Abstract
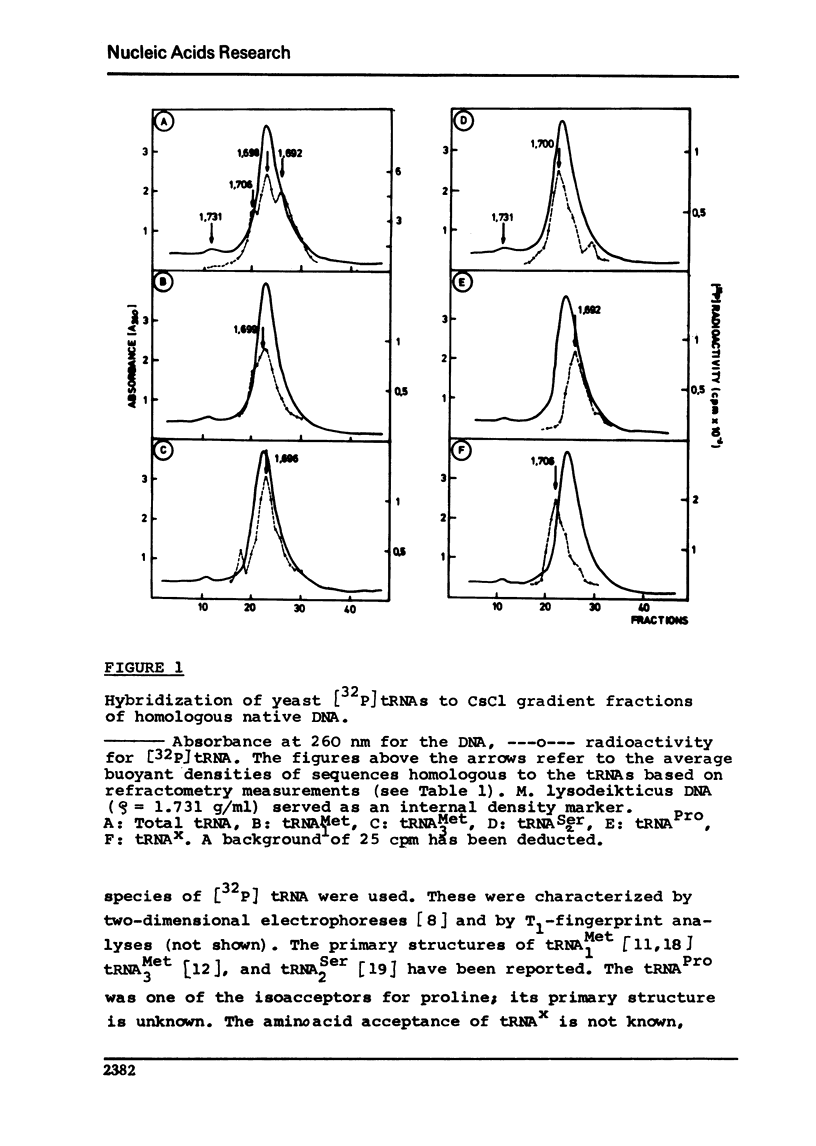
The redundancy and the arrangement of the genes for specific transfer ribonucleic acids in yeast were studied by the hybridization techniques developed by Birnstiel et al., e.g.[1]. The redundancy was found to be in the order of 10 genes for tRNA1Met, tRNA3Met, tRNA2Ser, and tRNA-Pro. High molecular weight yeast DNA was fractionated by density gradient centrifugation in cesium chloride and the [32p]tRNAs were hybridized to the single fractions. The results together with earlier findings [2] suggest that the cistrons for these tRNAs are arranged in tandem interspersed by 6 to 10 times longer segments of spacer DNA which varies in (G+C) content for the different tRNA species.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Blatt B., Feldmann H. Characterization of precursors to tRNA in yeast. FEBS Lett. 1973 Dec 1;37(2):129–133. doi: 10.1016/0014-5793(73)80441-7. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R., Hughes S. H., Wahl G. M. Yeast super-suppressors are altered tRNAs capable of translating a nonsense codon in vitro. Cell. 1975 Nov;6(3):269–277. doi: 10.1016/0092-8674(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Clarkson S. G., Birnstiel M. L., Purdom I. F. Clustering of transfer RNA genes of Xenopus laevis. J Mol Biol. 1973 Sep 15;79(2):411–429. doi: 10.1016/0022-2836(73)90014-4. [DOI] [PubMed] [Google Scholar]
- Fradin A., Gruhl H., Feldmann H. Mapping of yeast tRNAs by two-dimensional electrophoresis on polyacrylamide gels. FEBS Lett. 1975 Feb 1;50(2):185–189. doi: 10.1016/0014-5793(75)80485-6. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Wolfner M., Grisafi P., Fink G., Botstein D., Roth J. R. Yeast suppressors of UAA and UAG nonsense codons work efficiently in vitro via tRNA. Cell. 1976 Mar;7(3):381–390. doi: 10.1016/0092-8674(76)90167-7. [DOI] [PubMed] [Google Scholar]
- Gruhl H., Feldmann H. The primary structure of a non-initiating methionine specific tRNA from brewer's yeast. FEBS Lett. 1975 Sep 15;57(2):145–148. doi: 10.1016/0014-5793(75)80703-4. [DOI] [PubMed] [Google Scholar]
- Pirro G., Feldmann H. Characteristics of DNA fractionated on benzoylated DEAE-cellulose. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1693–1701. doi: 10.1515/bchm2.1975.356.2.1693. [DOI] [PubMed] [Google Scholar]
- Pirro G., Feldmann H. Purification of tDNA from yeast. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1703–1708. doi: 10.1515/bchm2.1975.356.2.1703. [DOI] [PubMed] [Google Scholar]
- RITOSSA F. M., SPIEGELMAN S. LOCALIZATION OF DNA COMPLEMENTARY TO RIBOSOMAL RNA IN THE NUCLEOLUS ORGANIZER REGION OF DROSOPHILA MELANOGASTER. Proc Natl Acad Sci U S A. 1965 Apr;53:737–745. doi: 10.1073/pnas.53.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Sulston J. E. Physical linkage of the 5 S cistrons to the 18 S and 28 S ribosomal RNA cistrons in Saccharomyces cerevisiae. J Mol Biol. 1973 Sep 25;79(3):521–530. doi: 10.1016/0022-2836(73)90403-8. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Schweizer E., MacKechnie C., Halvorson H. O. The redundancy of ribosomal and transfer RNA genes in Saccharomyces cerevisiae. J Mol Biol. 1969 Mar 14;40(2):261–277. doi: 10.1016/0022-2836(69)90474-4. [DOI] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L. The primary structure of yeast initiator transfer ribonucleic acid. Biochem Biophys Res Commun. 1972 Oct 17;49(2):508–515. doi: 10.1016/0006-291x(72)90440-8. [DOI] [PubMed] [Google Scholar]


