Abstract
Objective
An epileptic seizure is frequently the presenting sign of intracerebral hemorrhage (ICH) caused by stroke, head trauma, hypertension and a wide spectrum of disorders. However the cellular mechanisms responsible for occurrence of seizures during ICH have not been established. During intracerebral bleeding, blood constituents enter the neuronal tissue and produce both an acute and delayed effect on brain functioning. Among the blood components only thrombin has been shown to evoke seizures immediately after entering brain tissue. In the present study we tested the hypothesis that thrombin increase neuronal excitability in the immature brain through alteration of voltage-gated sodium channels.
Methods
The thrombin effect on neuronal excitability and voltage-gated sodium channels was assessed using extracellular and intracellular recording techniques in the hippocampal slice preparation of immature rats.
Results
We show that thrombin increased neuronal excitability in the immature hippocampus in an NMDA-independent manner. Application of thrombin did not alter transient voltage-gated sodium channels and action potential threshold. However thrombin significantly depolarized the membrane potential and produced a hyperpolarizing shift of TTX sensitive persistent voltage-gated sodium channel activation. This effect of thrombin was attenuated by application of protease-activated receptor-1 and protein kinase C antagonists.
Interpretation
Our data indicates that thrombin amplifies the persistent voltage-gated sodium current affecting resting membrane potential and seizure threshold at the network level. Our results provide a novel explanation as to how ICH in newborns results in seizures, which may provide avenues for therapeutic intervention in the prevention of post-ICH seizures.
INTRODUCTION
Intracerebral hemorrhage (ICH) is a significant cause of morbidity and mortality throughout the world. This condition is complicated by acute seizures which commonly accompany ICH in children as well as adult patients1–3. Newborns are at particularly high risk for ICH and seizures due to traumatic deliveries, stroke, subarachnoid and intraventricular hemorrhage and vitamin K deficiency4–6. Studies on premature infants show a highly significant correlation between intracranial hemorrhage and seizures4,7. Since seizures contribute to both mortality and morbidity, and patients with post-ICH seizures have worse outcomes than ICH patients without seizures2–4, understanding the cellular mechanisms responsible for the occurrence of seizures during ICH is important for developing proper medical treatment and management of ICH.
Following head and brain injury accompanied by ICH, concentrations of extracellular K+ and glutamate increase altering the overall excitability of neuronal environment in the vicinity of the injury locus8,9. Also it is generally acknowledged that the immediate seizures during intracerebral bleeding are associated with proepileptic effect of blood components. Among the possible blood components which can be involved in seizures generation after ICH (albumin, iron, thrombin) only thrombin has been shown to produce early-onset seizures10–12. Intracerebral infusion of thrombin into rat basal ganglia produces focal motor seizures in animals with behavioral seizures occurring immediately after recovery from isoflurane anesthesia11. However the mechanism by which thrombin leads to seizure generation is unclear.
Protease-activated receptors (PAR), which belong to the superfamily of seven transmembrane domain G protein-coupled receptors widely expressed in the brain, are activated by thrombin and upregulated during pathological conditions such as ischemia13,14. Activation of PAR by thrombin triggers a signal transduction cascade, resulting in the activation of phospholipase C and protein kinase C (PKC), as well as the inhibition of adenyl cyclase15. Thrombin potentiates the activity of N-methyl-D-aspartate (NMDA) receptors in hippocampal cells through PAR116. It has been proposed that epileptic activity induced by thrombin is secondary to its effect on NMDA receptors. However, while application of thrombin in vitro enhances the sensitivity of hippocampal tissue to epileptic seizures, this effect is not abated by the NMDA antagonists ifenprodil and (2R)-amino-5-phosphonopentanoate (D-APV)17, raising questions whether the seizure-enhancing effect of thrombin is solely explained by activation of NMDA receptors.
In the present study we tested the hypothesis that thrombin affects neuronal excitability of the immature brain through the alteration of voltage-gated sodium channels. We developed this hypothesis since there are several lines of evidence indicating that thrombin can affect neuronal voltage-gated sodium channels: i) application of thrombin produces a hyperpolarizing shift of activation of sodium channels in Chinese hamster ovary cells expressing the rat brain type IIA α subunit; ii) PKC activation increases excitability of neurons through modulation of voltage-gated sodium channels; iii) thrombin or PAR1 receptor agonists increase transient and persistent sodium currents in human cardiomyocytes18–21. However, there is no direct evidence that thrombin affects naïve neuronal voltage-gated sodium channels. Using a hippocampal slice preparation from immature rats we show that application of thrombin does not affect transient voltage-gated sodium current and AP characteristics. However, thrombin produces a significant hyperpolarizing shift in activation of the TTX-sensitive persistent voltage-gated sodium current thereby affecting membrane potential and seizure threshold at the network level.
METHODS
Sprague-Dawley rats (n=46) were used throughout the study and were treated in accordance with the guidelines set by the National Institute of Health and Dartmouth Medical School for the humane treatment of animals.
As thrombin acts through PAR, we used hippocampal slice preparations to eliminate enzymatic treatment. Rats at postnatal (P) days 6–7 were used to evaluate the effect of thrombin on transient voltage-gated sodium currents recorded in whole-cell configuration. All other experiments were done on P6-15 rats. Rats were anaesthetized with isoflurane and decapitated, brains were removed, and transversal hippocampal slices (500µm) were sectioned in a cold (4°C) oxygenated artificial cerebrospinal fluid (ACSF). All recordings were made from visually identified hippocampal CA3 pyramidal cells. To estimate effect of thrombin in voltage-clamp studies we used 10 U/ml of thrombin. Recordings were performed in outside-out and whole-cell configurations. In outside-out patches sodium current was elicited using the following protocol: holding potential −70 mV; 50 ms pulse to −130 mV; 30 ms test pulse to various test potentials; and step back to −70 mV. In whole-cell experiments sodium current was elicited during a 100 ms test pulse to various test potentials from a holding potential of −70 mV or −90 mV. Steady-state activation and inactivation were calculated as in our previous study22.
Membrane potential (Em) and AP threshold were recorded using current-clamp mode in whole-cell configuration. All recordings were performed in the presence of 10 µM 6,7-Dinitroquinoxaline-2,3-dione (DNQX), 50 µM D-APV and 10µM gabazine to block synaptic activity. Only cells with Em more negative than −60 mV were included into analysis. Thrombin effect (10 U/ml) on Em was estimated from Em held at −60 mV by steady current injection and determined as a mean value of Em obtained during 3–5 min recording under control conditions or in the presence of thrombin. The effect of thrombin was estimated five minutes after thrombin application. APs were evoked by injecting a depolarizing rectangular current pulses from Em held at −60 mV by steady current injection. Current strength was stepwise increased until spike failure occurred within the 100–300 ms duration pulse. Spike threshold was determined as in our previous study22. Extracellular recordings were performed from CA3 pyramidal cell layer using a differential amplifier (0.1 Hz-1 kHz; ×1000) and recordings were digitized (10 kHz) online.
The Shapiro-Wilk and Kolmogorov-Smirnov tests were used to estimate normality of the data for each group. Statistical analysis was performed using the two-tailed paired Student’s t-test when the data was assumed to be normally distributed. Otherwise statistical analysis of data was done using the non-parametric Wilcoxon matched-pairs signed rank test. All data in the text were expressed as the mean±SD. Errors for summarized voltage dependences of conductance and steady-state inactivation in figures were presented as mean±SE. The whiskers in the box graphs represented the 1st and 99th percentiles. The number of cells/slices was designated as “n”.
A more detailed description of the methods is provided in Supplementary Materials.
RESULTS
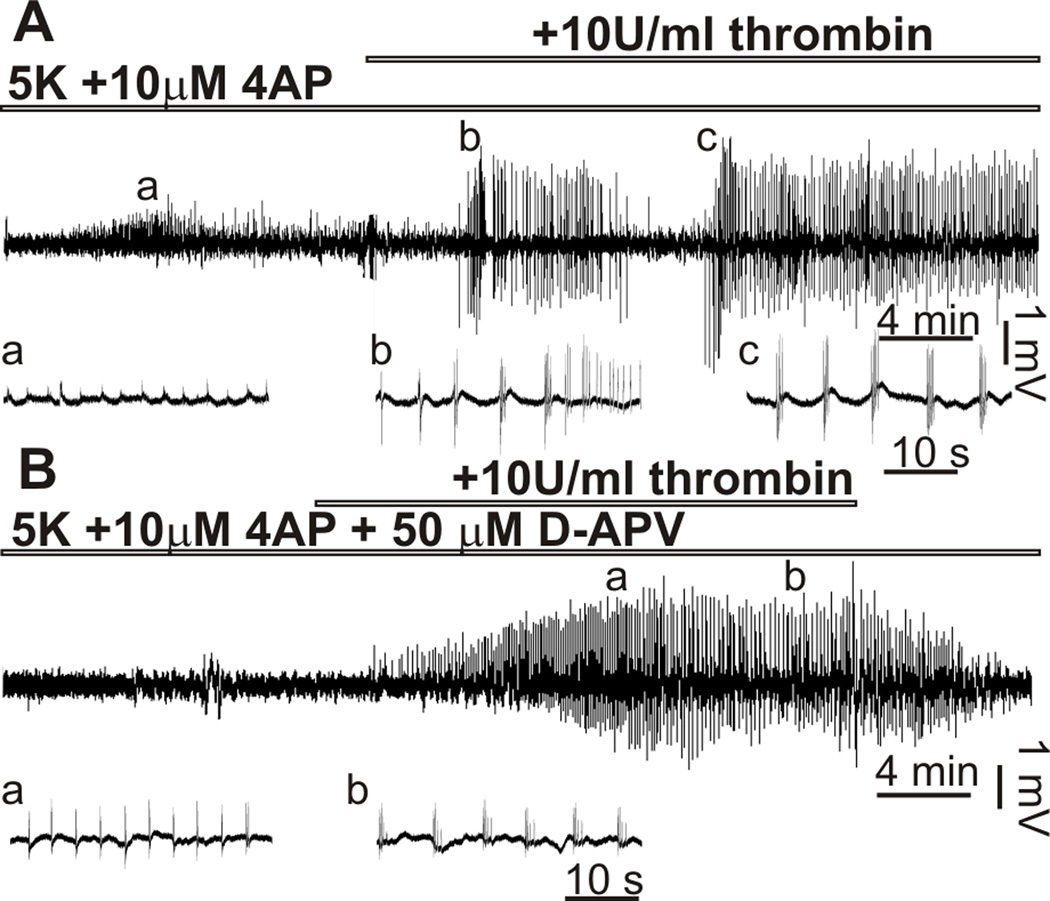
Application of thrombin increases spontaneous firing and facilitates the effects of proconvulsants in the hippocampal slice from adult rats17. Thus we initially determined if thrombin affects the immature neuronal network. In field potential recordings from CA3 hippocampal region of P6-15 rat we did not observe an increase in neuronal firing after application of 10 U/ml thrombin (n=8). In previous work on adult rats, Maggio and coauthors17 showed that thrombin evokes seizure-like activity (SLA) in the presence of 7.2 mM of K+ or 100 µM of glutamate in the extracellular solution. Increasing the concentration of K+ above 7 mM often evokes SLA in the hippocampus of young rats likely due to increased excitability of the immature brain23. Increasing extracellular concentration of K+ ([K+]o) to 5 mM and the addition of 10 µM 4-aminopyridine (4 AP) to the extracellular solution produced only a slight and transient (4.1±1.0 min) increase in synchronization (Fig 1A). Concentrations of K+ and 4AP used in our experiments were found in pilot studies. 10 U/ml thrombin was added after 25–30 min of superfusion with 5 mM [K+]o/10 µM 4AP ACSF. All of the slices superfused with 5 mM [K+]o/10 µM 4AP and 10 U/ml thrombin ACSF showed SLA (n=11). SLA was consistent during application of thrombin for 20–30 min and in 4 of 11 slices showed persistent epileptic activity even after thrombin washout. In slices preapplied with 50 µM D-APV and 5 mM [K+]o/10 µM 4AP ACSF we did not see pronounced synchronization, however the application of 10 U/ml thrombin evoked SLA in all tested slices (n=5) (Fig 1B). In 2 out of 5 slices SLA persisted after thrombin was removed from the perfusion solution. These observations were consistent at all ages (P6 to P15) studied with no apparent age-depended difference in SLA probability, duration and seizure manifestation. In in vivo studies Lee and colleagues (1997) used a concentration of thrombin 100–400 U/ml to induce seizures11. Increasing the thrombin concentration to 50 U/ml did not increase neuronal firing or produce further facilitation of the effect of the proepileptics in our study (data not shown).
Figure 1.
Thrombin evoked seizure-like activity in the hippocampal slices of immature rat. Extracellular field potential recordings from CA3 pyramidal cell layer. (A) Only initial bursting activity was evoked in the presence of 5[K+]o/10 µM 4AP. Application of 10 U/ml thrombin evoked seizure-like activity in P9 hippocampal slice. (B) Addition of 50µM D-APV did not prevent seizure-like activity induced by thrombin. Spontaneous discharges marked with a,b,c shown for A and B in expanded scales below each graph.
We next evaluated the effect of thrombin on Em and characteristics of AP in CA3 pyramidal cells. In current-clamp studies application of 10 U/ml thrombin consistently produced a small but significant depolarizing shift of Em from −59.9±1.4 mV to −57.7±1.6 mV, t10=8.8, p<.001). This effect of thrombin was blocked by 1 µM TTX (−61.2±1.2 mV before and −61.0±1.5 mV after application of thrombin, n=6) and PAR1 antagonist SCH79797 (3–5 µM) (−59.5±1.4 mV before and −59.3±1.1 mV after application of thrombin; n=7), but was observed in the presence of blockers of synaptic transmission D-APV, DNQX and gabazine. Application of thrombin did not change AP threshold (−39.9±2.8 mV in control vs −39.2±3.0 mV in the presence of thrombin, n=10), amplitude (74.0±11.6 mV in control vs 74.5±10.4 mV in the presence of thrombin) or half-width (1.6±0.2 ms in control vs 1.7±0.2 ms in the presence of thrombin).
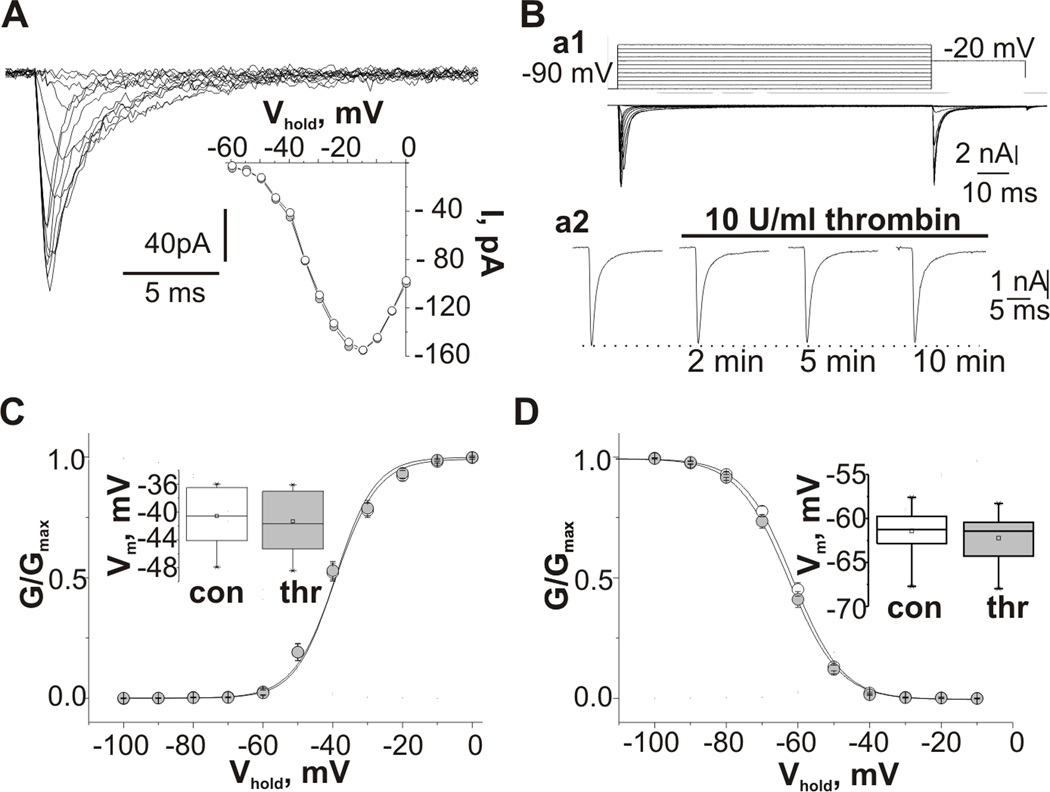
In voltage-clamp studies the effect of thrombin was first evaluated on TTX sensitive sodium transient currents (INat) recorded from outside out patches excised from CA3 pyramidal neurons of P6-15 rats. TTX sensitive INat was activated near −60 mV reaching its peak between −20 mV and −15 mV (Fig 2A). Application of 10 U/ml thrombin did not alter maximal INat amplitude (−76.9±35.6 pA vs −69.4±35.9 pA in the presence of thrombin, p=0.5, n=7). Midpoint potentials of the peak activation and inactivation curves were also unaffected by thrombin (activation: −35.8±1.7 mV vs −35.2±1.7 mV, inactivation: −68.4±1.4 mV vs −69.2±1.5 mV, n=7). As experiments in the outside out configuration can dissociate second messenger systems, we also performed experiments in the whole-cell configuration. Recordings in whole-cell configuration were performed from slices of P6-7 rats to reduce space-clamp errors22. INat was evoked by step depolarization (100 ms) from a holding potential of −90 mV (Fig 2Ba1). Figure 1Ba2 represents the effect of thrombin on INat amplitude. In 14 cells maximal INat amplitude was not changed in the presence of thrombin (−6.1±2.8 nA vs −5.9±2.7 nA, p=0.3). Thrombin did not change INat activation and inactivation (Fig 2C,D). In some experiments INat was evoked by step depolarization from the holding potential of −70 mV. In these conditions we also did not observe any significant effect of application of thrombin on the INat maximal amplitude (less than 5% decrease) and INat kinetic properties (n=6).
Figure 2.
Effect of thrombin on transient voltage-gated sodium currents. (A) Traces of INat recorded from a CA3 pyramidal cell outside out patch at different test pulse potentials. Insert shows current-voltage relationship of INat recorded from the same patch in control conditions (white) and in 10 min of the thrombin presence (grey); (B) Examples of whole-cell voltage-clamp recordings from CA3 pyramidal cell of P7 hippocampal slice. Ba1 upper panel pulse protocol: holding potential, −90 mV; 100 ms test pulse to membrane potentials between −90 and +20mV (10 mV increments); 30ms step to −20mV; and step back to holding potential. Lower panel traces of INat recorded using this protocol. Ba2 time course of effect of application of 10 U/ml thrombin on the maximal peak amplitude of INat. Recordings were made at depolarizing pulse to −20 mV. (C,D) Summarized voltage dependences of the normalized conductance (C) and steady-state inactivation (D) of INat recorded from CA3 pyramidal neurons in whole-cell configuration from control cells (white) and in the presence of thrombin (grey) fitted by a Boltzmann function. Inserts represent summarized box plots of mid-point potentials of the steady-state activation (C) and inactivation curves (D). Values are Mean±SE.
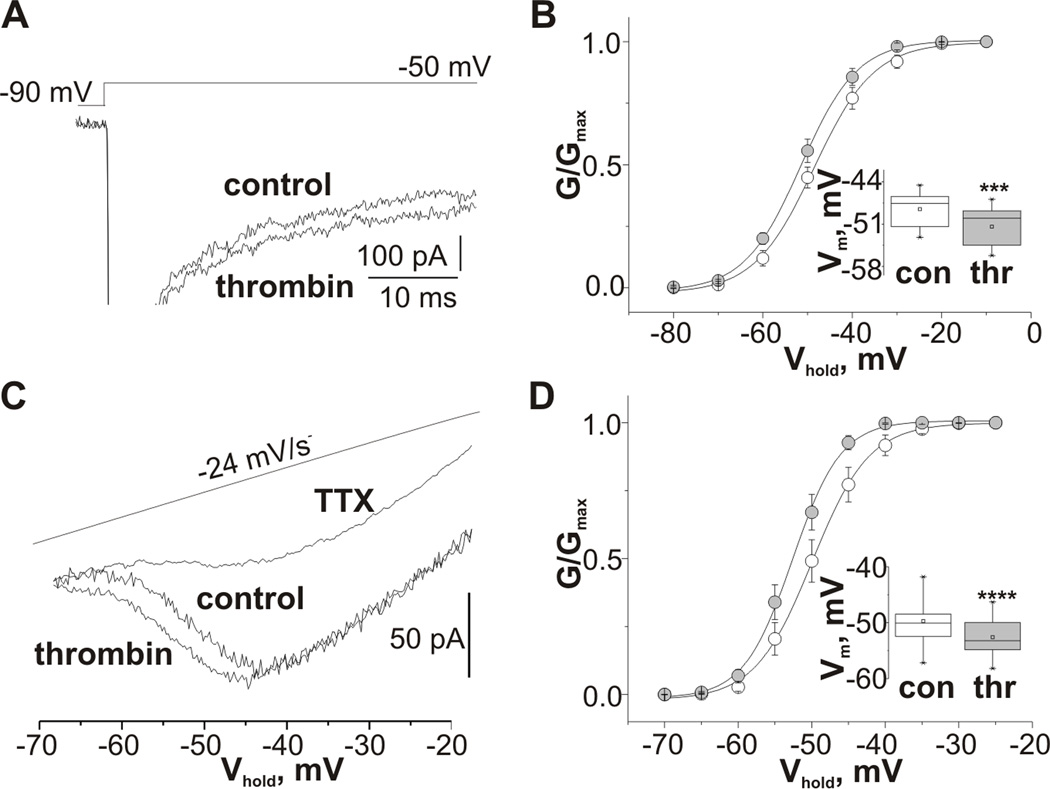
We next examined the effect of thrombin application on persistent sodium current (INap). Recordings of INap were performed using two different protocols. In nine cells INap was recorded in response to 100 ms depolarizing pulses from a holding potential of −90 mV. The INap amplitude was measured as the mean current calculated from 80 to 100 ms after the beginning of the depolarization to separate INap from INat 21. INap was activated near −70 mV reaching its peak at about −40 mV and completely abolished by application of 1 µM TTX. Thrombin did not change the maximal INap amplitude (130.2±59.3 pA in controls and 122.7±43.9 pA in the presence of thrombin, n=9), however thrombin increased INap amplitude recorded in response to more negative depolarization steps (Fig 3A). This effect can be attributed to a significant hyperpolarizing shift of steady-state activation of INap (Fig 3B). The voltage of half-maximal conductance of persistent sodium channels was shifted from −48.5±3.1 mV in controls to −51.4±3.6 mV in the presence of thrombin (p=0.02, t8=3.0). In twelve cells, INap was activated by depolarizing voltage ramps (−70 mV to 0 mV, 24 mV/s, Fig 3C). In agreement with previous reports24,25 INap activated at around −70 mV reaching its peak between −50 mV and −40 mV and at potentials more positive then −40 mV INap was superimposed with a large outward TTX-insensitive current. Thrombin did not change maximal INap amplitude (75.8±35.0 pA in controls and 76.6±22.2 pA in the presence of thrombin, n=12). However, INap activation curve shifted in the hyperpolarizing direction from −49.7±4.0 mV to −52.6±3.3 mV, t11=7.6, p<0.001) which produced an increase in INap amplitude at potentials more negative than −50 mV (Fig 2C,D). In the presence of TTX (1 µM) thrombin did not affect the remaining outward current (n=4). In addition, the effect of thrombin on INap was completely abolished in slices preincubated with specific PAR1 receptor antagonist SCH79797 (3 µM) (the potential for half-maximum conductance was −49.6±1.9 mV before and −50.2±2.5 mV after thrombin application, n=5). To verify that the effect of thrombin is mediated through PKC activation six slices were preincubated with H7 (10 µM), a blocker of PKC. Application of thrombin did not affect INap recorded from H7 pretreated slices (the potential for half-maximum conductance was −51.2±3.5 mV before and −51.6±3.5 mV after application of thrombin).
Figure 3.
Thrombin produces a hyperpolarizing shift of TTX-sensitive persistent sodium current activation. (A) Representative sodium current traces obtained under control conditions and in the presence of thrombin from CA3 pyramidal cell using the stimulus protocol shown in the upper panel. (B) Summarized voltage dependences of the normalized conductance of INap recorded using depolarizing step protocol from control cells (white) and in the presence of thrombin (grey) fitted by a Boltzmann function. (C) Representative sodium current traces obtained under control conditions, in the presence of thrombin or TTX from CA3 pyramidal cell using a slow ramp voltage protocol. (D) Summarized voltage dependences of the normalized conductance of INap recorded using a slow ramp voltage protocol from control cells (white) and in the presence of thrombin (grey) fitted by a Boltzmann function. Inserts represent summarized box plots of the mid-point potentials of the steady-state activation of INAp recorded using step (C) and ramp voltage protocols (D). Values are Mean ± SE.
Lidocaine in low concentrations has been shown to selectively block persistent sodium current26,27. In order to verify our hypothesis that thrombin decreases seizure threshold through facilitation of persistent sodium current, we preapplied 10 µM lidocaine with 5mM [K+]o/10 µM 4AP and 50 µM D-APV ACSF and tested the effect of thrombin on neuronal activity in field potential recordings from the CA3 hippocampal region (n=5). Adding 10 U/ml thrombin did not evoke either an increase of extracellular activity or SLA (data not shown).
DISCUSSION
In vivo and in vitro studies have demonstrated that thrombin entry into the brain is a contributing factor to seizures during ICH11,17, but the mechanism of the thrombin proepileptic action has not been established. Thrombin produces a long-lasting increase in spontaneous firing and lowered the threshold for generating epileptic seizures in hippocampal CA3 region in slice preparation from adult animals17. In our study we did not observe an increase in neuronal activity recorded from the same region of the hippocampus of P6-15 rats. However, thrombin markedly facilitated the effect of proconvulsants. To test the hypothesis whether thrombin provoked SLA through NMDA receptor activation, we blocked NMDA receptors by D-APV before thrombin application. We found that even in the absence of NMDA receptor signaling thrombin exerted a strong proepileptic influence on hippocampal neurons. Our data suggests that thrombin exerts a proepileptic effect on the immature hippocampus and similarly to investigations conducted on hippocampal slices from adult animals17, this effect cannot be explained solely by activation of NMDA receptors.
Application of thrombin results in a leftward shift of activation of TTX sensitive sodium current recorded in human cardiomyocytes and CHO cells transected with a cDNA encoding the a subunit of rat brain type IIA sodium channel18,21. Such effects on INat would affect the AP threshold and as a result increase neuronal firing and the ability of the network to synchronize28,29. In our voltage clamp experiments application of thrombin did not affect INat. As expected in current-clamp experiments with a fixed Em, we found no effect of thrombin on either threshold or other AP characteristics. To reduce space clamp errors in whole-cell configuration we performed voltage-clamp experiments on P6-7 animals. Current-clamp studies were performed on slices from P6-15 rats and no differences were found between recordings on cells from P6-7 and older rats in tested parameters. Our data indicate that increasing of neuronal activity by thrombin is not due to the effect of thrombin on the transient voltage-gated sodium channels. However, the depolarizing shift of Em caused by thrombin was not observed in the presence of TTX, suggesting that thrombin enhanced the noninactivating component of the TTX-sensitive sodium current.
Persistent TTX-sensitive voltage-gated sodium current is present in many cortical structures24,25,30. INap activated near resting membrane potential can affect Em and its activation leads to increase of neuronal bursting activity31,32. INap lasting several hundreds of milliseconds plays an important role during prolonged depolarization and in sustaining recurrent firing31,33. INap has been shown to be important in initiation and maintenance of ictal epileptiform activity34. In the present study thrombin application produced a small but significant hyperpolarizing shift of INap activation. This effect of thrombin on INap was attenuated by application of PAR1 and PKC antagonists, indicating that thrombin modulates INap through activation of PKC. PKC-mediated modulation of transient and persistent sodium channels has been demonstrated in different neuronal regions19,20,35,36. PKC dependent increase in neuronal excitability due to PKC modulation of sodium channels has also been shown in neocortical neurons19,20. As INap is activated at subthreshold potentials, the shift of its activation increases the probability of neurons discharging due to membrane depolarization. Thus, a lower depolarizing step is required to evoke AP. Our experiments with application of 10 µM lidocaine before thrombin perfusion suggest that thrombin decreases seizure threshold through a shift in INap activation.
In summary, the present study supports the idea that thrombin can increase seizure susceptibility of the immature hippocampus in an NMDA-independent manner17. We also show that in immature hippocampus thrombin increase the excitability of neuronal network through modulation of TTX-sensitive persistent sodium channels. This effect of thrombin at least partly explains the mechanism of development of epileptic activity following ICH in the immature brain. While this study was performed on immature animals, the similarity in the effect of thrombin in extracellular studies allows us to presume that thrombin can exert a similar action on INat and INap in adult preparations17. However future studies should evaluate the age-dependence of excitatory effect of thrombin.
Finally, the implications of our finding go beyond ICH-related seizures. Thrombin, its precursor and its receptors are widely distributed in the brain and play an important role in various neuronal processes such as response to injury, apoptosis, cell growth and regeneration37–39. Thrombin also serves as an endogenous mediator of neuroprotection40. Thus thrombin may be a double-edged sword, protecting the brain at low concentration but causing injury, including seizures at high concentrations. Our findings suggest that thrombin, through its effect on neuronal activity via alteration of the sodium channel properties; can have a critical role in both normal development of the brain and in seizures following ICH.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants (NS044295, NS073083) and State Foundation of Fundamental Research of Ukraine F46.2/001.
REFERENCES
- 1.Volpe JJ. Neurology of the newborn. 5th ed. Philadelphia: Saunders; 2008. [Google Scholar]
- 2.Dennis MS. Outcome after brain haemorrhage. Cerebrovasc Dis. 2003;16(Suppl 1):9–13. doi: 10.1159/000069935. [DOI] [PubMed] [Google Scholar]
- 3.Valeras P. Seizures in critical care: A guide to diagnosis and therapeutics (current clinical neurology) 2nd ed. New York: Humana Press; 2010. [Google Scholar]
- 4.Ohlweiler L, da Silva AR, Barros SV, et al. Influence of intracranial hemorrhage and neonatal seizures on the neurological and psychomotor development of premature infants at hospital de clinicas de porto alegre, brazil. Arq Neuropsiquiatr. 2003;61(4):902–905. doi: 10.1590/s0004-282x2003000600002. [DOI] [PubMed] [Google Scholar]
- 5.Zidan AS, Abdel-Hady H. Surgical evacuation of neonatal intracranial hemorrhage due to vitamin K deficiency bleeding. J Neurosurg Pediatr. 2011;7(3):295–299. doi: 10.3171/2010.12.PEDS10473. [DOI] [PubMed] [Google Scholar]
- 6.Silverstein FS, Jensen FE. Neonatal seizures. Ann Neurol. 2007;62(2):112–120. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- 7.Chadehumbe MA, Khatri P, Khoury JC, et al. Seizures are common in the acute setting of childhood stroke: A population-based study. J Child Neurol. 2009;24(1):9–12. doi: 10.1177/0883073808320756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinert M, Khaldi A, Zauner A, Doppenberg E, et al. High extracellular potassium and its correlates after severe head injury: relationship to high intracranial pressure. Neurosurg Focus. 2000;8(1):e10. doi: 10.3171/foc.2000.8.1.2027. [DOI] [PubMed] [Google Scholar]
- 9.Zauner A, Bullock R, Kuta AJ, Woodward J, et al. Glutamate release and cerebral blood flow after severe human head injury. Acta Neurochir Suppl. 1996;67:40–44. doi: 10.1007/978-3-7091-6894-3_9. [DOI] [PubMed] [Google Scholar]
- 10.Willmore LJ, Sypert GW, Munson JB. Recurrent seizures induced by cortical iron injection: A model of posttraumatic epilepsy. Ann Neurol. 1978;4(4):329–336. doi: 10.1002/ana.410040408. [DOI] [PubMed] [Google Scholar]
- 11.Lee KR, Drury I, Vitarbo E, Hoff JT. Seizures induced by intracerebral injection of thrombin: A model of intracerebral hemorrhage. J Neurosurg. 1997;87(1):73–78. doi: 10.3171/jns.1997.87.1.0073. [DOI] [PubMed] [Google Scholar]
- 12.Tomkins O, Friedman O, Ivens S, et al. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25(2):367–377. doi: 10.1016/j.nbd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Striggow F, Riek-Burchardt M, Kiesel A, et al. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14(4):595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in rat brain: Expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. J Neurosci. 1995;15(4):2906–2919. doi: 10.1523/JNEUROSCI.15-04-02906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung DT, Wong YH, Vu TK, Coughlin SR. The cloned platelet thrombin receptor couples to at least two distinct effectors to stimulate phosphoinositide hydrolysis and inhibit adenylyl cyclase. J Biol Chem. 1992;267(29):20831–20834. [PubMed] [Google Scholar]
- 16.Gingrich MB, Junge CE, Lyuboslavsky P, Traynelis SF. Potentiation of NMDA receptor function by the serine protease thrombin. J Neurosci. 2000;20(12):4582–4595. doi: 10.1523/JNEUROSCI.20-12-04582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggio N, Shavit E, Chapman J, Segal M. Thrombin induces long-term potentiation of reactivity to afferent stimulation and facilitates epileptic seizures in rat hippocampal slices: Toward understanding the functional consequences of cerebrovascular insults. J Neurosci. 2008;28(3):732–736. doi: 10.1523/JNEUROSCI.3665-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma JY, Li M, Catterall WA, Scheuer T. Modulation of brain na+ channels by a G-protein-coupled pathway. Proc Natl Acad Sci U S A. 1994;91(25):12351–12355. doi: 10.1073/pnas.91.25.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent na+ current in mouse neocortical slices. J Neurophysiol. 1998;80(3):1547–1551. doi: 10.1152/jn.1998.80.3.1547. [DOI] [PubMed] [Google Scholar]
- 20.Franceschetti S, Taverna S, Sancini G, et al. Protein kinase C-dependent modulation of na+ currents increases the excitability of rat neocortical pyramidal neurones. J Physiol. 2000;528(Pt 2):291–304. doi: 10.1111/j.1469-7793.2000.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinet C, Algalarrondo V, Sablayrolles S, et al. Protease-activated receptor-1 mediates thrombin-induced persistent sodium current in human cardiomyocytes. Mol Pharmacol. 2008;73(6):1622–1631. doi: 10.1124/mol.107.043182. [DOI] [PubMed] [Google Scholar]
- 22.Isaev D, Isaeva E, Shatskih T, Zhao Q, et al. Role of extracellular sialic acid in regulation of neuronal and network excitability in the rat hippocampus. J Neurosci. 2007;27(43):11587–11594. doi: 10.1523/JNEUROSCI.2033-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isaev D, Isaeva E, Khazipov R, Holmes GL. Shunting and hyperpolarizing GABAergic inhibition in the high-potassium model of ictogenesis in the developing rat hippocampus. Hippocampus. 2007;17(3):210–219. doi: 10.1002/hipo.20259. [DOI] [PubMed] [Google Scholar]
- 24.French CR, Sah P, Buckett KJ, Gage PW. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. J Gen Physiol. 1990;95(6):1139–1157. doi: 10.1085/jgp.95.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alzheimer C. A novel voltage-dependent cation current in rat neocortical neurones. J Physiol. 1994;479(Pt 2):199–205. doi: 10.1113/jphysiol.1994.sp020288. (Pt 2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammarstrom AK, Gage PW. Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J Physiol. 1998;510(Pt 3):735–741. doi: 10.1111/j.1469-7793.1998.735bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong H, Fan YH, Wang YY, Wang WT, et al. Lidocaine suppresses subthreshold oscillations by inhibiting persistent Na(+) current in injured dorsal root ganglion neurons. Physiol Res. 2008;57(4):639–645. doi: 10.33549/physiolres.931164. [DOI] [PubMed] [Google Scholar]
- 28.Spampanato J, Aradi I, Soltesz I, Goldin AL. Increased neuronal firing in computer simulations of sodium channel mutations that cause generalized epilepsy with febrile seizures plus. J Neurophysiol. 2004;91(5):2040–2050. doi: 10.1152/jn.00982.2003. [DOI] [PubMed] [Google Scholar]
- 29.Isaev D, Ivanchick G, Khmyz V, et al. Surface charge impact in low-magnesium model of seizure in rat hippocampus. J Neurophysiol. 2011 doi: 10.1152/jn.00574.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: Modulation by slow inactivation. Neuron. 2003;39(1):109–120. doi: 10.1016/s0896-6273(03)00360-x. [DOI] [PubMed] [Google Scholar]
- 31.Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- 32.Guatteo E, Franceschetti S, Bacci A, et al. A TTX-sensitive conductance underlying burst firing in isolated pyramidal neurons from rat neocortex. Brain Res. 1996;741(1–2):1–12. doi: 10.1016/s0006-8993(96)00866-9. [DOI] [PubMed] [Google Scholar]
- 33.Franceschetti S, Guatteo E, Panzica F, et al. Ionic mechanisms underlying burst firing in pyramidal neurons: Intracellular study in rat sensorimotor cortex. Brain Res. 1995;696(1–2):127–139. doi: 10.1016/0006-8993(95)00807-3. [DOI] [PubMed] [Google Scholar]
- 34.Segal MM, Douglas AF. Late sodium channel openings underlying epileptiform activity are preferentially diminished by the anticonvulsant phenytoin. J Neurophysiol. 1997;77(6):3021–3034. doi: 10.1152/jn.1997.77.6.3021. [DOI] [PubMed] [Google Scholar]
- 35.Colbert CM, Johnston D. Protein kinase C activation decreases activity-dependent attenuation of dendritic na+ current in hippocampal CA1 pyramidal neurons. J Neurophysiol. 1998;79(1):491–495. doi: 10.1152/jn.1998.79.1.491. [DOI] [PubMed] [Google Scholar]
- 36.Dascal N, Lotan I. Activation of protein kinase C alters voltage dependence of a na+ channel. Neuron. 1991;6(1):165–175. doi: 10.1016/0896-6273(91)90131-i. [DOI] [PubMed] [Google Scholar]
- 37.Rohatgi T, Sedehizade F, Reymann KG, Reiser G. Protease-activated receptors in neuronal development, neurodegeneration, and neuroprotection: Thrombin as signaling molecule in the brain. Neuroscientist. 2004;10(6):501–512. doi: 10.1177/1073858404269955. [DOI] [PubMed] [Google Scholar]
- 38.Niclou S, Suidan HS, Brown-Luedi M, Monard D. Expression of the thrombin receptor mRNA in rat brain. Cell Mol Biol. 1994;40(3):421–428. [PubMed] [Google Scholar]
- 39.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: Deleterious or protective? J Neurochem. 2003;84(1):3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 40.Turgeon VL, Salman N, Houenou LJ. Thrombin: a neuronal cell modulator. Thromb Res. 2000;99(5):417–412. doi: 10.1016/s0049-3848(00)00300-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.