Abstract
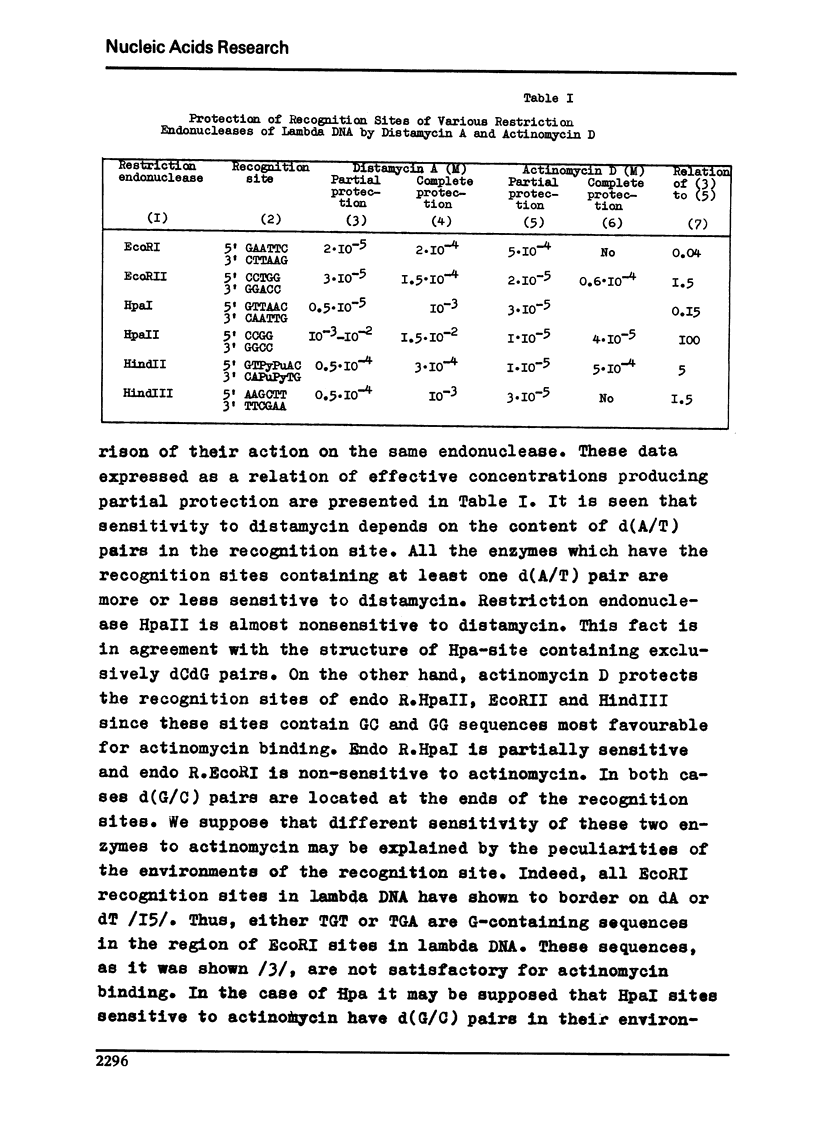
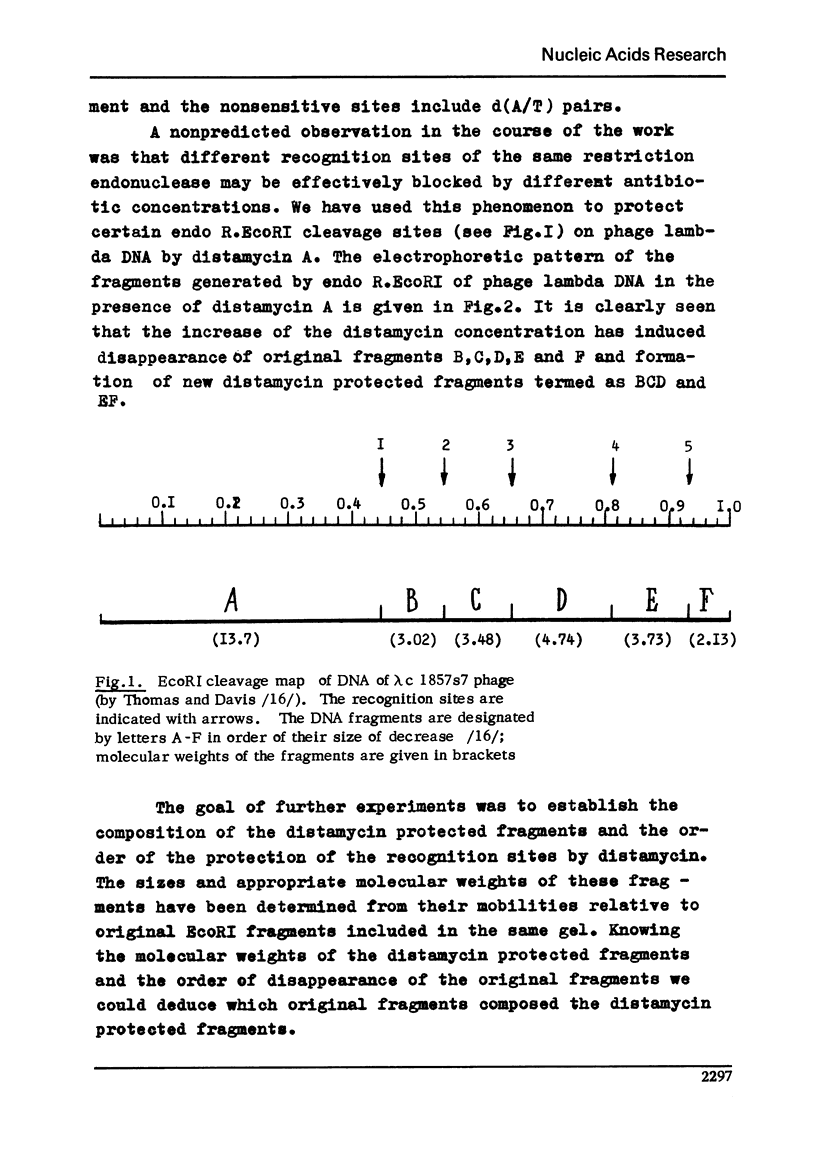
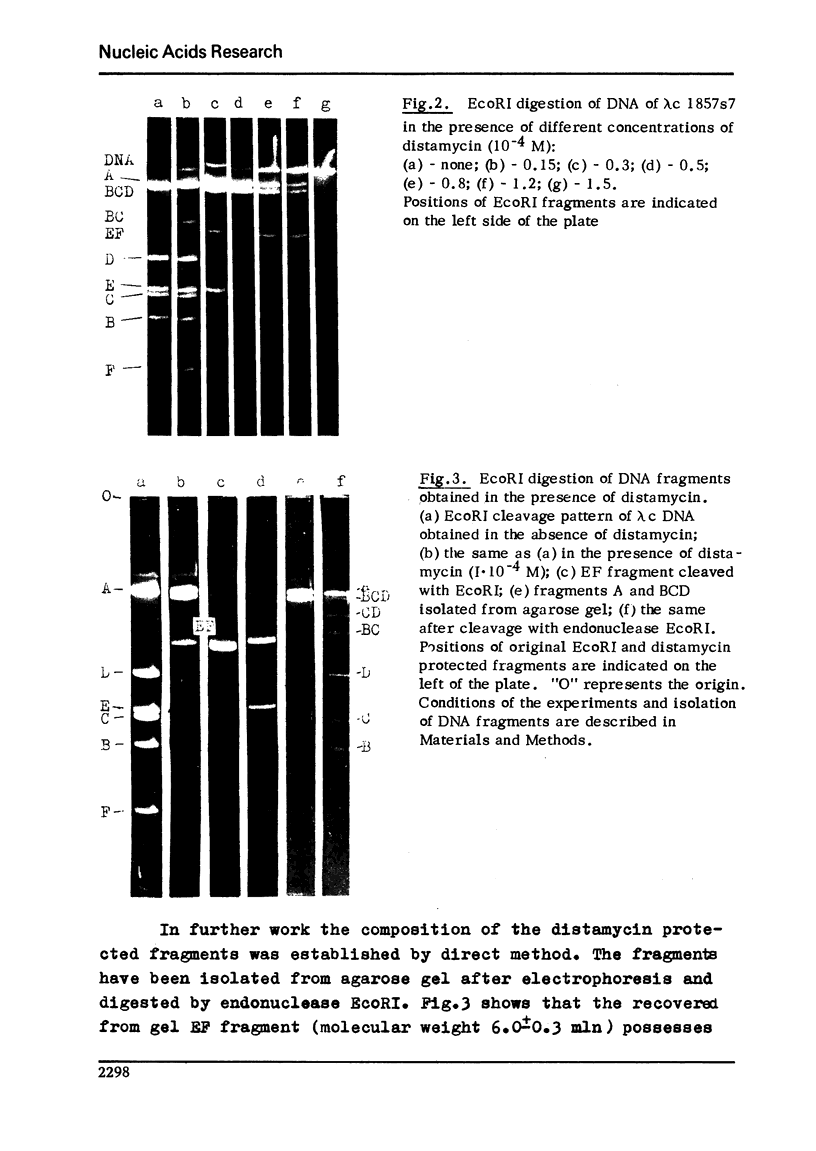
It is shown here that distamycin A and actinomycin D can protect the recognition sites of endo R.EcoRI, EcoRII, HindII, HindIII, HpaI and HpaII from the attack of these restriction endonucleases. At proper distamycin concentrations only two endo R.EcoRI sites of phage lambda DNA are available for the restriction enzyme--sRI1 and sRI4. This phenomenon results in the appearance of larger DNA fragments comprising several consecutive fragments of endo R.EcoRI complete cleavage. The distamycin fragments isolated from the agarose gels can be subsequently cleaved by endo R.EcoRI with the yield of the fragments of complete digestion. We have compared the effect of distamycin A and actinomycin D on a number of restriction endonucleases having different nucleotide sequences in the recognition sites and established that antibiotic action depends on the nucleotide sequences of the recognition sites and their closest environment
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampietro A. R., Vattuone de Sampietro M. A. Characterization of the agarolytic system of Agarbacterium pastinator. Biochim Biophys Acta. 1971 Jul 20;244(1):65–76. doi: 10.1016/0304-4165(71)90121-8. [DOI] [PubMed] [Google Scholar]
- Shaltiel S., Adler S. P., Purich D., Caban C., Senior P., Stadtman E. R. Omega-aminoalkyl agaroses in the resolution of enzymes involved in regulation of glutamine metabolism. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3397–3401. doi: 10.1073/pnas.72.9.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Reinert K. E., Luck G., Wähnert U., Löber G., Thrum H. Interaction of the oligopeptide antibiotics netropsin and distamycin A with nucleic acids. J Mol Biol. 1971 May 28;58(1):329–348. doi: 10.1016/0022-2836(71)90250-6. [DOI] [PubMed] [Google Scholar]