Abstract
Bioenergy homeostasis is crucial in maintaining normal cell function and survival and it is thus important to understand cellular mechanisms underlying its regulation. Neurons use a large amount of ATP to maintain membrane potential and synaptic communication, making the brain the most energy consuming organ in the body. Glutamate mediates a large majority of synaptic transmission which is responsible for the expression of neural plasticity and higher brain functions. Most of the energy cost is attributable to the glutamatergic system; under pathological conditions such as stroke and brain ischemia, neural energy depletion is accompanied by a massive release of glutamate. However, the specific cellular processes implicated in glutamate-dependent bioenergy dynamics are not well understood. We find that glutamate induces a rapid and dramatic reduction of ATP levels in neurons, through reduced ATP genesis and elevated consumption. ATP reduction depends on NMDA receptor activity, but is not a result of neuronal firing, gap junction-mediated leaking or intracellular signaling. Similar changes in ATP levels are also induced by synaptic glutamate accumulation following suppression of glutamate transporter activity. Furthermore, the glutamate-induced ATP down-regulation is blocked by the sodium pump inhibitor ouabain, suggesting the sodium pump as the primary energy consumer during glutamate stimulation. These data suggest the important role of glutamate in the control of cellular ATP homeostasis.
Keywords: glutamate, glutamatergic, energy, ATP, synapse, glutamate receptors
1. Introduction
The brain is the most energy consuming organ in our body. It accounts for 2% of our body weight, but uses more than 20% of the oxygen and ATP supply (Magistretti and Pellerin 1997; Magistretti et al. 1999; Attwell and Laughlin 2001; Raichle and Gusnard 2002; Rao et al. 2006). Therefore, neurons are extremely sensitive to bioenergy homeostasis. Within five minutes, the brain will cease to function if the oxygen supply stops. In contrast to peripheral tissues, neurons depend almost solely on glucose for ATP production (Attwell and Laughlin 2001). Among many neural processes, the excitatory glutamatergic system uses the most energy (Sibson et al. 1998; Shen et al. 1999; Raichle and Gusnard 2002), most probably due to postsynaptic events (Jolivet et al. 2009). Neuronal activity, manifested by fluctuation in membrane potential, represents a major ATP-consuming process. Other glutamate-related events, including glutamate synthesis, vesicle filling and release, transporter uptake and recycling, as well as receptor trafficking and signaling, are also energy consuming.
In the brain, the glutamatergic system is responsible for the majority of excitatory synaptic activity. In presynaptic terminals, glutamate is enriched in synaptic vesicles, powered indirectly by a proton pump on the vesicle membrane, at a concentration of 100 mM. During synaptic transmission, the release of a single vesicle can cause a rapid rise of glutamate in the cleft. At its peak, it can reach 1 mM (Danbolt 2001). Under normal conditions, ambient glutamate at the extracellular environment is maintained by the constant activity of glutamate transporters at the plasma membrane of both neurons and glia (Danbolt 2001; Tzingounis and Wadiche 2007). Transporters at the glia, which often surround synapses to ensure efficient uptake of released transmitter and to prevent glutamate spillover, are believed to play a major role in ensuring minimal ambient glutamate.
Glutamate functions through its specific receptors, which include ionotropic AMPA (AMPAR), NMDA (NMDAR) and kainate receptors (KR), as well as metabolic receptors (mGluRs) (Man et al. 2000; Collingridge et al. 2004; Newpher and Ehlers 2008). AMPARs are the major components responsible for synaptic transmission, whereas NMDARs play an essential role in the formation of synaptic plasticity, mainly via regulation of AMPAR trafficking and synaptic localization. More importantly, the high permeability of NMDAR to calcium enables the receptor to initiate a series of calcium-dependent signaling cascades, including energy-dependent protein modification and metabolic regulations (Kim et al. 2005). mGluRs are G-protein coupled receptors that, upon glutamate binding, regulate neuronal functions via PKA, PLC and PKC signaling cascades (Anwyl 2009; Gladding et al. 2009; Olive 2009).
Under normal conditions, high glutamate concentrations only occur at the synaptic cleft. The ambient glutamate concentration is maintained at very low levels (Herman and Jahr 2007). However, during traumatic brain injuries or stroke, massive glutamate release can lead to a marked increase in extracellular glutamate and hyperactivity of the overall glutamate system, causing additional acute and delayed neural pathology. A reduction in ATP is one of the first cellular abnormalities in stroke. Lack of energy will eventually cause the failure of the sodium pump, leading to a collapse of ion/electrical gradients and a complete loss of neuronal function. In addition, dysfunction in energy metabolism has been implicated in multiple neurological disorders including Alzheimer’s disease, Parkinson’s disease and Huntington disease (Ferreira et al.; Mochel et al.; Young-Collier et al.; Blass et al. 1988; Wallace 1994; Mattson et al. 1999; Parihar and Brewer 2007; Amato and Man). However, the cellular mechanisms by which glutamate negatively regulates ATP homeostasis remain less clear.
To this end, we examined the effect of glutamate application on cellular energy status in cultured cortical neurons. We find that glutamate triggers a rapid, significant reduction in neuronal ATP levels. The glutamate effect on ATP is dependent on the activity of NMDAR and the sodium pump, and can be triggered by synaptic glutamate accumulation. In contrast, neither firing of action potentials nor gap junction-mediated leaking is responsible for the ATP reduction. These findings suggest a process in which sodium influx via glutamate receptor channels stimulates sodium pump activity, leading to elevated energy consumption and ATP reduction in neurons.
2. Materials and methods
2.1. Primary cortical neuron culture
Primary neuronal cultures were prepared as described earlier (Zhang et al. 2009; Lin et al. 2011; Jarzylo and Man 2012). Briefly, rat brain cortices from E18 rat embryos were digested with papain (0.1 mg/ml in HBSS, 37°C for 20 min), washed and triturated with a serological pipette. To ensure high-quality cell adhesion and growth, 6-well plates were coated with poly-L-lysine (Sigma, 0.1 mg/ml) overnight and washed before being kept in plating medium (MEM containing 10% fetal bovine serum (FBS), 5% horse serum, 31 mg cystine, 5 mM glutamax and 1% P/S) until cell plating. Neurons were counted and plated onto 6-well dishes (1×106 per well). 24 hrs after plating, the culture medium was replaced with feeding medium (Neurobasal medium supplemented with 1% HS, 2% B-27, 2 mM glutamax and 1% P/S). Thereafter, cortical neurons were fed twice a week with 2 ml feeding medium/dish until experiments. To keep a low level of glial cells in the culture, the mitotic inhibitor 5-FDU (5 μM) was added to DIV 7 cultures to inhibit glial cell proliferation.
2.2. Glia culture
Cells from rat cortices were prepared as above, except that medium containing high serum (15%) was used for plating. Neurons do not survive high serum concentrations and die out gradually. Glia cultures were used for assays when cell confluency reached above 80%.
2.3. ATP assays
2 wk-old cultured cortical neurons grown in 6-well plates were used for ATP assays. Cells were treated with glutamate alone, or together with other drugs for 1 hr. On ice, culture medium was removed, and cells were washed 3 times with ACSF containing (in mM) 140 NaCl, 3 KCl, 1.5 MgCl2, 2.5 CaCl2, 11 Glucose, 10 HEPES, pH 7.4. After washing, 400 μl HEPES (20 mM in H2O) lysis buffer was added to each well, and cells were collected after thoroughly scraping the culture at the well bottom. Cell lysates were further triturated 5–6 times by repeated pippetting, boiled at 95°C for 10 min, sonicated in cold room for 2 s and followed by ATP assays using a Luminometer according to manufacturer’s instructions (ATP Determination Kit, Invitrogen).
2.4. Western blotting
Cultures were rinsed with cold PBS and resuspended in 200 μl modified RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP40, 1% SDOC and 0.1% SDS) containing mini cOmplete protease inhibitor (Roche). Lysates were further solubilized by sonication and 10 min incubation on ice followed by centrifugation for 10 min at 13,000 × g to remove insolubilities. Supernatants were mixed with 2x sample buffer and denatured on a 95°C heat block for 10 min. Lysate samples were separated by SDS-PAGE and transferred to PVDF membranes. The blot was then blocked at room temperature with 5% nonfat dry milk in TBST for one hr and incubated with primary antibody diluted in TBST overnight at 4°C, followed by incubation with HRP-conjugated secondary antibodies. The blot was developed using enhanced chemiluminescence (ECL) detection methods (Amersham).
2.5. Neuronal cell death detection
Cultured cortical neurons on coverslips were treated with 50 μM glutamate for 60 min at 37°C. Some treated cells were then washed and incubated at 37°C in normal drug-free medium for an additional 3 hr for recovery in order to detect possible delayed cell death. During the final 20 min of 1 hr glutamate treatment or of the 3 hr recovery, respectively, propidium iodide (1 μM) and Hoechst (1 μM) were added to the medium. After incubation, cells were washed 3 times with cold ACSF on the rocker. Following fixation, cells were mounted for cell death examination. Cells demonstrating nuclear condensation and positive PI staining were counted as cells undergoing cell death.
2.6. Data analysis and statistics
All values were reported as mean ± SEM. Statistical analysis was performed using the two-population student’s t test. N indicates the number of independent experiments. For westerns, the film was scanned and the optical intensities of the protein bands were quantified using NIH Image-J software.
3. Results
3.1. Glutamate induces rapid reduction in neuronal ATP
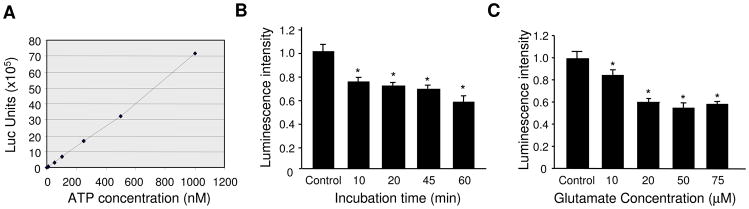
To examine the effect of glutamatergic activation on neuronal bioenergy homeostasis, 2 wk-old primary cortical neurons were incubated with glutamate (50 μM) in culture medium for varied periods of time. Neurons were then rinsed with ACSF, lysed in HEPES buffer and boiled at 95°C for 10 min. Equal amount of lysates were used for the luciferase-based ATP assay. As a control, a standard curve showed a linear relationship between ATP concentration and fluorescence intensity (Figure 1A). A 10 min glutamate treatment caused a 30% reduction in ATP level (0.74 ± 0.04, n=3). At 60 min, the ATP level was further reduced to 60% of control (0.59 ± 0.04, n=3) (Figure 1B). Next, we examined the dose response of glutamate incubation. 10 μM glutamate induced a modest, but significant ATP reduction (0.85 ± 0.04, n=3). A more drastic effect was obtained by a higher concentration, but the effect reached a plateau at 30–50 μM (Figure 1C). For the rest of the experiments, 50 μM glutamate for 60 min was adopted as a standard treatment protocol.
Figure 1.
Application of glutamate causes a reduction in ATP abundance in cultured cortical neurons. (A) A standard curve of ATP assay showing linear relationship between ATP levels and Luminometer measurement values. (B) Time course of glutamate treatment. 10 min glutamate incubation induced a significant reduction in ATP levels, which was further reduced by 60 min treatment. (C) Glutamate dose response. Significant changes in ATP amount could be induced by as low as 10 μM glutamate, and a plateau was reached by 50 μM glutamate. *P<0.05, student’s t test.
3.2. Glutamate regulates ATP production and utilization independent of neuronal firing
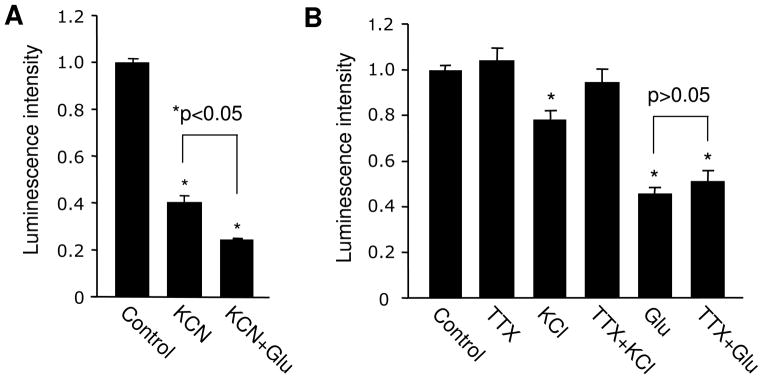
The reduction in ATP could result from suppression in energy production or enhancement in ATP consumption, or both. To test these possibilities, we used potassium cyanide (KCN) to block mitochondrial oxidative phosphorylation and ATP genesis. Cultured neurons were incubated with KCN for 15 min, then supplemented with glutamate for 60 min. Indeed, KCN alone in the same incubation time period (75 min) caused a drastic reduction in ATP (0.41 ± 0.02, n=5). When ATP synthesis was blocked by KCN, the addition of glutamate was able to induce a further reduction in ATP levels (0.25 ± 0.002, n=3) (Figure 2A), indicating enhanced ATP utilization by glutamatergic activation. However, glutamate caused only a 16% further reduction from that of KCN alone, much less than the total effect of glutamate alone (about 50%). Therefore, the glutamate effect likely resulted from a combination of inhibited production and facilitated consumption of cellular ATP.
Figure 2.
Glutamate effect in ATP abundance is not caused by elevated neuronal firing. (A) Blockade of energy synthesis process by KCN lead to a dramatic reduction in ATP levels. In the presence of KCN, glutamate treatment remained to be able to cause ATP reduction. (B) Changes in ATP abundance following KCl-induced neuronal excitation were blocked by TTX; in contrast, blockade of neuronal firing had no effect on glutamate-dependent ATP regulation. *P<0.05, student’s t test.
Application of glutamate should cause membrane depolarization and an increase in neuronal firing of action potentials, leading to elevated levels of network activity. To test whether the drop of ATP is caused by higher neuronal activation, cultured neurons were treated with KCl (40 mM) to trigger depolarization and firing. As expected, a 1 hr KCl incubation induced a significant drop in ATP. To further confirm the involvement of firing in ATP consumption, we applied tetrodotoxin (TTX, 1 μM) to block sodium channels and action potentials. Interestingly, no changes in the ATP level were detected by applying TTX alone, probably due to low levels of basal activity. Indeed, in our cultures, patch-clamp recording revealed that 2 wk-old neurons spike at a low frequency of about 0.5 Hz at resting condition (Hou et al. 2011). Consistent with the role of firing, we found that KCl-induced reduction in ATP abundance was completely abolished by TTX (KCl, 0.78 ± 0.04, n=3; KCl + TTX, 0.95 ± 0.06, n=3) (Figure 2B), confirming the contribution of cell firing in the KCl effect. By contrast, we found that in the presence of TTX, the glutamate treatment remained able to reduce ATP levels (Glutamate 0.46 ±0.02, n=3; glutamate plus TTX 0.51 ± 0.04, n=3) (Figure 2B), excluding cell firing as a major factor in the glutamate effect. This suggests that other cellular processes than neuronal electrical activity are responsible for the observed glutamate-dependent ATP depletion.
3.3. Involvement of glutamate receptors and signaling cascades in glutamate-induced ATP reduction
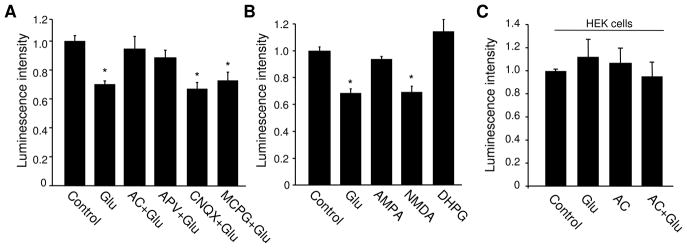
Glutamate affects neuronal activity via its receptors including ionotropic AMPARs and NMDARs, as well as metabotropic mGluRs. To examine the involvement of glutamate receptor activity, we mixed CNQX (20 μM), APV (50 μM) and MCPG (500 μM) to form an antagonist cocktail (AC) to block all glutamate receptors. In the presence of AC, glutamate failed to induce a change in ATP levels, indicating a requirement of glutamate receptor activation (Figure 3A). To dissect the receptor subtype(s) that mediates glutamate effect, a receptor antagonist was applied individually to selectively block AMPAR/KR (CNQX), NMDAR (APV) and mGluRs (MCPG), respectively. Glutamate effect on ATP was blocked by APV, but not by CNQX or MCPG (Figure 3A), indicating an important role of NMDARs. Consistently, when neurons were incubated with a specific agonist to activate individual receptor subtypes, we found that only NMDA, but neither AMPA nor DHPG, induced a reduction in ATP (Figure 3B). These data strongly indicate that the glutamate effect is mediated primarily via NMDARs.
Figure 3.
Glutamate effect in ATP regulation is mediated mainly by NMDARs. (A) Neurons were incubated with glutamate in the presence of antagonists against AMPAR, NMDAR and mGluR, applied individually for one type of receptor, or together as a mixed antagonist cocktail (AC) to block all receptors. AC and NMDAR antagonist APV, but not AMPAR antagonist CNQX or mGluR antagonist MCPG, abolished the glutamate effect, indicating a key role for NMDARs (n=4). (B) Neurons were treated with glutamate or an agonist specific for AMPAR (AMPA), NMDAR (NMDA) and mGluR (DHPG), respectively. NMDA induced an ATP reduction to a level similar to that of glutamate treatment, whereas AMPA and DHPG had no effect (n=3). (C) Glutamate and receptor antagonists had no effect on ATP levels in HEK cells (n=3). *P<0.05, student’s t test.
Next, we wanted to confirm that the ATP reduction is not a result of non-specific deleterious effects of glutamate on cell conditions, causing a failure of ATP synthesis. First, we tested whether glutamate could induce ATP reduction in other cell types. We found that when ATP was measured in HEK cells, glutamate treatment did not show any effect (Glutamate 1.12 ±0.05, n=3; glutamate plus AC 0.95 ± 0.12, n=3) (Figure 3C), indicating neuronal specificity in glutamatergic regulation of ATP homeostasis. Second, we performed cell death assays in glutamate treated neuronal cultures. Hoechst and propidium iodide (PI) dyes were added to the medium immediately after glutamate treatment, or after 1 hr recovery following glutamate treatment. Nucleus condensation and fragmentation in PI staining, as well as positive fluorescence signals in PI staining would indicate cell death. Examination showed a minimal level of cell death in non-treated controls (0%, n=850 cells). We found no significant change in cell death immediately after 1 hr glutamate treatment (0%, n=861 cells), and only a modest increase in cell death after 1 hr recovery (9.2%, n=1201 cells). In contrast, as a positive control, a typical cell death protocol (100 μM glutamate for 1 hr, and 5 hr recovery) induced a drastic rate of cell death (42%, n=935 cells). Thus, alterations in cell health condition did not seem to contribute significantly to the glutamate effect on ATP.
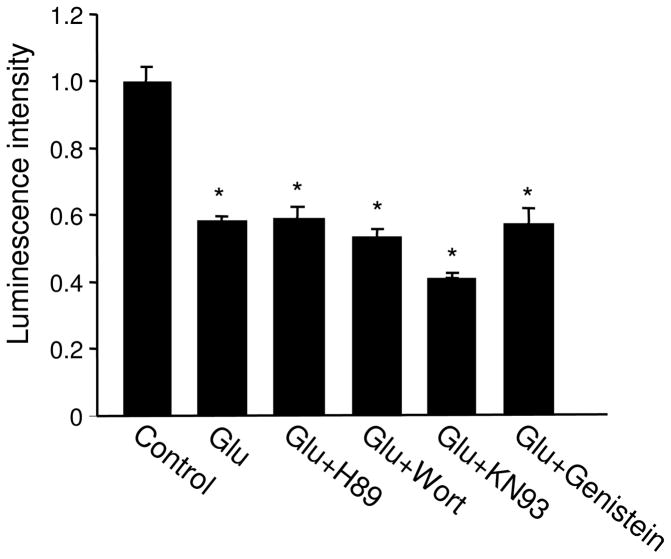
We then examined the involvement of multiple major signaling pathways related to glutamate activation including protein kinase A (PKA), PI3-kinase (PI3K), calcium/calmodulin-dependent kinase II (CaMKII), and tyrosine kinase. In the presence of antagonists specific to each of the individual kinases, we found that glutamate induced a reduction in ATP to levels comparable to glutamate alone (Figure 4), excluding these signaling molecules from a role in bioenergy regulation.
Figure 4.
Involvement of signaling pathways in glutamate effects. Neurons were treated with glutamate in the presence of varied drugs to inhibitor the activity of PKA, PI3K, CaMKII and tyrosine kinases. None of the inhibitors showed effect on the glutamate-induced ATP reduction. *P<0.05, student’s t test.
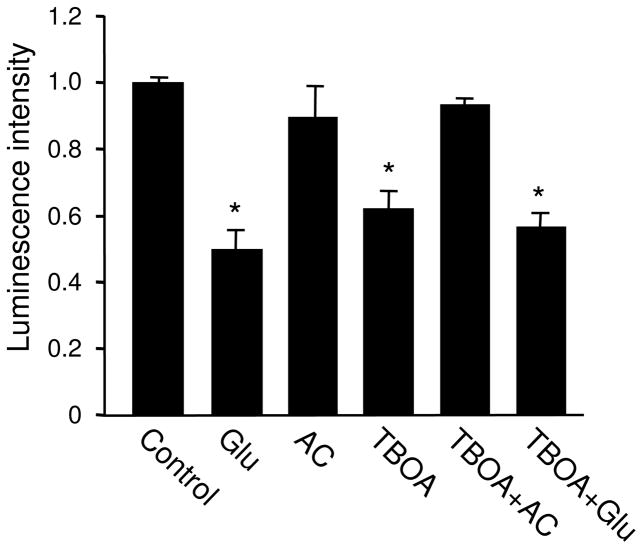
3.4. Synaptic glutamate regulates ATP in a receptor activity-dependent manner
Application of glutamate in the medium should activate all receptors at the cell surface, including those localized at synaptic and non-synaptic sites. We wondered whether an increase in glutamate selectively at the synaptic sites also regulates cellular energy homeostasis. At basal conditions, glutamate release can be triggered by action potentials, or can occur spontaneously. The presynaptically released glutamate is rapidly removed by diffusion and activities of excitatory amino acid transporters (EAATs) (Gegelashvili et al. 2000; Danbolt 2001; Nieoullon et al. 2006; Tzingounis and Wadiche 2007). Inhibition of EAATs slows down transmitter clearance, leading to glutamate synaptic accumulation, AMPAR internalization and degradation and alterations in synaptic transmission (Danbolt 2001; Tzingounis and Wadiche 2007). To examine the role of synaptic glutamate in ATP status, we incubated neurons with TBOA, a non-transportable glutamate transporter inhibitor. Following 1 hr TBOA treatment (100 μM), the ATP assays revealed a dramatic reduction in ATP amount. Similar to the effect of glutamate application, the TBOA effect on ATP abundance was completely blocked by the glutamate receptor antagonist cocktail (TBOA 0.62 ± 0.05, n=3; TBOA plus AC 0.94 ± 0.01, n=3) (Figure 5). Interestingly, glutamate application in the presence of TBOA resulted in a drop in ATP to a level similar to that of glutamate alone or TBOA alone (Glutamate 0.50 ± 0.05, n=2; TBOA 0.62 ± 0.05, n=3; TBOA plus glutamate 0.57 ± 0.04, n=3) (Figure 5), indicating a saturation and/or shared processes on ATP regulation by synaptic vs. global glutamate stimulation.
Figure 5.
Effect of synaptic glutamate accumulation on ATP abundance. Incubation of cultured neurons with glutamate transporter inhibitor TBOA caused a marked reduction in ATP levels, which was blocked by receptor antagonist cocktail (AC). Co-application of TBOA and glutamate reduced ATP to a level similar to that by either glutamate or TBOA alone, suggesting a saturation by a single treatment, or a shared cellular process triggered by global and synaptic glutamate stimulation. *P<0.05, student’s t test.
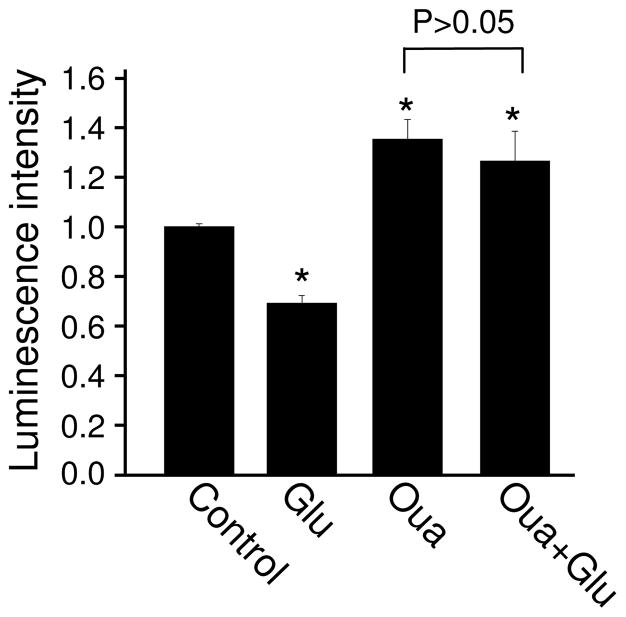
3.5. Sodium pump is involved in glutamate-induced ATP reduction
During glutamate application, the activity of glutamate receptors, transporters and voltage-gated sodium channels permit a large amount of sodium into neurons. The maintenance of ion gradients, which are necessary for the proper functioning of neurons, depends on the activity of the Na+/K+-ATPase (NKA), or sodium pump. For each cycle, NKA takes in 2 K+ and brings out 3 Na+, powered by the hydrolysis of ATP (Hernandez 1992; Schoner and Scheiner-Bobis 2007). Through intracellular sodium, sodium pumps may be involved in other physiological functions including synaptic transmission, and channel and receptor expression (Nanou et al. 2008; Zhang et al. 2009, Desfrere, 2009 #3681). Therefore, glutamate treatment should stimulate the NKA, leading to an enhanced consumption of ATP. To test this hypothesis, neurons were incubated with glutamate in the presence of NKA inhibitor ouabain (50 μM). Consistent with energy consumption under basal conditions, ouabain caused a modest, but significant increase in ATP amount (Figure 6). Importantly, in the presence of ouabain, glutamate incubation failed to induce a decrease in ATP abundance (Glutamate 1.0 ± 0.01; Glutamate 0.69 ± 0.03; Ouabain 1.35 ± 0.08; Ouabain + glutamate 1.26 ± 0.01; n=4) (Figure 6). This data indicates that NKA activity is essential in glutamate-dependent down-regulation of ATP homeostasis.
Figure 6.
The role of sodium pumps in glutamate regulation of ATP abundance. Blocking of sodium pump by ouabain (Oua) caused a marked increase in ATP amount. In the presence of ouabain, glutamate failed to induce a reduction in ATP amount. *P<0.05, student’s t test.
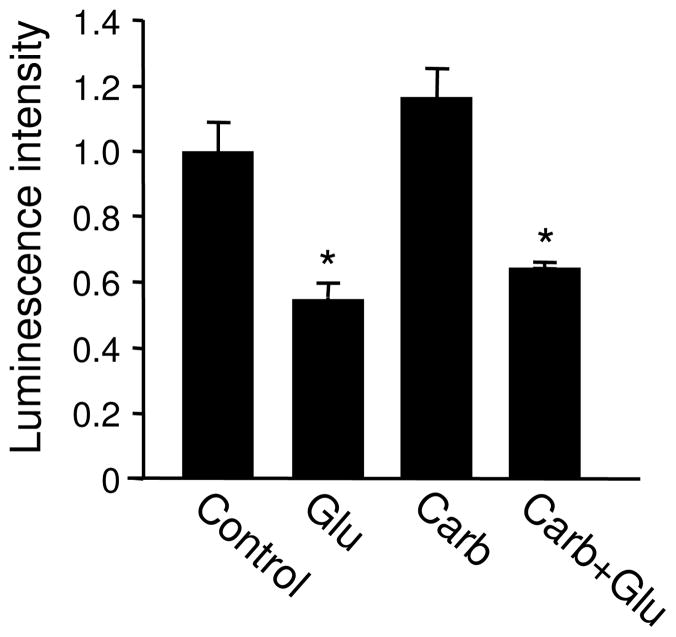
3.6. Glutamate incubation does not cause ATP leaking via gap junction
Gap junctions are molecular tunnels that directly connect neighboring cells, through which ions and small molecules with a molecular weight below 1000 Da are allowed to be exchanged (Zoidl et al. 2008; Dobrowolski and Willecke 2009). Gap junctions exist in glia (Seifert and Steinhauser; Giaume and Venance 1998), and to a lesser extent, in neurons (Bennett and Zukin 2004; Connors and Long 2004; Hughes and Crunelli 2006). Studies have shown that cellular ATP can be released from glia via gap junctions (Garre et al.; Orellana et al.; Frenguelli et al. 2007; Blum et al. 2008; Gourine et al. 2010). It is thus possible that glutamate stimulation opens the gap junction channels as a consequence of membrane depolarization or signaling cascades, causing diffusion of intracellular ATP into the extracellular solution. To this end, we treated cultured neurons with the gap junction inhibitor carbenoxolene (100 μM) for 1 hr. With the blockade of the gap junctions, glutamate still caused a significant decrease in ATP amount (Glutamate 0.55 ± 0.04; Carb 1.17 ± 0.08; Glutamate+Carb 0.64 ± 0.01; n=3 each) (Figure 7). We then measured ATP levels in the medium of 2 wk-old cultured neurons under basal conditions and following glutamate treatment. The ATP assay revealed that in control culture medium, only background levels of signal were detected, and no difference was found following glutamate treatment (data not shown). These results indicate that gap junctions are not implicated in ATP reduction during glutamate incubation.
Figure 7.
Glutamate effect on ATP does not result from gap junction-mediated ATP release. In the presence of a gap junction blocker carbenoxolene (Carb), glutamate incubation reduced ATP to a level comparable to that by glutamate alone. *P<0.05, student’s t test.
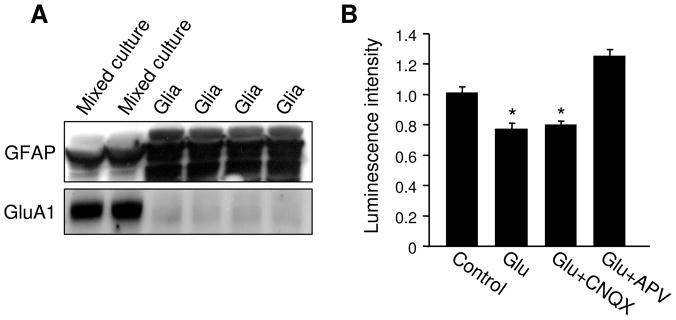
3.7. Glia are not the major target in glutamate-induced ATP regulation
The primary cell cultures used in this study contain both neurons and glia, in order to ensure normal maturation of neuronal connections (Christopherson et al. 2005). In the brain, glia play a critical role in energy metabolism, which accounts for approximately 30% of oxygen consumption in brain cortext (Dienel and Cruz 2004; Hertz et al. 2007; Hertz 2011). In this study, it is not clear whether the glutamate-induced ATP reduction occurs only in neurons or in both cell types. Given that glutamate transporters and glutamate receptors are expressed in glia (Zhou et al.; Conti et al. 1996; Verkhratsky and Steinhauser 2000; Ge et al. 2006; De Biase et al. 2011), glutamatergic stimulation could be detected by glia to regulate their energetic metabolism (Prebil et al.). To examine the possible involvement of glia in glutamate-dependent bioenergy regulation, we eliminated neurons from the culture by incubating the newly-plated cells with medium containing high serum, resulting in cultures of pure glia. Western blots showed that the regular mixed cultures of neuron and glia expressed low levels of the glial marker protein GFAP (glial fibrillary acidic protein) and high levels of AMPARs, indicating a dominant amount of neuronal cells. In contrast, glial cultures expressed much higher GFAP, but only a negligible amount of AMPARs (Figure 8A). This result indicated an efficient elimination of neurons from the culture. Using this glia culture, we found that glutamate stimulation remained able to cause a significant reduction in ATP (Glutamate 0.78 ± 0.03, n=3) (Figure 8B). However, compared to the effect on normal mixed culture (up to 50% ATP reduction), the effect on glia was markedly weaker (20% ATP reduction). Further, since glia growth was routinely inhibited with FDU in our regular neuronal culture, the relative proportion of total cellular ATP in glia was much lower than that of neurons. These results suggest that neurons, rather than glia, are the major target of the glutamate effect in terms of ATP regulation. Similar to neuronal culture, the glutamate effect was blocked by NMDAR antagonist APV (Glutamate+CNQX 0.80 ± 0.02, n=3; Glutamate+APV 1.26 ± 0.04, n=3), but not affected by AMPAR antagonist CNQX (Figure 8B), indicating that in glia, glutamate-caused ATP reduction is also mediated by NMDARs.
Figure 8.
Effect of glutamate on glial ATP. (A) Regular neuronal cultures (containing both neurons and glia) and glial cultures (neuron free) were lysed and analyzed by western blotting. Regular mixed neuronal cultures contained high levels of AMPAR subunit GluA1, but only a low level of the glial marker protein GFAP. In contrast, the glial cultures were enriched in GFAP, but only expressed minimal levels of glutamate receptors. (B) In glial cultures, glutamate induced a modest, but significant reduction in ATP amount. The change was blocked by NMDAR antagonist APV, but not AMPAR antagonist CNQX. *P<0.05, student’s t test.
4. Discussion
In this study, we found that neuronal bioenergy levels are controlled by glutamatergic activity. Application of glutamate induces a rapid and dramatic reduction in cellular ATP abundance. This regulation is independent of neuronal firing, and is not a result of gap junction-mediated ATP transmembrane diffusion. We find that glutamate regulates ATP in both neuronal and glial cells. Neurons produce and utilize most of the ATP in the brain (Magistretti et al. 1999; Pellerin and Magistretti 2003), but a more active role for glia in brain energetics has been increasingly appreciated (Hertz et al. 2007; Hertz 2011). In our study, since glial cells were maintained at a minimal level by routine application of the mitotic inhibitor 5-FDU in the medium, the observed ATP reduction by glutamate incubation seems mainly attributable to the neuronal component. In addition, the effect of glutamate on bioenergy homeostasis is not a non-specific side effect since glutamate treatment does not cause significant cell death, and incubation of HEK cells with the same concentration of glutamate does not change ATP levels.
There are three types of ionotropic glutamate receptors including AMPARs, NMDARs and KRs. Activation of these receptors will lead to membrane depolarization, firing of action potentials and thus elevated neuronal network activity, which are all energy consuming processes. Glutamate can also stimulate metabotropic mGluRs, triggering intracellular signaling pathways. The glutamate induced ATP reduction in neurons as well as in glia was blocked only by NMDAR antagonist APV, indicating that the glutamate effect is mediated solely by NMDARs.
In addition to receptors, glutamate also binds to its transporters for removal. To date, five EAATs (EAAT1–5) have been identified in glia and neurons. The glial transporters EAAT1–2 are primarily localized to the plasma membrane of specialized domains in astrocytic processes that wrap the synapse and are believed to be the major players in glutamate removal during synaptic transmission (Rothstein et al. 1994; Chaudhry et al. 1995). The distribution of neuronal transporters shows cell type specificity. EAAT3 is expressed in most neurons, including hippocampal and cortical neurons, whereas EAAT4 is mainly localized in cerebellar Purkinje cells and EAAT5 only in retinal ganglion neurons (Rothstein et al. 1994). Given spontaneous neuronal activation and synaptic activity, EAAT suppression leads to glutamate accumulation in the synaptic cleft. We found that blockade of EAATs results in a decrease in ATP amount, which can be completely blocked by the glutamate receptor antagonist cocktail, indicating that local glutamate stimulation at synaptic sites causes similar ATP regulation as global glutamate application. Since the transporter blockade can cause glutamate spillover and activation of parasynaptic NR2B-containing NMDARs, the TBOA effect could also result from the stimulation of non-synaptic NMDARs (Tzingounis and Wadiche 2007; Scimemi et al. 2009; Chalifoux and Carter 2011; Jarzylo and Man 2012). Also, TBOA and glutamate effects are not additive, because in the presence of EAAT inhibitors, glutamate application fails to induce further reduction in ATP, suggesting the utilization of share cellular processes.
The sodium pump is a major energy user in neurons. During neuronal activity, a large amount of sodium fluxes into the cell via multiple routes, but mainly through glutamate receptors and sodium channels. At steady states, the intracellular sodium concentration is about 10 mM. In hippocampal neurons one action potential can increase spine sodium to 35–40 mM, and a typical protocol for the induction of long-term potentiation (100 Hz stimulation for 1 s) increases sodium in the spine to more than 100 mM (Rose and Konnerth 2001). The frequent rises of cellular sodium must be efficiently rebalanced by the activity of the sodium pump, accompanied by a large amount of ATP consumption. We found that NKA suppression abolished glutamate-induced ATP reduction, supporting a hypothesis that glutamate-dependent sodium rises lead to elevated sodium pump activity and unbalanced ATP consumption. Sodium influxes mainly via ionotropic glutamate receptors and voltage-gated sodium channels. However, blockage of sodium channels by TTX had no effect on ATP reduction, indicating glutamate receptors, especially the NMDARs, are the primary source of intracellular sodium. Given that AMPARs contribute the most in synaptic currents, it seems a surprise that NMDARs, rather than AMPARs, are responsible for presumably sodium/sodium pump-dependent ATP reduction. Although NMDARs show high permeability to calcium and are often mistakenly considered a calcium channel, more than 80% of NMDA currents are actually carried also by sodium (Skeberdis et al. 2006). Further, due to its long-lasting time course, albeit with a lower amplitude compared to that of AMPAR, NMDA current can confer a large amount of sodium during synaptic activities. Alternatively, the requirement of NMDARs may be, at least partially, due to calcium-dependent regulatory events.
Glucose is the sole source for ATP production in neurons (Attwell and Laughlin 2001). Therefore, glutamate might alter neuronal energy balance through an inhibition of glucose uptake. It has been shown that glutamate treatment leads to an increase in glucose uptake in glia (Loaiza et al. 2003), but a decrease in neurons (Porras et al. 2004). However, the reduction of neuronal glucose uptake is AMPAR-dependent, whereas the glutamate-induced ATP reduction is independent of glutamate receptor activity, suggesting a negligible role for changes in the cellular supply of glucose.
Energy depletion has been implicated in glutamate-induced neurotoxicity (Baltan et al.; Nicholls and Budd 1998; Del Rio et al. 2007; Nicholls et al. 2007) and neurological disorders (Ferreira et al.; Mochel et al.; Blass et al. 1988; Wallace 1994; Mattson et al. 1999; Parihar and Brewer 2007; Amato and Man). Glutamate stimulation causes more severe cell death when cellular energy homeostasis is impaired (Del Rio et al. 2007). In the brain, massive exposure to glutamate often occurs during neural trauma and stroke. Under these circumstances, a drop in cellular energy supply is one of the first pathological events. A lack of sufficient ATP undermines a large number of energy-dependent cellular processes including kinase/enzymatic activity, proteasomal protein turnover, and the operation of the sodium pump, all leading to a collapse of cellular functional integrity and deterioration of cell conditions. In stroke, the decrease in energy level is believed to be the consequence of a ceased supply of oxygen and glucose; the role of glutamate in the control of bioenergy homeostasis has not been fully appreciated. Findings of current work suggest that a massive release of glutamate during the early stages of stroke is a crucial factor leading to energy disturbance. Therefore, early intervention in glutamate accumulation should be important in preventing a disruption in bioenergy homeostasis and subsequent cell death.
Highlights.
glutamate stimulation causes dramatic reduction in neuronal ATP
ATP reduction is due to both suppressed production and elevated consumption
glutamate-induced ATP reduction is mediated mainly by activation of NMDA receptors
Sodium pump activity is required for glutamate-dependent ATP reduction
Acknowledgments
We thank members of the Man lab for helpful discussions and comments on the manuscript. This work was supported in part by the US National Institutes of Health grant MH079407 (H.Y.M.) and a NARSAD Young Investigator Award (H.Y.M).
Footnotes
Conflict of interest
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato S, Man HY. Bioenergy sensing in the brain: The role of AMP-activated protein kinase in neuronal metabolism, development and neurological diseases. Cell Cycle. 2011;10:3452–3460. doi: 10.4161/cc.10.20.17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Baltan S, Murphy SP, Danilov CA, Bachleda A, Morrison RS. Histone deacetylase inhibitors preserve white matter structure and function during ischemia by conserving ATP and reducing excitotoxicity. J Neurosci. 31:3990–3999. doi: 10.1523/JNEUROSCI.5379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Blass JP, Sheu RK, Cedarbaum JM. Energy metabolism in disorders of the nervous system. Rev Neurol (Paris) 1988;144:543–563. [PubMed] [Google Scholar]
- Blum AE, Joseph SM, Przybylski RJ, Dubyak GR. Rho-family GTPases modulate Ca(2+) -dependent ATP release from astrocytes. Am J Physiol Cell Physiol. 2008;295:C231–241. doi: 10.1152/ajpcell.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. Glutamate spillover promotes the generation of NMDA spikes. J Neurosci. 2011;31:16435–16446. doi: 10.1523/JNEUROSCI.2777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Conti F, DeBiasi S, Minelli A, Melone M. Expression of NR1 and NR2A/B subunits of the NMDA receptor in cortical astrocytes. Glia. 1996;17:254–258. doi: 10.1002/(SICI)1098-1136(199607)17:3<254::AID-GLIA7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci. 2011;31:12650–12662. doi: 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio P, Montiel T, Chagoya V, Massieu L. Exacerbation of excitotoxic neuronal death induced during mitochondrial inhibition in vivo: relation to energy imbalance or ATP depletion? Neuroscience. 2007;146:1561–1570. doi: 10.1016/j.neuroscience.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int. 2004;45:321–351. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, Willecke K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid Redox Signal. 2009;11:283–295. doi: 10.1089/ars.2008.2128. [DOI] [PubMed] [Google Scholar]
- Ferreira IL, Resende R, Ferreiro E, Rego AC, Pereira CF. Multiple Defects in Energy Metabolism in Alzheimer’s Disease. Curr Drug Targets. doi: 10.2174/1389450111007011193. [DOI] [PubMed] [Google Scholar]
- Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem. 2007;101:1400–1413. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garre JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Saez JC, Bennett MV, Abudara V. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci U S A. 107:22659–22664. doi: 10.1073/pnas.1013793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, Duan S. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312:1533–1537. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Dehnes Y, Danbolt NC, Schousboe A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem Int. 2000;37:163–170. doi: 10.1016/s0197-0186(00)00019-x. [DOI] [PubMed] [Google Scholar]
- Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- Gladding CM, Fitzjohn SM, Molnar E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol Rev. 2009;61:395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RJ. Na+/K(+)-ATPase regulation by neurotransmitters. Neurochem Int. 1992;20:1–10. doi: 10.1016/0197-0186(92)90119-c. [DOI] [PubMed] [Google Scholar]
- Hertz L. Astrocytic energy metabolism and glutamate formation--relevance for 13C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking. Magn Reson Imaging. 2011;29:1319–1329. doi: 10.1016/j.mri.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hou Q, Gilbert J, Man HY. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron. 2011;72:806–818. doi: 10.1016/j.neuron.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Hardwiring goes soft: long-term modulation of electrical synapses in the mammalian brain. Cellscience. 2006;2:1–9. [PMC free article] [PubMed] [Google Scholar]
- Jarzylo LA, Man HY. Parasynaptic NMDA receptor signaling couples neuronal glutamate transporter function to AMPA receptor synaptic distribution and stability. J Neurosci. 2012;32:2552–2563. doi: 10.1523/JNEUROSCI.3237-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet R, Magistretti PJ, Weber B. Deciphering neuron-glia compartmentalization in cortical energy metabolism. Front Neuroenergetics. 2009;1:4. doi: 10.3389/neuro.14.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Lin A, Hou Q, Jarzylo L, Amato S, Gilbert J, Shang F, Man HY. Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Metabolic coupling during activation. A cellular view. Adv Exp Med Biol. 1997;413:161–166. doi: 10.1007/978-1-4899-0056-2_18. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Man HY, Ju W, Ahmadian G, Wang YT. Intracellular trafficking of AMPA receptors in synaptic plasticity. Cell Mol Life Sci. 2000;57:1526–1534. doi: 10.1007/PL00000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann N Y Acad Sci. 1999;893:154–175. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- Mochel F, Durant B, Meng X, O’Callaghan J, Yu H, Brouillet E, Wheeler VC, Humbert S, Schiffmann R, Durr A. Early alterations of brain cellular energy homeostasis in Huntington disease models. J Biol Chem. doi: 10.1074/jbc.M111.309849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanou E, Kyriakatos A, Bhattacharjee A, Kaczmarek LK, Paratcha G, El Manira A. Na+-mediated coupling between AMPA receptors and KNa channels shapes synaptic transmission. Proc Natl Acad Sci U S A. 2008;105:20941–20946. doi: 10.1073/pnas.0806403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal glutamate excitotoxicity. Biochim Biophys Acta. 1998;1366:97–112. doi: 10.1016/s0005-2728(98)00123-6. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Johnson-Cadwell L, Vesce S, Jekabsons M, Yadava N. Bioenergetics of mitochondria in cultured neurons and their role in glutamate excitotoxicity. J Neurosci Res. 2007;85:3206–3212. doi: 10.1002/jnr.21290. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S. The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? J Neurochem. 2006;98:1007–1018. doi: 10.1111/j.1471-4159.2006.03978.x. [DOI] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Figueroa XF, Sanchez HA, Contreras-Duarte S, Velarde V, Saez JC. Hemichannels in the neurovascular unit and white matter under normal and inflamed conditions. CNS Neurol Disord Drug Targets. 10:404–414. doi: 10.2174/187152711794653869. [DOI] [PubMed] [Google Scholar]
- Parihar MS, Brewer GJ. Mitoenergetic failure in Alzheimer disease. Am J Physiol Cell Physiol. 2007;292:C8–23. doi: 10.1152/ajpcell.00232.2006. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. How to balance the brain energy budget while spending glucose differently. J Physiol. 2003;546:325. doi: 10.1113/jphysiol.2002.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras OH, Loaiza A, Barros LF. Glutamate mediates acute glucose transport inhibition in hippocampal neurons. J Neurosci. 2004;24:9669–9673. doi: 10.1523/JNEUROSCI.1882-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prebil M, Jensen J, Zorec R, Kreft M. Astrocytes and energy metabolism. Arch Physiol Biochem. 117:64–69. doi: 10.3109/13813455.2010.539616. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA. Appraising the brain’s energy budget. Proc Natl Acad Sci U S A. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J, Oz G, Seaquist ER. Regulation of cerebral glucose metabolism. Minerva Endocrinol. 2006;31:149–158. [PubMed] [Google Scholar]
- Rose CR, Konnerth A. NMDA receptor-mediated Na+ signals in spines and dendrites. J Neurosci. 2001;21:4207–4214. doi: 10.1523/JNEUROSCI.21-12-04207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7:173–189. doi: 10.2165/00129784-200707030-00004. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Tian H, Diamond JS. Neuronal transporters regulate glutamate clearance, NMDA receptor activation, and synaptic plasticity in the hippocampus. J Neurosci. 2009;29:14581–14595. doi: 10.1523/JNEUROSCI.4845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Steinhauser C. Neuron-astrocyte signaling and epilepsy. Exp Neurol. doi: 10.1016/j.expneurol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OA, Shulman GI, Shulman RG, Rothman DL. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA mutations in diseases of energy metabolism. J Bioenerg Biomembr. 1994;26:241–250. doi: 10.1007/BF00763096. [DOI] [PubMed] [Google Scholar]
- Young-Collier KJ, McArdle M, Bennett JP. The Dying of the Light: Mitochondrial Failure in Alzheimer’s Disease. J Alzheimers Dis. doi: 10.3233/JAD-2011-111487. [DOI] [PubMed] [Google Scholar]
- Zhang D, Hou Q, Wang M, Lin A, Jarzylo L, Navis A, Raissi A, Liu F, Man HY. Na,K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J Neurosci. 2009;29:4498–4511. doi: 10.1523/JNEUROSCI.6094-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Li HL, Zhao R, Yang LT, Dong Y, Yue X, Ma YY, Wang Z, Chen J, Cui CL, Yu AC. Astrocytes express N-methyl-D-aspartate receptor subunits in development, ischemia and post-ischemia. Neurochem Res. 35:2124–2134. doi: 10.1007/s11064-010-0325-x. [DOI] [PubMed] [Google Scholar]
- Zoidl G, Kremer M, Zoidl C, Bunse S, Dermietzel R. Molecular diversity of connexin and pannexin genes in the retina of the zebrafish Danio rerio. Cell Commun Adhes. 2008;15:169–183. doi: 10.1080/15419060802014081. [DOI] [PubMed] [Google Scholar]