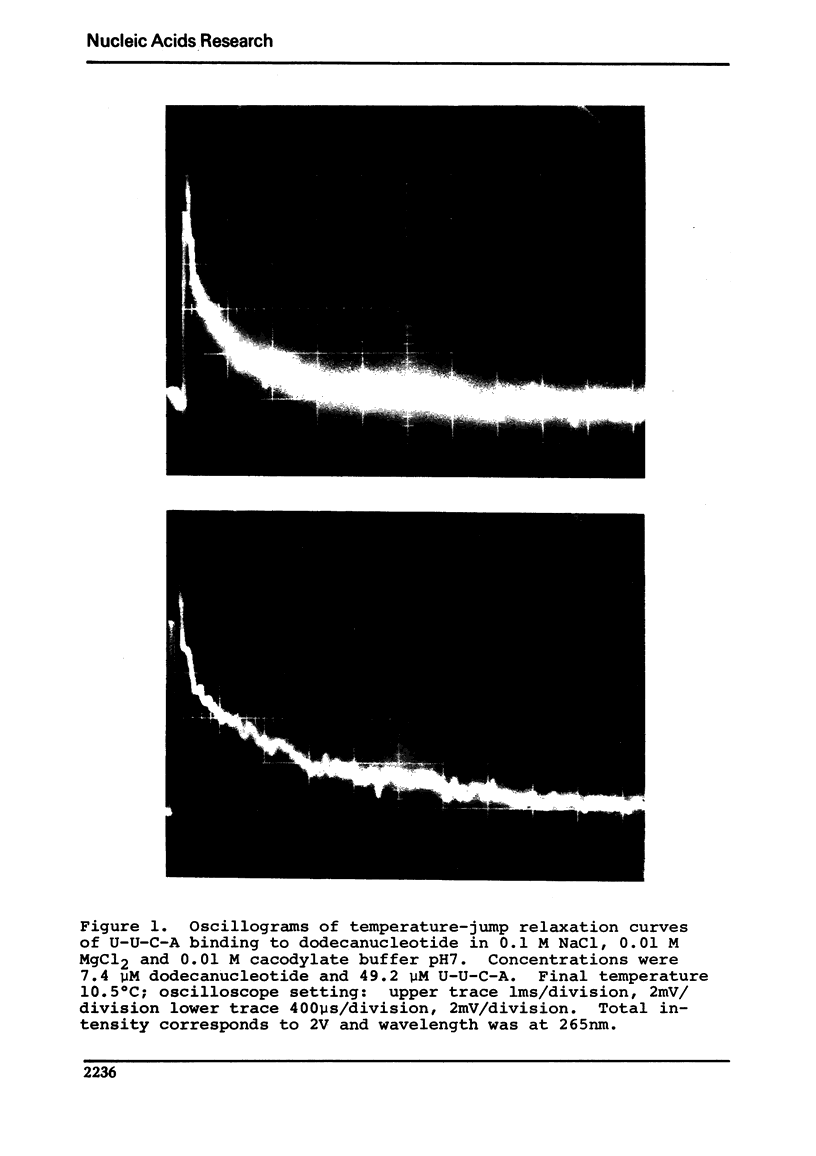
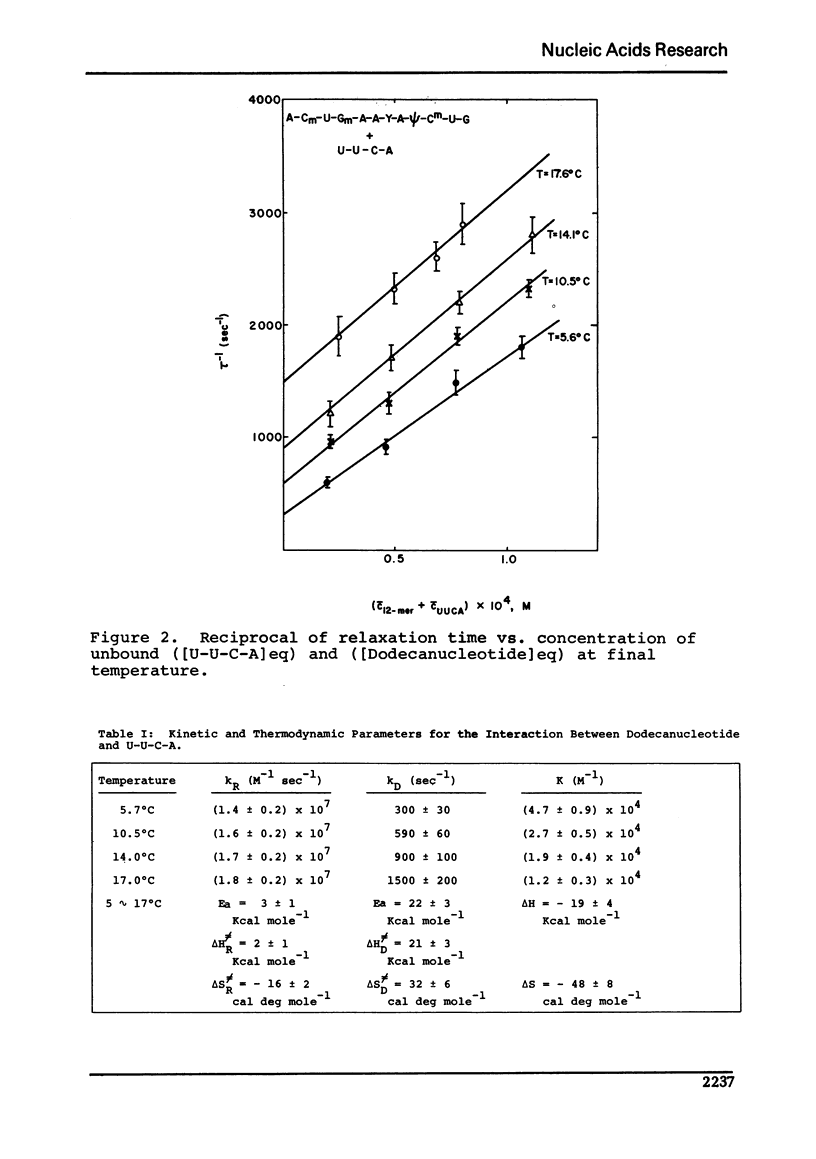
Abstract
The kinetics of U-U-C-A binding to the dodecanucleotide (A-Cm-U-Gm-A-A-Y-A-psi-m5C-U-Gp) isolated from the anticodon region of yeast tRNA-Phe are similar to the kinetics of binding of U-U-C-A to intact tRNA-Phe. A large enhancement in binding constant over that predicted for U-U-C-A-U-G-A-A is observed for both the complexes of dodecanucleotide and tRNA-Phe with U-U-C-A. This strongly suggests that both the anticodon loop in tRNA-Phe and the dodecanucleotide can form four base pairs with U-U-C-A. Furthermore, the enhanced stability cannot be attributed to a special conformation of the anticodon loop, but instead the anticodon loop is probably flexible. A likely explanation for the increased binding is the effect of non-base-paired ends. This increased thermodynamic stability comes from a larger entropy gain rather than a larger enthalpy decrease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beardsley K., Tao T., Cantor C. R. Studies on the conformation of the anticodon loop of phenylalanine transfer ribonucleic acid. Effect of environment on the fluorescence of the Y base. Biochemistry. 1970 Sep 1;9(18):3524–3532. doi: 10.1021/bi00820a005. [DOI] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Drake A. F., Mason S. F., Trim A. R. Optical studies of the base-stacking properties of 2'-O-methylated dinucleoside monophosphates. J Mol Biol. 1974 Jul 15;86(4):727–739. doi: 10.1016/0022-2836(74)90349-0. [DOI] [PubMed] [Google Scholar]
- Eisinger J. Complex formation between transfer RNA'S with complementary anticodons. Biochem Biophys Res Commun. 1971 May 21;43(4):854–861. doi: 10.1016/0006-291x(71)90695-4. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Feuer B., Yamane T. Codon-anticodon binding in tRNAphe. Nat New Biol. 1971 May 26;231(21):126–128. doi: 10.1038/newbio231126a0. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Gross N. The anticodon-anticodon complex. J Mol Biol. 1974 Sep 5;88(1):165–174. doi: 10.1016/0022-2836(74)90302-7. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Spahr P. F. Binding of complementary pentanucleotides to the anticodon loop of transfer RNA. J Mol Biol. 1973 Jan;73(1):131–137. doi: 10.1016/0022-2836(73)90165-4. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Söll D. G., Crothers D. M. Studies of the complex between transfer RNAs with complementary anticodons. I. Origins of enhanced affinity between complementary triplets. J Mol Biol. 1976 May 25;103(3):499–519. doi: 10.1016/0022-2836(76)90214-x. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Cantor C. R., Tinoco I., Jr Association of complementary oligoribonucleotides in aqueous solution. Biochemistry. 1968 Sep;7(9):3164–3178. doi: 10.1021/bi00849a020. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Atomic co-ordinates for yeast phenylalanine tRNA. Nucleic Acids Res. 1975 Sep;2(9):1629–1637. doi: 10.1093/nar/2.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelicke A., von der Haar F., Cramer F. Spectroscopic properties of oligonucleotides excised from the anticodon region of phenylalanine tRNA from yeast. Biopolymers. 1973;12(1):27–43. doi: 10.1002/bip.1973.360120104. [DOI] [PubMed] [Google Scholar]
- Martin F. H., Uhlenbeck O. C., Doty P. Self-complementary oligoribonucleotides: adenylic acid-uridylic acid block copolymers. J Mol Biol. 1971 Apr 28;57(2):201–215. doi: 10.1016/0022-2836(71)90341-x. [DOI] [PubMed] [Google Scholar]
- Ohashi Ziro, Harada Fumio, Nishimura Susumu. Primary sequence of glutamic acid tRNA II from Escherichia coli. FEBS Lett. 1972 Feb 1;20(2):239–241. doi: 10.1016/0014-5793(72)80804-4. [DOI] [PubMed] [Google Scholar]
- Pongs O., Reinwald E. Function of Y in codon-anticodon interaction of tRNA Phe . Biochem Biophys Res Commun. 1973 Jan 23;50(2):357–363. doi: 10.1016/0006-291x(73)90848-6. [DOI] [PubMed] [Google Scholar]
- Pörchke D. The nature of stacking interations in polynucleotides. Molecular states in Oligo- and polyribocytidylic acids by relaxation analysis. Biochemistry. 1976 Apr 6;15(7):1495–1499. doi: 10.1021/bi00652a021. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Seeman N. C., Wang A. H., Suddath F. L., Rich A. Yeast phenylalanine transfer RNA: atomic coordinates and torsion angles. Nucleic Acids Res. 1975 Dec;2(12):2329–2341. doi: 10.1093/nar/2.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Hillier B. Oligonucleotide studies: optical rotatory dispersion of normal and 2'-O-methylated diribonucleoside monophosphates. Biopolymers. 1971;10(12):2445–2457. doi: 10.1002/bip.360101208. [DOI] [PubMed] [Google Scholar]
- Yoon K., Turner D. H., Tinoco I., Jr The kinetics of codon-anticodon interaction in yeast phenylalanine transfer RNA. J Mol Biol. 1975 Dec 25;99(4):507–518. doi: 10.1016/s0022-2836(75)80169-0. [DOI] [PubMed] [Google Scholar]