Abstract
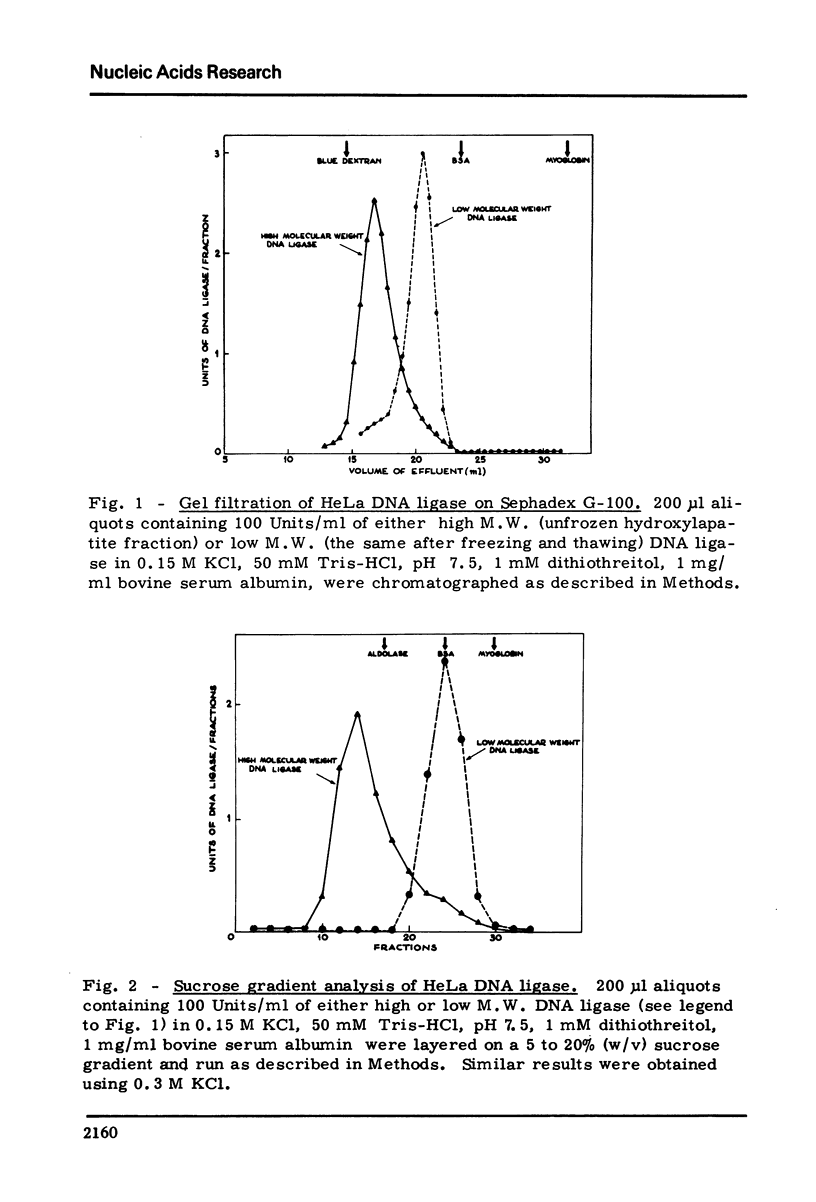
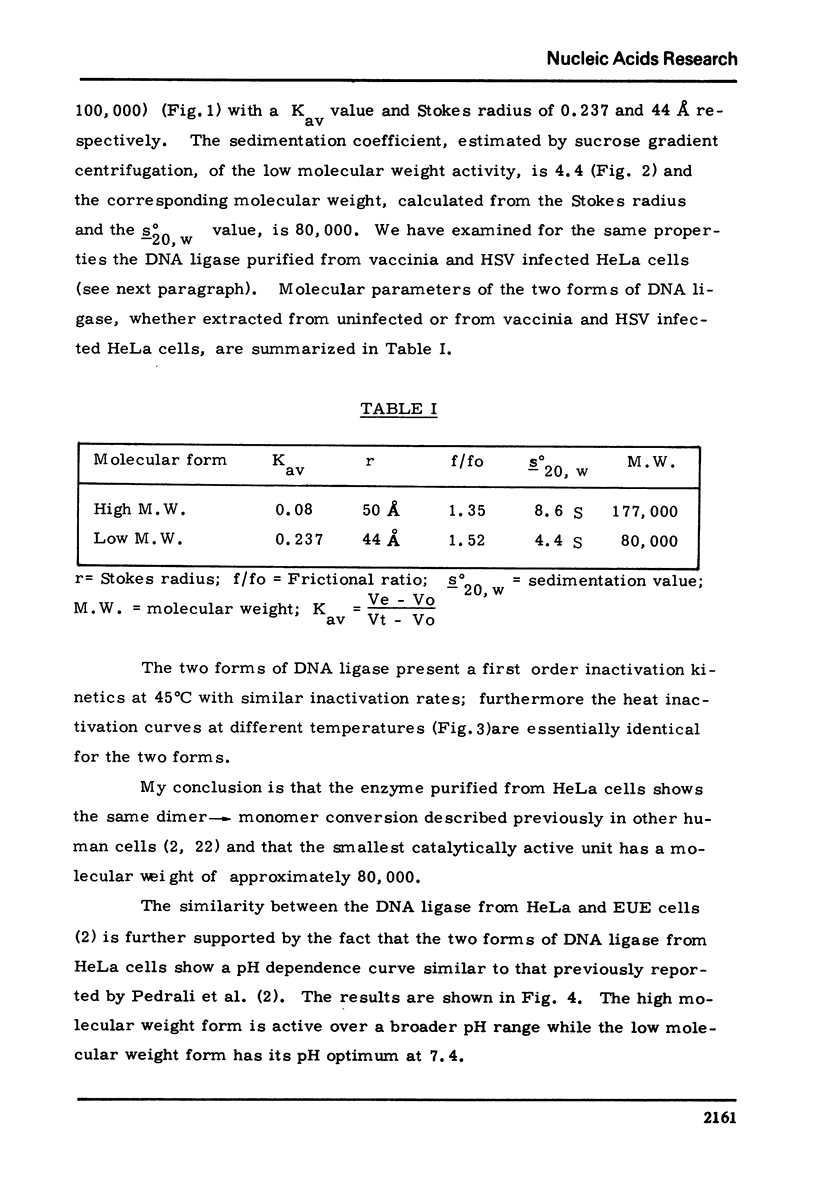
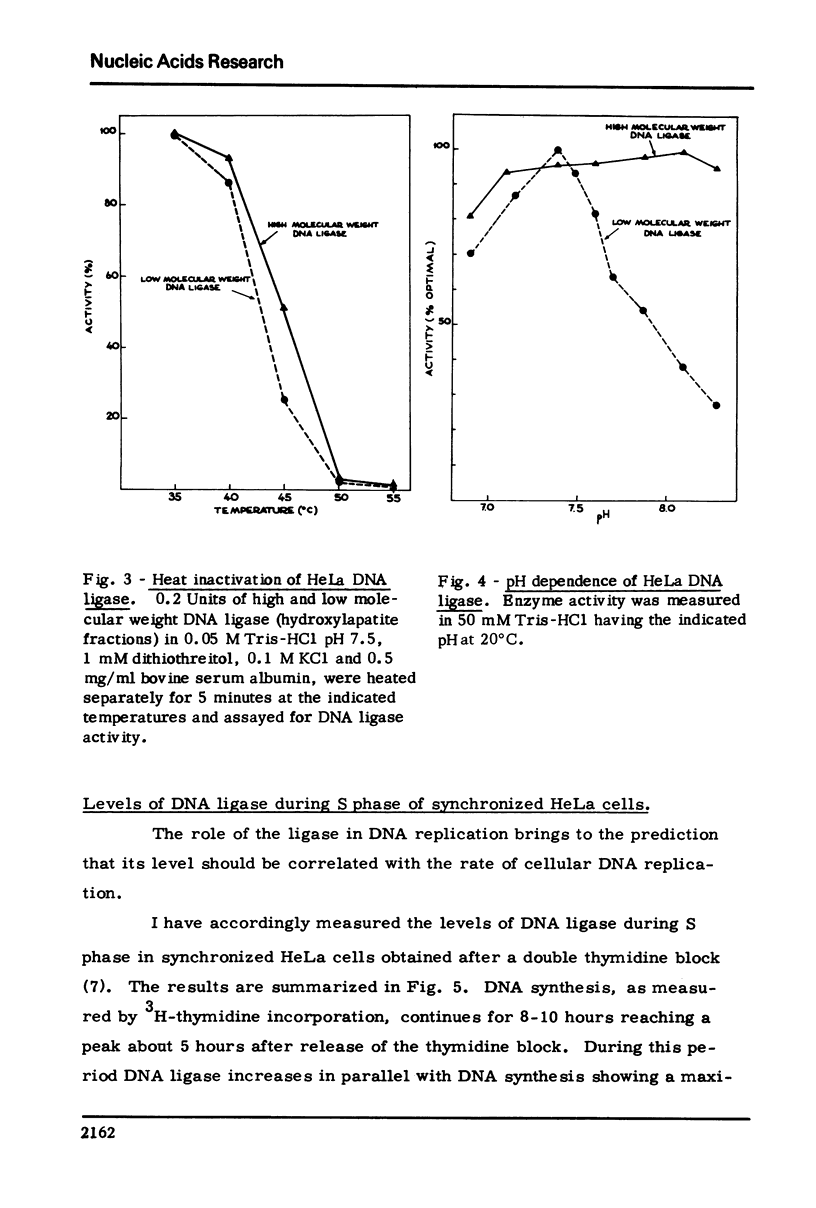
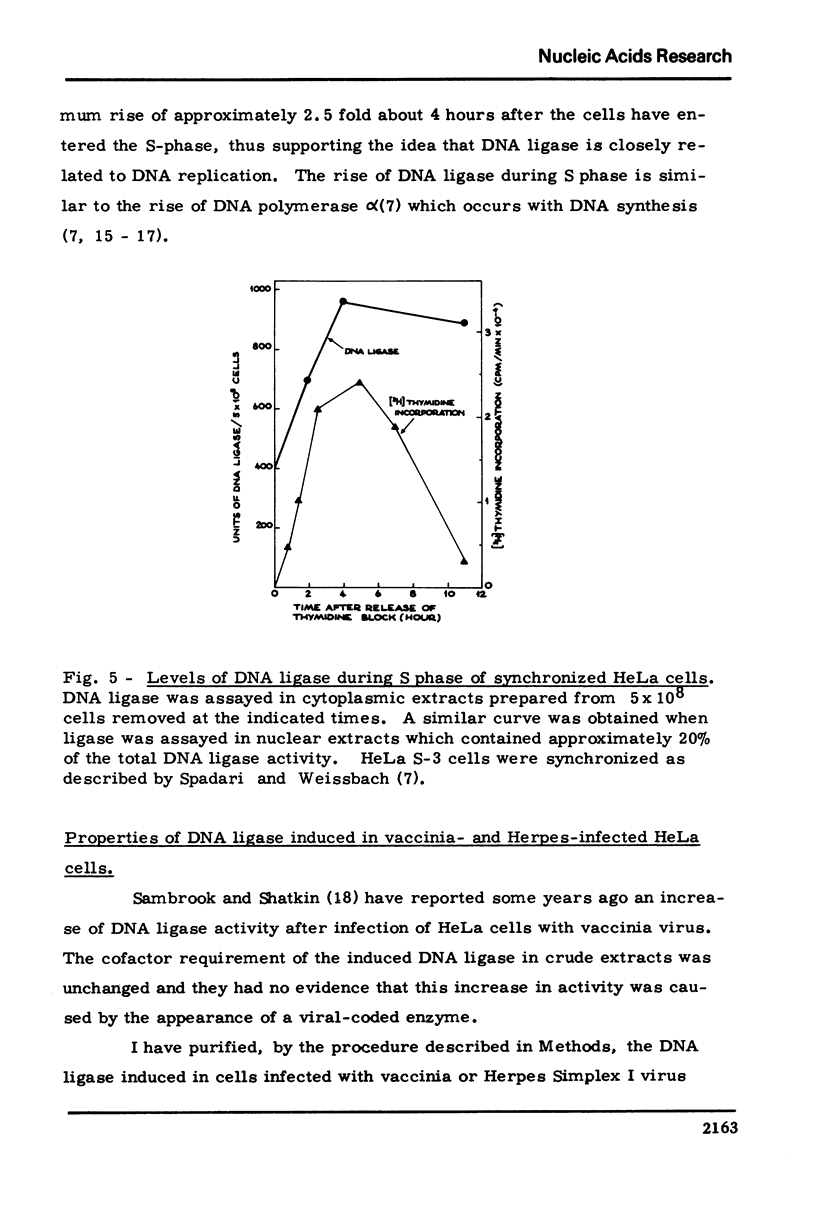
HeLa cells contain a high M.W. form of DNA ligase which can be completely converted to a low M.W. form. Stokes radius, frictional ratio, sedimentation coefficient, molecular weight, pH dependence, and heat inactivation rate of the two forms have been studied. The major properties of the two forms of DNA ligase in HeLa cells (in particular molecular weights and pH dependence) resemble those of the "dimer" and "monomer" structures described in cultured human cells (Pedrali, G., Spadari, S., Ciarrocchi, G., Pedrini, M., Falaschi, A. (1973) Eur. J. Biochem., 39 343) .In synchronized HeLa cells, the DNA ligase shows a two fold increase during S phase and parallels the increase in the DNA synthesis rate. DNA ligase increases in parallel with viral DNA synthesis after infection of HeLa cells with vaccinia and Herpes virus but its cofactor requirements and physical properties (including the dimer leads to monomer conversion) are unchanged, suggesting that the newly formed ligase is not virus-coded.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baril E. F., Jenkins M. D., Brown O. E., Laszlo J., Morris H. P. DNA polymerases I and II in regenerating rat liver and Morris hepatomas. Cancer Res. 1973 Jun;33(6):1187–1193. [PubMed] [Google Scholar]
- Berkowitz D. M., Kakefuda T., Sporn M. A simple and rapid method for the isolation of enzymatically active HeLa cell nuclei. J Cell Biol. 1969 Sep;42(3):851–854. doi: 10.1083/jcb.42.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzoni U., Mathelet M., Campagnari F. Purification and properties of a polynucleotide ligase from calf thymus glands. Biochim Biophys Acta. 1972 Dec 22;287(3):404–414. doi: 10.1016/0005-2787(72)90284-5. [DOI] [PubMed] [Google Scholar]
- Bertazzoni U., Stefanini M., Noy G. P., Giulotto E., Nuzzo F., Falaschi A., Spadari S. Variations of DNA polymerase-alpha and -beta during prolonged stimulation of human lymphocytes. Proc Natl Acad Sci U S A. 1976 Mar;73(3):785–789. doi: 10.1073/pnas.73.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. V. Homogeneous terminal deoxynucleotidyl transferase. J Biol Chem. 1971 Feb 25;246(4):909–916. [PubMed] [Google Scholar]
- Chang L. M., Brown M., Bollum F. J. Induction of DNA polymerase in mouse L cells. J Mol Biol. 1973 Feb 15;74(1):1–8. doi: 10.1016/0022-2836(73)90349-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaColla P., Weissbach A. Vaccinia virus infection of HeLa cells. I. Synthesis of vaccinia DNA in host cell nuclei. J Virol. 1975 Feb;15(2):305–315. doi: 10.1128/jvi.15.2.305-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Pedrali Noy G. C., Spadari S., Ciarrocchi G., Pedrini A. M., Falaschi A. Two forms of the DNA ligase of human cells. Eur J Biochem. 1973 Nov 15;39(2):343–351. doi: 10.1111/j.1432-1033.1973.tb03132.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Shatkin A. J. Polynucleotide ligase activity in cells infected with simian virus 40, polyoma virus, or vaccinia virus. J Virol. 1969 Nov;4(5):719–726. doi: 10.1128/jvi.4.5.719-726.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Spadari S., Ciarrocchi G., Falaschi A. Purification and properties of a polynucleotide ligase from human cell cultures. Eur J Biochem. 1971 Sep 13;22(1):75–78. doi: 10.1111/j.1432-1033.1971.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Spadari S., Muller R., Weissbach A. The dissimilitude of the low and high molecular weight deoxyribonucleic acid-dependent deoxyribonucleic acid polymerases of HeLa cells. J Biol Chem. 1974 May 10;249(9):2991–2992. [PubMed] [Google Scholar]
- Spadari S., Weissbach A. HeLa cell R-deoxyribonucleic acid polymerases. Separation and characterization of two enzymatic activities. J Biol Chem. 1974 Sep 25;249(18):5809–5815. [PubMed] [Google Scholar]
- Spadari S., Weissbach A. RNA-primed DNA synthesis: specific catalysis by HeLa cell DNA polymerase alpha. Proc Natl Acad Sci U S A. 1975 Feb;72(2):503–507. doi: 10.1073/pnas.72.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadari S., Weissbach A. The interrelation between DNA synthesis and various DNA polymerase activities in synchronized HeLa cells. J Mol Biol. 1974 Jun 15;86(1):11–20. doi: 10.1016/s0022-2836(74)80003-3. [DOI] [PubMed] [Google Scholar]
- Söderhäll S., Lindahl T. Mammalian DNA ligases. Serological evidence for two separate enzymes. J Biol Chem. 1975 Nov 10;250(21):8438–8444. [PubMed] [Google Scholar]
- Söderhäll S., Lindahl T. Two DNA ligase activities from calf thymus. Biochem Biophys Res Commun. 1973 Aug 6;53(3):910–916. doi: 10.1016/0006-291x(73)90178-2. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]


