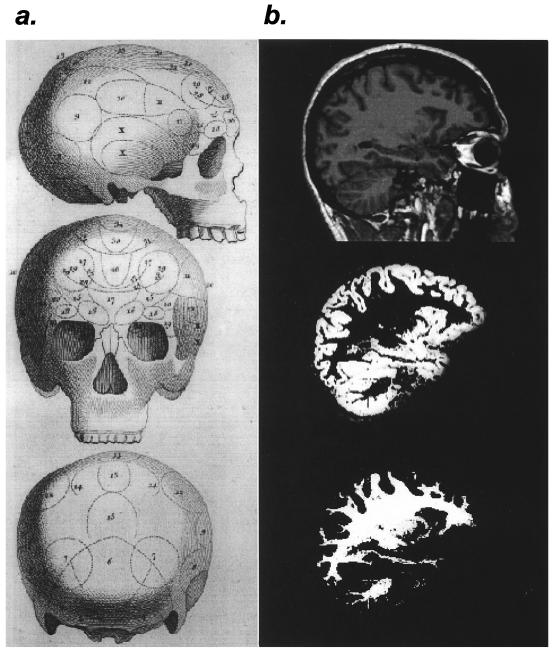
Does the human brain actually grow or shrink to reflect the cognitive demands of the environment? The paper by Maguire et al. (1) claims exactly this. Using structural magnetic resonance imaging and sophisticated image analysis techniques, the investigators demonstrated enlarged posterior hippocampal gray matter volume in London taxi drivers, a group required to undergo extensive navigational training to maintain their licenses. At first blush, it might seem that their approach is simply a high-tech reincarnation of the failed methods used by the phrenologists (Fig. 1) of the late 18th century. Although this chapter in the history of scientific thought often is ridiculed, the basic approach of correlating behavioral skills (or deficits) with the size of different brain areas still is very much used today. An extreme case is the recent attribution of Albert Einstein's genius to his relatively large parietal cortex (2).
Figure 1.
Structural studies of the brain, past and present. (a) Phrenologist's map from the end of the 18th century. Bumps on the skull were thought to reflect the size of the underlying brain. [Reproduced with permission from John van Whye, The History of Phrenology on the Web (http://www.jmvanwyhe.freeserve.co.uk), March 20, 2000. Originally published in The Philosophical Magazine (1802), Vol. 14.] (b) Example of voxel-based morphology as used in the Maguire et al. (1) study. From top to bottom, T1-weighted anatomical image in a sagittal plane containing the hippocampus, segmented gray matter from the same image, and segmented white matter from the same image. [Images courtesy of Timothy M. Ellmore, Laboratory of Brain and Cognition, National Institute of Mental Health, Bethesda, MD.]
Of course, what really separates us from our phrenologist predecessors is that today there exist technologically advanced tools with which to measure the gross morphology of the living human brain. Faster scanner hardware, higher field strengths, and ever more sophisticated software for analyzing brain images will continue to appear at a blistering pace. But what do these tools really buy us? Have we merely changed our focus from the bumps on the skull to the bumps in the skull?
For several important reasons, the Maguire et al. (1) study is more than simple neophrenology. First and foremost, the investigators were guided by a strong neuroanatomical hypothesis. Since the 1970s, O'Keefe and Nadel (3) have held that the essential function of the hippocampus is to form an internal map of the environment, subserving such cognitive functions as shortcut taking, detection of environmental novelty, and memory for events in their spatial contexts. The strong interpretation of O'Keefe and Nadel's proposal is hotly debated with the central issue being whether the hippocampus performs exclusively spatial computations or encompasses spatial and nonspatial functions (4, 5).
Maguire et al. work from a principled cross-species hypothesis as well. Experiments with food-storing birds have shown expansion and contraction of the hippocampus according to the seasonal need to store and retrieve seeds (6). Other studies have linked hippocampal volume to the evolutionary demands to navigate. For example, polygamous male voles exhibit both larger navigational territories and hippocampal volumes than do females of the same species (7). In contrast, monogamous voles, a species in which neither the male nor the female navigates large territories, do not exhibit the same sexual dimorphism for hippocampal volume. Lesion experiments with primates (8) and postmortem studies with humans (9) are further supportive of the idea that structural changes in hippocampal gray matter may be fairly common across species. It should be noted here, however, that although the structure of the hippocampus does appear to be fairly conserved among birds, rodents, nonhuman primates, and humans, it remains to be determined whether the computational function is conserved as well. Despite this caveat, the notion that the human hippocampus may also respond similarly to increased functional demands is at least plausible.
Given Maguire et al.'s (1) principled hypothesis, then, it is worthwhile to ask whether the currently available neuroimaging tools are sufficient to observe these subtle changes in cellular morphology. It is not clear whether this is the case. Neuroimaging data undergo an arduous journey before attaining their ultimate form. Among other things, the images are digitally resampled and transformed into a standardized three-dimensional brain space (a process known as spatial normalization) and later smoothed with a filter. Although perhaps necessary for interpretation, all of these steps come with a cost, namely loss of detail and anatomical specificity. One important step in the analysis of Maguire et al. (1) is brain tissue segmentation, the process of delineating distinct regions of gray matter, white matter, and cerebrospinal fluid. There are numerous technical challenges inherent to tissue segmentation, and at least two approaches are currently en vogue (10, 11). These methods differ with respect to statistical corrections, the demands on the user (no small issue), and computational efficiency, and they can produce quite different results.
Perhaps the greatest challenge in measuring learning-related structural changes with magnetic resonance imaging is that it is currently impossible to dissociate the specific contributions of neurons and glia to the observed gray matter volume. Although it is true that widespread dendritic sprouting (with a concomitant increase in supportive glia) would be reflected in increased gray matter volume, it is also true that pathological changes such as proliferation of microglia or swelling of neurons could increase gray matter volume as well.
Details of the imaging methodology aside, the Maguire et al. (1) study highlights how many of the same fundamental challenges faced by the phrenologists two centuries ago will continue to be challenges to new generations of scientists doing structure/function research. There are many reasons why London taxi drivers, above and beyond their well-honed navigational abilities, might not be reflective of the general population. Lifestyle differences, exposure to poor air, and high stress levels, for example, come to mind. These factors could well produce the same observed gray matter changes and therefore present a reasonable alternative explanation to the data. But an even more fundamental issue can be raised. The authors conclude that increased navigational experience alone is the reason for the increased posterior hippocampal gray matter observed in their sample of taxi drivers. However, no attempt was made to measure the hippocampi of other groups having similarly high demands placed on their nonspatial knowledge. What pattern of changes would be observable in law or medical students who encounter high memory and cognitive demands that are not fundamentally spatial in nature? The results, indeed, are generally consistent with O'Keefe and Nadel's (3) position, but they are also consistent with other theories of hippocampal function as well.
Despite these concerns, the Maguire et al. (1) study brings up new questions that await further experimentation. Do the hippocampi of retired cabbies revert to a more “normal” structural pattern over time? Do taxi drivers gain spatial knowledge at the expense of some other form of knowledge? Further, is the capacity of the hippocampus as a whole fixed, or can both the anterior and posterior hippocampi grow?
One rather surprising result from the Maguire et al. (1) study is that the posterior hippocampal gray matter volume is largest in drivers with greater than 20 years experience. Drivers with less experience had gray matter measurements that were substantially below the values predicted from the linear fit (figure 3b of ref. 1). We would argue that the most likely time to see learning-related changes in gray matter structure would be much earlier, during the intensive part of the training regimen when knowledge acquisition is greatest. Issues regarding the time scale of the changes serve to highlight one of the important, but largely underutilized, capabilities of magnetic resonance imaging, that is the ability to do repeated measurements of individual subjects over time. Although longitudinal studies have the distinct disadvantage of taking a long time to complete, they do control for the substantial individual variability in brain shape and allow for characterization of the time scale over which the changes occur. Much stronger evidence of learning-related changes in hippocampal gray matter would result if individual subjects were scanned before and after attainment of their spatial expertise.
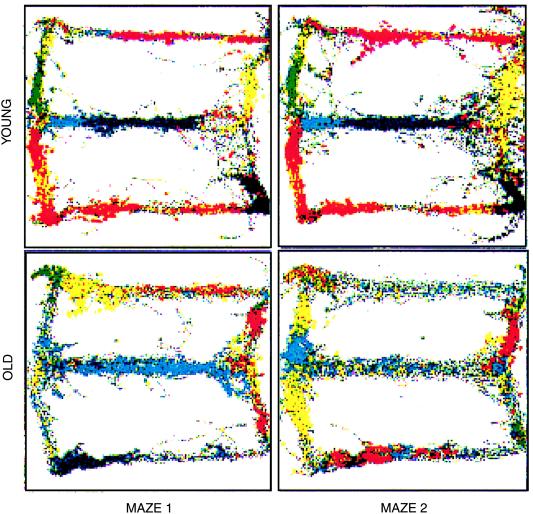
Developing a cross-species understanding of hippocampal function is a laudable goal and is likely to benefit research in numerous debilitating mental disorders such as schizophrenia, Alzheimer's disease, and posttraumatic stress disorder, in which the hippocampus is a central focus (12–14). A particularly good example of this potential is in the area of cognitive aging, where both behavioral and neurophysiological data seem to converge. Rats, like monkeys and humans (15–17), become impaired in spatial memory tasks as they age. The neural correlates of these deficits have been studied by Barnes et al. (18), who showed that the representation of space in the hippocampus of older rats does not achieve the same stability with repeated experience of an environment as it does in younger animals (Fig. 2). These and other data coupled with Maguire et al.'s (1) results would seem to open the door to futuristic behavioral therapies such as using virtual reality navigational games to increase the viability of the hippocampus in older people. Indeed, the oldest subject in the study also had the largest posterior gray matter volume score. Along these lines, it would be informative to know whether older cabbies show less evidence of the spatial memory deficits that so often accompany aging and, similarly, whether navigational training in older rats can reduce the propensity toward multistabile spatial representations.
Figure 2.
Multistability of place representation in old rats. Parallel recordings of single units in hippocampal area CA1 made on two consecutive experiences of a “Figure 8” maze (Maze 1, Maze 2; see figure 8 of ref. 18) for one old and one young rat. Between each recording session, the rats were removed from the room. Each color represents the spikes of a single unit recorded as the rat traversed the maze. The rat's movements through the maze are represented by the underlying gray traces. For the young rat, the spatial representation is highly consistent between visits. In contrast, the older rat exhibits an almost complete redistribution of the firing fields between the two visits. [Reproduced with permission from Barnes et al. (1997) Nature (London) 388, 272–275 (Copyright 1997, Macmillan Magazines Ltd).]
In many ways psychology continues to debate the same questions that it has for centuries. The question still remains, can we really determine cognitive function from the physical structure of the human brain?
Acknowledgments
We thank Lynn Nadel and Carol Barnes for insightful comments on this commentary. The research was supported by National Institute of Mental Health Grants MHO1565 and MH46823 (to B.L.M.) and National Institute on Aging Grant AG12609 (to Carol Barnes).
Footnotes
See companion article on page 4398 in issue 8 of volume 97.
References
- 1.Maguire E A, Gadian D G, Johnsrude I S, Good C D, Ashburner J, Frackowiak R S J, Frith C D. Proc Natl Acad Sci USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witelson S F, Kigar D L, Harvey T. Lancet. 1999;353:2149–2153. doi: 10.1016/S0140-6736(98)10327-6. [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 4.Squire L. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 5.Cohen N J, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 6.Clayton N S, Krebs J R. Proc Natl Acad Sci USA. 1994;91:7410–7414. doi: 10.1073/pnas.91.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs L F, Gaulin S J, Sherry D F, Hoffman G E. Proc Natl Acad Sci USA. 1990;87:6349–6352. doi: 10.1073/pnas.87.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonard B W, Amaral D G, Squire L R, Zola-Morgan S. J Neurosci. 1995;15:5637–5659. doi: 10.1523/JNEUROSCI.15-08-05637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson P S, Perfilieva E, Bjork-Eriksson T, Alborn A M, Nordborg C, Peterson D A, Gage F H. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 10.Wright I C, McGuire P K, Poline J B, Travere J M, Murray R M, Frith C D, Frackowiak R S, Friston K J. Neuroimage. 1995;2:244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- 11.Dale A M, Fischl B, Sereno M I. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 12.Weinberger D R. Biol Psychiatry. 1999;45:395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]
- 13.Deweer B, Lehericy S, Pillon B, Baulac M, Chiras J, Marsault C, Agid Y, Dubois B. J Neurol Neurosurg Psychiatry. 1995;58:590–597. doi: 10.1136/jnnp.58.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bremner J D. Biol Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 15.Barnes C A, Nadel L, Honig W K. Can J Psychol. 1980;34:29–39. doi: 10.1037/h0081022. [DOI] [PubMed] [Google Scholar]
- 16.Rapp P R, Kansky M T, Roberts J A. Neuroreport. 1997;8:1923–1928. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- 17.Uttl B, Graf P. Psychol Aging. 1993;8:257–273. doi: 10.1037//0882-7974.8.2.257. [DOI] [PubMed] [Google Scholar]
- 18.Barnes C A, Suster M S, Shen J, McNaughton B L. Nature (London) 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]