Google Scholar]
- 14.Alquier T, Leloup C, Arnaud E, Magnan C, Penicaud L. Altered Glut4 mRNA levels in specific brain areas of hyperglycemic-hyperinsulinemic rats. Neurosci Lett. 2001;308:75–78. doi: 10.1016/s0304-3940(01)01936-x. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez J, Lee DC, Baldwin SA, Chapman D. Fourier transform infrared spectroscopic study of the structure and conformational changes of the human erythrocyte glucose transporter. J Biol Chem. 1987;262:3502–3509. [PubMed] [Google Scholar]
- 16.Amato PA, Loizzi RF. The effects of cytochalasin B on glucose transport and lactose synthesis in lactating mammary gland slices. Eur J Cell Biol. 1979;20:150–155. [PubMed] [Google Scholar]
- 17.Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. ACTIVITY OF SINGLE NEURONS IN THE HYPOTHALAMIC FEEDING CENTERS: EFFECT OF GLUCOSE. Am J Physiol. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- 18.Ando M, Edamatsu M, Fukuizumi S, Takeuchi S. Cellular localization of facilitated glucose transporter 1 (GLUT-1) in the cochlear stria vascularis: its possible contribution to the transcellular glucose pathway. Cell Tissue Res. 2008;331:763–769. doi: 10.1007/s00441-007-0495-2. [DOI] [PubMed] [Google Scholar]
- 19.Angulo C, Rauch MC, Droppelmann A, Reyes AM, Slebe JC, Delgado-Lopez F, Guaiquil VH, Vera JC, Concha II. Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J Cell Biochem. 1998;71:189–203. [PubMed] [Google Scholar]
- 20.Antonetti DA, Wolpert EB. Isolation and characterization of retinal endothelial cells. Methods Mol Med. 2003;89:365–374. doi: 10.1385/1-59259-419-0:365. [DOI] [PubMed] [Google Scholar]
- 21.Apelt J, Mehlhorn G, Schliebs R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res. 1999;57:693–705. [PubMed] [Google Scholar]
- 22.Appleman JR, Lienhard GE. Rapid kinetics of the glucose transporter from human erythrocytes. Detection and measurement of a half-turnover of the purified transporter. J. Biol. Chem. 1985;260:4575–4578. [PubMed] [Google Scholar]
- 23.Arbuckle MI, Kane S, Porter LM, Seatter MJ, Gould GW. Structure-function analysis of liver-type (GLUT2) and brain-type (GLUT3) glucose transporters: expression of chimeric transporters in Xenopus oocytes suggests an important role for putative transmembrane helix 7 in determining substrate selectivity. Biochemistry. 1996;35:16519–16527. doi: 10.1021/bi962210n. [DOI] [PubMed] [Google Scholar]
- 24.Arden C, Harbottle A, Baltrusch S, Tiedge M, Agius L. Glucokinase is an integral component of the insulin granules in glucose-responsive insulin secretory cells and does not translocate during glucose stimulation. Diabetes. 2004;53:2346–2352. doi: 10.2337/diabetes.53.9.2346. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong DT, Greep RO. Effect of gonadotrophic hormones on glucose metabolism by luteinized rat ovaries. Endocrinology. 1962;70:701–710. doi: 10.1210/endo-70-5-701. [DOI] [PubMed] [Google Scholar]
- 26.Asano T, Katagiri H, Takata K, Lin JL, Ishihara H, Inukai K, Tsukuda K, Kikuchi M, Hirano H, Yazaki Y. The role of N-glycosylation of GLUT1 for glucose transport activity. J Biol Chem. 1991;266:24632–24636. [PubMed] [Google Scholar]
- 27.Asplin C, Raghu P, Dornan T, Palmer JP. Glucose regulation of glucagon secretion independent of B cell activity. Metabolism. 1983;32:292–295. doi: 10.1016/0026-0495(83)90195-6. [DOI] [PubMed] [Google Scholar]
- 28.Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004;279:16229–16236. doi: 10.1074/jbc.M312226200. [DOI] [PubMed] [Google Scholar]
- 29.Augustin R, Riley J, Moley KH. GLUT8 contains a [DE]XXXL[LI] sorting motif and localizes to a late endosomal/lysosomal compartment. Traffic. 2005;6:1196–1212. doi: 10.1111/j.1600-0854.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 30.Bachelard HS. Deoxyglucose and brain glycolysis. Biochem J. 1972;127:83P. doi: 10.1042/bj1270083pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker GF, Widdas WF. The asymmetry of the facilitated transfer system for hexoses in human red cells and the simple kinetics of a two component model. J Physiol. 1973;231:143–165. doi: 10.1113/jphysiol.1973.sp010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker GF, Naftalin RJ. Evidence of multiple operational affinities for D-glucose inside the human erythrocyte membrane. Biochim. Biophys. Acta. 1979;550:474–484. doi: 10.1016/0005-2736(79)90150-0. [DOI] [PubMed] [Google Scholar]
- 33.Baker PF, Carruthers A. 3-O-methylglucose transport in internally dialysed giant axons of Loligo. J. Physiol. (Lond.) 1981;316:503–525. doi: 10.1113/jphysiol.1981.sp013803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakirtzi K, Belfort G, Lopez-Coviella I, Kuruppu D, Cao L, Abel ED, Brownell AL, Kandror KV. Cerebellar neurons possess a vesicular compartment structurally and functionally similar to Glut4-storage vesicles from peripheral insulin-sensitive tissues. J Neurosci. 2009;29:5193–5201. doi: 10.1523/JNEUROSCI.0858-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balint E, Szabo P, Marshall CF, Sprague SM. Glucose-induced inhibition of in vitro bone mineralization. Bone. 2001;28:21–28. doi: 10.1016/s8756-3282(00)00426-9. [DOI] [PubMed] [Google Scholar]
- 36.Barathi S, Angayarkanni N, Sumantran VN. GLUT-1 expression in bovine retinal capillary endothelial cells and pericytes exposed to advanced glycation end products. Invest Ophthalmol Vis Sci. 2010;51:6810–6814. doi: 10.1167/iovs.10-5312. [DOI] [PubMed] [Google Scholar]
- 37.Barbosa FB, Capito K, Kofod H, Thams P. Pancreatic islet insulin secretion and metabolism in adult rats malnourished during neonatal life. Br J Nutr. 2002;87:147–155. doi: 10.1079/BJN2001489. [DOI] [PubMed] [Google Scholar]
- 38.Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci. 1999;318:36–42. doi: 10.1097/00000441-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Barnett JE, Holman GD, Chalkley RA, Munday KA. Evidence for two asymmetric conformational states in the human erythrocyte sugar-transport system. Biochem J. 1975;145:417–429. doi: 10.1042/bj1450417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnett JE, Holman GD, Munday KA. Structural requirements for binding to the sugartransport system of the human erythrocyte. Biochem J. 1973;131:211–221. doi: 10.1042/bj1310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett EJ, Ferrannini E, Gusberg R, Bevilacqua S, DeFronzo RA. Hepatic and extrahepatic splanchnic glucose metabolism in the postabsorptive and glucose fed dog. Metabolism. 1985;34:410–420. doi: 10.1016/0026-0495(85)90205-7. [DOI] [PubMed] [Google Scholar]
- 42.Barros LF, Courjaret R, Jakoby P, Loaiza A, Lohr C, Deitmer JW. Preferential transport and metabolism of glucose in Bergmann glia over Purkinje cells: a multiphoton study of cerebellar slices. Glia. 2009;57:962–970. doi: 10.1002/glia.20820. [DOI] [PubMed] [Google Scholar]
- 43.Basketter DA, Widdas WF. Asymmetry of the hexose transfer system in human erythrocytes. Comparison of the effects of cytochalasin B, phloretin and maltose as competitive inhibitors. J Physiol. 1978;278:389–401. doi: 10.1113/jphysiol.1978.sp012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baumann MU, Deborde S, Illsley NP. Placental glucose transfer and fetal growth. Endocrine. 2002;19:13–22. doi: 10.1385/ENDO:19:1:13. [DOI] [PubMed] [Google Scholar]
- 45.Bell AW, Hay WWJ, Ehrhardt RA. Placental transport of nutrients and its implications for fetal growth. J Reprod Fertil Suppl. 1999;54:401–410. [PubMed] [Google Scholar]
- 46.Belyantseva IA, Adler HJ, Curi R, Frolenkov GI, Kachar B. Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J Neurosci. 2000;20:RC116. doi: 10.1523/JNEUROSCI.20-24-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benirschke K, Kaufmann P. Pathology of human placenta. New York: Springer- Verlag; 2006. [Google Scholar]
- 48.Bentley J, Itchayanan D, Barnes K, McIntosh E, Tang X, Downes CP, Holman GD, Whetton AD, Owen-Lynch PJ, Baldwin SA. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J Biol Chem. 2003;278:39337–39348. doi: 10.1074/jbc.M305689200. [DOI] [PubMed] [Google Scholar]
- 49.Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71:140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 50.Betz AL, Bowman PD, Goldstein GW. Hexose transport in microvascular endothelial cells cultured from bovine retina. Exp Eye Res. 1983;36:269–277. doi: 10.1016/0014-4835(83)90011-8. [DOI] [PubMed] [Google Scholar]
- 51.Betz AL, Iannotti F. Simultaneous determination of regional cerebral blood flow and blood--brain glucose transport kinetics in the gerbil. J Cereb Blood Flow Metab S. 1983;3:193–199. doi: 10.1038/jcbfm.1983.26. [DOI] [PubMed] [Google Scholar]
- 52.Bianchi J, Rose RC. Glucose-independent transport of dehydroascorbic acid in human erythrocytes. Proc Soc Exp Biol Med. 1986;181:333–337. doi: 10.3181/00379727-181-42261. [DOI] [PubMed] [Google Scholar]
- 53.Biggee BA, Blinn CM, Nuite M, Silbert JE, McAlindon TE. Effects of oral glucosamine sulphate on serum glucose and insulin during an oral glucose tolerance test of subjects with osteoarthritis. Ann Rheum Dis. 2007;66:260–262. doi: 10.1136/ard.2006.058222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bilezikian JP, Raisz LG, Martin TJ. Principles of Bone Biology, Two-Volume Set Volume 1–2, Third Edition (Bilezikian, Principles of Bone Biology 2 Vol Set) Academic Press; 2008. [Google Scholar]
- 55.Birnbaum MJ, Haspel HC, Rosen OM. Cloning and characterization of a cDNA encoding the rat brain glucose- transporter protein. Proc. Natl. Acad. Sci. USA. 1986;83:5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Birnbaum MJ. Activating AMP-Activated Protein Kinase without AMP. Mol Cell. 2005;19:289–290. doi: 10.1016/j.molcel.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 57.Blanc L, Liu J, Vidal M, Chasis JA, An X, Mohandas N. The water channel aquaporin-1 partitions into exosomes during reticulocyte maturation: implication for the regulation of cell volume. Blood. 2009;114:3928–3934. doi: 10.1182/blood-2009-06-230086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bloch R. Inhibition of glucose transport in the human erythrocyte by cytochalasin B. Biochemistry. 1973;12:4799–4801. doi: 10.1021/bi00747a036. [DOI] [PubMed] [Google Scholar]
- 59.Blodgett DM, Carruthers A. Quench-Flow Analysis Reveals Multiple Phases of GluT1- Mediated Sugar Transport. Biochemistry. 2005;44:2650–2660. doi: 10.1021/bi048247m. [DOI] [PubMed] [Google Scholar]
- 60.Blodgett DM, Carruthers A. Conventional transport assays underestimate sugar transport rates in human red cells. Blood Cells Mol Dis. 2004;32:401–407. doi: 10.1016/j.bcmd.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 61.Blodgett DM, De Zutter JK, Levine KB, Karim P, Carruthers A. Structural Basis of GLUT1 Inhibition by Cytoplasmic ATP. J Gen Physiol. 2007;130:157–168. doi: 10.1085/jgp.200709818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blodgett DM, Graybill C, Carruthers A. Analysis of glucose transporter topology and structural dynamics. J Biol Chem. 2008;283:36416–36424. doi: 10.1074/jbc.M804802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boado RJ, Pardridge WM. Glucose deprivation and hypoxia increase the expression of the GLUT1 glucose transporter via a specific mRNA cis-acting regulatory element. J Neurochem. 2002;80:552–554. doi: 10.1046/j.0022-3042.2001.00756.x. [DOI] [PubMed] [Google Scholar]
- 64.Boado RJ, Wu D, Windisch M. In vivo upregulation of the blood-brain barrier GLUT1 glucose transporter by brain-derived peptides. Neurosci Res. 1999;34:217–224. doi: 10.1016/s0168-0102(99)00056-5. [DOI] [PubMed] [Google Scholar]
- 65.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes. 1995;44:180–184. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- 67.Broer S, Schneider HP, Broer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J. 1998;333:167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002;30:258–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 69.Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- 70.Bucci D, Isani G, Spinaci M, Tamanini C, Mari G, Zambelli D, Galeati G. Comparative immunolocalization of GLUTs 1, 2, 3 and 5 in boar, stallion and dog spermatozoa. Reprod Domest Anim. 2010;45:315–322. doi: 10.1111/j.1439-0531.2008.01307.x. [DOI] [PubMed] [Google Scholar]
- 71.Bucci D, Rodriguez-Gil JE, Vallorani C, Spinaci M, Galeati G, Tamanini C. GLUTs and Mammalian Sperm Metabolism. J Androl. 2010 doi: 10.2164/jandrol.110.011197. [DOI] [PubMed] [Google Scholar]
- 72.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 73.Burant CF, Takeda J, Brot LE, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. Journal of Biological Chemistry. 1992;267:14523–14526. [PubMed] [Google Scholar]
- 74.Burant CF, Bell GI. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry. 1992;31:10414–10420. doi: 10.1021/bi00157a032. [DOI] [PubMed] [Google Scholar]
- 75.Burcelin R, Crivelli V, Perrin C, Da Costa A, Mu J, Kahn BB, Birnbaum MJ, Kahn CR, Vollenweider P, Thorens B. GLUT4, AMP kinase, but not the insulin receptor, are required for hepatoportal glucose sensor-stimulated muscle glucose utilization. J Clin Invest. 2003;111:1555–1562. doi: 10.1172/JCI16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burcelin R, del Carmen Munoz M, Guillam MT, Thorens B. Liver hyperplasia and paradoxical regulation of glycogen metabolism and glucose-sensitive gene expression in GLUT2-null hepatocytes. Further evidence for the existence of a membrane-based glucose release pathway. J Biol Chem. 2000;275:10930–10936. doi: 10.1074/jbc.275.15.10930. [DOI] [PubMed] [Google Scholar]
- 77.Burcelin R, Dolci W, Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes. 2000;49:1643–1648. doi: 10.2337/diabetes.49.10.1643. [DOI] [PubMed] [Google Scholar]
- 78.Burcelin R, Dolci W, Thorens B. Portal glucose infusion in the mouse induces hypoglycemia: evidence that the hepatoportal glucose sensor stimulates glucose utilization. Diabetes. 2000;49:1635–1642. doi: 10.2337/diabetes.49.10.1635. [DOI] [PubMed] [Google Scholar]
- 79.Burcelin R, Thorens B. Evidence that extrapancreatic GLUT2-dependent glucose sensors control glucagon secretion. Diabetes. 2001;50:1282–1289. doi: 10.2337/diabetes.50.6.1282. [DOI] [PubMed] [Google Scholar]
- 80.Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 81.Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burnol AF, Leturque A, Loizeau M, Postic C, Girard J. Glucose transporter expression in rat mammary gland. Biochem J. 1990;270:277–279. doi: 10.1042/bj2700277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cabantchik ZI, Ginsburg H. Transport of uridine in human red blood cells. Demonstration of a simple carrier-mediated process. J Gen Physiol. 1977;69:75–96. doi: 10.1085/jgp.69.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camps M, Vilaro S, Testar X, Palacin M, Zorzano A. High and polarized expression of GLUT1 glucose transporters in epithelial cells from mammary gland: acute downregulation of GLUT1 carriers by weaning. Endocrinology. 1994;134:924–934. doi: 10.1210/endo.134.2.8299587. [DOI] [PubMed] [Google Scholar]
- 85.Camps M, Castello A, Munoz P, Monfar M, Testar X, Palacin M, Zorzano A. Effect of diabetes and fasting on GLUT-4 (muscle/fat) glucose-transporter expression in insulinsensitive tissues. Heterogeneous response in heart, red and white muscle. Biochemical Journal. 1992 doi: 10.1042/bj2820765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cani PD, Holst JJ, Drucker DJ, Delzenne NM, Thorens B, Burcelin R, Knauf C. GLUT2 and the incretin receptors are involved in glucose-induced incretin secretion. Mol Cell Endocrinol. 2007;276:18–23. doi: 10.1016/j.mce.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH. GLUT8 is a glucose transporter responsible for insulinstimulated glucose uptake in the blastocyst. Proc Natl Acad Sci U S A. 2000;97:7313–7318. doi: 10.1073/pnas.97.13.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carayannopoulos MO, Schlein A, Wyman A, Chi M, Keembiyehetty C, Moley KH. GLUT9 Is Differentially Expressed and Targeted in the Preimplantation Embryo. Endocrinology. 2004;145:1435–1443. doi: 10.1210/en.2003-1264. [DOI] [PubMed] [Google Scholar]
- 89.Carbo R, Guarner V. Insulin effect on glucose transport in thymocytes and splenocytes from rats with metabolic syndrome. Diabetol Metab Syndr. 2010;2:64. doi: 10.1186/1758-5996-2-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carruthers A, Naftalin RJ. Altered GLUT1 Substrate Selectivity in Human Erythropoiesis? Cell. 2009;137:200–201. doi: 10.1016/j.cell.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 91.Carruthers A. Anomalous asymmetric kinetics of human red cell hexose transfer: role of cytosolic adenosine 5'-triphosphate. Biochemistry. 1986;25:3592–3602. doi: 10.1021/bi00360a018. [DOI] [PubMed] [Google Scholar]
- 92.Carruthers A. ATP regulation of the human red cell sugar transporter. J. Biol. Chem. 1986;261:11028–11037. [PubMed] [Google Scholar]
- 93.Carruthers A. Facilitated diffusion of glucose. Physiol. Rev. 1990;70:1135–1176. doi: 10.1152/physrev.1990.70.4.1135. [DOI] [PubMed] [Google Scholar]
- 94.Carruthers A. Mechanisms for the facilitated diffusion of substrates across cell membranes. Biochemistry. 1991;30:3898–3906. doi: 10.1021/bi00230a014. [DOI] [PubMed] [Google Scholar]
- 95.Carruthers A, Helgerson AL. The human erythrocyte sugar transporter is also a nucleotide binding protein. Biochemistry. 1989;28:8337–8346. doi: 10.1021/bi00447a011. [DOI] [PubMed] [Google Scholar]
- 96.Carruthers A, Helgerson AL. Inhibitions of sugar transport produced by ligands binding at opposite sides of the membrane. Evidence for simultaneous occupation of the carrier by maltose and cytochalasin B. Biochemistry. 1991;30:3907–3915. doi: 10.1021/bi00230a015. [DOI] [PubMed] [Google Scholar]
- 97.Carruthers A, Melchior DL. Transport of α- and β-D-glucose by the intact human red cell. Biochemistry. 1985;24:4244–4250. doi: 10.1021/bi00336a065. [DOI] [PubMed] [Google Scholar]
- 98.Carruthers A, Zottola RJ. Erythrocyte sugar transport. In: Konings HRKJSL WN, editor. Handbook of Biological Physics "Transport Processes in Eukaryotic and Prokaryotic Organisms".(2) Amsterdam: Elsevier; 1996. pp. 311–342. [Google Scholar]
- 99.Casey JR, Reithmeier RA. Analysis of the oligomeric state of Band 3, the anion transport protein of the human erythrocyte membrane, by size exclusion high performance liquid chromatography. Oligomeric stability and origin of heterogeneity. J. Biol. Chem. 1991;266:15726–15737. [PubMed] [Google Scholar]
- 100.Castellini MA, Costa DP, Castellini JM. Blood glucose distribution, brain size and diving in small odontocetes. Marine Mammal Science. 1992;8:294–298. [Google Scholar]
- 101.Castro MA, Beltran FA, Brauchi S, Concha II. A metabolic switch in brain: glucose and lactate metabolism modulation by ascorbic acid. J Neurochem. 2009;110:423–440. doi: 10.1111/j.1471-4159.2009.06151.x. [DOI] [PubMed] [Google Scholar]
- 102.Caulfield MJ, Munroe PB, O'Neill D, Witkowska K, Charchar FJ, Doblado M, Evans S, Eyheramendy S, Onipinla A, Howard P, Shaw-Hawkins S, Dobson RJ, Wallace C, Newhouse SJ, Brown M, Connell JM, Dominiczak A, Farrall M, Lathrop GM, Samani NJ, Kumari M, Marmot M, Brunner E, Chambers J, Elliott P, Kooner J, Laan M, Org E, Veldre G, Viigimaa M, Cappuccio FP, Ji C, Iacone R, Strazzullo P, Moley KH, Cheeseman C. SLC2A9 Is a High-Capacity Urate Transporter in Humans. PLoS Med. 2008;5:e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL Homolog from Mycobacterium tuberculosis: A Gated Mechanosensitive Ion Channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- 104.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10:65–71. doi: 10.2119/2005-00029.Saltiel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheeseman C. GLUT7: a new intestinal facilitated hexose transporter. Am J Physiol Endocrinol Metab. 2008;295:E238–E241. doi: 10.1152/ajpendo.90394.2008. [DOI] [PubMed] [Google Scholar]
- 107.Chen G, Liu P, Thurmond DC, Elmendorf JS. Glucosamine-induced insulin resistance is coupled to O-linked glycosylation of Munc18c. FEBS Lett. 2003;534:54–60. doi: 10.1016/s0014-5793(02)03774-2. [DOI] [PubMed] [Google Scholar]
- 108.Chen SY, Pan CJ, Nandigama K, Mansfield BC, Ambudkar SV, Chou JY. The glucose- 6-phosphate transporter is a phosphate-linked antiporter deficient in glycogen storage disease type Ib and Ic. FASEB J. 2008;22:2206–2213. doi: 10.1096/fj.07-104851. [DOI] [PubMed] [Google Scholar]
- 109.Cheng A, van Hoek AN, Yeager M, Verkman AS, Mitra AK. Three-dimensional organization of a human water channel. Nature. 1997;387:627–630. doi: 10.1038/42517. [DOI] [PubMed] [Google Scholar]
- 110.Chin E, Zhou J, Bondy C. Anatomical and developmental patterns of facilitative glucose transporter gene expression in the rat kidney. J Clin Invest. 1993;91:1810–1815. doi: 10.1172/JCI116392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chin JJ, Jung EK, Chen V, Jung CY. Structural basis of human erythrocyte glucose transporter function in proteoliposome vesicles: circular dichroism measurements. Proc Natl Acad Sci U S A. 1987;84:4113–4116. doi: 10.1073/pnas.84.12.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chin JJ, Jung EK, Jung CY. Structural basis of human erythrocyte glucose transporter function in reconstituted vesicles. J Biol Chem. 1986;261:7101–7104. [PubMed] [Google Scholar]
- 113.Choeiri C, Staines W, Messier C. Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neuroscience. 2002;111:19–34. doi: 10.1016/s0306-4522(01)00619-4. [DOI] [PubMed] [Google Scholar]
- 114.Choi YK, Kim KW. Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep. 2008;41:345–352. doi: 10.5483/bmbrep.2008.41.5.345. [DOI] [PubMed] [Google Scholar]
- 115.Clarke DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Siegel GJ, Agranoff BW, Albers RW, editors. Basic neurochemistry. Philadelphia: Lippincott-Raven; 1999. pp. 637–669. [Google Scholar]
- 116.Cloherty EK, Diamond DL, Heard KS, Carruthers A. Regulation of GLUT1-mediated sugar transport by an antiport/uniport switch mechanism. Biochemistry. 1996;35:13231–13239. doi: 10.1021/bi961208t. [DOI] [PubMed] [Google Scholar]
- 117.Cloherty EK, Heard KS, Carruthers A. Human erythrocyte sugar transport is incompatible with available carrier models. Biochemistry. 1996;35:10411–10421. doi: 10.1021/bi953077m. [DOI] [PubMed] [Google Scholar]
- 118.Cloherty EK, Levine KB, Carruthers A. The red blood cell glucose transporter presents multiple, nucleotide-sensitive sugar exit sites. Biochemistry. 2001;40:15549–15561. doi: 10.1021/bi015586w. [DOI] [PubMed] [Google Scholar]
- 119.Cloherty EK, Sultzman LA, Zottola RJ, Carruthers A. Net sugar transport is a multistep process. Evidence for cytosolic sugar binding sites in erythrocytes. Biochemistry. 1995;34:15395–15406. doi: 10.1021/bi00047a002. [DOI] [PubMed] [Google Scholar]
- 120.Coderre PE, Cloherty EK, Zottola RJ, Carruthers A. Rapid substrate translocation by the multisubunit, erythroid glucose transporter requires subunit associations but not cooperative ligand binding. Biochemistry. 1995;34:9762–9773. doi: 10.1021/bi00030a014. [DOI] [PubMed] [Google Scholar]
- 121.Coerver KA, Gray SM, Barnes JE, Armstrong DL, McCabe ER. Developmental expression of hexokinase 1 and 3 in rats. Histochem Cell Biol. 1998;109:75–86. doi: 10.1007/s004180050204. [DOI] [PubMed] [Google Scholar]
- 122.Colville CA, Seatter MJ, Gould GW. Analysis of the structural requirements of sugar binding to the liver, brain and insulin-responsive glucose transporters expressed in oocytes. Biochemical Journal. 1993 doi: 10.1042/bj2940753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Colville CA, Seatter MJ, Jess TJ, Gould GW, Thomas HM. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J. 1993:701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Combes B, Adams RH, Strickland W, Madison LL. The physiological significance of the secretion of endogenous insulin into the portal circulation. IV. Hepatic uptake of glucose during glucose infusion in non-diabetic dogs. J Clin Invest. 1961;40:1706–1718. doi: 10.1172/JCI104393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Concha II, Velasquez FV, Martinez JM, Angulo C, Droppelmann A, Reyes AM, Slebe JC, Vera JC, Golde DW. Human erythrocytes express GLUT5 and transport fructose. Blood. 1997;89:4190–4195. [PubMed] [Google Scholar]
- 126.Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic Bcells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- 127.Coombe NB, Smith RH. Absorption of glucose and galactose and digestion and absorption of lactose by the prepruminant calf. Br J Nutr. 1973;30:331–344. doi: 10.1079/bjn19730037. [DOI] [PubMed] [Google Scholar]
- 128.Coomber BL, Stewart PA. Morphometric analysis of CNS microvascular endothelium. Microvasc Res. 1985;30:99–115. doi: 10.1016/0026-2862(85)90042-1. [DOI] [PubMed] [Google Scholar]
- 129.Cooper DR, Khalakdina A, Watson JE. Chronic effects of glucose on insulin signaling in A-10 vascular smooth muscle cells. Archives of Biochemistry & Biophysics. 1993;302:490–498. doi: 10.1006/abbi.1993.1244. [DOI] [PubMed] [Google Scholar]
- 130.Cope DL, Holman GD, Baldwin SA, Wolstenholme AJ. Domain assembly of the GLUT1 glucose transporter. Biochem J. 1994;300:291–294. doi: 10.1042/bj3000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cornford EM, Hyman S, Pardridge WM. An electron microscopic immunogold analysis of developmental up-regulation of the blood-brain barrier GLUT1 glucose transporter. Journal of Cerebral Blood Flow & Metabolism. 1993;13:841–854. doi: 10.1038/jcbfm.1993.106. [DOI] [PubMed] [Google Scholar]
- 132.Cornford EM, Nguyen EV, Landaw EM. Acute upregulation of blood-brain barrier glucose transporter activity in seizures. Am J Physiol Heart Circ Physiol S. 2000;279:H1346–H1354. doi: 10.1152/ajpheart.2000.279.3.H1346. [DOI] [PubMed] [Google Scholar]
- 133.Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, Fox JE, Mancini GMS, Kambouris M, Gardella R, Facchetti F, Willems PJ, Forsyth R, Dietz HC, Barlati S, Colombi M, Loeys B, De Paepe A. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet. 2006;38:452–457. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- 134.Craik JD, Cheeseman CI, Young JD. Rapid entry of D-glucose into erythrocytes from bottlenose dolphins (Tursiops truncatus) Marine Mammal Science. 1995;11:584–589. [Google Scholar]
- 135.Craik JD, Elliott KR. Kinetics of 3-O-methyl-D-glucose transport in isolated rat hepatocytes. Biochem J S. 1979;182:503–508. doi: 10.1042/bj1820503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Craik JD, Markovich D. Rapid GLUT-1 mediated glucose transport in erythrocytes from the grey-headed fruit bat (Pteropus poliocephalus) Comp Biochem Physiol A Mol Integr Physiol. 2000;126:45–55. doi: 10.1016/s1095-6433(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 137.Craik JD, Stewart M, Cheeseman CI. GLUT-3 (brain-type) glucose transporter polypeptides in human blood platelets. Thromb Res. 1995;79:461–469. doi: 10.1016/0049-3848(95)00136-f. [DOI] [PubMed] [Google Scholar]
- 138.Craik JD, Young JD, Cheeseman CI. GLUT-1 mediation of rapid glucose transport in dolphin (Tursiops truncatus) red blood cells. Am J Physiol. 1998;274:R112–R119. doi: 10.1152/ajpregu.1998.274.1.R112. [DOI] [PubMed] [Google Scholar]
- 139.Cramer SC, Pardridge WM, Hirayama BA, Wright EM. Colocalization of GLUT2 glucose transporter, sodium/glucose cotransporter, and gamma-glutamyl transpeptidase in rat kidney with double-peroxidase immunocytochemistry. Diabetes. 1992;41:766–770. doi: 10.2337/diab.41.6.766. [DOI] [PubMed] [Google Scholar]
- 140.Crone C. Facilitated transfer of glucose from blood into brain tissue. J Physiol. 1965;181:103–113. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cunningham P, Afzal-Ahmed I, Naftalin RJ. Docking studies show that D-glucose and quercetin slide through the transporter GLUT1. J Biol Chem. 2006;281:5797–5803. doi: 10.1074/jbc.M509422200. [DOI] [PubMed] [Google Scholar]
- 142.Cura AJ, Carruthers A. Acute modulation of sugar transport in brain capillary endothelial cell cultures during activation of the metabolic stress pathway. J Biol Chem. 2010;285:15430–15439. doi: 10.1074/jbc.M110.110593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.D'Angelo G. Evidence for an Erythrocyte Glucose Transport System in the Belukha Whale (Delphinapterus Leucas) Biological Systems; 1982. [Google Scholar]
- 144.Dang S, Sun L, Huang Y, Lu F, Liu Y, Gong H, Wang J, Yan N. Structure of a fucose transporter in an outward-open conformation. Nature. 2010;467:734–738. doi: 10.1038/nature09406. [DOI] [PubMed] [Google Scholar]
- 145.Darakhshan F, Hajduch E, Kristiansen S, Richter EA, Hundal HS. Biochemical and functional characterization of the GLUT5 fructose transporter in rat skeletal muscle. Biochem J. 1998;336:361–366. doi: 10.1042/bj3360361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Datta NS, Abou-Samra AB. PTH and PTHrP signaling in osteoblasts. Cell Signal. 2009;21:1245–1254. doi: 10.1016/j.cellsig.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Davidson NO, Hausman AM, Ifkovits CA, Buse JB, Gould GW, Burant CF, Bell GI. Human intestinal glucose transporter expression and localization of GLUT5. Am J Physiol. 1992;262:C795–C800. doi: 10.1152/ajpcell.1992.262.3.C795. [DOI] [PubMed] [Google Scholar]
- 148.Davis AJ, Fleet IR, Goode JA, Hamon MH, Walker FM, Peaker M. Changes in mammary function at the onset of lactation in the goat: correlation with hormonal changes. J Physiol. 1979;288:33–44. [PMC free article] [PubMed] [Google Scholar]
- 149.Dawson PA, Mychaleckyj JC, Fossey SC, Mihic SJ, Craddock AL, Bowden DW. Sequence and functional analysis of glut10: a glucose transporter in the type 2 diabeteslinked region of chromosome 20q12-13.1. Mol Genet Metab. 2001;74:186–199. doi: 10.1006/mgme.2001.3212. [DOI] [PubMed] [Google Scholar]
- 150.de Graaf RA, Pan JW, Telang F, Lee JH, Brown P, Novotny EJ, Hetherington HP, Rothman DL. Differentiation of glucose transport in human brain gray and white matter. J Cereb Blood Flow Metab S. 2001;21:483–492. doi: 10.1097/00004647-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 151.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 152.Dhar-Mascareno M, Chen J, Zhang RH, Carcamo JM, Golde DW. Granulocytemacrophage colony-stimulating factor signals for increased glucose transport via phosphatidylinositol 3-kinase- and hydrogen peroxide-dependent mechanisms. J Biol Chem. 2003;278:11107–11114. doi: 10.1074/jbc.M212541200. [DOI] [PubMed] [Google Scholar]
- 153.Diamond D, Carruthers A. Metabolic control of sugar transport by derepression of cell surface glucose transporters: an insulin-independent, recruitment-independent mechanism of regulation. J. Biol. Chem. 1993;268:6437–6444. [PubMed] [Google Scholar]
- 154.Dick AP, Harik SI, Klip A, Walker DM. Identification and characterization of the glucose transporter of the blood-brain barrier by cytochalasin B binding and immunological reactivity. Proc Natl Acad Sci U S A. 1984;81:7233–7237. doi: 10.1073/pnas.81.22.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RGJ. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. 2000;278:H1294–H1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 156.Doblado M, Moley KH. Facilitative glucose transporter 9, a unique hexose and urate transporter. Am J Physiol Endocrinol Metab. 2009;297:E831–E835. doi: 10.1152/ajpendo.00296.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Doege H, Bocianski A, Joost HG, Schurmann A. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar-transport facilitators predominantly expressed in brain and leucocytes. Biochem J. 2000;350 Pt 3:771–776. [PMC free article] [PubMed] [Google Scholar]
- 158.Doege H, Schurmann A, Bahrenberg G, Brauers A, Joost HG. GLUT8, a novel member of the sugar transport facilitator family with glucose transport activity. J Biol Chem. 2000;275:16275–16280. doi: 10.1074/jbc.275.21.16275. [DOI] [PubMed] [Google Scholar]
- 159.Doege H, Schurmann A, Ohnimus H, Monser V, Holman GD, Joost HG. Serine-294 and threonine-295 in the exofacial loop domain between helices 7 and 8 of glucose transporters (GLUT) are involved in the conformational alterations during the transport process. Biochem J. 1998;329:289–293. doi: 10.1042/bj3290289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Doege H, Bocianski A, Scheepers A, Axer H, Eckel J, Joost HG, Schurmann A. Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar-transport facilitator specifically expressed in heart and skeletal muscle. Biochem J. 2001;359:443–49.. doi: 10.1042/0264-6021:3590443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dolznig H, Boulme F, Stangl K, Deiner EM, Mikulits W, Beug H, Mullner EW. Establishment of normal, terminally differentiating mouse erythroid progenitors: molecular characterization by cDNA arrays. FASEB J. 2001;15:1442–1444. doi: 10.1096/fj.00-0705fje. [DOI] [PubMed] [Google Scholar]
- 162.Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab. 2008;295:E227–E237. doi: 10.1152/ajpendo.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Drewes LR, Horton RW, Betz AL, Gilboe DD. Cytochalasin B inhibition of brain glucose transport and the influence of blood components on inhibitor concentration. Biochim Biophys Acta. 1977;471:477–486. doi: 10.1016/0005-2736(77)90051-7. [DOI] [PubMed] [Google Scholar]
- 164.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 165.Duara R, Grady C, Haxby J, Ingvar D, Sokoloff L, Margolin RA, Manning RG, Cutler NR, Rapoport SI. Human brain glucose utilization and cognitive function in relation to age. Ann Neurol. 1984;16:703–713. doi: 10.1002/ana.410160613. [DOI] [PubMed] [Google Scholar]
- 166.Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. News Physiol Sci. 2001;16:71–76. doi: 10.1152/physiologyonline.2001.16.2.71. [DOI] [PubMed] [Google Scholar]
- 167.Duelli R, Maurer MH, Staudt R, Sokoloff L, Kuschinsky W. Correlation between local glucose transporter densities and local 3-O-methylglucose transport in rat brain. Neurosci Lett. 2001;310:101–104. doi: 10.1016/s0304-3940(01)02060-2. [DOI] [PubMed] [Google Scholar]
- 168.Dwyer DS. Model of the 3-D structure of the GLUT3 glucose transporter and molecular dynamics simulation of glucose transport. Proteins. 2001;42:531–41.. [PubMed] [Google Scholar]
- 169.Edamatsu M, Kondo Y, Ando M. Multiple expression of glucose transporters in the lateral wall of the cochlear duct studied by quantitative real-time PCR assay. Neurosci Lett. 2011;490:72–77. doi: 10.1016/j.neulet.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 170.Eddy EM, Toshimori K, O'Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–115. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 171.El Messari S, Leloup C, Quignon M, Brisorgueil MJ, Penicaud L, Arluison M. Immunocytochemical localization of the insulin-responsive glucose transporter 4 (Glut4) in the rat central nervous system. J Comp Neurol. 1998;399:492–512. doi: 10.1002/(sici)1096-9861(19981005)399:4<492::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 172.Engel A, Fujiyoshi Y, Agre P. The importance of aquaporin water channel protein structures. Embo J. 2000;19:800–806. doi: 10.1093/emboj/19.5.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ericsson A, Hamark B, Jansson N, Johansson BR, Powell TL, Jansson T. Hormonal regulation of glucose and system A amino acid transport in first trimester placental villous fragments. Am J Physiol Regul Integr Comp Physiol. 2005;288:R656–R662. doi: 10.1152/ajpregu.00407.2004. [DOI] [PubMed] [Google Scholar]
- 174.Estrada DE, Elliott E, Zinman B, Poon I, Liu Z, Klip A, Daneman D. Regulation of glucose transport and expression of GLUT3 transporters in human circulating mononuclear cells: studies in cells from insulin-dependent diabetic and nondiabetic individuals. Metabolism. 1994;43:591–598. doi: 10.1016/0026-0495(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 175.Evans SA, Doblado M, Chi MM, Corbett JA, Moley KH. Facilitative glucose transporter 9 expression affects glucose sensing in pancreatic beta-cells. Endocrinology. 2009;150:5302–5310. doi: 10.1210/en.2009-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Farrell CL, Pardridge WM. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci U S A. 1991;88:5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Faulkner A, Blatchford DR, Pollock HT. The transport of hexoses across the apical membrane of the mammary gland of the goat. Biochemical Society Transactions. 1985;13:689–690. [Google Scholar]
- 178.Faulkner A, Chaiyabutr N, Peaker M, Carrick DT, Kuhn NJ. Metabolic significance of milk glucose. J Dairy Res. 1981;48:51–56. doi: 10.1017/s0022029900021440. [DOI] [PubMed] [Google Scholar]
- 179.Fawcett HA, Baldwin SA, Flint DJ. Hormonal regulation of the glucose transporter GLUT I in the lactating rat mammary gland. Biochemical Society Transactions. 1992;20 doi: 10.1042/bst020017s. [DOI] [PubMed] [Google Scholar]
- 180.Fazakerley DJ, Holman GD, Marley A, James DE, Stockli J, Coster AC. Kinetic evidence for unique regulation of GLUT4 trafficking by insulin and AMP-activated protein kinase activators in L6 myotubes. J Biol Chem. 2010;285:1653–1660. doi: 10.1074/jbc.M109.051185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fernando RN, Albiston AL, Chai SY. The insulin-regulated aminopeptidase IRAP is colocalised with GLUT4 in the mouse hippocampus--potential role in modulation of glucose uptake in neurones? Eur J Neurosci. 2008;28:588–598. doi: 10.1111/j.1460-9568.2008.06347.x. [DOI] [PubMed] [Google Scholar]
- 182.Fernando RN, Larm J, Albiston AL, Chai SY. Distribution and cellular localization of insulin-regulated aminopeptidase in the rat central nervous system. J Comp Neurol. 2005;487:372–390. doi: 10.1002/cne.20585. [DOI] [PubMed] [Google Scholar]
- 183.Fernando RN, Luff SE, Albiston AL, Chai SY. Sub-cellular localization of insulinregulated membrane aminopeptidase, IRAP to vesicles in neurons. J Neurochem. 2007;102:967–976. doi: 10.1111/j.1471-4159.2007.04659.x. [DOI] [PubMed] [Google Scholar]
- 184.Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci. 2006;7:19–29. doi: 10.1038/nrn1828. [DOI] [PubMed] [Google Scholar]
- 185.Flier JS, Mueckler M, McCall AL, Lodish HF. Distribution of glucose transporter messenger RNA transcripts in tissues of rat and man. J Clin Invest. 1987;79:657–661. doi: 10.1172/JCI112864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ford WC. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update. 2006;12:269–274. doi: 10.1093/humupd/dmi053. [DOI] [PubMed] [Google Scholar]
- 187.Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006;572:5–15. doi: 10.1113/jphysiol.2005.104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- 189.Frizzell RT, Jones EM, Davis SN, Biggers DW, Myers SR, Connolly CC, Neal DW, Jaspan JB, Cherrington AD. Counterregulation during hypoglycemia is directed by widespread brain regions. Diabetes. 1993;42:1253–1261. doi: 10.2337/diab.42.9.1253. [DOI] [PubMed] [Google Scholar]
- 190.Frohnert PP, Hohmann B, Zwiebel R, Baumann K. Free flow micropuncture studies of glucose transport in the rat nephron. Pflugers Arch. 1970;315:66–85. doi: 10.1007/BF00587238. [DOI] [PubMed] [Google Scholar]
- 191.Fry DC, Kuby SA, Mildvan AS. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotidebinding proteins. Proc Natl Acad Sci U S A. 1986;83:907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fu Y, Maianu L, Melbert BR, Garvey WT. Facilitative glucose transporter gene expression in human lymphocytes, monocytes, and macrophages: a role for GLUT isoforms 1, 3, and 5 in the immune response and foam cell formation. Blood Cells Mol Dis. 2004;32:182–190. doi: 10.1016/j.bcmd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 193.Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5'AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- 194.Gaposchkin CG, Garcia-Diaz JF. Modulation of cultured brain, adrenal, and aortic endothelial cell glucose transport. Biochim Biophys Acta S. 1996;1285:255–266. doi: 10.1016/s0005-2736(96)00172-1. [DOI] [PubMed] [Google Scholar]
- 195.Garcia MA, Millan C, Balmaceda-Aguilera C, Castro T, Pastor P, Montecinos H, Reinicke K, Zuniga F, Vera JC, Onate SA, Nualart F. Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J Neurochem. 2003;86:709–724. doi: 10.1046/j.1471-4159.2003.01892.x. [DOI] [PubMed] [Google Scholar]
- 196.Gardner TW, Antonetti DA, Barber AJ, Lieth E, Tarbell JA. The molecular structure and function of the inner blood-retinal barrier. Penn State Retina Research Group. Doc Ophthalmol. 1999;97:229–237. doi: 10.1023/a:1002140812979. [DOI] [PubMed] [Google Scholar]
- 197.Geleoc GS, Casalotti SO, Forge A, Ashmore JF. A sugar transporter as a candidate for the outer hair cell motor. Nat Neurosci. 1999;2:713–719. doi: 10.1038/11174. [DOI] [PubMed] [Google Scholar]
- 198.Gerhart DZ, Broderius MA, Borson ND, Drewes LR. Neurons and microvessels express the brain glucose transporter protein GLUT3. Proc Natl Acad Sci U S A. 1992;89:733–737. doi: 10.1073/pnas.89.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gerhart DZ, Leino RL, Borson ND, Taylor WE, Gronlund KM, McCall AL, Drewes LR. Localization of glucose transporter GLUT 3 in brain: comparison of rodent and dog using species-specific carboxyl-terminal antisera. Neuroscience. 1995;66:237–246. doi: 10.1016/0306-4522(94)00544-f. [DOI] [PubMed] [Google Scholar]
- 200.Gerhart DZ, Leino RL, Taylor WE, Borson ND, Drewes LR. GLUT1 and GLUT3 gene expression in gerbil brain following brief ischemia: an in situ hybridization study. Brain Res Mol Brain Res. 1994;25:313–322. doi: 10.1016/0169-328x(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 201.Gerhart DZ, LeVasseur RJ, Broderius MA, Drewes LR. Glucose transporter localization in brain using light and electron immunocytochemistry. J Neurosci Res. 1989;22:464–472. doi: 10.1002/jnr.490220413. [DOI] [PubMed] [Google Scholar]
- 202.Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24:382–391. doi: 10.2337/diacare.24.2.382. [DOI] [PubMed] [Google Scholar]
- 203.Gerritsen ME, Burke TM, Allen LA. Glucose starvation is required for insulin stimulation of glucose uptake and metabolism in cultured microvascular endothelial cells. Microvasc Res. 1988;35:153–166. doi: 10.1016/0026-2862(88)90059-3. [DOI] [PubMed] [Google Scholar]
- 204.Gillespie DS. OVERVIEW OF SPECIES NEEDING DIETARY VITAMIN C. Journal of Zoo Animal Medicine. 1980;11:88–91. [Google Scholar]
- 205.Ginsburg H, Stein WD. Zero-trans and infinite-cis uptake of galactose in human erythrocytes. Biochim Biophys Acta. 1975;382:353–368. doi: 10.1016/0005-2736(75)90277-1. [DOI] [PubMed] [Google Scholar]
- 206.Gjedde A. Bradbury M. Physiology and pharmacology of the blood brain barrier. New York: Springer-Verlag; 1992. Blood brain glucose transfer; pp. 65–117. [Google Scholar]
- 207.Golay A, DeFronzo RA, Ferrannini E, Simonson DC, Thorin D, Acheson K, Thiebaud D, Curchod B, Jequier E, Felber JP. Oxidative and non-oxidative glucose metabolism in non-obese type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1988;31:585–591. doi: 10.1007/BF00264764. [DOI] [PubMed] [Google Scholar]
- 208.Gomez O, Romero A, Terrado J, Mesonero JE. Differential expression of glucose transporter GLUT8 during mouse spermatogenesis. Reproduction. 2006;131:63–70. doi: 10.1530/rep.1.00750. [DOI] [PubMed] [Google Scholar]
- 209.Gonzalez JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA, Burdakov D. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur J Neurosci. 2009;30:57–64. doi: 10.1111/j.1460-9568.2009.06789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Goodyear LJ, Hirshman MF, Valyou PM, Horton ES. Glucose transporter number, function, and subcellular distribution in rat skeletal muscle after exercise training. Diabetes. 1992;41:1091–1099. doi: 10.2337/diab.41.9.1091. [DOI] [PubMed] [Google Scholar]
- 211.Gorga FR, Lienhard GE. Equilibria and kinetics of ligand binding to the human erythrocyte glucose transporter. Evidence for an alternating conformation model for transport. Biochemistry. 1981;20:5108–5113. doi: 10.1021/bi00521a003. [DOI] [PubMed] [Google Scholar]
- 212.Gorga FR, Lienhard GE. Changes in the intrinsic fluorescence of the human erythrocyte monosaccharide transporter upon ligand binding. Biochemistry. 1982;21:1905–1908. doi: 10.1021/bi00537a031. [DOI] [PubMed] [Google Scholar]
- 213.Gorga FR, Baldwin SA, Lienhard GE. The monosaccharide transporter from human erythrocytes is heterogeneously glycosylated. Biochemical and Biophysical Research Communications. 1979;91:955–961. doi: 10.1016/0006-291x(79)91972-7. [DOI] [PubMed] [Google Scholar]
- 214.Gorus FK, Malaisse WJ, Pipeleers DG. Differences in glucose handling by pancreatic A- and B-cells. J Biol Chem. 1984;259:1196–1200. [PubMed] [Google Scholar]
- 215.Graybill C, van Hoek AN, Desai D, Carruthers AM, Carruthers A. Ultrastructure of Human Erythrocyte GLUT1. Biochemistry. 2006;45:8096–8107. doi: 10.1021/bi060398x. [DOI] [PubMed] [Google Scholar]
- 216.Grdisa M, White MK. Regulation of glucose transport in differentiating HD3 cells. Cell Biochem Funct. 2000;18:293–297. doi: 10.1002/1099-0844(200012)18:4<293::AID-CBF885>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 217.Grdisa M, White MK. Erythrocytic differentiation and glyceraldehydes-3-phosphate dehydrogenase expression are regulated by protein phosphorylation and cAMP in HD3 cells. Int J Biochem Cell Biol. 2000;32:589–595. doi: 10.1016/s1357-2725(99)00148-x. [DOI] [PubMed] [Google Scholar]
- 218.Grdisa M, White MK. Molecular and biochemical events during differentiation of the HD3 chicken erythroblastic cell line. Int J Biochem Cell Biol. 2003;35:422–431. doi: 10.1016/s1357-2725(02)00281-9. [DOI] [PubMed] [Google Scholar]
- 219.Gromada J, Bokvist K, Ding WG, Barg S, Buschard K, Renstrom E, Rorsman P. Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. J Gen Physiol. 1997;110:217–228. doi: 10.1085/jgp.110.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gronowicz G, Swift H, Steck TL. Maturation of the reticulocyte in vitro. J Cell Sci. 1984;71:177–197. doi: 10.1242/jcs.71.1.177. [DOI] [PubMed] [Google Scholar]
- 221.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–779. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 222.Gruetter R, Novotny EJ, Boulware SD, Rothman DL, Shulman RG. 1H NMR studies of glucose transport in the human brain. J Cereb Blood Flow Metab S. 1996;16:427–438. doi: 10.1097/00004647-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 223.Gruetter R, Novotny EJ, Boulware SD, Rothman DL, Shulman RG. 1H NMR studies of glucose transport in the human brain. J Cereb Blood Flow Metab. 1996;16:427–438. doi: 10.1097/00004647-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 224.Gruetter R, Ugurbil K, Seaquist ER. Steady-state cerebral glucose concentrations and transport in the human brain. J Neurochem. 1998;70:397–408. doi: 10.1046/j.1471-4159.1998.70010397.x. [DOI] [PubMed] [Google Scholar]
- 225.Gude NM, Stevenson JL, Rogers S, Best JD, Kalionis B, Huisman MA, Erwich JJ, Timmer A, King RG. GLUT12 expression in human placenta in first trimester and term. Placenta. 2003;24:566–570. doi: 10.1053/plac.2002.0925. [DOI] [PubMed] [Google Scholar]
- 226.Guillam MT, Burcelin R, Thorens B. Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes. Proc Natl Acad Sci U S A. 1998;95:12317–12321. doi: 10.1073/pnas.95.21.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Haber RS, Weinstein SP, O'Boyle E, Morgello S. Tissue distribution of the human GLUT3 glucose transporter. Endocrinology. 1993;132:2538–2543. doi: 10.1210/endo.132.6.8504756. [DOI] [PubMed] [Google Scholar]
- 228.Hahn TJ, Westbrook SL, Sullivan TL, Goodman WG, Halstead LR. Glucose transport in osteoblast-enriched bone explants: characterization and insulin regulation. J Bone Miner Res. 1988;3:359–365. doi: 10.1002/jbmr.5650030317. [DOI] [PubMed] [Google Scholar]
- 229.Hamill S, Cloherty EK, Carruthers A. The human erythrocyte sugar transporter presents two sugar import sites. Biochemistry. 1999;38:16974–16983. doi: 10.1021/bi9918792. [DOI] [PubMed] [Google Scholar]
- 230.Haney PM. Localization of the GLUT1 glucose transporter to brefeldin A-sensitive vesicles of differentiated CIT3 mouse mammary epithelial cells. Cell Biol Int. 2001;25:277–288. doi: 10.1006/cbir.2000.0649. [DOI] [PubMed] [Google Scholar]
- 231.Haney PM. Glucose transport in lactation. Adv Exp Med Biol. 2004;554:253–261. doi: 10.1007/978-1-4757-4242-8_21. [DOI] [PubMed] [Google Scholar]
- 232.Hankin BL, Lieb WR, Stein WD. Rejection criteria for the asymmetric carrier and their application to glucose transport in the human red blood cell. Biochim. Biophys. Acta. 1972;288:114–126. doi: 10.1016/0005-2736(72)90229-5. [DOI] [PubMed] [Google Scholar]
- 233.Hansen P, Gulve E, Gao J, Schluter J, Mueckler M, Holloszy J. Kinetics of 2- deoxyglucose transport in skeletal muscle: effects of insulin and contractions. American Journal of Physiology. 1995;268:C30–C35. doi: 10.1152/ajpcell.1995.268.1.C30. [DOI] [PubMed] [Google Scholar]
- 234.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206–1237. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 235.Harik SI. Changes in the glucose transporter of brain capillaries. Can J Physiol Pharmacol. 1992;70:S113–S117. doi: 10.1139/y92-252. [DOI] [PubMed] [Google Scholar]
- 236.Harik SI, Behmand RA, Murphy JR. Stability of the glucose transporter in plasma membranes of human erythrocytes [letter] Diabetologia. 1994;37:730. doi: 10.1007/BF00417702. [DOI] [PubMed] [Google Scholar]
- 237.Harik SI, Kalaria RN, Andersson L, Lundahl P, Perry G. Immunocytochemical localization of the erythroid glucose transporter: abundance in tissues with barrier functions. Journal of Neuroscience. 1990;10:3862–3872. doi: 10.1523/JNEUROSCI.10-12-03862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Harik SI, Kalaria RN, Whitney PM, Andersson L, Lundahl P, Ledbetter SR, Perry G. Glucose transporters are abundant in cells with "occluding" junctions at the blood-eye barriers. Proc Natl Acad Sci U S A. 1990;87:4261–4264. doi: 10.1073/pnas.87.11.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Harkness DR, Grayson V. Erythrocyte metabolism in the bottle-nosed dolphin, Tursiops truncatus. Comparative Biochemistry and Physiology. 1969;28:1289–1301. doi: 10.1016/0010-406x(69)90567-2. [DOI] [PubMed] [Google Scholar]
- 240.Hasselbalch SG, Holm S, Pedersen HS, Svarer C, Knudsen GM, Madsen PL, Paulson OB. The (18)F-fluorodeoxyglucose lumped constant determined in human brain from extraction fractions of (18)F-fluorodeoxyglucose and glucose. J Cereb Blood Flow Metab S. 2001;21:995–1002. doi: 10.1097/00004647-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 241.Hauguel-de Mouzon S, Challier JC, Kacemi A, Cauzac M, Malek A, Girard J. The GLUT3 glucose transporter isoform is differentially expressed within human placental cell types. J Clin Endocrinol Metab. 1997;82:2689–2694. doi: 10.1210/jcem.82.8.4147. [DOI] [PubMed] [Google Scholar]
- 242.Haworth RA, Berkoff HA. The control of sugar uptake by metabolic demand in isolated heart cells. Circ Res. 1986;58:157–165. doi: 10.1161/01.res.58.1.157. [DOI] [PubMed] [Google Scholar]
- 243.Heard KS, Diguette M, Heard AC, Carruthers A. Membrane-bound glyceraldehydes-3- phosphate dehydrogenase and multiphasic erythrocyte sugar transport. Exp Physiol. 1998;83:195–202. doi: 10.1113/expphysiol.1998.sp004103. [DOI] [PubMed] [Google Scholar]
- 244.Heard KS, Fidyk N, Carruthers A. ATP-dependent substrate occlusion by the human erythrocyte sugar transporter. Biochemistry. 2000;39:3005–3014. doi: 10.1021/bi991931u. [DOI] [PubMed] [Google Scholar]
- 245.Hebert DN, Carruthers A. Cholate-solubilized erythrocyte glucose transporters exist as a mixture of homodimers and homotetramers. Biochemistry. 1991;30:4654–4658. doi: 10.1021/bi00233a003. [DOI] [PubMed] [Google Scholar]
- 246.Hebert DN, Carruthers A. Glucose transporter oligomeric structure determines transporter function. Reversible redox-dependent interconversions of tetrameric and dimeric GLUT1. J. Biol. Chem. 1992;267:23829–23838. [PubMed] [Google Scholar]
- 247.Hebert DN, Carruthers A. Uniporters and anion antiporters. Curr Opin Cell Biol. 1991;3:702–709. doi: 10.1016/0955-0674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- 248.Heijnen HF, Oorschot V, Sixma JJ, Slot JW, James DE. Thrombin stimulates glucose transport in human platelets via the translocation of the glucose transporter GLUT-3 from alpha-granules to the cell surface. J Cell Biol. 1997;138:323–330. doi: 10.1083/jcb.138.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Heimberg H, De Vos A, Pipeleers D, Thorens B, Schuit F. Differences in glucose transporter gene expression between rat pancreatic alpha- and beta-cells are correlated to differences in glucose transport but not in glucose utilization. J Biol Chem. 1995;270:8971–8975. doi: 10.1074/jbc.270.15.8971. [DOI] [PubMed] [Google Scholar]
- 250.Heimberg H, De Vos A, Vandercammen A, Van Schaftingen E, Pipeleers D, Schuit F. Heterogeneity in glucose sensitivity among pancreatic beta-cells is correlated to differences in glucose phosphorylation rather than glucose transport. EMBO J. 1993;12:2873–2879. doi: 10.1002/j.1460-2075.1993.tb05949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Helgerson AL, Carruthers A. Equilibrium ligand binding to the human erythrocyte sugar transporter. Evidence for two sugar-binding sites per carrier. J. Biol. Chem. 1987;262:5464–5475. [PubMed] [Google Scholar]
- 252.Helgerson AL, Carruthers A. Analysis of protein-mediated 3-O-methylglucose transport in rat erythrocytes: rejection of the alternating conformation carrier model for sugar transport. Biochemistry. 1989;28:4580–4594. doi: 10.1021/bi00437a012. [DOI] [PubMed] [Google Scholar]
- 253.Helgerson AL, Hebert DN, Naderi S, Carruthers A. Characterization of two independent modes of action of ATP on human erythrocyte sugar transport. Biochemistry. 1989;28:6410–6417. doi: 10.1021/bi00441a038. [DOI] [PubMed] [Google Scholar]
- 254.Hellwig B, Joost HG. Differentiation of erythrocyte-(GLUT1), liver-(GLUT2), and adipocyte-type (GLUT4) glucose transporters by binding of the inhibitory ligands cytochalasin B, forskolin, dipyridamole, and isobutylmethylxanthine. Mol Pharmacol. 1991;40:383–389. [PubMed] [Google Scholar]
- 255.Henderson PJ, Maiden MC. Homologous sugar transport proteins in Escherichia coli and their relatives in both prokaryotes and eukaryotes. [Review] Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1990;326:391–410. doi: 10.1098/rstb.1990.0020. [DOI] [PubMed] [Google Scholar]
- 256.Henderson PJF. The homologous glucose transport proteins of prokaryotes and eukaryotes. Res Microbiol. 1990;141:316–328. doi: 10.1016/0923-2508(90)90005-b. [DOI] [PubMed] [Google Scholar]
- 257.Henderson PJF, Maiden MCJ. Homologous Sugar Transport Proteins in Escherichia coli and Their Relatives in Both Prokaryotes and Eukaryotes. Philos Trans R Soc Lond B Biol Sci. 1990;326:391–410. doi: 10.1098/rstb.1990.0020. [DOI] [PubMed] [Google Scholar]
- 258.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 259.Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Frontiers in Human Neuroscience. 2009;3 doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hermansen L, Hultman E, Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand. 1967;71:129–139. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- 261.Hinkle PC, Sogin DC, Wheeler TJ, Teleford JN. Studies of the glucose transporter from human erythrocytes reconstituted in liposomes. In: Quagliariello Eea., editor. Function and Molecular Aspect of Biomembrane Transport. Amsterdam: Elsevier/North-Holland Biomedical Press; 1979. pp. 487–494. [Google Scholar]
- 262.Hirai T, Heymann JA, Shi D, Sarker R, Maloney PC, Subramaniam S. Threedimensional structure of a bacterial oxalate transporter. Nat Struct Biol. 2002;9:597–600. doi: 10.1038/nsb821. [DOI] [PubMed] [Google Scholar]
- 263.Ho YY, Yang H, Klepper J, Fischbarg J, Wang D, De Vivo DC. Glucose transporter type 1 deficiency syndrome (Glut1DS): methylxanthines potentiate GLUT1 haploinsufficiency in vitro. Pediatr Res S. 2001;50:254–260. doi: 10.1203/00006450-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 264.Holloszy JO, Narahara HT. Studies on tissue permeability. X. Changes in permeability to 3-O-methylglucose associated with contraction in frog muscle. J. Biol. Chem. 1975;240:349–355. [PubMed] [Google Scholar]
- 265.Holman GD, Kozka IJ, Clark AE, Flower CJ, Saltis J, Habberfield AD, Simpson IA, Cushman SW. Cell surface labeling of glucose transporter isoform GLUT4 by bismannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J. Biol. Chem. 1990;265:18172–18179. [PubMed] [Google Scholar]
- 266.Holman GD. An allosteric pore model for sugar transport in human erythrocytes. Biochim Biophys Acta. 1980;599:202–213. doi: 10.1016/0005-2736(80)90068-1. [DOI] [PubMed] [Google Scholar]
- 267.Holyoake J, Caulfeild V, Baldwin SA, Sansom MS. Modeling, docking, and simulation of the major facilitator superfamily. Biophysical journal. 2006;91:L84–L86. doi: 10.1529/biophysj.106.093971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hosoya K, Tachikawa M. Inner blood-retinal barrier transporters: role of retinal drug delivery. Pharm Res. 2009;26:2055–2065. doi: 10.1007/s11095-009-9930-2. [DOI] [PubMed] [Google Scholar]
- 269.Hosoya K, Tomi M. Inner Blood Retinal Barrier: Transport Biology and Methodology. Drug Absorption Studies. 2008:321–338. [Google Scholar]
- 270.Hresko RC, Kruse M, Strube M, Mueckler M. Topology of the Glut 1 glucose transporter deduced from glycosylation scanning mutagenesis. J Biol Chem. 1994;269:20482–20488. [PubMed] [Google Scholar]
- 271.Hubin F, Humblet C, Belaid Z, Lambert C, Boniver J, Thiry A, Defresne MP. Murine bone marrow stromal cells sustain in vivo the survival of hematopoietic stem cells and the granulopoietic differentiation of more mature progenitors. Stem Cells. 2005;23:1626–1633. doi: 10.1634/stemcells.2005-0041. [DOI] [PubMed] [Google Scholar]
- 272.Hudson ER, MA LS, Wilde CJ, Flint DJ, Baldwin SA. Regulation of GLUT1 expression in the mammary gland. Biochem Soc Trans. 1997;25:464S. doi: 10.1042/bst025464s. [DOI] [PubMed] [Google Scholar]
- 273.Hui H, Huang D, McArthur D, Nissen N, Boros LG, Heaney AP. Direct spectrophotometric determination of serum fructose in pancreatic cancer patients. Pancreas. 2009;38:706–712. doi: 10.1097/MPA.0b013e3181a7c6e5. [DOI] [PubMed] [Google Scholar]
- 274.Hussar P, Tserentsoodol N, Koyama H, Yokoo-Sugawara M, Matsuzaki T, Takami S, Takata K. The glucose transporter GLUT1 and the tight junction protein occludin in nasal olfactory mucosa. Chem Senses. 2002;27:7–11. doi: 10.1093/chemse/27.1.7. [DOI] [PubMed] [Google Scholar]
- 275.Ibberson M, Riederer BM, Uldry M, Guhl B, Roth J, Thorens B. Immunolocalization of GLUTX1 in the testis and to specific brain areas and vasopressin-containing neurons. Endocrinology. 2002;143:276–284. doi: 10.1210/endo.143.1.8587. [DOI] [PubMed] [Google Scholar]
- 276.Ibberson M, Uldry M, Thorens B. GLUTX1 (GLUT8), a Novel Mammalian Glucose Transporter Expressed in the Central Nervous System and Insulin-sensitive Tissues. J Biol Chem. 2000;275:4607–4612. doi: 10.1074/jbc.275.7.4607. [DOI] [PubMed] [Google Scholar]
- 277.Ikemoto A, Bole DG, Ueda T. Glycolysis and glutamate accumulation into synaptic vesicles. Role of glyceraldehyde phosphate dehydrogenase and 3-phosphoglycerate kinase. J Biol Chem. 2003;278:5929–5940. doi: 10.1074/jbc.M211617200. [DOI] [PubMed] [Google Scholar]
- 278.Illsley NP. Glucose transporters in the human placenta. Placenta. 2000;21:14–22. doi: 10.1053/plac.1999.0448. [DOI] [PubMed] [Google Scholar]
- 279.Ingermann RL, Stankova L, Bigley RH. Role of monosaccharide transporter in vitamin C uptake by placentalmembrane vesicles. Am. J. Physiol. 1986;250:C637–C641. doi: 10.1152/ajpcell.1986.250.4.C637. [DOI] [PubMed] [Google Scholar]
- 280.Ituarte EA, Ituarte HG, Iida-Klein A, Hahn TJ. Characterization of insulin binding in the UMR-106 rat osteoblastic osteosarcoma cell. J Bone Miner Res. 1989;4:69–73. doi: 10.1002/jbmr.5650040110. [DOI] [PubMed] [Google Scholar]
- 281.Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66:27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose uptake is limiting in T cell activation and requires CD28-mediated Aktdependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jacquez JA. Modulation of glucose transport in human red blood cells by ATP. Biochim Biophys Acta. 1983;727:367–378. doi: 10.1016/0005-2736(83)90422-4. [DOI] [PubMed] [Google Scholar]
- 284.Jacquez JA. Red blood cell as glucose carrier: significance for placental and cerebral glucose transfer. Am J Physiol. 1984;246:R289–R298. doi: 10.1152/ajpregu.1984.246.3.R289. [DOI] [PubMed] [Google Scholar]
- 285.James DE, Burleigh KM, Kraegen EW. Time dependence of insulin action in muscle and adipose tissue in the rat in vivo. An increasing response in adipose tissue with time. Diabetes. 1985;34:1049–1054. doi: 10.2337/diab.34.10.1049. [DOI] [PubMed] [Google Scholar]
- 286.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gutexpressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77:1554–1562. doi: 10.1210/jcem.77.6.8263141. [DOI] [PubMed] [Google Scholar]
- 288.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 289.Jay TM, Dienel GA, Cruz NF, Mori K, Nelson T, Sokoloff L. Metabolic stability of 3- O-methyl-D-glucose in brain and other tissues. J Neurochem. 1990;55:989–1000. doi: 10.1111/j.1471-4159.1990.tb04588.x. [DOI] [PubMed] [Google Scholar]
- 290.Jeukendrup AE. Regulation of fat metabolism in skeletal muscle. Ann N Y Acad Sci. 2002;967:217–235. doi: 10.1111/j.1749-6632.2002.tb04278.x. [DOI] [PubMed] [Google Scholar]
- 291.Jolivet R, Allaman I, Pellerin L, Magistretti PJ, Weber B. Comment on recent modeling studies of astrocyte-neuron metabolic interactions. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Jones HN, Powell TL, Jansson T. Regulation of placental nutrient transport--a review. Placenta. 2007;28:763–774. doi: 10.1016/j.placenta.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 293.Jones PM, George AM. Symmetry and structure in P-glycoprotein and ABC transporters. European Journal of Biochemistry. 2000;267:5298–5305. doi: 10.1046/j.1432-1327.2000.01628.x. [DOI] [PubMed] [Google Scholar]
- 294.Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schurmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab. 2002;282:E974–E976. doi: 10.1152/ajpendo.00407.2001. [DOI] [PubMed] [Google Scholar]
- 295.Joost H-G, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members. Mol Membr Biol. 2001;18:247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 296.Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Mol Membr Biol. 2001;18:247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 297.Jordens I, Molle D, Xiong W, Keller SR, McGraw TE. Insulin-regulated aminopeptidase is a key regulator of GLUT4 trafficking by controlling the sorting of GLUT4 from endosomes to specialized insulin-regulated vesicles. Mol Biol Cell. 2010;21:2034–2044. doi: 10.1091/mbc.E10-02-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Jung CY, Hsu TL, Hah JS, Cha C, Haas MN. Glucose transport carrier of human erythrocytes. Radiation-target size of glucose-sensitive cytochalasin B binding protein. J. Biol. Chem. 1980;255:361–364. [PubMed] [Google Scholar]
- 299.Jung CY, Carlson LM, Whaley DA. Glucose transport carrier activities in extensively washed human red cell ghosts. Biochim. Biophys. Acta. 1971;241:613–627. doi: 10.1016/0005-2736(71)90059-9. [DOI] [PubMed] [Google Scholar]
- 300.Jungermann K, Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989;69:708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- 301.Kachar B, Brownell WE, Altschuler R, Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986;322:365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- 302.Kaczmarczyk SJ, Andrikopoulos S, Favaloro J, Domenighetti AA, Dunn A, Ernst M, Grail D, Fodero-Tavoletti M, Huggins CE, Delbridge LM, Zajac JD, Proietto J. Threshold effects of glucose transporter-4 (GLUT4) deficiency on cardiac glucose uptake and development of hypertrophy. J Mol Endocrinol. 2003;31:449–459. doi: 10.1677/jme.0.0310449. [DOI] [PubMed] [Google Scholar]
- 303.Kalaria RN, Gravina SA, Schmidley JW, Perry G, Harik SI. The glucose transporter of the human brain and blood-brain barrier. Ann Neurol. 1988;24:757–764. doi: 10.1002/ana.410240610. [DOI] [PubMed] [Google Scholar]
- 304.Kandror KV, Pilch PF. The sugar is sIRVed; sorting Glut4 and its fellow travelers. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01175.x. [DOI] [PubMed] [Google Scholar]
- 305.Kane S, Seatter MJ, Gould GW. Functional studies of human GLUT5: effect of pH on substrate selection and an analysis of substrate interactions. Biochem Biophys Res Commun. 1997;238:503–505. doi: 10.1006/bbrc.1997.7204. [DOI] [PubMed] [Google Scholar]
- 306.Karlish SJD, Lieb WR, Ram D, Stein WD. Kinetic Parameters of glucose efflux from human red blood cells under zero-trans conditions. Biochim. Biophys. Acta. 1972;255:126–132. doi: 10.1016/0005-2736(72)90014-4. [DOI] [PubMed] [Google Scholar]
- 307.Kasahara M, Hinkle PC. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977;252:7384–7390. [PubMed] [Google Scholar]
- 308.Katz EB, Stenbit AE, Hatton K, DePinhot R, Charron MJ. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature. 1995;377:151–155. doi: 10.1038/377151a0. [DOI] [PubMed] [Google Scholar]
- 309.Kayano T, Burant CF, Fukumoto H, Gould GW, Fan YS, Eddy RL, Byers MG, Shows TB, Seino S, Bell GI. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6) J. Biol. Chem. 1990;265:13276–13282. [PubMed] [Google Scholar]
- 310.Keenan TW, Morre DJ, Cheetham RD. Lactose synthesis by a golgi apparatus fraction from rat mammary gland. Nature. 1970;228:1105–1106. doi: 10.1038/2281105a0. [DOI] [PubMed] [Google Scholar]
- 311.Keller DM. Glucose excretion in man and dog. Nephron. 1968;5:43–66. doi: 10.1159/000179616. [DOI] [PubMed] [Google Scholar]
- 312.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35–54. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- 313.Khera PK, Joiner CH, Carruthers A, Lindsell CJ, Smith EP, Franco RS, Holmes YR, Cohen RM. Evidence for interindividual heterogeneity in the glucose gradient across the human red blood cell membrane and its relationship to hemoglobin glycation. Diabetes. 2008;57:2445–2452. doi: 10.2337/db07-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kim JM, Jeong D, Kang HK, Jung SY, Kang SS, Min BM. Osteoclast precursors display dynamic metabolic shifts toward accelerated glucose metabolism at an early stage of RANKL-stimulated osteoclast differentiation. Cell Physiol Biochem. 2007;20:935–946. doi: 10.1159/000110454. [DOI] [PubMed] [Google Scholar]
- 315.Kim YB, Peroni OD, Aschenbach WG, Minokoshi Y, Kotani K, Zisman A, Kahn CR, Goodyear LJ, Kahn BB. Muscle-specific deletion of the Glut4 glucose transporter alters multiple regulatory steps in glycogen metabolism. Mol Cell Biol. 2005;25:9713–9723. doi: 10.1128/MCB.25.21.9713-9723.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kinne R, Murer H, Kinne-Saffran E, Thees M, Sachs G. Sugar transport by renal plasma membrane vesicles. Characterization of the systems in the brush-border microvilli and basal-lateral plasma membranes. J Membr Biol. 1975;21:375–395. doi: 10.1007/BF01941077. [DOI] [PubMed] [Google Scholar]
- 317.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Klepper J, Leiendecker B. GLUT1 deficiency syndrome - 2007 update. Developmental medicine and child neurology. 2007;49:707–716. doi: 10.1111/j.1469-8749.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- 319.Klepper J, Voit T. Facilitated glucose transporter protein type 1 (GLUT1) deficiency syndrome: impaired glucose transport into brain-- a review. Eur J Pediatr. 2002;161:295–304. doi: 10.1007/s00431-002-0939-3. [DOI] [PubMed] [Google Scholar]
- 320.Knott RM, Robertson M, Muckersie E, Forrester JV. Regulation of glucose transporters (GLUT-1 and GLUT-3) in human retinal endothelial cells. Biochem J. 1996;318:313–317. doi: 10.1042/bj3180313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kobayashi M, Nikami H, Morimatsu M, Saito M. Expression and localization of insulin-regulatable glucose transporter (GLUT4) in rat brain. Neurosci Lett. 1996;213:103–106. doi: 10.1016/0304-3940(96)12845-7. [DOI] [PubMed] [Google Scholar]
- 322.Kokk K, Verajankorva E, Laato M, Wu XK, Tapfer H, Pollanen P. Expression of insulin receptor substrates 1–3, glucose transporters GLUT-1-4, signal regulatory protein 1alpha, phosphatidylinositol 3-kinase and protein kinase B at the protein level in the human testis. Anat Sci Int. 2005;80:91–96. doi: 10.1111/j.1447-073x.2005.00091.x. [DOI] [PubMed] [Google Scholar]
- 323.Kokk K, Verajankorva E, Wu XK, Tapfer H, Poldoja E, Simovart HE, Pollanen P. Expression of insulin signaling transmitters and glucose transporters at the protein level in the rat testis. Ann N Y Acad Sci. 2007;1095:262–273. doi: 10.1196/annals.1397.030. [DOI] [PubMed] [Google Scholar]
- 324.Kol S, Ben-Shlomo I, Ruutiainen K, Ando M, Davies-Hill TM, Rohan RM, Simpson IA, Adashi EY. The midcycle increase in ovarian glucose uptake is associated with enhanced expression of glucose transporter 3. Possible role for interleukin-1, a putative intermediary in the ovulatory process. J Clin Invest. 1997;99:2274–2283. doi: 10.1172/JCI119403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Komatsu T, Arashiki N, Otsuka Y, Sato K, Inaba M. Extrusion of Na,K-ATPase and transferrin receptor with lipid raft-associated proteins in different populations of exosomes during reticulocyte maturation in dogs. Jpn J Vet Res. 2010;58:17–27. [PubMed] [Google Scholar]
- 326.Komori T, Morikawa Y, Tamura S, Doi A, Nanjo K, Senba E. Subcellular localization of glucose transporter 4 in the hypothalamic arcuate nucleus of ob/ob mice under basal conditions. Brain Res. 2005;1049:34–42. doi: 10.1016/j.brainres.2005.04.079. [DOI] [PubMed] [Google Scholar]
- 327.Kondo T, Beutler E. Developmental changes in glucose transport of guinea pig erythrocytes. J Clin Invest. 1980;65:1–4. doi: 10.1172/JCI109638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Koumanov F, Jin B, Yang J, Holman GD. Insulin signaling meets vesicle traffic of GLUT4 at a plasma-membrane-activated fusion step. Cell Metab. 2005;2:179–189. doi: 10.1016/j.cmet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 329.Koury MJ, Sawyer ST, Brandt SJ. New insights into erythropoiesis. Curr Opin Hematol. 2002;9:93–100. doi: 10.1097/00062752-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 330.Kraegen EW, Sowden JA, Halstead MB, Clark PW, Rodnick KJ, Chisholm DJ, James DE. Glucose transporters and in vivo glucose uptake in skeletal and cardiac muscle: fasting, insulin stimulation and immunoisolation studies of GLUT1 and GLUT4. Biochem J. 1993;295:287–293. doi: 10.1042/bj2950287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Krupka RM, Devés R. An experimental test for cyclic versus linear transport models. The mechanism of glucose and choline transport in erythrocytes. J. Biol. Chem. 1981;256:5410–5416. [PubMed] [Google Scholar]
- 332.Kumagai AK. Glucose transport in brain and retina: implications in the management and complications of diabetes. Diabetes Metab Res Rev. 1999;15:261–273. doi: 10.1002/(sici)1520-7560(199907/08)15:4<261::aid-dmrr43>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 333.Kumagai AK, Kang YS, Boado RJ, Pardridge WM. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes. 1995;44:1399–1404. doi: 10.2337/diab.44.12.1399. [DOI] [PubMed] [Google Scholar]
- 334.Lachaal M, Rampal AL, Ryu J, Lee W, Hah J-S, Jung CY. Characterization and partial purification of liver glucose transporter GLUT2. Biochim Biophys Acta. 2000;1466:379–389. doi: 10.1016/s0005-2736(00)00205-4. [DOI] [PubMed] [Google Scholar]
- 335.Lacko L, Wittke B, Kromphardt H. Eur. J. Biochem. 1972;25:447–454. doi: 10.1111/j.1432-1033.1972.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 336.Lamarche L, Yamaguchi N, Peronnet F. Hepatic denervation reduces adrenal catecholamine secretion during insulin-induced hypoglycemia. Am J Physiol. 1995;268:R50–R57. doi: 10.1152/ajpregu.1995.268.1.R50. [DOI] [PubMed] [Google Scholar]
- 337.Lamb CA, McCann RK, Stockli J, James DE, Bryant NJ. Insulin-regulated trafficking of GLUT4 requires ubiquitination. Traffic. 2010;11:1445–1454. doi: 10.1111/j.1600-0854.2010.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Larsen KI, Falany M, Wang W, Williams JP. Glucose is a key metabolic regulator of osteoclasts; glucose stimulated increases in ATP/ADP ratio and calmodulin kinase II activity. Biochem Cell Biol. 2005;83:667–673. doi: 10.1139/o05-136. [DOI] [PubMed] [Google Scholar]
- 339.Laughery M, Todd M, Kaplan JH. Oligomerization of the Na,K-ATPase in cell membranes. J Biol Chem. 2004;279:36339–36348. doi: 10.1074/jbc.M402778200. [DOI] [PubMed] [Google Scholar]
- 340.Lauritzen HP, Galbo H, Brandauer J, Goodyear LJ, Ploug T. Large GLUT4 vesicles are stationary while locally and reversibly depleted during transient insulin stimulation of skeletal muscle of living mice: imaging analysis of GLUT4-enhanced green fluorescent protein vesicle dynamics. Diabetes. 2008;57:315–324. doi: 10.2337/db06-1578. [DOI] [PubMed] [Google Scholar]
- 341.Lauritzen HP, Galbo H, Toyoda T, Goodyear LJ. Kinetics of contraction-induced GLUT4 translocation in skeletal muscle fibers from living mice. Diabetes. 2010;59:2134–2144. doi: 10.2337/db10-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Lauritzen HP, Ploug T, Ai H, Donsmark M, Prats C, Galbo H. Denervation and high-fat diet reduce insulin signaling in T-tubules in skeletal muscle of living mice. Diabetes. 2008;57:13–23. doi: 10.2337/db07-0516. [DOI] [PubMed] [Google Scholar]
- 343.Lauritzen HP, Ploug T, Prats C, Tavare JM, Galbo H. Imaging of insulin signaling in skeletal muscle of living mice shows major role of T-tubules. Diabetes. 2006;55:1300–1306. doi: 10.2337/db05-1216. [DOI] [PubMed] [Google Scholar]
- 344.Le KA, Tappy L. Metabolic effects of fructose. Curr Opin Clin Nutr Metab Care. 2006;9:469–475. doi: 10.1097/01.mco.0000232910.61612.4d. [DOI] [PubMed] [Google Scholar]
- 345.Leach L, Firth JA. Fine structure of the paracellular junctions of terminal villous capillaries in the perfused human placenta. Cell Tissue Res. 1992;268:447–452. doi: 10.1007/BF00319151. [DOI] [PubMed] [Google Scholar]
- 346.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Lee WH, Bondy CA. Ischemic injury induces brain glucose transporter gene expression. Endocrinology. 1993;133:2540–2544. doi: 10.1210/endo.133.6.8243275. [DOI] [PubMed] [Google Scholar]
- 348.LeFevre PG, Marshall JK. The attachment of phloretin and analogues to human erythrocytes in connection with inhibition of sugar transport. J. Biol. Chem. 1959;234:3022–3027. [PubMed] [Google Scholar]
- 349.Leino RL, Gerhart DZ, van Bueren AM, McCall AL, Drewes LR. Ultrastructural localization of GLUT 1 and GLUT 3 glucose transporters in rat brain. J Neurosci Res S. 1997;49:617–626. doi: 10.1002/(SICI)1097-4547(19970901)49:5<617::AID-JNR12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 350.Leitch JM, Carruthers A. ATP-dependent sugar transport complexity in human erythrocytes. American journal of physiology Cell physiology. 2007;292:C974–C986. doi: 10.1152/ajpcell.00335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Leitch JM, Carruthers A. Alpha- and Beta-Monosaccharide transport in human erythrocytes. Am J Physiol Cell Physiol. 2009;296:C151–C161. doi: 10.1152/ajpcell.00359.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Leloup C, Arluison M, Kassis N, Lepetit N, Cartier N, Ferre P, Penicaud L. Discrete brain areas express the insulin-responsive glucose transporter GLUT4. Brain Res Mol Brain Res. 1996;38:45–53. doi: 10.1016/0169-328x(95)00306-d. [DOI] [PubMed] [Google Scholar]
- 353.Lemieux MJ, Song J, Kim MJ, Huang Y, Villa A, Auer M, Li XD, Wang DN. Threedimensional crystallization of the Escherichia coli glycerol-3-phosphate transporter: A member of the major facilitator superfamily. Protein Sci. 2003;12:2748–2756. doi: 10.1110/ps.03276603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Lemieux MJ. Eukaryotic major facilitator superfamily transporter modeling based on the prokaryotic GlpT crystal structure (Review) Molecular membrane biology. 2007;24:333–341. doi: 10.1080/09687680701496507. [DOI] [PubMed] [Google Scholar]
- 355.Leoncini G, Maresca M. Glucose transport across plasma membrane in human platelets. Ital J Biochem. 1986;35:287–295. [PubMed] [Google Scholar]
- 356.Levine KB, Carruthers A. Boles E, Krämer R. Topics in Current Genetics Molecular mechanisms controling transmembrane transport(9) 2004. Regulation of carrier-mediated sugar transport by transporter quaternary structure; pp. 67–694. [Google Scholar]
- 357.Levine KB, Cloherty EK, Fidyk NJ, Carruthers A. Structural and physiologic determinants of human erythrocyte sugar transport regulation by adenosine triphosphate. Biochemistry. 1998;37:12221–12232. doi: 10.1021/bi980585y. [DOI] [PubMed] [Google Scholar]
- 358.Levine KB, Cloherty EK, Hamill S, Carruthers A. Molecular determinants of sugar transport regulation by ATP. Biochemistry. 2002;41:12629–12638. doi: 10.1021/bi0258997. [DOI] [PubMed] [Google Scholar]
- 359.Levine KB, Hamill S, Cloherty EK, Carruthers A. Alanine scanning mutagenesis of the human erythrocyte glucose transporter putative ATP binding domain. Blood Cells Mol Dis. 2001;27:139–142. doi: 10.1006/bcmd.2000.0359. [DOI] [PubMed] [Google Scholar]
- 360.Levine KB, Robichaud TK, Hamill S, Sultzman LA, Carruthers A. Properties of the human erythrocyte glucose transport protein are determined by cellular context. Biochemistry. 2005;44:5606–5616. doi: 10.1021/bi0477541. [DOI] [PubMed] [Google Scholar]
- 361.Levine KB, DeZutter JK, Carruthers A. Analysis of GLUT1-oligomerization determinants by helix-swapping mutagenesis. Blood Cells Mol Dis. 2011 Submitted. [Google Scholar]
- 362.Li Q, Manolescu A, Ritzel M, Yao S, Slugoski M, Young JD, Chen XZ, Cheeseman CI. Cloning and functional characterization of the human GLUT7 isoform SLC2A7 from the small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G236–G242. doi: 10.1152/ajpgi.00396.2003. [DOI] [PubMed] [Google Scholar]
- 363.Lieb WR, Stein WD. Testing and characterizing the simple carrier. Biochim Biophys Acta. 1974;373:178–196. doi: 10.1016/0005-2736(74)90144-8. [DOI] [PubMed] [Google Scholar]
- 364.Lieb WR, Stein WD. Is there a high affinity site for sugar transport at the inner face of the human red cell membrane? J. Theor. Biol. 1977;69:311–319. doi: 10.1016/0022-5193(77)90140-0. [DOI] [PubMed] [Google Scholar]
- 365.Lieb WR, Stein WD. Testing and characterizing the simple carrier. Biochim. Biophys. Acta. 1974;373:178–196. doi: 10.1016/0005-2736(74)90144-8. [DOI] [PubMed] [Google Scholar]
- 366.Lin S, Spudich JA. Biochemical studies on the mechanism of action of cytochalasin B. Cytochalasin B binding to red cell membranes in relation to glucose transport. J. Biol. Chem. 1974;249:5778–5783. [PubMed] [Google Scholar]
- 367.Lind AR, Williams CA. The control of blood flow through human forearm muscles following brief isometric contractions. J Physiol. 1979;288:529–547. [PMC free article] [PubMed] [Google Scholar]
- 368.Linder BL, Chernoff A, Kaplan KL, Goodman DS. Release of platelet-derived growth factor from human platelets by arachidonic acid. Proc Natl Acad Sci U S A. 1979;76:4107–4111. doi: 10.1073/pnas.76.8.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Lisinski I, Schurmann A, Joost HG, Cushman SW, Al-Hasani H. Targeting of GLUT6 (formerly GLUT9) and GLUT8 in rat adipose cells. Biochem J. 2001;358:517–22.. doi: 10.1042/0264-6021:3580517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol. 1997;273:C1596–C1604. doi: 10.1152/ajpcell.1997.273.5.C1596. [DOI] [PubMed] [Google Scholar]
- 371.Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol. 2005;169:481–489. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Loike JD, Cao L, Brett J, Ogawa S, Silverstein SC, Stern D. Hypoxia induces glucose transporter expression in endothelial cells. Am J Physiol S. 1992;263:C326–C333. doi: 10.1152/ajpcell.1992.263.2.C326. [DOI] [PubMed] [Google Scholar]
- 374.Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta. 1994;1213:263–276. doi: 10.1016/0005-2760(94)00082-4. [DOI] [PubMed] [Google Scholar]
- 375.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol. 1991;261:H1698–H1705. doi: 10.1152/ajpheart.1991.261.6.H1698. [DOI] [PubMed] [Google Scholar]
- 376.Lowe AG, Walmsley AR. The kinetics of glucose transport in human red blood cells. Biochim. Biophys. Acta. 1986;857:146–154. doi: 10.1016/0005-2736(86)90342-1. [DOI] [PubMed] [Google Scholar]
- 377.Lund-Andersen H. Transport of glucose from blood to brain. Physiol Rev. 1979;59:305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- 378.Lundahl P, Mascher E, Andersson L, Englund AK, Greijer E, Kameyama K, Takagi T. Active and monomeric human red cell glucose transporter after high performance molecular-sieve chromatography in the presence of octyl glucoside and phosphatidylserine or phosphatidylcholine. Biochim Biophys Acta. 1991;1067:177–186. doi: 10.1016/0005-2736(91)90041-6. [DOI] [PubMed] [Google Scholar]
- 379.Lutsenko S, Anderko R, Kaplan JH. Membrane disposition of the M5-M6 hairpin of Na+,K(+)−ATPase alpha subunit is ligand dependent. Proc Natl Acad Sci U S A S. 1995;92:7936–7940. doi: 10.1073/pnas.92.17.7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Lynch RM, Paul RJ. Energy metabolism and transduction in smooth muscle. Experientia. 1985;41:970–977. doi: 10.1007/BF01952116. [DOI] [PubMed] [Google Scholar]
- 381.MacDonald PE, De Marinis YZ, Ramracheya R, Salehi A, Ma X, Johnson PR, Cox R, Eliasson L, Rorsman P. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 2007;5:e143. doi: 10.1371/journal.pbio.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Macheda ML, Williams ED, Best JD, Wlodek ME, Rogers S. Expression and localisation of GLUT1 and GLUT12 glucose transporters in the pregnant and lactating rat mammary gland. Cell Tissue Res. 2003;311:91–97. doi: 10.1007/s00441-002-0661-5. [DOI] [PubMed] [Google Scholar]
- 383.Maddox DA, Gennari FJ. The early proximal tubule: a high-capacity deliveryresponsive reabsorptive site. Am J Physiol. 1987;252:F573–F584. doi: 10.1152/ajprenal.1987.252.4.F573. [DOI] [PubMed] [Google Scholar]
- 384.Madison LL. Role of insulin in the hepatic handling of glucose. Arch Intern Med. 1969;123:284–292. [PubMed] [Google Scholar]
- 385.Madon RJ, Martin S, Davies A, Fawcett HA, Flint DJ, Baldwin SA. Identification and characterization of glucose transport proteins in plasma membrane- and Golgi vesicleenriched fractions prepared from lactating rat mammary gland. Biochem J. 1990;272:99–105. doi: 10.1042/bj2720099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Magistretti PJ. Brain energy metabolism. In: Squire LR, Bloom FE, McConnell SK, editors. Fundamental Neuroscience. San Diego: Academic Press; 2003. pp. 339–360. [Google Scholar]
- 387.Magnani P, Cherian PV, Gould GW, Greene DA, Sima AA, Brosius FCr. Glucose transporters in rat peripheral nerve: paranodal expression of GLUT1 and GLUT3. Metabolism S. 1996;45:1466–1473. doi: 10.1016/s0026-0495(96)90174-2. [DOI] [PubMed] [Google Scholar]
- 388.Maher F, Davies-Hill TM, Lysko PG, Henneberry RC, Simpson IA. Expression of two glucose transporters, GLUT1 and GLUT3, in cultured cerebellar neurons: Evidence for neuron-specific expression of GLUT3. Mol Cell Neurosci. 1991;2:351–360. doi: 10.1016/1044-7431(91)90066-w. [DOI] [PubMed] [Google Scholar]
- 389.Maher F, Davies-Hill TM, Simpson IA. Substrate specificity and kinetic parameters of GLUT3 in rat cerebellar granule neurons. Biochem J. 1996;315:827–831. doi: 10.1042/bj3150827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Maher F, Vannucci S, Takeda J, Simpson IA. Expression of mouse-GLUT3 and human-GLUT3 glucose transporter proteins in brain. Biochem Biophys Res Commun. 1992;182:703–711. doi: 10.1016/0006-291x(92)91789-s. [DOI] [PubMed] [Google Scholar]
- 391.Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J. 1994;8:1003–1011. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
- 392.Maiden MC, Davis EO, Baldwin SA, Moore DC, Henderson PJ. Mammalian and bacterial sugar transport proteins are homologous. Nature. 1987;325:641–643. doi: 10.1038/325641a0. [DOI] [PubMed] [Google Scholar]
- 393.Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini JL. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell. 2003;115:449–459. doi: 10.1016/s0092-8674(03)00881-x. [DOI] [PubMed] [Google Scholar]
- 394.Mangia S, DiNuzzo M, Giove F, Carruthers A, Simpson IA, Vannucci SJ. Response to “Comment on recent modeling studies of astrocyte-neuron metabolic interactions”: much ado about nothing. Cerebral Blood Flow and Metabolism. 2011 doi: 10.1038/jcbfm.2011.29. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Mangia S, Simpson IA, Vannucci SJ, Carruthers A. The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem. 2009;109 Suppl 1:55–62. doi: 10.1111/j.1471-4159.2009.06003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev S. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- 397.Mann GV, Newton P. The membrane transport of ascorbic acid. Ann N Y Acad Sci. 1975;258:243–252. doi: 10.1111/j.1749-6632.1975.tb29285.x. [DOI] [PubMed] [Google Scholar]
- 398.Manolescu AR, Augustin R, Moley K, Cheeseman C. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol. 2007;24:455–463. doi: 10.1080/09687680701298143. [DOI] [PubMed] [Google Scholar]
- 399.Manolescu AR, Witkowska K, Kinnaird A, Cessford T, Cheeseman C. Facilitated hexose transporters: new perspectives on form and function. Physiology (Bethesda) 2007;22:234–240. doi: 10.1152/physiol.00011.2007. [DOI] [PubMed] [Google Scholar]
- 400.Mantych GJ, James DE, Devaskar SU. Jejunal/kidney glucose transporter isoform (Glut-5) is expressed in the human blood-brain barrier. Endocrinology. 1993;132:35–40. doi: 10.1210/endo.132.1.8419132. [DOI] [PubMed] [Google Scholar]
- 401.Mantych GJ, James DE, Chung HD, Devaskar SU. Cellular localization and characterization of Glut 3 glucose transporter isoform in human brain. Neurosci Lett. 1992;310:101–104. doi: 10.1210/endo.131.3.1505464. [DOI] [PubMed] [Google Scholar]
- 402.Maratou E, Dimitriadis G, Kollias A, Boutati E, Lambadiari V, Mitrou P, Raptis SA. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Invest. 2007;37:282–290. doi: 10.1111/j.1365-2362.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 403.Marcus DC, Thalmann R, Marcus NY. Respiratory rate and ATP content of stria vascularis of guinea pig in vitro. Laryngoscope. 1978;88:1825–1835. doi: 10.1288/00005537-197811000-00011. [DOI] [PubMed] [Google Scholar]
- 404.Marcus DC, Thalmann R, Marcus NY. Respiratory quotient of stria vascularis of guinea pig in vitro. Arch Otorhinolaryngol. 1978;221:97–103. doi: 10.1007/BF00455880. [DOI] [PubMed] [Google Scholar]
- 405.Marette A, Burdett E, Douen A, Vranic M, Klip A. Insulin induces the translocation of GLUT4 from a unique intracellular organelle to transverse tubules in rat skeletal muscle. Diabetes. 1992;41:1562–1569. doi: 10.2337/diab.41.12.1562. [DOI] [PubMed] [Google Scholar]
- 406.Marger MD, Saier Jr MH. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 407.Marin P, Hogh-Kristiansen I, Jansson S, Krotkiewski M, Holm G, Bjorntorp P. Uptake of glucose carbon in muscle glycogen and adipose tissue triglycerides in vivo in humans. Am J Physiol. 1992;263:E473–E480. doi: 10.1152/ajpendo.1992.263.3.E473. [DOI] [PubMed] [Google Scholar]
- 408.Martin JR, Novin D, Vanderweele DA. Loss of glucagon suppression of feeding after vagotomy in rats. Am J Physiol. 1978;234:E314–E318. doi: 10.1152/ajpendo.1978.234.3.E314. [DOI] [PubMed] [Google Scholar]
- 409.Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I, Binnert C, Beermann F, Thorens B. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J Clin Invest. 2005;115:3545–3553. doi: 10.1172/JCI26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 2007;22:241–251. doi: 10.1152/physiol.00010.2007. [DOI] [PubMed] [Google Scholar]
- 411.Masud MM, Fujimoto T, Miyake M, Watanuki S, Itoh M, Tashiro M. Redistribution of whole-body energy metabolism by exercise: a positron emission tomography study. Ann Nucl Med. 2009;23:81–88. doi: 10.1007/s12149-008-0212-6. [DOI] [PubMed] [Google Scholar]
- 412.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 413.Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K, Wiriyasermkul P, Kikuchi Y, Oda T, Nishiyama J, Nakamura T, Morimoto Y, Kamakura K, Sakurai Y, Nonoyama S, Kanai Y, Shinomiya N. Mutations in Glucose Transporter 9 Gene SLC2A9 Cause Renal Hypouricemia. Am J Hum Genet. 2008;83:744–751. doi: 10.1016/j.ajhg.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.May JM, Qu ZC, Whitesell RR. Ascorbic acid recycling enhances the antioxidant reserve of human erythrocytes. Biochemistry. 1995;34:12721–12728. doi: 10.1021/bi00039a031. [DOI] [PubMed] [Google Scholar]
- 415.Mazella J, Zsurger N, Navarro V, Chabry J, Kaghad M, Caput D, Ferrara P, Vita N, Gully D, Maffrand JP, Vincent JP. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273:26273–26276. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- 416.McCall AL, Fixman LB, Fleming N, Tornheim K, Chick W, Ruderman NB. Chronic hypoglycemia increases brain glucose transport. Am J Physiol. 1986;251:E442–E447. doi: 10.1152/ajpendo.1986.251.4.E442. [DOI] [PubMed] [Google Scholar]
- 417.McCall AL, van Bueren AM, Huang L, Stenbit A, Celnik E, Charron MJ. Forebrain endothelium expresses GLUT4, the insulin-responsive glucose transporter. Brain Res. 1997;744:318–326. doi: 10.1016/S0006-8993(96)01122-5. [DOI] [PubMed] [Google Scholar]
- 418.McCall AL, Van Bueren AM, Moholt-Siebert M, Cherry NJ, Woodward WR. Immunohistochemical localization of the neuron-specific glucose transporter (GLUT3) to neuropil in adult rat brain. Brain Res. 1994;659:292–297. doi: 10.1016/0006-8993(94)90896-6. [DOI] [PubMed] [Google Scholar]
- 419.McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 420.McLean P, Brown J, Walters E, Greenslade K. Effect of alloxan-diabetes on multiple forms of hexokinase in adipose tissue and lung. Biochem J. 1967;105:1301–1305. doi: 10.1042/bj1051301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.McVie-Wylie AJ, Lamson DR, Chen YT. Molecular cloning of a novel member of the GLUT family of transporters, SLC2a10 (GLUT10), localized on chromosome 20q13.1: a candidate gene for NIDDM susceptibility. Genomics. 2001;72:113–117. doi: 10.1006/geno.2000.6457. [DOI] [PubMed] [Google Scholar]
- 422.Mehlhorn RJ. Ascorbate- and dehydroascorbic acid-mediated reduction of free radicals in the human erythrocyte. J. Biol. Chem. 1991;266:2724–2731. [PubMed] [Google Scholar]
- 423.Mendiratta S, Qu ZC, May JM. Erythrocyte ascorbate recycling: antioxidant effects in blood. Free Radic Biol Med. 1998;24:789–797. doi: 10.1016/s0891-5849(97)00351-1. [DOI] [PubMed] [Google Scholar]
- 424.Michelle Furtado L, Poon V, Klip A. GLUT4 activation: thoughts on possible mechanisms. Acta Physiol Scand. 2003;178:287–296. doi: 10.1046/j.1365-201X.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- 425.Miki K. Energy metabolism and sperm function. Soc Reprod Fertil Suppl. 2007;65:309–325. [PubMed] [Google Scholar]
- 426.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Miller C, Nguitragool W. A provisional transport mechanism for a chloride channeltype Cl−/H+ exchanger. Philos Trans R Soc Lond B Biol Sci. 2009;364:175–180. doi: 10.1098/rstb.2008.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Miller DM. The kinetics of selective biological transport. III. Erythrocytemonosaccharide transport data. Biophys. J. 1968;8:1329–1338. doi: 10.1016/s0006-3495(68)86559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Miller DM. The kinetics of selective biological transport. IV. Assessment of three carrier systems using the erythrocyte-monosaccharide transport data. Biophys. J. 1968;8:1339–1352. doi: 10.1016/S0006-3495(68)86560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Miller DM. The kinetics of selective biological transport. V. Further data on the erythrocyte monosaccharide transport system. Biophys. J. 1971;11:915–923. doi: 10.1016/S0006-3495(71)86263-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Minokoshi Y, Kahn CR, Kahn BB. Tissue-specific ablation of the GLUT4 glucose transporter or the insulin receptor challenges assumptions about insulin action and glucose homeostasis. J Biol Chem. 2003;278:33609–33612. doi: 10.1074/jbc.R300019200. [DOI] [PubMed] [Google Scholar]
- 432.Montel-Hagen A, Blanc L, Boyer-Clavel M, Jacquet C, Vidal M, Sitbon M, Taylor N. The Glut1 and Glut4 glucose transporters are differentially expressed during perinatal and postnatal erythropoiesis. Blood. 2008;112:4729–4738. doi: 10.1182/blood-2008-05-159269. [DOI] [PubMed] [Google Scholar]
- 433.Montel-Hagen A, Kinet S, Manel N, Mongellaz C, Prohaska R, Battini JL, Delaunay J, Sitbon M, Taylor N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132:1039–1048. doi: 10.1016/j.cell.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 434.Montel-Hagen A, Sitbon M, Taylor N. Erythroid glucose transporters. Curr Opin Hematol. 2009;16:165–172. doi: 10.1097/MOH.0b013e328329905c. [DOI] [PubMed] [Google Scholar]
- 435.Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab. 2009;297:E1358–E1365. doi: 10.1152/ajpendo.00412.2009. [DOI] [PubMed] [Google Scholar]
- 436.Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 437.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMPactivated protein kinase in contraction- and hypoxia- regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–194.. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 438.Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 439.Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science. 1985;229:941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- 440.Mueckler M, Makepeace C. Transmembrane segment 12 of the Glut1 glucose transporter is an outer helix and is not directly involved in the transport mechanism. The Journal of biological chemistry. 2006;281:36993–36998. doi: 10.1074/jbc.M608158200. [DOI] [PubMed] [Google Scholar]
- 441.Mueckler M, Makepeace C. Model of the exofacial substrate-binding site and helical folding of the human Glut1 glucose transporter based on scanning mutagenesis. Biochemistry. 2009;48:5934–5942. doi: 10.1021/bi900521n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science. 1985;229:941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- 443.Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem. 2009;284:21036–21046. doi: 10.1074/jbc.M109.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Naemsch LN, Weidema AF, Sims SM, Underhill TM, Dixon SJ. P2X(4) purinoceptors mediate an ATP-activated, non-selective cation current in rabbit osteoclasts. J Cell Sci. 1999;112:4425–4435. doi: 10.1242/jcs.112.23.4425. [DOI] [PubMed] [Google Scholar]
- 445.Naftalin RJ, Rist RJ. 3-O-methyl-D-glucose transport in rat red cells: effects of heavy water. Biochimica et Biophysica Acta. 1991;1064:37–48. doi: 10.1016/0005-2736(91)90409-2. [DOI] [PubMed] [Google Scholar]
- 446.Naftalin RJ, Holman GD. Transport of sugars in human red cells. In: In: Ellory JC, Lew VL, editors. Membrane transport in red cells. New York: Academic Press; 1977. pp. 257–300. [Google Scholar]
- 447.Naftalin RJ. Alternating Carrier Models of Asymmetric Glucose Transport Violate the Energy Conservation Laws. Biophys J. 2008;95:4300–4314. doi: 10.1529/biophysj.108.136366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Nagamatsu S, Sawa H, Kamada K, Nakamichi Y, Yoshimoto K, Hoshino T. Neuronspecific glucose transporter (NSGT): CNS distribution of GLUT3 rat glucose transporter (RGT3) in rat central neurons. Febs Letters. 1993;334:289–295. doi: 10.1016/0014-5793(93)80697-s. [DOI] [PubMed] [Google Scholar]
- 449.Nakazawa K, Spicer SS, Schulte BA. Postnatal expression of the facilitated glucose transporter, GLUT 5, in gerbil outer hair cells. Hear Res. 1995;82:93–99. doi: 10.1016/0378-5955(94)00162-j. [DOI] [PubMed] [Google Scholar]
- 450.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 451.Niijima A. The effect of D-glucose on the firing rate of glucose-sensitive vagal afferents in the liver in comparison with the effect of 2-deoxy-D-glucose. J Auton Nerv Syst. 1984;10:255–260. doi: 10.1016/0165-1838(84)90021-3. [DOI] [PubMed] [Google Scholar]
- 452.Nishimoto H, Matsutani R, Yamamoto S, Takahashi T, Hayashi KG, Miyamoto A, Hamano S, Tetsuka M. Gene expression of glucose transporter (GLUT) 1, 3 and 4 in bovine follicle and corpus luteum. J Endocrinol. 2006;188:111–119. doi: 10.1677/joe.1.06210. [DOI] [PubMed] [Google Scholar]
- 453.Nishimura H, Pallardo FV, Seidner GA, Vannucci S, Simpson IA, Birnbaum MJ. Kinetics of GLUT1 and GLUT4 glucose transporters expressed in Xenopus oocytes. Journal of Biological Chemistry. 1993;268:8514–8520. [PubMed] [Google Scholar]
- 454.Nualart F, Godoy A, Reinicke K. Expression of the hexose transporters GLUT1 and GLUT2 during the early development of the human brain. Brain Res. 1999;824:97–104. doi: 10.1016/s0006-8993(99)01078-1. [DOI] [PubMed] [Google Scholar]
- 455.Ogawa E, Hishiyama N. Japanese Shiba dogs possessing erythrocytes with high Glut-1 activity and high ascorbic acid recycling capacity. Comparative Clinical Pathology. 2011 1618-565X. [Google Scholar]
- 456.Oka Y, Asano T, Shibasaki Y, Lin JL, Tsukuda K, Katagiri H, Akanuma Y, Takaku F. C-terminal truncated glucose transporter is locked into an inward-facing form without transport activity. Nature. 1990;345:550–553. doi: 10.1038/345550a0. [DOI] [PubMed] [Google Scholar]
- 457.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 458.Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996;16:235–256. doi: 10.1146/annurev.nu.16.070196.001315. [DOI] [PubMed] [Google Scholar]
- 459.Palfreyman RW, Clark AE, Denton RM, Holman GD, Kozka IJ. Kinetic resolution of the separate GLUT1 and GLUT4 glucose transport activities in 3T3-L1 cells. Biochem J. 1992;284:275–282. doi: 10.1042/bj2840275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Pao SS, Paulsen IT, Saier MH., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Pardridge WM. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983;63:1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- 462.Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. Journal of Biological Chemistry. 1990;265:18035–18040. [PubMed] [Google Scholar]
- 463.Pardridge WM. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983;63:1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- 464.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52:289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Pascual JM, Wang D, Lecumberri B, Yang H, Mao X, Yang R, De Vivo DC. GLUT1 deficiency and other glucose transporter diseases. Eur J Endocrinol. 2004;150:627–633. doi: 10.1530/eje.0.1500627. [DOI] [PubMed] [Google Scholar]
- 466.Patel JR, Brewer GJ. Age-related changes in neuronal glucose uptake in response to glutamate and beta-amyloid. J Neurosci Res. 2003;72:527–536. doi: 10.1002/jnr.10602. [DOI] [PubMed] [Google Scholar]
- 467.Pawagi AB, Deber CM. D-glucose binding increases secondary structure of human erythrocyte monosaccharide transport protein. Biochem Biophys Res Commun. 1987;145:1087–1091. doi: 10.1016/0006-291x(87)91548-8. [DOI] [PubMed] [Google Scholar]
- 468.Pellerin L, Bonvento G, Chatton JY, Pierre K, Magistretti PJ. Role of neuron-glia interaction in the regulation of brain glucose utilization. Diabetes Nutr Metab. 2002;15:268–273. discussion 273. [PubMed] [Google Scholar]
- 469.Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Pessino A, Hebert DN, Woon CW, Harrison SA, Clancy BM, Buxton JM, Carruthers A, Czech MP. Evidence that functional erythrocyte-type glucose transporters are oligomers. J. Biol. Chem. 1991;266:20213–20217. [PubMed] [Google Scholar]
- 471.Peters BJ, Rillema JA. Effect of prolactin on 2-deoxyglucose uptake in mouse mammary gland explants. Am J Physiol. 1992;262:E627–E630. doi: 10.1152/ajpendo.1992.262.5.E627. [DOI] [PubMed] [Google Scholar]
- 472.Petersen KF, Krssak M, Navarro V, Chandramouli V, Hundal R, Schumann WC, Landau BR, Shulman GI. Contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in cirrhosis. American Journal of Physiology- Endocrinology And Metabolism. 1999;276:E529. doi: 10.1152/ajpendo.1999.276.3.E529. [DOI] [PubMed] [Google Scholar]
- 473.Pham T, Cornea A, Blick KE, Jenkins A, Scofield RH. Oral glucosamine in doses used to treat osteoarthritis worsens insulin resistance. Am J Med Sci. 2007;333:333–339. doi: 10.1097/MAJ.0b013e318065bdbe. [DOI] [PubMed] [Google Scholar]
- 474.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 475.Ploug T, van Deurs B, Ai H, Cushman SW, Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J Cell Biol. 1998;142:1429–1446. doi: 10.1083/jcb.142.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Porras OH, Loaiza A, Barros LF. Glutamate mediates acute glucose transport inhibition in hippocampal neurons. J Neurosci S. 2004;24:9669–9673. doi: 10.1523/JNEUROSCI.1882-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Preitner F, Bonny O, Laverrière A, Rotman S, Firsov D, Da Costa A, Metref S, Thorens B. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A. 2009;106:15501–15506. doi: 10.1073/pnas.0904411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Prosser CG. Mechanism of the decrease in hexose transport by mouse mammary epithelial cells caused by fasting. Biochem J. 1988;249:149–154. doi: 10.1042/bj2490149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Prosser CG, Topper YJ. Changes in the rate of carrier-mediated glucose transport by mouse mammary epithelial cells during ontogeny: hormone dependence delineated in vitro. Endocrinology. 1986;119:91–96. doi: 10.1210/endo-119-1-91. [DOI] [PubMed] [Google Scholar]
- 480.Quesada I, Todorova MG, Soria B. Different metabolic responses in alpha-, beta-, and delta-cells of the islet of Langerhans monitored by redox confocal microscopy. Biophys J. 2006;90:2641–2650. doi: 10.1529/biophysj.105.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Quesada I, Tuduri E, Ripoll C, Nadal A. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol. 2008;199:5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- 482.Quistorff B, Secher NH, Van Lieshout JJ. Lactate fuels the human brain during exercise. FASEB J. 2008;22:3443–3449. doi: 10.1096/fj.08-106104. [DOI] [PubMed] [Google Scholar]
- 483.Rabilloud T. Membrane proteins ride shotgun. Nat Biotech. 2003;21:508–510. doi: 10.1038/nbt0503-508. [DOI] [PubMed] [Google Scholar]
- 484.Randle PJ, Garland PB, HALES CN, Newsholme EA. The glucose fattyacid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 485.Rayner DV, Thomas ME, Trayhurn P. Glucose transporters (GLUTs 1-4) and their mRNAs in regions of the rat brain: insulin-sensitive transporter expression in the cerebellum. Can J Physiol Pharmacol. 1994;72:476–479. doi: 10.1139/y94-069. [DOI] [PubMed] [Google Scholar]
- 486.Regen DM, Morgan HE. STUDIES OF THE GLUCOSE-TRANSPORT SYSTEM IN THE RABBIT ERYTHROCYTE. Biochim Biophys Acta. 1964;79:151–166. doi: 10.1016/0926-6577(64)90048-8. [DOI] [PubMed] [Google Scholar]
- 487.Reimann F. Molecular mechanisms underlying nutrient detection by incretin-secreting cells. Int Dairy J. 2010;20:236–242. doi: 10.1016/j.idairyj.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Richardson S, Neama G, Phillips T, Bell S, Carter SD, Moley KH, Moley JF, Vannucci SJ, Mobasheri A. Molecular characterization and partial cDNA cloning of facilitative glucose transporters expressed in human articular chondrocytes; stimulation of 2- deoxyglucose uptake by IGF-I and elevated MMP-2 secretion by glucose deprivation. Osteoarthritis Cartilage. 2003;11:92–101. doi: 10.1053/joca.2002.0858. [DOI] [PubMed] [Google Scholar]
- 489.Richter EA. Glucose Utilization. John Wiley & Sons, Inc.; 2010. [Google Scholar]
- 490.Rieu S, Geminard C, Rabesandratana H, Sainte-Marie J, Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1. Eur J Biochem. 2000;267:583–590. doi: 10.1046/j.1432-1327.2000.01036.x. [DOI] [PubMed] [Google Scholar]
- 491.Riquelme G. Review: Placental syncytiotrophoblast membranes - domains, subdomains and microdomains. Placenta. 2011 doi: 10.1016/j.placenta.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 492.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 493.Rivas C, Zúñiga F, Salas-Burgos A, Mardones L, Ormazabal V, Vera J. Vitamin C transporters. J Physiol Biochem. 2008;64:357–375. doi: 10.1007/BF03174092. [DOI] [PubMed] [Google Scholar]
- 494.Rogers S, Chandler JD, Clarke AL, Petrou S, Best JD. Glucose transporter GLUT12- functional characterization in Xenopus laevis oocytes. Biochem Biophys Res Commun. 2003;308:422–426. doi: 10.1016/s0006-291x(03)01417-7. [DOI] [PubMed] [Google Scholar]
- 495.Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD. Identification of a novel glucose transporter-like protein--- GLUT-12. Am J Physiol Endocrinol Metab. 2002;282:E733–E738. doi: 10.1152/ajpendo.2002.282.3.E733. [DOI] [PubMed] [Google Scholar]
- 496.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 497.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 498.Rossetti L, Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990;85:1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- 500.Rowland AF, Fazakerley DJ, James DE. Mapping Insulin/GLUT4 Circuitry. Traffic. 2011;12:672–681. doi: 10.1111/j.1600-0854.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 501.Rumsey SC, Daruwala R, Al-Hasani H, Zarnowski MJ, Simpson IA, Levine M. Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. J Biol Chem. 2000;275:28246–28253. doi: 10.1074/jbc.M000988200. [DOI] [PubMed] [Google Scholar]
- 502.Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. 1997;272:18982–18989. doi: 10.1074/jbc.272.30.18982. [DOI] [PubMed] [Google Scholar]
- 503.Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helixhelix association. J Mol Biol. 2000;296:911–99.. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- 504.Saier MH., Jr Families of proteins forming transmembrane channels. J Membr Biol. 2000;175:165–180. doi: 10.1007/s00232001065. [DOI] [PubMed] [Google Scholar]
- 505.Saier MH, Jr, Beatty JT, Goffeau A, Harley KT, Heijne WH, Huang SC, Jack DL, Jahn PS, Lew K, Liu J, Pao SS, Paulsen IT, Tseng TT, Virk PS. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1:257–279. [PubMed] [Google Scholar]
- 506.Saier MH, Jr, Eng BH, Fard S, Garg J, Haggerty DA, Hutchinson WJ, Jack DL, Lai EC, Liu HJ, Nusinew DP, Omar AM, Pao SS, Paulsen IT, Quan JA, Sliwinski M, Tseng TT, Wachi S, Young GB. Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim Biophys Acta. 1999;1422:1–56. doi: 10.1016/s0304-4157(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 507.Saito T, Jones CC, Huang S, Czech MP, Pilch PF. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J Biol Chem. 2007;282:32280–32287. doi: 10.1074/jbc.M704150200. [DOI] [PubMed] [Google Scholar]
- 508.Salas-Burgos A, Iserovich P, Zuniga F, Vera JC, Fischbarg J. Predicting the threedimensional structure of the human facilitative glucose transporter glut1 by a novel evolutionary homology strategy: insights on the molecular mechanism of substrate migration, and binding sites for glucose and inhibitory molecules. Biophys J. 2004;87:2990–2999. doi: 10.1529/biophysj.104.047886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–173. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- 510.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 511.Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. American Journal of Physiology- Renal Physiology. 2009;296:F947. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Sato M, Mueckler M. A conserved amino acid motif (R-X-G-R-R) in the glut1 glucose transporter is an important determinant of membrane topology [In Process Citation] J. Biol. Chem. 1999;274:24721–24725. doi: 10.1074/jbc.274.35.24721. [DOI] [PubMed] [Google Scholar]
- 513.Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol. 2007;292:H1–H18. doi: 10.1152/ajpheart.00662.2006. [DOI] [PubMed] [Google Scholar]
- 514.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 515.Schmidt S, Joost HG, Schurmann A. GLUT8, the enigmatic intracellular hexose transporter. Am J Physiol Endocrinol Metab. 2009;296:E614–E618. doi: 10.1152/ajpendo.91019.2008. [DOI] [PubMed] [Google Scholar]
- 516.Schmidt U, Briese S, Leicht K, Schurmann A, Joost HG, Al-Hasani H. Endocytosis of the glucose transporter GLUT8 is mediated by interaction of a dileucine motif with the beta2-adaptin subunit of the AP-2 adaptor complex. J Cell Sci. 2006;119:2321–2331. doi: 10.1242/jcs.02943. [DOI] [PubMed] [Google Scholar]
- 517.Schmitt M. Influences of hepatic portal receptors on hypothalamic feeding and satiety centers. Am J Physiol. 1973;225:1089–1095. doi: 10.1152/ajplegacy.1973.225.5.1089. [DOI] [PubMed] [Google Scholar]
- 518.Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 519.Schurmann A. Insight into the "odd" hexose transporters GLUT3, GLUT5, and GLUT7. Am J Physiol Endocrinol Metab. 2008;295:E225–E226. doi: 10.1152/ajpendo.90406.2008. [DOI] [PubMed] [Google Scholar]
- 520.Seatter MJ, De la Rue SA, Porter LM, Gould GW. QLS motif in transmembrane helix VII of the glucose transporter family interacts with the C-1 position of D-glucose and is involved in substrate selection at the exofacial binding site. Biochemistry. 1998;37:1322–1326. doi: 10.1021/bi972322u. [DOI] [PubMed] [Google Scholar]
- 521.Seidner G, Alvarez MG, Yeh JI, O'Driscoll KR, Klepper J, Stump TS, Wang D, Spinner NB, Birnbaum MJ, De Vivo DC. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat Genet. 1998;18:188–191. doi: 10.1038/ng0298-188. [DOI] [PubMed] [Google Scholar]
- 522.Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- 523.Sekine N, Cirulli V, Regazzi R, Brown LJ, Gine E, Tamarit-Rodriguez J, Girotti M, Marie S, MacDonald MJ, Wollheim CB, et a. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- 524.Sergeant S, Kim HD. Inhibition of 3-O-methylglucose transport in human erythrocytes by forskolin. J Biol Chem. 1985;260:14677–14682. [PubMed] [Google Scholar]
- 525.Shanahan MF, Morris DP, Edwards BM. [3H]forskolin. Direct photoaffinity labeling of the erythrocyte D-glucose transporter. J Biol Chem. 1987;262:5978–5984. [PubMed] [Google Scholar]
- 526.Shen J, Cross ST, Tang-Liu DD, Welty DF. Evaluation of an immortalized retinal endothelial cell line as an in vitro model for drug transport studies across the bloodretinal barrier. Pharm Res. 2003;20:1357–1363. doi: 10.1023/a:1025789606885. [DOI] [PubMed] [Google Scholar]
- 527.Shennan DB, Beechey RB. Mechanisms involved in the uptake of D-glucose into the milk producing cells of rat mammary tissue. Biochem Biophys Res Commun. 1995;211:986–990. doi: 10.1006/bbrc.1995.1908. [DOI] [PubMed] [Google Scholar]
- 528.Shennan DB, Peaker M. Transport of milk constituents by the mammary gland. Physiol Rev. 2000;80:925–951. doi: 10.1152/physrev.2000.80.3.925. [DOI] [PubMed] [Google Scholar]
- 529.Shetty M, Loeb JN, Vikstrom K, Ismail BF. Rapid activation of GLUT-1 glucose transporter following inhibition of oxidative phosphorylation in clone 9 cells. Journal of Biological Chemistry. 1993;268:17225–17232. [PubMed] [Google Scholar]
- 530.Shewan AM, Marsh BJ, Melvin DR, Martin S, Gould GW, James DE. The cytosolic Cterminus of the glucose transporter GLUT4 contains an acidic cluster endosomal targeting motif distal to the dileucine signal. Biochem J. 2000;350 Pt 1:99–107. [PMC free article] [PubMed] [Google Scholar]
- 531.Shillingford JM, Wood IS, Shennan DB, Shirazi-Beechey SP, Beechey RB. Determination of the sequence of a mRNA from lactating sheep mammary gland that encodes a protein identical to the Na(+)-dependent glucose transporter (SGLT1) Biochem Soc Trans. 1997;25:467S. doi: 10.1042/bst025467s. [DOI] [PubMed] [Google Scholar]
- 532.Shimizu N, Oomura Y, Novin D, Grijalva CV, Cooper PH. Functional correlations between lateral hypothalamic glucose-sensitive neurons and hepatic portal glucosesensitive units in rat. Brain Res. 1983;265:49–54. doi: 10.1016/0006-8993(83)91332-x. [DOI] [PubMed] [Google Scholar]
- 533.Shin BC, Fujikura K, Suzuki T, Tanaka S, Takata K. Glucose transporter GLUT3 in the rat placental barrier: a possible machinery for the transplacental transfer of glucose. Endocrinology. 1997;138:3997–4004. doi: 10.1210/endo.138.9.5369. [DOI] [PubMed] [Google Scholar]
- 534.Shiota C, Rocheleau JV, Shiota M, Piston DW, Magnuson MA. Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Am J Physiol Endocrinol Metab. 2005;289:E570–E577. doi: 10.1152/ajpendo.00102.2005. [DOI] [PubMed] [Google Scholar]
- 535.Simon RR, Marks V, Leeds AR, Anderson JW. A comprehensive review of oral glucosamine use and effects on glucose metabolism in normal and diabetic individuals. Diabetes Metab Res Rev. 2011;27:14–27. doi: 10.1002/dmrr.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Simpson IA, Vannucci SJ, DeJoseph MR, Hawkins RA. Glucose transporter asymmetries in the bovine blood-brain barrier. J. Biol. Chem. 2001;276:12725–1279.. doi: 10.1074/jbc.M010897200. [DOI] [PubMed] [Google Scholar]
- 537.Simpson IA, Cushman SW. Hormonal regulation of mammalian glucose transport. Ann. Rev. Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- 538.Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem. 1999;72:238–247. doi: 10.1046/j.1471-4159.1999.0720238.x. [DOI] [PubMed] [Google Scholar]
- 539.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–E253. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Sivitz W, DeSautel S, Walker PS, Pessin JE. Regulation of the glucose transporter in developing rat brain. Endocrinology. 1989;124:1875–1880. doi: 10.1210/endo-124-4-1875. [DOI] [PubMed] [Google Scholar]
- 542.Sixma JJ, Nievelstein PF, Houdijk WP, Van Breugel H, Hindriks G, de Groot PG. Adhesion of blood platelets to isolated components of the vessel wall. Ann N Y Acad Sci. 1987;509:103–117. doi: 10.1111/j.1749-6632.1987.tb30988.x. [DOI] [PubMed] [Google Scholar]
- 543.Slot JW, Geuze HJ, Gigengack S, James DE, Lienhard GE. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci U S A. 1991;88:7815–7819. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Sogin DC, Hinkle PC. Characterization of the glucose transporter from human erythrocytes. J Supramol Struct. 1978;8:447–453. doi: 10.1002/jss.400080407. [DOI] [PubMed] [Google Scholar]
- 545.Staal RG, Mosharov EV, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nat Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- 546.Steck TL, Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1:220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- 547.Stein WD. Transport and diffusion across cell membranes. New York: Academic Press; 1986. [Google Scholar]
- 548.Stenbit AE, Burcelin R, Katz EB, Tsao TS, Gautier N, Charron MJ, Le Marchand- Brustel Y. Diverse effects of Glut 4 ablation on glucose uptake and glycogen synthesis in red and white skeletal muscle. J Clin Invest. 1996;98:629–634. doi: 10.1172/JCI118833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Stuart CA, Howell ME, Zhang Y, Yin D. Insulin-stimulated translocation of glucose transporter (GLUT) 12 parallels that of GLUT4 in normal muscle. J Clin Endocrinol Metab. 2009;94:3535–3542. doi: 10.1210/jc.2009-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Stuart CA, Yin D, Howell ME, Dykes RJ, Laffan JJ, Ferrando AA. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab. 2006;291:E1067–E1073. doi: 10.1152/ajpendo.00250.2006. [DOI] [PubMed] [Google Scholar]
- 551.Stumpel F, Jungermann K. Sensing by intrahepatic muscarinic nerves of a portalarterial glucose concentration gradient as a signal for insulin-dependent glucose uptake in the perfused rat liver. FEBS Lett. 1997;406:119–122. doi: 10.1016/s0014-5793(97)00254-8. [DOI] [PubMed] [Google Scholar]
- 552.Sultzman LA, Carruthers A. Stop-flow analysis of cooperative interactions between GLUT1 sugar import and export sites. Biochemistry. 1999;38:6640–6650. doi: 10.1021/bi990130o. [DOI] [PubMed] [Google Scholar]
- 553.Summers SA, Yin VP, Whiteman EL, Garza LA, Cho H, Tuttle RL, Birnbaum MJ. Signaling pathways mediating insulin-stimulated glucose transport. Ann N Y Acad Sci. 1999;892:169–186. doi: 10.1111/j.1749-6632.1999.tb07795.x. [DOI] [PubMed] [Google Scholar]
- 554.Suzuki M, Kobayashi Y, Kurata M, Agar NS. Substrates for glutathione regeneration in mammalian erythrocytes. COMPARATIVE HAEMATOLOGY INTERNATIONAL. 1997;7:70–73. [Google Scholar]
- 555.Suzuki M, Kurata M. Effects of ATP level on glutathione regeneration in rabbit and guinea-pig erythrocytes. Comp Biochem Physiol B. 1992;103:859–862. doi: 10.1016/0305-0491(92)90205-6. [DOI] [PubMed] [Google Scholar]
- 556.Swarthout JT, D'Alonzo RC, Selvamurugan N, Partridge NC. Parathyroid hormonedependent signaling pathways regulating genes in bone cells. Gene. 2002;282:1–17. doi: 10.1016/s0378-1119(01)00798-3. [DOI] [PubMed] [Google Scholar]
- 557.Takagi H, Tanihara H, Seino Y, Yoshimura N. Characterization of glucose transporter in cultured human retinal pigment epithelial cells: gene expression and effect of growth factors. Invest Ophthalmol Vis Sci. 1994;35:170–177. [PubMed] [Google Scholar]
- 558.Takakura Y, Kuentzel SL, Raub TJ, Davies A, Baldwin SA, Borchardt RT. Hexose uptake in primary cultures of bovine brain microvessel endothelial cells. I. Basic characteristics and effects of D-glucose and insulin. Biochimica et Biophysica Acta. 1991;1070:1–10. doi: 10.1016/0005-2736(91)90139-y. [DOI] [PubMed] [Google Scholar]
- 559.Takanaga H, Frommer WB. Facilitative plasma membrane transporters function during ER transit. FASEB J. 2010;24:2849–2858. doi: 10.1096/fj.09-146472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Takarada T, Hinoi E, Kambe Y, Sahara K, Kurokawa S, Takahata Y, Yoneda Y. Osteoblast protects osteoclast devoid of sodium-dependent vitamin C transporters from oxidative cytotoxicity of ascorbic acid. Eur J Pharmacol. 2007;575:1–11. doi: 10.1016/j.ejphar.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 561.Takata K, Hirano H, Kasahara M. Transport of glucose across the blood-tissue barriers. Int Rev Cytol S. 1997;172:1–53. doi: 10.1016/s0074-7696(08)62357-8. [DOI] [PubMed] [Google Scholar]
- 562.Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Erythrocyte/HepG2-type glucose transporter is concentrated in cells of blood-tissue barriers. Biochem Biophys Res Commun S. 1990;173:67–73. doi: 10.1016/s0006-291x(05)81022-8. [DOI] [PubMed] [Google Scholar]
- 563.Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Erythrocyte/HepG2-type glucose transporter is concentrated in cells of blood-tissue barriers. Biochemical & Biophysical Research Communications. 1990;173:67–73. doi: 10.1016/s0006-291x(05)81022-8. [DOI] [PubMed] [Google Scholar]
- 564.Takeuchi S, Ando M. Marginal cells of the stria vascularis of gerbils take up glucose via the facilitated transporter GLUT: application of autofluorescence. Hear Res. 1997;114:69–74. doi: 10.1016/s0378-5955(97)00157-3. [DOI] [PubMed] [Google Scholar]
- 565.Tal M, Schneider DL, Thorens B, Lodish HF. Restricted expression of the erythroid/brain glucose transporter isoform to perivenous hepatocytes in rats. Modulation by glucose. J Clin Invest. 1990;86:986–992. doi: 10.1172/JCI114801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Tappy L, Jequier E, Schneiter P. Autoregulation of Glucose Production. News Physiol Sci. 2000;15:198–202. doi: 10.1152/physiologyonline.2000.15.4.198. [DOI] [PubMed] [Google Scholar]
- 567.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 568.Tappy L, Le KA, Tran C, Paquot N. Fructose and metabolic diseases: new findings, new questions. Nutrition. 2010;26:1044–1049. doi: 10.1016/j.nut.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 569.Taverna RD, Langdon RG. Glucose transport in white erythrocyte ghosts and membrane derived vesicles. Biochim. Biophys. Acta. 1973;298:422–428. doi: 10.1016/0005-2736(73)90369-6. [DOI] [PubMed] [Google Scholar]
- 570.Taverna RD, Langdon RG. Reversible association of cytochalasin B with the human erythrocyte membrane. Inhibition of glucose transport and the stoichiometry of cytochalasin binding. Biochim Biophys Acta. 1973;323:207–219. doi: 10.1016/0005-2736(73)90145-4. [DOI] [PubMed] [Google Scholar]
- 571.Taylor LP, Holman GD. Symmetrical kinetic parameters for 3-O-methyl-D-glucose transport in adipocytes in the presence and in the absence of insulin. Biochim. Biophys. Acta. 1981;642:325–335. doi: 10.1016/0005-2736(81)90449-1. [DOI] [PubMed] [Google Scholar]
- 572.Taylor LP, Holman GD. Symmetrical kinetic parameters for 3-O-methyl-D-glucose transport in adipocytes in the presence and in the absence of insulin. Biochim Biophys Acta. 1981;642:325–335. doi: 10.1016/0005-2736(81)90449-1. [DOI] [PubMed] [Google Scholar]
- 573.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 574.Thomas DM, Maher F, Rogers SD, Best JD. Expression and regulation by insulin of GLUT 3 in UMR 106-01, a clonal rat osteosarcoma cell line. Biochem Biophys Res Commun. 1996;218:789–793. doi: 10.1006/bbrc.1996.0140. [DOI] [PubMed] [Google Scholar]
- 575.Thorens B. Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes. Am J Physiol. 1996;270:G541–G553. doi: 10.1152/ajpgi.1996.270.4.G541. [DOI] [PubMed] [Google Scholar]
- 576.Thorens B. A gene knockout approach in mice to identify glucose sensors controlling glucose homeostasis. Pflugers Arch. 2003;445:482–490. doi: 10.1007/s00424-002-0954-2. [DOI] [PubMed] [Google Scholar]
- 577.Thorens B, Guillam MT, Beermann F, Burcelin R, Jaquet M. Transgenic reexpression of GLUT1 or GLUT2 in pancreatic beta cells rescues GLUT2-null mice from early death and restores normal glucose-stimulated insulin secretion. J Biol Chem. 2000;275:23751–23758. doi: 10.1074/jbc.M002908200. [DOI] [PubMed] [Google Scholar]
- 578.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–E145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Thorens B, Sarkar HK, Kaback HR, Lodish HF. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and 2− pancreatic islet cells. Cell. 1988;55:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- 580.Threadgold LC, Coore HG, Kuhn NJ. Monosaccharide transport into lactating-rat mammary acini. Biochem J. 1982;204:493–501. doi: 10.1042/bj2040493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Threadgold LC, Kuhn NJ. Monosaccharide transport in the mammary gland of the intact lactating rat. Biochem J. 1984;218:213–219. doi: 10.1042/bj2180213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Topping DL, Mayes PA. The concentration of fructose, glucose and lactate in the splanchnic blood vessels of rats absorbing fructose. Nutr Metab. 1971;13:331–338. doi: 10.1159/000175352. [DOI] [PubMed] [Google Scholar]
- 583.Toyoda N, Flanagan JE, Kono T. Reassessment of insulin effects on the Vmax and Km values of hexose transport in isolated rat epididymal adipocytes. J. Biol. Chem. 1987;262:2737–2745. [PubMed] [Google Scholar]
- 584.Travis AJ, Tutuncu L, Jorgez CJ, Ord TS, Jones BH, Kopf GS, Williams CJ. Requirements for glucose beyond sperm capacitation during in vitro fertilization in the mouse. Biol Reprod. 2004;71:139–145. doi: 10.1095/biolreprod.103.025809. [DOI] [PubMed] [Google Scholar]
- 585.Tschritter O, Stumvoll M, Machicao F, Holzwarth M, Weisser M, Maerker E, Teigeler A, Haring H, Fritsche A. The prevalent Glu23Lys polymorphism in the potassium inward rectifier 6.2 (KIR6.2) gene is associated with impaired glucagon suppression in response to hyperglycemia. Diabetes. 2002;51:2854–2860. doi: 10.2337/diabetes.51.9.2854. [DOI] [PubMed] [Google Scholar]
- 586.Tserentsoodol N, Shin B, Koyama H, Suzuki T, Takata K. Immunolocalization of Tight Junction Proteins, Occludin and ZO-1, and Glucose Transporter GLUT1 in the Cells of the Blood-Nerve Barrier. Archives Histol Cytol. 1999;62:459–469. doi: 10.1679/aohc.62.459. [DOI] [PubMed] [Google Scholar]
- 587.Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T. QUANTITATIVE TARGETED ABSOLUTE PROTEOMICS OF HUMAN BLOODBRAIN BARRIER TRANSPORTERS AND RECEPTORS. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- 588.Ueki M, Linn F, Hossmann KA. Functional activation of cerebral blood flow and metabolism before and after global ischemia of rat brain. J Cereb Blood Flow Metab S. 1988;8:486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- 589.Uldry M, Ibberson M, Horisberger JD, Chatton JY, Riederer BM, Thorens B. Identification of a mammalian H(+)-myo-inositol symporter expressed predominantly in the brain. EMBO J. 2001;20:4467–4477. doi: 10.1093/emboj/20.16.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447:480–489. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- 591.Uldry M, Ibberson M, Hosokawa M, Thorens B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002;524:199–203. doi: 10.1016/s0014-5793(02)03058-2. [DOI] [PubMed] [Google Scholar]
- 592.Uldry M, Steiner P, Zurich M-G, Beguin P, Hirling H, Dolci W, Thorens B. Regulated exocytosis of an H+/myo-inositol symporter at synapses and growth cones. EMBO J. 2004;23:531–540. doi: 10.1038/sj.emboj.7600072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447:480–489. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- 594.Vannucci SJ. Developmental expression of GLUT1 and GLUT3 glucose transporters in rat brain. Journal of Neurochemistry. 1994;62:240–246. doi: 10.1046/j.1471-4159.1994.62010240.x. [DOI] [PubMed] [Google Scholar]
- 595.Vannucci SJ, Willing LB, Vannucci RC. Developmental expression of glucose transporters, GLUT1 and GLUT3, in postnatal rat brain. Adv Exp Med Biol. 1993;331:3–7. doi: 10.1007/978-1-4615-2920-0_1. [DOI] [PubMed] [Google Scholar]
- 596.Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain Res. 1998;797:1–11. doi: 10.1016/s0006-8993(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 597.Vannucci SJ, Maher F, Koehler E, Simpson IA. Altered expression of GLUT-1 and GLUT-3 glucose transporters in neurohypophysis of water-deprived or diabetic rats. Am J Physiol. 1994;267:E605–E611. doi: 10.1152/ajpendo.1994.267.4.E605. [DOI] [PubMed] [Google Scholar]
- 598.Vardhana PA, Illsley NP. Transepithelial glucose transport and metabolism in BeWo choriocarcinoma cells. Placenta. 2002;23:653–660. doi: 10.1053/plac.2002.0857. [DOI] [PubMed] [Google Scholar]
- 599.Vera JC, Rivas CI, Velasquez FV, Zhang RH, Concha II, Golde DW. Resolution of the facilitated transport of dehydroascorbic acid from its intracellular accumulation as ascorbic acid. J. Biol. Chem. 1995;270:23706–23712. doi: 10.1074/jbc.270.40.23706. [DOI] [PubMed] [Google Scholar]
- 600.Vera JC, Rivas CI, Zhang RH, Farber CM, Golde DW. Human HL-60 myeloid leukemia cells transport dehydroascorbic acid via the glucose transporters and accumulate reduced ascorbic acid. Blood. 1994;84:1628–1634. [PubMed] [Google Scholar]
- 601.Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- 602.Vieira E, Salehi A, Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia. 2007;50:370–379. doi: 10.1007/s00125-006-0511-1. [DOI] [PubMed] [Google Scholar]
- 603.Vollers S, Carruthers A. Molecular determinants of GLUT1-mediated accelerated exchange. unpublished. 2011 doi: 10.1074/jbc.M112.369587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Wadhwani KC, Rapoport SI. Transport properties of vertebrate blood-nerve barrier: comparison with blood-brain barrier. Prog Neurobiol. 1994;43:235–279. doi: 10.1016/0301-0082(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 605.Walmsley AR, Lowe AG. Comparison of the kinetics and thermodynamics of the carrier systems for glucose and leucine in human red blood cells. Biochim Biophys Acta. 1987;901:229–238. doi: 10.1016/0005-2736(87)90119-2. [DOI] [PubMed] [Google Scholar]
- 606.Walz T, Hirai T, Murata K, Heymann JB, Mitsuoka K, Fujiyoshi Y, Smith BL, Agre P, Engel A. The three-dimensional structure of aquaporin-1. Nature. 1997;387:624–627. doi: 10.1038/42512. [DOI] [PubMed] [Google Scholar]
- 607.Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- 608.Watson RT, Pessin JE. Bridging the GAP between insulin signaling and GLUT4 translocation. Trends Biochem Sci. 2006;31:215–222. doi: 10.1016/j.tibs.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 609.Weidema AF, Dixon SJ, Sims SM. Activation of P2Y but not P2X(4) nucleotide receptors causes elevation of [Ca2+]i in mammalian osteoclasts. Am J Physiol Cell Physiol. 2001;280:C1531–C1539. doi: 10.1152/ajpcell.2001.280.6.C1531. [DOI] [PubMed] [Google Scholar]
- 610.Weiser MB, Razin M, Stein WD. Kinetic tests of models for sugar transport in human erythrocytes and a comparison of fresh and cold stored cells. Biochim. Biophys. Acta. 1983;727:379–388. doi: 10.1016/0005-2736(83)90423-6. [DOI] [PubMed] [Google Scholar]
- 611.Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes. 2004;53:1038–1045. doi: 10.2337/diabetes.53.4.1038. [DOI] [PubMed] [Google Scholar]
- 612.Wheeler TJ, Hinkle PC. Kinetic properties of the reconstituted glucose transporter from human erythrocytes. J. Biol. Chem. 1981;256:8907–8914. [PubMed] [Google Scholar]
- 613.Wheeler TJ, Simpson IA, Sogin DC, Hinkle PC, Cushman SW. Detection of the rat adipose cell glucose transporter with antibody against the human red cell glucose transporter. Biochem Biophys Res Commun. 1982;105:89–95. doi: 10.1016/s0006-291x(82)80014-4. [DOI] [PubMed] [Google Scholar]
- 614.White MD, Kuhn NJ, Ward S. Permeability of lactating-rat mammary gland Golgi membranes to monosaccharides. Biochem J. 1980;190:621–624. doi: 10.1042/bj1900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Widdas WF. Facilitated transfer of hexoses across the human erythrocyte membrane. J. Physiol. (Lond.) 1954;125:163–180. doi: 10.1113/jphysiol.1954.sp005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Widdas WF. Inability of diffusion to account for placental glucose transfer in the sheep and consideration of the kinetics of a possible carrier transfer. J. Physiol. (London) 1952;118:23–39. doi: 10.1113/jphysiol.1952.sp004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Widdas WF. The asymmetry of the hexose transfer system in the human red cell membrane. Curr. Top. Memb. Transp. 1980;14:165–223. [Google Scholar]
- 618.Widmer M, Uldry M, Thorens B. GLUT8 Subcellular Localization and Absence of Translocation to the Plasma Membrane in PC12 Cells and Hippocampal Neurons. Endocrinology. 2005;146:4727–4736. doi: 10.1210/en.2005-0668. [DOI] [PubMed] [Google Scholar]
- 619.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Wightman RM, Haynes CL. Synaptic vesicles really do kiss and run. Nat Neurosci. 2004;7:321–322. doi: 10.1038/nn0404-321. [DOI] [PubMed] [Google Scholar]
- 621.Williams CA, Phillips T, Macdonald I. The influence of glucose on serum galactose levels in man. Metabolism. 1983;32:250–256. doi: 10.1016/0026-0495(83)90189-0. [DOI] [PubMed] [Google Scholar]
- 622.Williams JP, Blair HC, McDonald JM, McKenna MA, Jordan SE, Williford J, Hardy RW. Regulation of osteoclastic bone resorption by glucose. Biochem Biophys Res Commun. 1997;235:646–651. doi: 10.1006/bbrc.1997.6795. [DOI] [PubMed] [Google Scholar]
- 623.Williams SA, Blache D, Martin GB, Foot R, Blackberry MA, Scaramuzzi RJ. Effect of nutritional supplementation on quantities of glucose transporters 1 and 4 in sheep granulosa and theca cells. Reproduction. 2001;122:947–956. [PubMed] [Google Scholar]
- 624.Wilson-O'Brien AL, Dehaan CL, Rogers S. Mitogen-Stimulated and Rapamycin- Sensitive Glucose Transporter 12 Targeting and Functional Glucose Transport in Renal Epithelial Cells. Endocrinology. 2008;149:917–924. doi: 10.1210/en.2007-0985. [DOI] [PubMed] [Google Scholar]
- 625.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 626.Wilson A, Laurenti E, Trumpp A. Balancing dormant and self-renewing hematopoietic stem cells. Curr Opin Genet Dev. 2009;19:461–468. doi: 10.1016/j.gde.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 627.Wilson CM, Cushman SW. Insulin stimulation of glucose transport activity in rat skeletal muscle: increase in cell surface GLUT4 as assessed by photolabelling. Biochem J. 1994:755–759. doi: 10.1042/bj2990755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Wittrant Y, Gorin Y, Woodruff K, Horn D, Abboud HE, Mohan S, Abboud-Werner SL. High d(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone. 2008;42:1122–1130. doi: 10.1016/j.bone.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- 631.Wood IS, Hunter L, Trayhurn P. Expression of Class III facilitative glucose transporter genes (GLUT-10 and GLUT-12) in mouse and human adipose tissues. Biochem Biophys Res Commun. 2003;308:43–49. doi: 10.1016/s0006-291x(03)01322-6. [DOI] [PubMed] [Google Scholar]
- 632.Wright EM. The intestinal Na+/glucose cotransporter. [Review] Annual Review of Physiology. 1993;55:575–589. doi: 10.1146/annurev.ph.55.030193.003043. [DOI] [PubMed] [Google Scholar]
- 633.Wright EM. Diseases of Renal Glucose Handling. Genetic diseases of the kidney. 2009:131. [Google Scholar]
- 634.Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280:F10–F18. doi: 10.1152/ajprenal.2001.280.1.F10. [DOI] [PubMed] [Google Scholar]
- 635.Wu X, Freeze HH. GLUT14, a duplicon of GLUT3, is specifically expressed in testis as alternative splice forms. Genomics. 2002;80:553–557. doi: 10.1006/geno.2002.7010. [DOI] [PubMed] [Google Scholar]
- 636.Wu X, Li W, Sharma V, Godzik A, Freeze HH. Cloning and characterization of glucose transporter 11, a novel sugar transporter that is alternatively spliced in various tissues. Mol Genet Metab. 2002;76:37–45. doi: 10.1016/s1096-7192(02)00018-5. [DOI] [PubMed] [Google Scholar]
- 637.Xing AY, Challier JC, Lepercq J, Cauzac M, Charron MJ, Girard J, Hauguel-de Mouzon S. Unexpected expression of glucose transporter 4 in villous stromal cells of human placenta. J Clin Endocrinol Metab. 1998;83:4097–4101. doi: 10.1210/jcem.83.11.5290. [DOI] [PubMed] [Google Scholar]
- 638.Yang J, Holman GD. Insulin and contraction stimulate exocytosis, but increased AMPactivated protein kinase activity resulting from oxidative metabolism stress slows endocytosis of GLUT4 in cardiomyocytes. J Biol Chem. 2005;280:4070–4078. doi: 10.1074/jbc.M410213200. [DOI] [PubMed] [Google Scholar]
- 639.Yang XJ, Kow LM, Funabashi T, Mobbs CV. Hypothalamic glucose sensor: similarities to and differences from pancreatic beta-cell mechanisms. Diabetes. 1999;48:1763–1772. doi: 10.2337/diabetes.48.9.1763. [DOI] [PubMed] [Google Scholar]
- 640.Yang XJ, Kow LM, Pfaff DW, Mobbs CV. Metabolic pathways that mediate inhibition of hypothalamic neurons by glucose. Diabetes. 2004;53:67–73. doi: 10.2337/diabetes.53.1.67. [DOI] [PubMed] [Google Scholar]
- 641.Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Structure of the multidrug transporter EmrD from Escherichia coli. Science (New York, NY) 2006;312:741–744. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Yoshihara T, Satoh M, Yamamura Y, Itoh H, Ishii T. Ultrastructural localization of glucose transporter 1 (GLUT1) in guinea pig stria vascularis and vestibular dark cell areas: an immunogold study. Acta Otolaryngol. 1999;119:336–340. doi: 10.1080/00016489950181350. [DOI] [PubMed] [Google Scholar]
- 643.Yu J, Fischman DA, Steck TL. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1:233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]
- 644.Zhao C, Wilson MC, Schuit F, Halestrap AP, Rutter GA. Expression and distribution of lactate/monocarboxylate transporter isoforms in pancreatic islets and the exocrine pancreas. Diabetes. 2001;50:361–366. doi: 10.2337/diabetes.50.2.361. [DOI] [PubMed] [Google Scholar]
- 645.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113–128. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Zhao FQ, Dixon WT, Kennelly JJ. Localization and gene expression of glucose transporters in bovine mammary gland. Comp Biochem Physiol B Biochem Mol Biol. 1996;115:127–134. doi: 10.1016/0305-0491(96)00043-0. [DOI] [PubMed] [Google Scholar]
- 647.Zhao FQ, Glimm DR, Kennelly JJ. Distribution of mammalian facilitative glucose transporter messenger RNA in bovine tissues. Int J Biochem. 1993;25:1897–1903. doi: 10.1016/0020-711x(88)90322-9. [DOI] [PubMed] [Google Scholar]
- 648.Zhao FQ, Keating AF. Expression and regulation of glucose transporters in the bovine mammary gland. J Dairy Sci. 2007;90(Suppl 1):E76–E86. doi: 10.3168/jds.2006-470. [DOI] [PubMed] [Google Scholar]
- 649.Zhou J, Bondy CA. Placental glucose transporter gene expression and metabolism in the rat. J Clin Invest. 1993;91:845–852. doi: 10.1172/JCI116305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med. 2000;6:924–928. doi: 10.1038/78693. [DOI] [PubMed] [Google Scholar]
- 651.Zoccoli MA, Baldwin SA, Lienhard GE. The monosaccharide transport system of the human erythrocyte. Solubilization and characterization on the basis of cytochalasin B binding. J. Biol. Chem. 1978;253:6923–6930. [PubMed] [Google Scholar]
- 652.Zoidis E, Ghirlanda-Keller C, Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem. 2011;348:33–42. doi: 10.1007/s11010-010-0634-z. [DOI] [PubMed] [Google Scholar]
- 653.Zola H, Swart B, Nicholson I, Voss E. Leukocyte and Stromal Cell Molecules: The CD Markers. Wiley; 007. [Google Scholar]
- 654.Zottola RJ, Cloherty EK, Coderre PE, Hansen A, Hebert DN, Carruthers A. Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promotes GLUT1 tetramerization. Biochemistry. 1995;34:9734–9747. doi: 10.1021/bi00030a011. [DOI] [PubMed] [Google Scholar]