Abstract
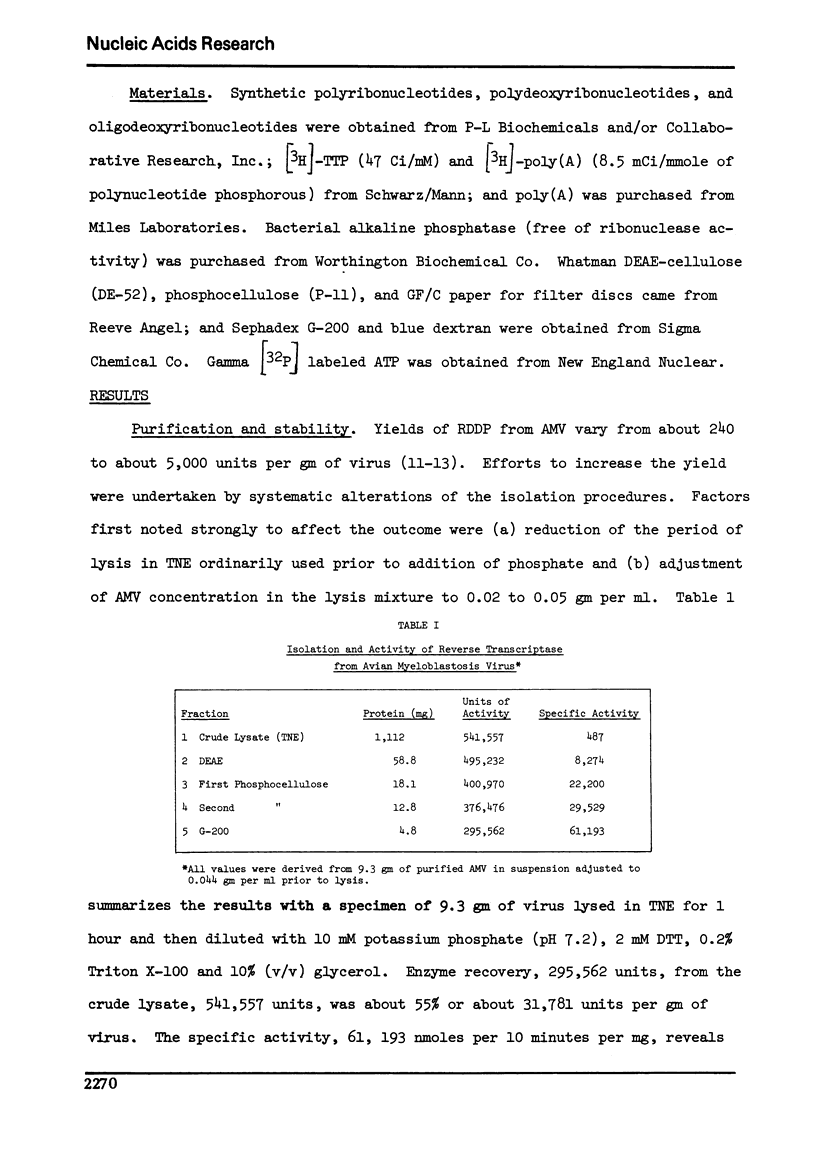
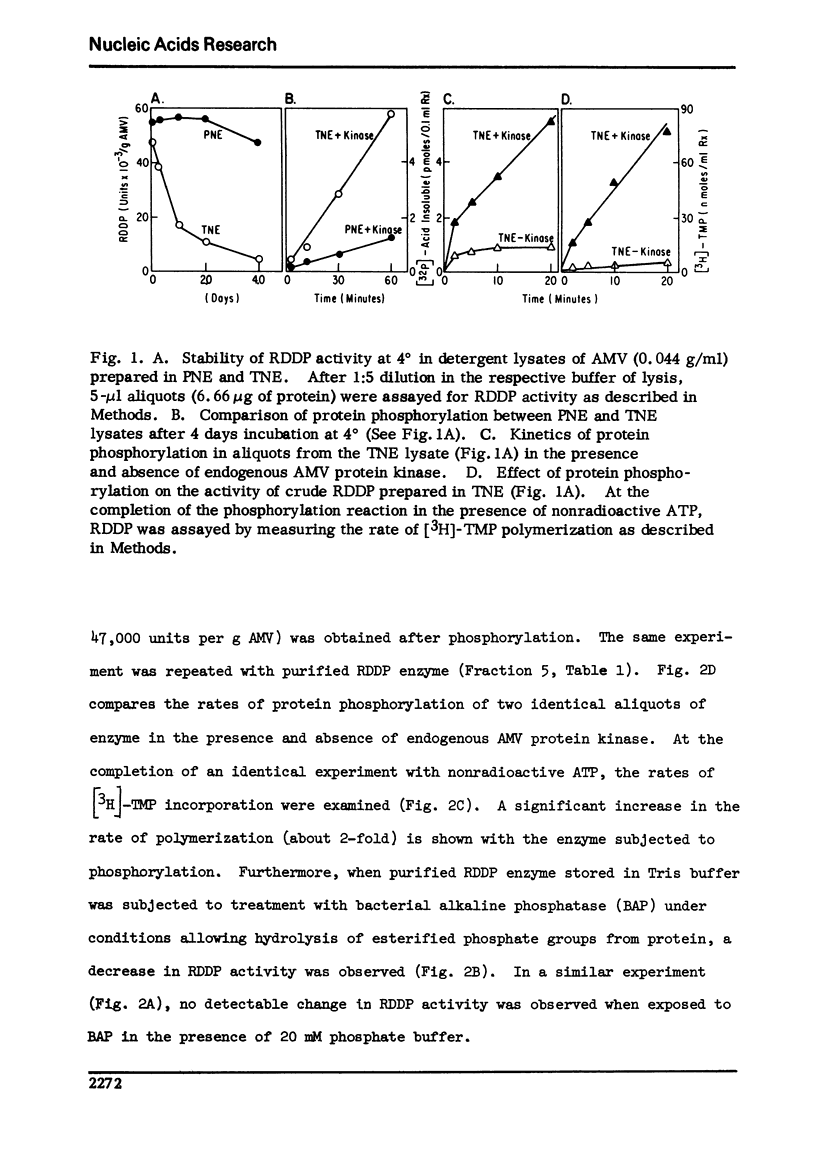
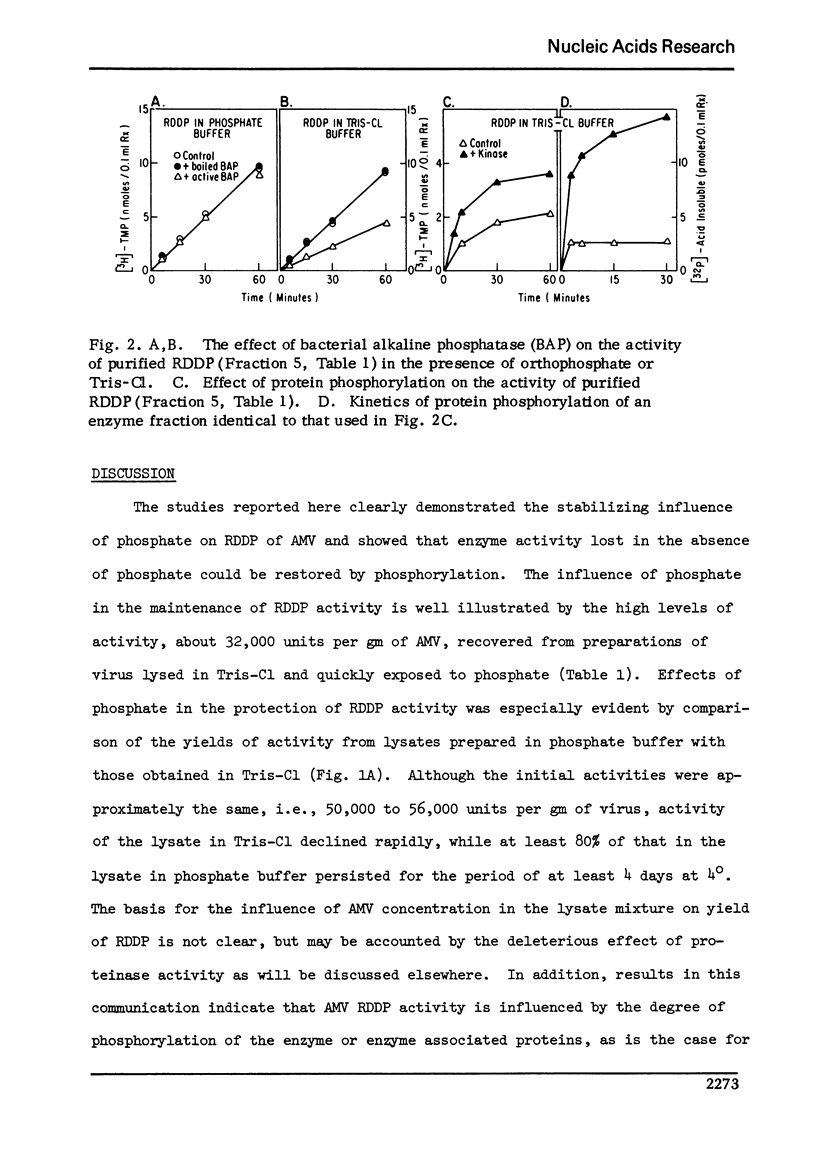
Activity of RNA-dependent DNA polymerase (RDDP) from avian myeloblastosis virus (AMV), either in purified form or in virus lysates, was increased by phosphorylation. Stability of RDDP in lysates buffered with phosphate was much greater (no loss of activity in 48 hours at 4 degrees) than that in lysates buffered with Tris-Cl (76% loss). Activity lost in the Tris-buffered extracts was completely restored by phosphorylation. The findings suggested that AMV RDDP activity is influenced by the degree of phosphorylation of the enzyme or enzyme-associated proteins and that this chemical modification is mediated by protein phosphokinase and phosphoprotein phosphatase present in crude extracts of purified AMV. Application of these results provided the basis of procedures whereby RDDP can be recovered in significantly higher yield and purity than formerly.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAUDREAU G. S., BECKER C. Virus of avian myeloblastosis. X. Photometric microdetermination of adenosinetriphosphatase activity. J Natl Cancer Inst. 1958 Feb;20(2):339–349. [PubMed] [Google Scholar]
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maxam A. M., Maniatis T. Enzymatic in vitro synthesis of globin genes. Cell. 1976 Feb;7(2):279–288. doi: 10.1016/0092-8674(76)90027-1. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Gerard G. F. RNA-directed DNA polymerase--properties and functions in oncogenic RNA viruses and cells. Prog Nucleic Acid Res Mol Biol. 1974;14(0):187–334. [PubMed] [Google Scholar]
- Hurwitz J., Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor viruses. I. Directing influence of DNA in the reaction. J Virol. 1972 Jan;9(1):116–129. doi: 10.1128/jvi.9.1.116-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Spiegelman S. Purification and detection of reverse transcriptase in viruses and cells. Methods Enzymol. 1974;29:150–173. doi: 10.1016/0076-6879(74)29018-9. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Krueger L. J., Weiss G. V., Merrick W. C., Lloyd M. A., Anderson W. F. Cycling of RNA-directed DNA polymerase on natural and synthetic RNA templates. Nature. 1976 Mar 25;260(5549):363–365. doi: 10.1038/260363a0. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Miceli M. V., Jungmann R. A., Hung P. P. Protein kinase and its regulatory effect on reverse transcriptase activity of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2945–2949. doi: 10.1073/pnas.72.8.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal H. L. Enzymatic interconversion of active and inactive forms of enzymes. Science. 1973 Apr 6;180(4081):25–32. doi: 10.1126/science.180.4081.25. [DOI] [PubMed] [Google Scholar]
- Silberstein H., August J. T. Phosphorylation of animal virus proteins by a virion protein kinase. J Virol. 1973 Sep;12(3):511–522. doi: 10.1128/jvi.12.3.511-522.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]


