Abstract
Using palladium-catalyzed decarboxylation, several cascade reactions of allyl and prenyl nitroalkanoates that lead to nitro-containing chemical building blocks are described. A nitronate Michael addition/Tsuji-Trost allylation cascade was developed, leading to functionally dense chemical building blocks. Likewise, a Tsuji-Trost/decarboxylative protonation sequence was developed for the synthesis of orthogonally functionalized 2° nitroalkanes. The latter method provides rapid access to the indolizidine core.
Keywords: Decarboxylative Coupling, Pd-Catalysis, Multi-Component Reactions, Tsuji-Trost Allylation, Indolizidine Alkaloids
1. Introduction
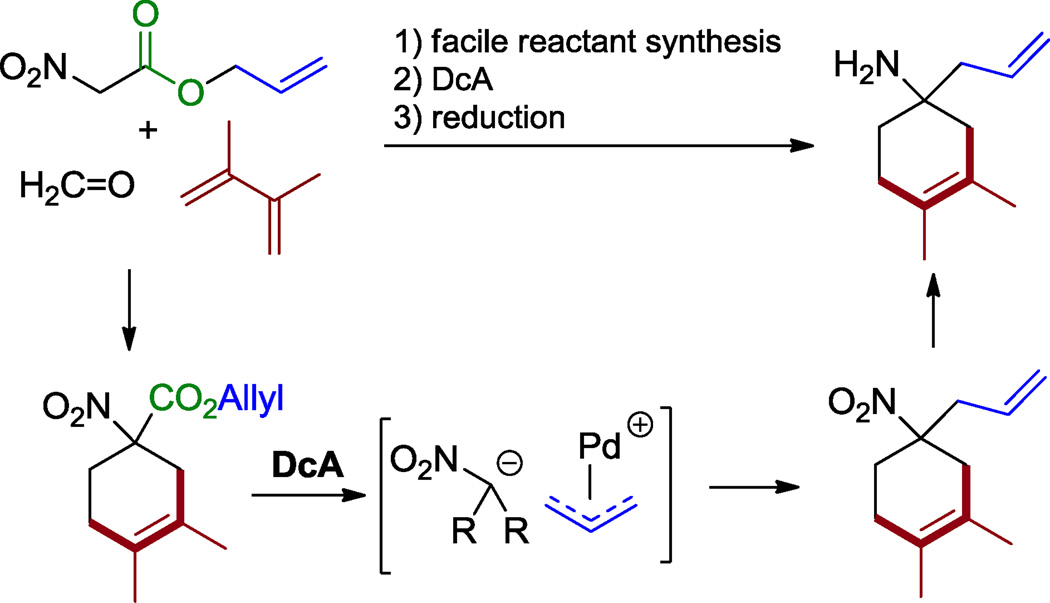
Palladium-catalyzed decarboxylative allylation (DcA) is a convenient method to generate functionalized chemical building blocks with only CO2 as a byproduct.1,2 Using this chemical reactivity, various methods have been developed for the synthesis of nitrogen-containing chemical building blocks.3 This is of significance since nitrogen-containing materials often exhibit interesting biological activities. In this regard, we reported the rapid decarboxylative allylation of nitroalkanes (Scheme 1).4 Nitroalkanes readily allow the incorporation of nitrogen into alkaloids and other biologically active nitrogenous compounds because they have the advantageous chemical properties of a relatively low pKa (~10 in H2O)5 and facile reducibility to amines. As shown in Scheme 1, nitroacetic esters are readily functionalized by α-alkylation.6,7 Decarboxylative allylation then provides tertiary nitroalkanes that are readily reduced to amines.
Scheme 1.
DcA of allyl nitroalkanoates.
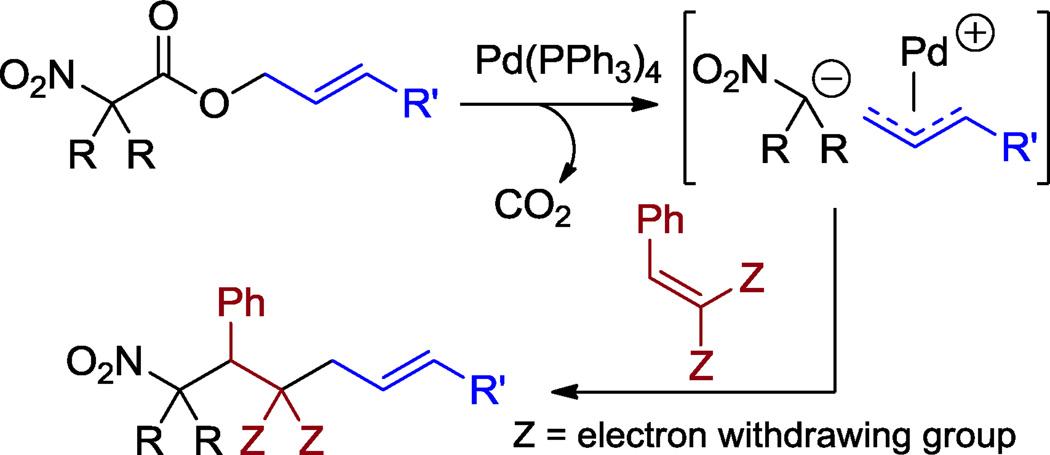
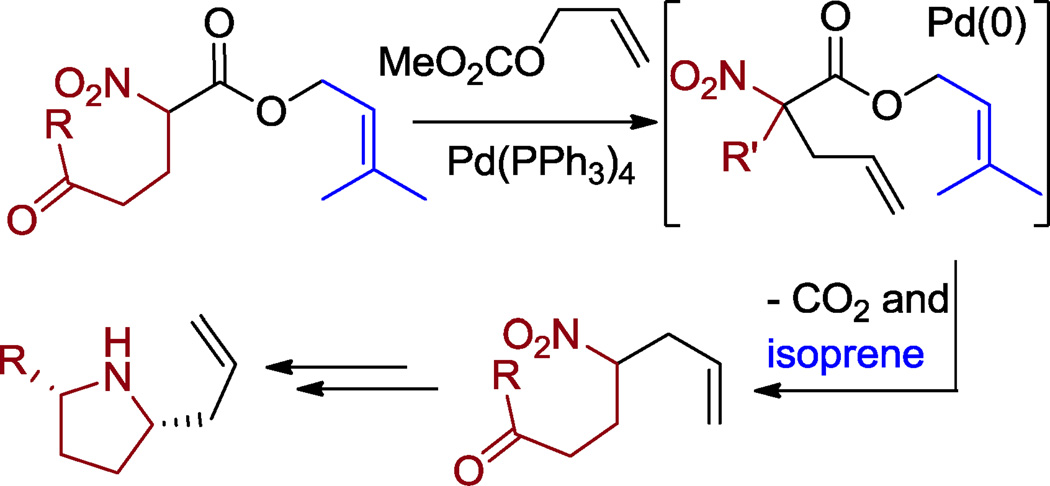
One advantage of the DcA of nitroalkanes is that it allows the generation of reactive nucleophiles and electrophiles in situ. We and others have previously demonstrated that these nucleophilic and electrophilic coupling partners can be funneled down alternate reaction pathways such as Michael-addition/Tsuji-Trost allylation cascades (interceptive DcA)1,8 or capture by protonation.9 Herein we report that allyl nitroalkanoates can participate in similar cascade reactions. We present a Michaeladdition/Tsuji-Trost cascade leading to functionally dense nitro group-containing compounds (Scheme 2) as well as a Tsuji-Trost/decarboxylative protonation cascade strategy to access functional allylated 2° nitroalkanes (Scheme 3).
Scheme 2.
Cascade Michael addition/Tsuji-Trost allylation initiated by decarboxylation of allyl nitroalkanoates.
Scheme 3.
Cascade Tsuji-Trost/Decarboxylative Protonation of allyl nitroalkanoates.
2. Michael addition/Tsuji-Trost allylation cascades
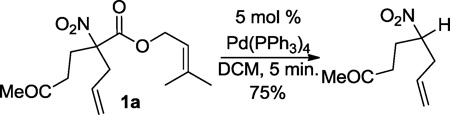
To begin, we treated allyl nitroalkanoates under similar conditions to those developed for the successful DcA reaction of allyl nitroalkanoate (5 mol% Pd(PPh3)4, DCM),4 however an equivalent of benzylidenemalononitrile was included in the reaction mixture. Gratifylingly, the intermediate allyl electrophile and nitronate nucleophiles were intercepted with the benzylidene malononitrile to form highly functionalized nitroalkanes (Table 1). The intercepted DcA reaction was not nearly as rapid as the standard DcA reaction, requiring 12 h for completion. The uninterrupted decarboxylative allylation reaction of allyl nitroalkanoates required < 5 minutes to achieve completion under the same conditions.4 The slower rate of interceptive DcA is easily explained by the coordination of benzylidene malononitrile to Pd(0), rendering the catalyst less electron-rich and less prone to undergo oxidative addition with the allylic carboxylate. Nonetheless, various allyl nitroalkanoates were excellent coupling partners (2a–d). α,α-Dialkyl nitroalkanes (2a–b), including Michael (2c)7 and Knoevenagel/Diels-Alder (2d) adducts6 were compatible coupling partners. It was unfortunate, though not surprising, that Diels-Alder adduct 2d was formed with no diastereoselection; changing the solvent from DCM to toluene did not improve the diastereoselectivity, but the cascade reaction progressed comparably well. Aside from allyl nitroalkanoate (2a–d), cinnamyl, hexenyl, and prenyl nitroalkanoates were excellent coupling partners (2e–g), giving exclusively the linear product. Although 2e–g were formed with no diastereoselection, the diastereomers of 2g could be chromatographically separated. The successful synthesis of prenylated product 2g was particularly gratifying given that attempted decarboxylative prenylation of 1a led primarily to the protonation product (eq. 1). While palladium-π prenyl complexes often undergo β-elimination instead of the desired C–C linkage, prenylation methodologies have been developed for some nucleophiles.10
Table 1.
Interceptive decarboxylative allylation of allyl nitroalkanoates with benzylidenemalononitrile.
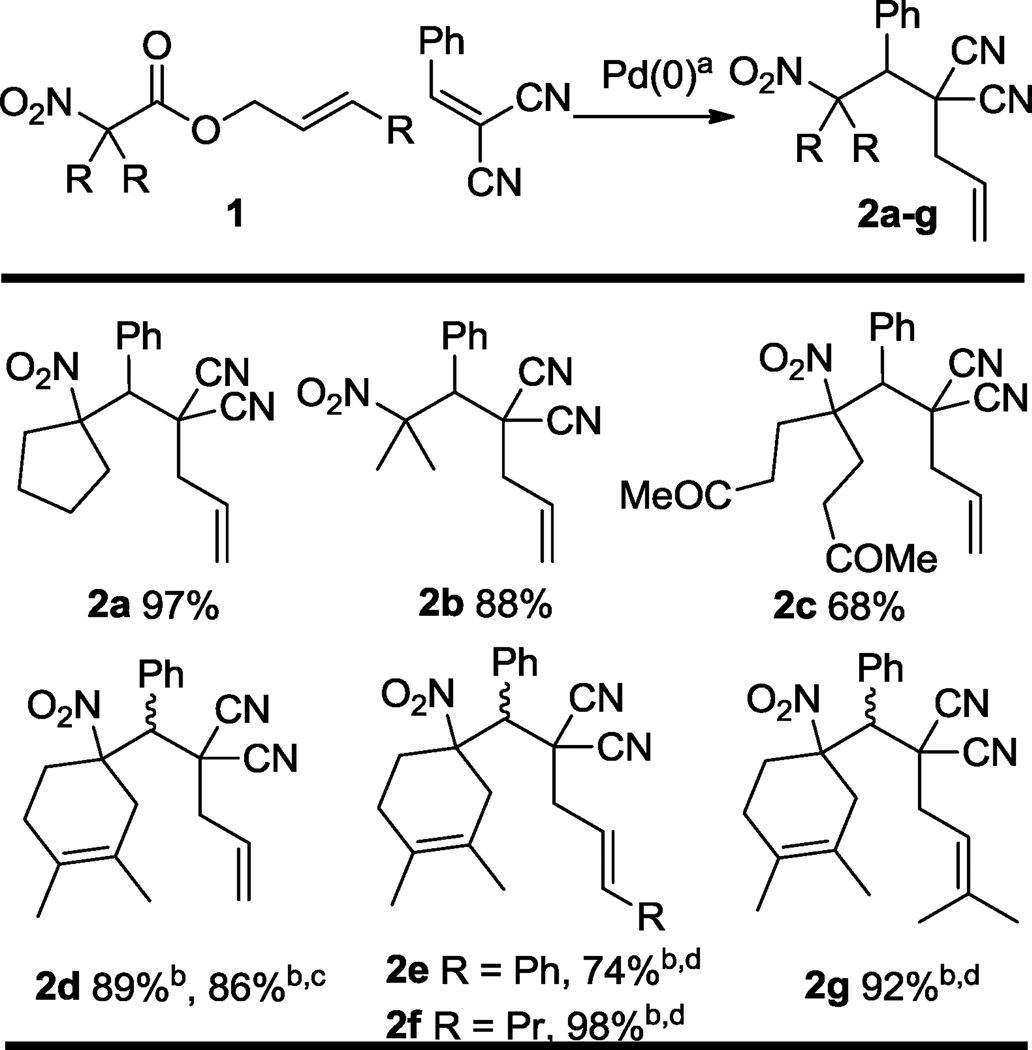 |
reaction conditions: 1:1 1:benzylidenemalononitrile, 5 mol % Pd(PPh3)4, DCM, rt, 12h
1:1 d.r.
toluene in lieu of DCM, rt, 12h
>20:1 linear:branched
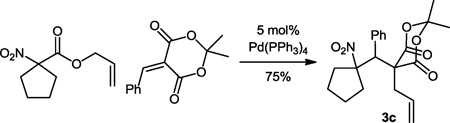
Having demonstrated that the Michael addition/Tsuji-Trost cascade process was successful with benzylidenemalononitrile, we wished to extend this methodology to Michael acceptors derived from Meldrum’s acid (Table 2). Surprisingly, the reaction failed to produce any of the desired product under the same conditions developed for benzylidenemalononitrile (Table 2, entry 1). Furthermore, heating the reactants at various temperatures in chlorinated solvents failed to give a desirable result (Table 2, entries 2–3). Fortunately, good yields could be achieved in THF (entry 4) or toluene (entry 5). Interestingly, a modest diastereoselectivity was observed, though different solvents did not affect this ratio. Aside from simple unsubstituted allyl esters, the alkyl-substitute hexenyl nitroalkanoate provided a modest yield of the Michael addition/Tsuji-Trost coupling product (entry 6). Simple cyclopentyl allyl nitroalkanoate could also undergo a clean reaction in 75% isolated yield (eq. 2, 3c)
Table 2.
Interceptive decarboxylative allylation of allyl nitroalkanoates with Meldrum’s acid derived Michael acceptors.
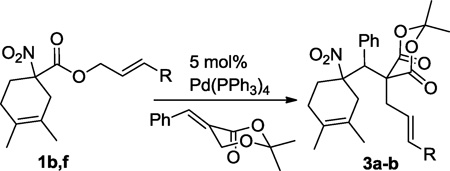 | |||||
|---|---|---|---|---|---|
| Entry | R | Solvent | Temp (°C) |
Time (h) |
% Yield (dr)a |
| 1 | H | DCM | rt | 12 | trace |
| 2 | H | DCM | 40 | 12 | trace |
| 3 | H | DCE | 80 | 12 | trace |
| 4 | H | THF | rt | 12 | 74 (7:3) |
| 5 | H | Tol | rt | 12 | 70 (7:3) |
| 6 | n-Pr | THF | 60 | 1 | 55 (7:3) |
The relative configuration of the major diastereomer is not known.
 |
(1) |
 |
(2) |
We also attempted to utilize nitrostyrenes as coupling partners for interceptive DcA (eq. 3). Unfortunately, there appears to be no driving force for the Michael addition to form 4, and DcA to produce 5 was the only reaction pathway observed (eq. 3).4 In the successful examples of interceptive DcA, the anion generated upon Michael addition is always more stable than that of the initial nucleophile. Thus, there is a thermodynamic driving force for reaction progression. Comparison of the relevant pKa values (in DMSO) further illuminates the driving force for nitronate (pKa ~ 17) addition to malononitriles (pKa ~ 12) and Meldrum’s acid adducts (pKa ~ 7.5).5 Moreover, our results trend with Mayr’s observation that Michael acceptors derived from malononitrile and Melrum’s acid are more electrophilic than a Pd-π-allyl complex,11 thus addition of nitronates to benzylidene malononitriles is expected to be kinetically faster than allylation.
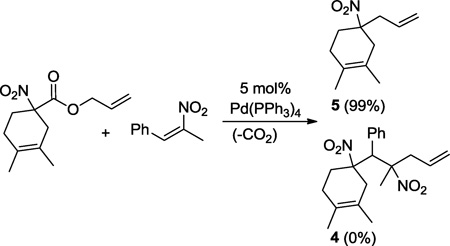 |
(3) |
3. Michael addition/Tsuji-Trost allylation cascades
In addition to the development of the Michael addition/Tsuji-Trost cascades initiated by decarboxylation, we were intrigued by the clean conversion of the prenyl nitroalkanoate into the protonated 2° nitroalkane product (eq. 1). Historically, allylated 2° nitroalkanes can be challenging to access due to competing over alkylation. Thus, the nitroalkane nucleophile is commonly used in excess to selectively give the 2° nitroalkane.12 Clearly, this is an unattractive solution if one wishes to utilize precious nitroalkane reactants. Since nitroalkanoates are excellent Tsuji-Trost substrates,13 we proposed that a single pot Tsuji-Trost allylation/decarboxylative protonation strategy could quickly lead to synthetically useful 2° nitroalkanes (Scheme 3). Moreover, with appropriate substitution, functional groups can be paired to quickly access cis-1,5-dialkyl pyrrolidines and the indolizidine core.14,15
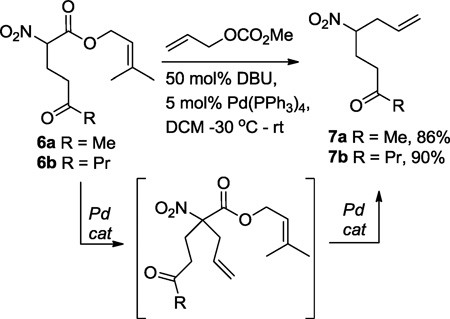 |
(4) |
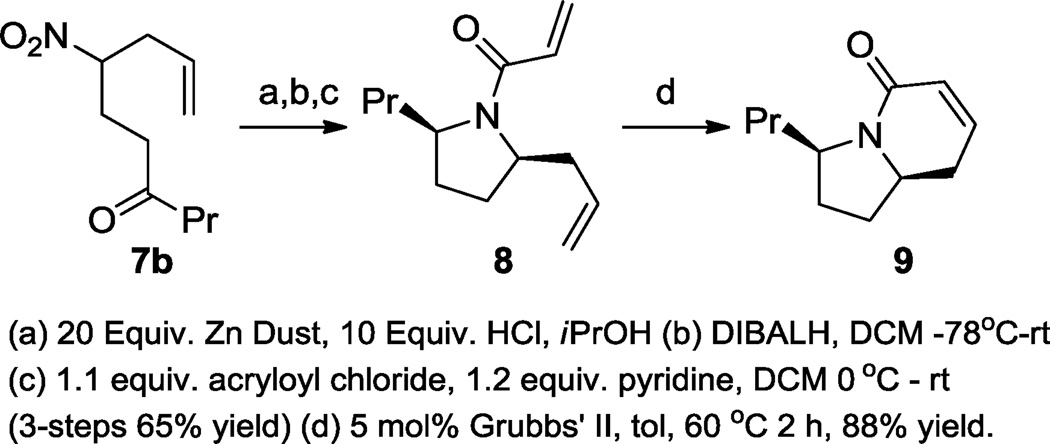
We began by synthesizing substrates 6 from prenyl nitroalkanoate using Yb(OTf)3-catalyzed Michael additions (eq. 4).7b Once the nitroalkanoates were alkylated with the vinyl ketone, the substrates 6 were allowed to react with allyl carbonate in the presence of catalytic amounts of DBU (50 mol%) and Pd(PPh3)4 (5 mol%) at −30 °C. The palladium catalyst first effects the Tsuji-Trost allylation of the nitroalkanoate. Upon warming, this reaction is followed by decarboxylative protonation to yield secondary nitroalkanes 7a and 7b in good yields. Moreover, synthetically useful quantities (>1 g) of 7b were prepared for further chemical manipulation.
To demonstrate the utility of this process, compound 7b was then converted to the indolizidine core in 4 steps (Scheme 4). Upon reduction of the nitro group,6 spontaneous condensation to the imine occurred.11 This imine was immediately reduced to the cis-1,5-pyrrolidine as a single diastereomer.15 As purification of this secondary amine was deemed too challenging, it was not purified until after acylation with acryloyl chloride. This 3-step process progressed in 65% overall yield. Interestingly, this amide exists as a 1:1.2 mixture of rotamers about the amide-bond. Fortunately, heating with Grubbs’ second generation catalyst (5 mol%), the diastereomers underwent convergent metathesis leading to a single ring-closed product 9 in 88% yield.
Scheme 4.
Synthesis of the indolizidine core.
In conclusion, we have shown that nitronates and Pd-π-allyl complexes derived from allyl nitroalkanoates can be diverted from decarboxylative allylation (DcA) through reaction pathways including Michael addition/Tsuji-Trost cascades and Tsuji-Trost/decarboxylative protonation reactions.
Supplementary Material
Acknowledgments
We acknowledge the National Institute of General Medical Sciences (NIGMS 1RO1GM079644) for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information
Please see the supplemental information for detailed experimental analysis and spectral analysis including 1H, 13C, and HRMS or GC-MS.
References
- 1.Weaver JD, Recio A, III, Grenning AJ, Tunge JA. Chem. Rev. 2011;111:1846. doi: 10.1021/cr1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For select examples of decarboxylative allylation of carbonnucleophiles see: Shimizu I, Yamada T, Tsuji J. Tetrahedron Lett. 1980;21:3199. Tsuda T, Chujo Y, Nishi S, Tawara K, Saegusa T. J. Am. Chem. Soc. 1980;102:6381. Recio A, III, Tunge JA. Org. Lett. 2009;11:5630. doi: 10.1021/ol902065p. Weaver JD, Tunge JA. Org. Lett. 2008;10:4657. doi: 10.1021/ol801951e. Weaver JD, Ka BJ, Morris DK, Thompson W, Tunge JA. J. Am. Chem. Soc. 2010;132:12179. doi: 10.1021/ja104196x. Burger EC, Tunge JA. J. Am. Chem. Soc. 2006;128:10002. doi: 10.1021/ja063115x. Trost BM, Bream RN, Xu J. Angew. Chem. Int. Ed. 2006;45:3109. doi: 10.1002/anie.200504421. Hong AY, Krout MR, Jensen T, Bennett NB, Harned AM, Stoltz BM. Angew. Chem. Int. Ed. 2011;50:2756. doi: 10.1002/anie.201007814. Mohr JT, Behenna DC, Harned AM, Stoltz BM. Angew. Chem. Int. Ed. 2005;44:6924. doi: 10.1002/anie.200502018. Trost BM, Xu J, Schmidt T. J. Am. Chem. Soc. 2009;131:18343. doi: 10.1021/ja9053948.
- 3.(a) Burger EC, Tunge JA. J. Am. Chem. Soc. 2006;128:10002. doi: 10.1021/ja063115x. [DOI] [PubMed] [Google Scholar]; (b) Yeagley AA, Chruma JJ. Org. Lett. 2007;9:2979. doi: 10.1021/ol071080f. [DOI] [PubMed] [Google Scholar]; (c) Fields WH, Khan AK, Sabat M, Chruma JJ. Org. Lett. 2008;10:5131. doi: 10.1021/ol801986m. [DOI] [PubMed] [Google Scholar]; (d) Waetzig SR, Tunge JA. J. Am. Chem. Soc. 2007;129:14860. doi: 10.1021/ja077070r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Waetzig SR, Tunge JA. J. Am. Chem. Soc. 2007;129:4138. doi: 10.1021/ja077070r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grenning AJ, Tunge JA. Org. Lett. 2010;12:740. doi: 10.1021/ol902828p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prof. Hans Reich has compiled a useful list of pKa’s in DMSO: http://www.chem.wisc.edu/areas/reich/pkatable/index.htm.
- 6.Nitroalkanoates undergo facile Knoevenagel/Diels-Alder reactions: Wade PA, Murray JK, Shah-Patel S, Carroll PJ. Tetrahedron Lett. 2002;43:2585. Butt NA, Moody CJ. Org. Lett. 2011;13:2224. doi: 10.1021/ol200477s. Jayakanthan K, Vankar YD. Org. Lett. 2005;7:5441. doi: 10.1021/ol052190u.
- 7.For examples of Michael addition to Nitroalkanoates: Keller E, Feringa BL. Synlett. 1997:842. Aplander K, Ding R, Krasavin M, Lindstrom UM, Wennerberg J. Eur. J. Org. Chem. 2009:810.
- 8.See reference 1 for a review of IDcA. For leading references on IDcA see: Nokami J, Watanabe H, Mandai T, Kawada M, Tsuji J. Tetrahedron Lett. 1989;30:4829. Wang C, Tunge JA. J. Am. Chem. Soc. 2008;130:8118. doi: 10.1021/ja801742h. Patil NT, Yamamoto Y. Synlett. 2007:1994. Shim J-G, Nakamura H, Yamamoto Y. J. Org. Chem. 1998;63:8470. doi: 10.1021/jo980818r. Shintani R, Park S, Hayashi T. J. Am. Chem. Soc. 2007;129:14866. doi: 10.1021/ja077236o. Streuff J, White DE, Virgil SC, Stoltz BM. Nature Chem. 2010;2:192. doi: 10.1038/nchem.518.
- 9.(a) Mohr JT, Nishimata T, Behenna DC, Stoltz BM. J. Am. Chem. Soc. 2006;128:11348. doi: 10.1021/ja063335a. [DOI] [PubMed] [Google Scholar]; (b) Marinescu SC, Nishimata T, Mohr JT, Stoltz BM. Org. Lett. 2008;10:1039. doi: 10.1021/ol702821j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prenylation can often be challenging due to competing elimination but works best with highly stabilized anions: (a) reference 1 and references therein. Trost BM, Malhotra S, Chan WH. J. Am. Chem. Soc. 2011;133:7328. doi: 10.1021/ja2020873., and references therein.
- 11.(a) Mayr H, Kempf B, Ofial AR. Acc. Chem. Res. 2003;36:66. doi: 10.1021/ar020094c. [DOI] [PubMed] [Google Scholar]; (b) Kaumanns O, Mayr H. J. Org. Chem. 2008;73:2738–2745. doi: 10.1021/jo702590s. [DOI] [PubMed] [Google Scholar]; (c) Zenz I, Mayr H. J. Org. Chem. . 2011;76:9370. doi: 10.1021/jo201678u. [DOI] [PubMed] [Google Scholar]
- 12.(a) Aleksandrowicz P, Piotrowska H, Sas W. Tetrahedron. 1982;38:1321. [Google Scholar]; (b) Wade P/A, Morrow SD, Hardinger SA. J. Org. Chem. 1982;47:365. [Google Scholar]; (c) Deardorff DR, Savin KA, Justman CJ, Karanjawala ZE, Sheppeck JE, II, Harger DC, Aydin N. J. Org. Chem. 1996;61:3616. doi: 10.1021/jo951510s. [DOI] [PubMed] [Google Scholar]; (d) Trost BM, Surivet JP. J. Am. Chem. Soc. 2000;122:6291. [Google Scholar]; (e) Trost BM, Surivet J-P. Angew. Chem. Int. Ed. 2000;39:3122. doi: 10.1002/1521-3773(20000901)39:17<3122::aid-anie3122>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]; (f) Maki K, Kanai M, Shibasaki M. Tetrahedron. 2007;63:4250. [Google Scholar]
- 13.(a) Ono N, Hamamoto I, Kaji A. J. Org. Chem. 1986;51:2832. [Google Scholar]; (b) Genet JP, Ferroud D. Tetrahedron Lett. 1984;25:3579. [Google Scholar]; (c) Fu Y, Etienne MA, Hammer RP. J. Org. Chem. 2003;68:9854. doi: 10.1021/jo034885j. [DOI] [PubMed] [Google Scholar]; (d) Tsuji J, Yamada T, Minami I, Yuhara M, Nisar M, Shimizu I. J. Org. Chem. 1987;52:2988. [Google Scholar]
- 14.Comer E, Rohan E, Deng L, Porco JA. Org. Lett. 2007;9:2123. doi: 10.1021/ol070606t. [DOI] [PubMed] [Google Scholar]
- 15.Bacos D, Célérier JP, Marx E, Saliou C, Lhommet G. Tetrahedron Lett. 1989;30:1081. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.