Abstract
Tumor-associated neoangiogenesis and suppression of antitumor immunity are hallmarks of tumor development and progression. Death receptor 6 (DR6) has been reported to be associated with suppression of antitumor immunity and tumor progression in several malignancies. However, expression of DR6 by malignant ovarian epithelial tumors at an early stage is unknown. The goals of this study were to determine whether DR6 is expressed by malignant ovarian epithelial tumors at an early stage and to examine whether DR6 expression is associated with ovarian cancer (OVCA) progression in a laying hen model of spontaneous OVCA. Expression of DR6 was examined in normal and malignant ovaries, normal ovarian surface epithelial (OSE) cells, or malignant epithelial cells and in serum of 3-year-old hens. The population of microvessels expressing DR6 was significantly higher in hens with early-stage OVCA than hens with normal ovaries (P < .01) and increased further in late-stage OVCA. The results of this study showed that, in addition to microvessels, tumor cells in the ovary also express DR6 with a significantly higher intensity than normal OSE cells. Similar patterns of DR6 expression were also observed by immunoblot analysis and gene expression studies. Furthermore, DR6 was also detected in the serum of hens. In conclusion, DR6 expression is associated with OVCA development and progression in laying hens. This study may be helpful to examine the feasibility of DR6 as a useful surrogate marker of OVCA, a target for antitumor immunotherapy and molecular imaging and thus provide a foundation for clinical studies.
Introduction
Ovarian cancer (OVCA) is the most lethal tumor among gynecologic malignancies, with an estimated yearly incidence rates of 22,000 in the United States and 42,000 in Europe [1,2]. In most cases, OVCA is detected at advanced stages, and despite the remarkable improvements in treatment strategies, most of these patients have recurrences. The reasons for failure to detect and treat OVCA at an early stage as well as its high rate of recurrences are the lack of an effective early detection test, suppression of antitumor immunity by the tumor, and resistance to drugs [3–5]. OVCA differs from other malignancies in its specific dissemination pattern, which is characterized by tumor spread in a diffuse intrapelvic and abdominal manner [5]. Thus, the local tumor microenvironment including tumor-associated neoangiogenesis and suppression of antitumor immunity play important roles in the development and progression of ovarian tumors. However, the way tumor establishes neoangiogenesis and escapes antitumor immune surveillance is not well understood. Information on factors related to the development of tumor-associated angiogenesis and immune suppression in the tumor microenvironment is important because it may offer opportunities to establish an early detection test as well as targeted antitumor therapy. Death receptor 6 (DR6) has been suggested to be one of such factors because of its expression by blood vessels and its involvement in immunoregulation [6,7].
DR6 is a member of the tumor necrosis factor α receptor super-family (TNFRSF21) [8,9]. Although DR6 has been shown to be involved in apoptotic cell death, elevated expression of DR6 has been observed in several tumors in humans [10]. DR6 expression was increased in tumor tissues from patients with late-stage prostate and breast cancers compared with its level in normal tissues [10]. Recently, DR6 concentration in the serum has been shown to be elevated in patients with late-stage OVCA [6]. In addition, DR6 has been demonstrated to be expressed by blood vessels in tumor tissues [11]. All these reports suggest that increased DR6 expression is associated with advanced stages of several malignancies including OVCA. However, its association with early-stage OVCA including tumor-associated angiogenesis is not known.
Suppression of antitumor immunity has been suggested as one of the mechanisms of tumor survival and progression [5]. Despite the presentation of antigens by malignant cells, which should induce immune-mediated rejection, spontaneous rejection of established tumor is rare [5]. Inefficient tumor rejection by the immune system is not only a passive result of insufficient effector cells [12,13] because tumors induce immune-suppressive mechanisms that protect them against eradication [3,4]. Compared with other solid tumors, studies on immunosuppression by ovarian tumors are very few. As in other epithelial malignancies, antitumor immune responses were reported to be elicited against ovarian tumors [14–16], but these responses were not effective enough to eliminate bulky tumor [5]. Moreover, the distinctive type of disease dissemination (peritoneal spread and metastasis) makes OVCA unique compared to other solid tumors. Although the precise mechanism(s) of inadequate or defective antitumor immune responses are not well understood, expression or secretion of immunosuppressive factors by the tumor has been suggested as a potential strategy for immune evasion. DR6 has been reported to alter normal differentiation of monocyte to immature dendritic cells rather than mature dendritic cells [17] and immature dendritic cells have been demonstrated to induce tolerance [18]. Furthermore, because of its inhibitory roles in T- and B-cell proliferation and migration, DR6 has been proposed to be immunosuppressive and may be involved in tumor cell survival and immune evasion [7]. However, expression of DR6 by ovarian tumors at early stages as well as inhibition of antitumor immunity by DR6 in OVCA patients is not known.
To develop and improve the efficacy of an antitumor immunotherapy, more insight into the interaction between ovarian cancer (OVCA) and the immune system is needed. Information on DR6 expression by ovarian tumors may lead to the identification of additional targets, which may allow opportunities for developing new therapeutic approaches to inhibit tumor progression. Studies on the immune response against tumors or immune suppression by ovarian tumors at early stages are lacking. Furthermore, if DR6 is expressed by ovarian tumor-associated neoangiogenic microvessels, it may be a useful target for the early detection of OVCA by Doppler ultrasound imaging. The difficulty in identifying patients with OVCA at an early stage and the limited access to tumor tissue are significant barriers to the study and to the development of an effective immunotherapy against OVCA. Laying hens are the only widely available and easily accessible animals that develop OVCA spontaneously with high incidence rates and remarkably similar histologic subtypes and tumor markers to human OVCA [19–21]. In addition, avian DR6 has been reported to be orthologous to human DR6 (70% homology) and is expressed by the hen ovaries [22]. Thus, the objective of the present study was to explore whether DR6 is expressed by ovarian tumors in hens and, if so, whether its expression changes in association with the stage of the tumors and histologic subtypes.
Materials and Methods
Animals
A flock of 3-year-old commercial strains of White Leghorn laying hens (Gallus domesticus) was maintained under standard poultry husbandry practices. Hens (n = 120) were selected based on their egg laying rates (normal or low) and transvaginal ultrasound scanning as reported previously [23]. The incidence of OVCA in hens of this age group is approximately 15% to 20% and is associated with low or complete cessation of egg laying [19,23]. All experimental procedures were performed according to the institutional animal care and use committee-approved protocol.
Tissue Collection and Processing
Serum samples. Blood was obtained from brachial veins of all hens before euthanasia and centrifuged (1000g for 20 minutes), and serum samples were stored at -80°C.
Ovarian morphology and histopathology. Ovarian pathology and tumor staging were performed by gross and histologic examination as reported previously [19]. Each normal and malignant ovary (tumor bearing ovary) was divided into four portions for protein extraction, total RNA collection, paraffin and frozen embedding for routine histology, and immunohistochemical studies as reported previously [24]. Normal ovarian surface epithelial (OSE) cells or tumor cells (malignant epithelial cells of the tumor) in hens with OVCA were collected as reported earlier [25,26]. Samples were divided into three groups including normal and early- and late-stage OVCA based on gross inspection and microscopy as previously reported [19].
Preparation of Ovarian Specimen for Biochemical Analysis
Snap-frozen ovarian tissues as well as normal OSE cells and tumor cells from hens with normal ovaries or hens with OVCA were homogenized with a Polytron homogenizer (Brinkman Instruments, Westbury, NY) as reported previously [27], were centrifuged; the supernatant was collected and the protein content of the extract was measured and stored at -80°C.
Immunohistochemistry
Rabbit polyclonal anti-chicken DR6 antibodies were used as primary antibodies, and immunoreactions were determined using Vectastain Elite ABC kit (Universal, RTU; Vector Laboratories, Inc, Burlingame, CA). Normal or malignant ovaries (n = 15 hens each for normal, early, and late stages) were selected randomly for immunohistochemical study. The number of hens for each group for immunohistochemistry was determined based on the power analysis to achieve significant differences in different parameters among the normal or malignant groups. Briefly, after deparaffinization, antigens on the sections were unmasked by heat treatment, endogenous peroxidase in the sections was inactivated, and nonspecific staining was blocked by incubating with 0.3% hydrogen peroxide in methanol and normal horse serum, respectively. Sections were then incubated for 2 hours with primary antibodies (1:100 dilution) followed by 1 hour of incubation with secondary antibodies (Vectastain Elite ABC kit; Vector Laboratories). Immune reaction products on the sections were visualized by incubating with diaminobenzidine and hydrogen peroxide mixture (DAB Peroxidase Substrate Kit, 3,3′-diaminobenzidine; Vector Laboratories). Sections were then counterstained with hematoxylin, dehydrated, and covered. Control staining was carried out simultaneously in which the first antibodies were omitted and normal serum was used. No staining was found in these control slides.
Sections were then examined under a light microscope attached to digital imaging software (MicroSuite version 5; Olympus Corporation, Tokyo, Japan). The population of microvessels expressing DR6 as well as the intensity of DR6 staining by the normal ovarian stroma or stroma (around the tumor) in malignant ovaries was determined. Three sections per ovary and five regions of interest with the highest immunoreactivity (20,000 µm2 per region at an objective of x40 and ocular magnification of x10) per section were selected. Using the software, the intensity of the DR6 immunostaining in each region was measured and recorded as pixel values in 20,000 µm2 of the section as reported previously [28]. The mean of pixel values of these five regions in a section was considered as the intensity of DR6 in a 20,000-µm2 area of each section. The mean intensity of three sections was considered as the DR6 staining intensity in a 20,000-µm2 area of each normal or malignant ovary. The groupwise DR6 staining intensity (normal or tumor groups) was expressed as mean ± SD in a 20,000-µm2 area of ovaries in normal or malignant groups. Similarly, and using the same software, the population of microvessels expressing DR6 in the section was counted and reported as the frequency (mean ± SD) of DR6-expressing microvessels in a 20,000-µm2 area of the stroma of normal or malignant ovaries as reported previously [29].
One-dimensional Western Blot
Ovarian expression of DR6 was confirmed by immunoblot analysis using homogenates of normal (n = 5) or malignant ovaries as well as normal OSE cells or tumor cells. Twelve samples (four from each of the serous, endometrioid, and mucinous samples) at early and late stages of OVCA were selected for immunoblot analysis based on their immunoreactivity for DR6 in immunohistochemistry. Immunoreactions on the membrane were visualized as a chemiluminescence product (Super Dura West substrate; Pierce/Thermo Fisher, Rockford, IL), and the image was captured using a Chemidoc XRS (Bio-Rad, Hercules, CA). Similarly, serum samples from the same hens used for ovarian DR6 expression were selected for immunoblot analysis to examine the presence of DR6 in serum. Serum samples were filtered by acetonitrile and chloroform-methanol precipitation before using for immunoblot analysis [27].
Reverse Transcription-Polymerase Chain Reaction
DR6 mRNA expression was assessed by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) as reported previously [30]. For RT-PCR analyses, serum and tissue samples from 5 hens with normal ovaries, from 12 hens with early stages of OVCA (4 for each histologic subtype), and from 12 hens with late stages of OVCA (4 for each histologic subtype) were selected based on their reactivity in immunohistochemistry and immunoblot analysis. Hen-specific DR6 primers were designed by OligoPerfect Designer software (Invitrogen, Carlsbad, CA) using the DR6 sequence from the National Center for Biotechnology Information (accession no. A1980074) as reported earlier [22]. The forward primer was 5′-GAT GGA GGA CAC CAC GCC-3′ and the reverse primer was 5′-TCG GGG TTG AGG ATG TGC-3′. β-Actin was used as the endogenous control, with a forward primer of TGCGTGACATCAAGGAGAAG and a reverse primer of ATGCCAGGGTACATTGTGGT. The expected base pair size for the DR6 amplicon was 384 bp and that for β-actin was 300 bp. PCR amplicons were visualized in a 3% agarose gel (Pierce/Thermo Fisher) in Tris-acetate-EDTA (TAE) buffer and stained with ethidium bromide. The image was captured using a ChemiDoc XRS system (Bio-Rad).
Statistical Analysis
The differences in the intensity of DR6 immunostaining and the number of microvessels expressing DR6 in normal ovaries and malignant ovaries were assessed by analysis of variance, F tests, and the alternative nonparametric Kruskal-Wallis tests. Subsequently, pairwise comparison between the groups (normal and early- and late-stage OVCAs) by two-sample t tests and alternative Mann-Whitney tests were performed. All reported P values are two-sided, and P <.05 was considered significant. Statistical analyses were performed with SPSS (PASW) version 18 software (IBM, Inc, Armonk, NY).
Results
Ovarian Morphology
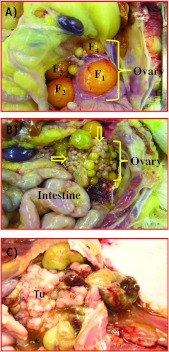
In laying hens, only the left ovary becomes functional, and the rate of egg production declines with aging. A fully functional ovary contains five to six developing large preovulatory follicles (Figure 1A). As the hen ages, the rate of egg production decreases. The ovary of an older (>3 years old) healthy hen with a low rate of egg production contains fewer than three large preovulatory follicles. In apparently healthy hens that have stopped laying eggs, the ovaries were atrophied and the oviducts were smaller. Solid tissue masses either limited to a small part or the entire ovary, with or without ascites, were observed in 12 hens. These hens were diagnosed with early-stage OVCA (Figure 1B). In 16 hens, tumor had metastasized to the abdominal organs with moderate to profuse ascites. These hens were diagnosed with late-stage OVCA (Figure 1C).
Figure 1.
Gross morphology of normal and malignant ovaries in laying hens. (A) Fully functional normal ovary in a laying hen. Laying hens ovulate and subsequently lay eggs once a day for 5 or 6 days in a week in a continuous manner and then take a pause for 1 day before laying resumes. Thus, the ovary in a fully functional laying hen contains a set of multiple large and growing preovulatory follicles arranged in a hierarchy of sizes (F1–F5). The largest follicle (F1) is destined to ovulate soon and then the second largest follicle (F2) becomes F1 and a small growing follicle is recruited from the ovarian stroma to the hierarchy to maintain the laying rates. (B) Ovarian tumor at an early stage in a hen showing solid tumor mass (arrows) limited to the ovary. (C) Ovarian tumor at a late stage. The2 tumor (Tu) appears like a cauliflower and has metastasized to other organs with accompanied profuse ascites (*).
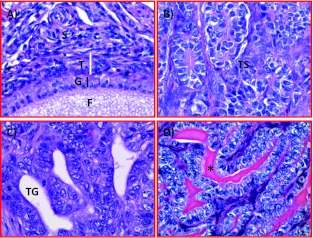
Histopathology. Cortical follicles with or without distinguishable granulosa cell and theca layer were embedded in the ovarian stroma of hens with normal ovaries (Figure 2A). Tumors were confirmed in all hens displaying gross ovarian solid masses (12 hens at early stage with solid masses limited to the ovaries or in 16 hens with late-stage OVCA) by routine histology (Figure 2, B–D). However, tumor-related microscopic changes (including focal lesions containing large cells with irregular shapes and pleomorphic nuclei) were also found during histologic examinations in 11 additional hens that had no gross ovarian tumor and were grouped in early-stage OVCA. Thus, a total of 23 (12 + 11) hens had early-stage OVCA, 16 had late-stage OVCA, whereas 81 hens had normal ovaries. Tumors were typed as serous (n =17), endometrioid (n = 12), mucinous (n = 8), clear cell (n =1), as well as mixed (n = 1, seromucinous) as reported previously [19].
Figure 2.
Microscopic features of hen ovaries. Paraffin-embedded sections from normal or malignant ovaries with tumors were stained with hematoxylin and eosin. (A) Section of a normal ovary showing a developing follicle embedded in the ovarian stroma. (B) Section of an ovarian serous carcinoma showing a solid sheet of tumor surrounded by fibromuscular tissue. The tumor contains a labyrinth of slitlike glandular spaces lined by cells with large pleomorphic nuclei and mitotic figures. (C) Section showing endometrioid carcinoma displaying confluent back-to-back glands. Glands contain a single layer of epithelial cells with sharp luminal margins. (D) Section of a mucinous carcinoma. Glands in clusters with scarce intervening stroma lined by columnar and goblet cells with intracytoplasmic mucin. Original magnification, x40.
Tissue Expression of DR6
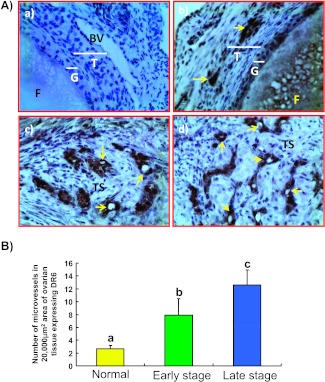
Microvessels expressing DR6 were detected in both normal and malignant ovaries (Figure 3). Most of the DR6-expressing ovarian microvessels in hens with normal ovaries had thick, complete, and continuous vessel walls with intense staining. These vessels were located in the theca layer of the follicles and a few vessels were in the ovarian stroma. In contrast, most of the DR6-expressing vessels in malignant ovaries were discontinuous or incomplete with thin vessel walls (Figure 3A). The number of DR6-expressing microvessels was significantly higher (P < .01, exact Mann-Whitney test) in hens with early (mean ± SD = 8.0 ± 2.29 microvessels per 20,000-µm2 area of stroma) and late (13.0 ± 2.37 microvessels per 20,000-µm2 area of stroma in malignant ovaries) stages of OVCA than in hens with normal ovaries (3.0 ± 0.52 microvessels per 20,000-µm2 area of stroma in normal ovaries; Figure 3B). However, significant differences in the population of DR6-expressing microvessels among the histologic subtypes were not observed.
Figure 3.
(A) Immunohistochemical detection of DR6-expressing microvessels in hen ovaries with or without tumor. (a) Section of a normal ovarian stroma immunostained by omitting primary antibodies used as control. No immunopositive vessel is seen. (b) Serial section from the same normal ovary immunostained with primary antibodies. Very few DR6-expressing vessels are seen. (c) An ovarian section from a hen with early-stage OVCA. Compared to the normal ovary, many DR6-expressing microvessels are seen in the stroma between tumors. (d) Section of a malignant tumor from a hen with late-stage OVCA. Many DR6-expressing microvessels are localized in the tumor stroma. BV indicates blood vessel; F, follicle; G, granulosa layer; T, theca layer; TS, tumor stroma. Arrows indicate DR6-expressing microvessels. Original magnification, x40. (B) Changes in the frequency of DR6-expressing ovarian microvessels relative to ovarian tumor development and progression in hens. The frequency of microvessels expressing DR6 in a 20,000-µm2 area of normal (n = 15) and malignant ovaries (expressed as the mean ± SD, n = 15 each for early and late stages). Compared to the normal ovary, the frequency of microvessels expressing DR6 was significantly (P < .001) higher in hens with early-stage OVCA cancer and increased further (P < .001) as the disease progressed to a late stage in hens. Each bar with a different letter indicates significant differences (P < .001) between normal and tumor groups.
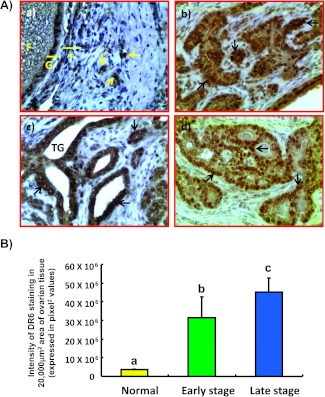
Tumor cells (Figure 4A) as well as a few normal OSE cells expressed DR6. In addition, rounded (T lymphocyte like) to irregularly shaped (macrophage-like) DR6 cells were also detected in the stroma of the normal ovaries or malignant ovaries. Compared with normal ovaries (mean ± SD = 3.6 x 106 ± 3.8 x 105 in a 20,000-µm2 area), the intensities of DR6 staining increased approximately 9-fold (P < .001) in hens with early-stage OVCA and 13-fold (P < .001) in hens with late-stage OVCA, respectively (Figure 4B). However, significant differences in DR6 staining intensities were not observed among three different histologic subtypes of OVCA in hens.
Figure 4.
(A) Expression of DR6 by tumor cells in malignant ovaries in hens. (a) Section of a normal ovary immunostained for DR6 expression. Very few immunopositive cells are present in the ovarian stroma. (b-d) Sections of different histologic subtypes of OVCA in hens including serous (b), endometrioid (c), and mucinous (d) at early stages immunostained for DR6 expression. Compared with the normal OSE cells, tumor cells in all three histologic subtypes stained intensely. Original magnification, x40. (B) Changes in the DR6 staining intensity relative to ovarian tumor development and progression in hens. The intensity of DR6 staining is expressed as the pixel values (mean ± SD) in a 20,000-µm2 area in normal ovarian stroma (n = 15) or in malignant ovaries. Compared to the normal ovary, the intensity of DR6 staining was significantly (P < .001) higher in hens with early-stage OVCA (n = 15) and increased further (P < .001) in late-stage OVCA (n = 15). Each bar with different letter indicates significant differences (P < .001) among normal or OVCA stages.
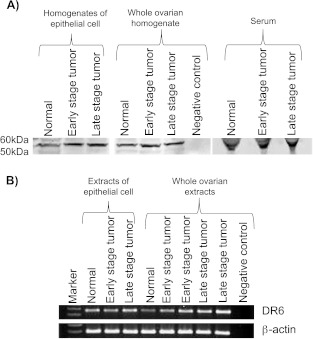
Immunoblot analysis for DR6 protein in ovarian tissues and serum samples. Immunohistochemical expression of DR6 in normal or malignant ovaries was confirmed by immunoblot analysis using homogenates of OSE cells (from normal ovaries) or tumor cells (from malignant ovaries) as well as homogenates from whole normal or malignant ovaries. A band of 50 to 60 kDa was detected in the homogenates of cells (normal OSE cells or tumor cells) and tissues (whole normal or tumor ovaries; Figure 5A). In addition, immunoreactive 50-to 60-kDa DR6 protein was also detected by immunoblot analysis in the serum of hens with normal ovaries or those with OVCA (Figure 5A). Compared with the whole ovarian homogenates or serum from hens with normal ovaries, immunoreactivity for DR6 protein was intense in the homogenates of malignant ovaries or serum from hens with OVCA (Figure 5A). These results support the immunohistochemical observation that OSE in hens with normal ovaries and tumor cells in hens with OVCA express DR6 protein. Moreover, these epithelial cells may be a source of DR6 proteins in the circulation of laying hens because DR6 was detected in the serum of hens with normal ovaries or those with OVCA.
Figure 5.
Immunoreactive (A) DR6 protein or (B) mRNA in serum and ovaries of hens with normal ovaries or those with OVCA. (A) One-dimensional Western blot analysis: Immunoreactive DR6 proteins of 50-to 60-kDa molecular weight were detected in the homogenates of normal OSE cells or tumor cells, in homogenates of whole normal or malignant ovaries, as well as in serum of hens by one-dimensional Western blot. Compared to the hens with normal ovaries, relatively stronger immunoreactive bands for DR6 proteins were observed in serum and ovaries of hens with early and late stages of OVCA. No immunoreactive band was detected in the negative control in which protein sample was omitted. (B) Semiquantitative RT-PCR: mRNA expression for DR6 was detected in the extracts of normal OSE cells or tumor cells and in extracts of normal and malignant ovaries by semiquantitative PCR. Compared to the weak expression by normal ovaries and OSE, strong amplification for DR6 mRNA was observed in the extracts of malignant ovaries and tumor cells in hens with early- and late-stage OVCA. No DR6 mRNA expression was detected in the negative control in which mRNA sample was omitted.
Expression of DR6 messenger RNA. DR6 messenger RNA (mRNA) expression confirmed the observed variations in ovarian DR6 expression among hens with normal ovaries or those with OVCA. Although the patterns of DR6 mRNA expression were similar between normal OSE and tumor cells from early-stage OVCA, it was stronger for tumor cells from late-stage OVCA. Compared with the hens with normal ovaries, strong amplification of signal for DR6 (Figure 5B) was observed in the ovarian extracts from hens with early-stage OVCA and the amplification was stronger in hens with late-stage OVCA. However, differences in DR6 mRNA expression were not observed among different histologic subtypes of OVCA at the same stage (early or late). Overall, compared to hens with normal ovaries, strong amplification of DR6 mRNA was observed in hens with OVCA as observed for immunoreactivities in immunohistochemistry and immunoblot analysis.
Discussion
This is the first report on the expression of death receptor (DR6) by tumor cells of malignant ovaries in hens. The expression of DR6 was significantly higher in the tumor cells of malignant ovaries than OSE cells of normal ovaries. Furthermore, the population of ovarian micro-vessels expressing DR6 was significantly higher in hens with early-stage OVCA than hens with normal ovaries and increased further in hens with late-stage OVCA. In addition, DR6 was also detected in the serum of hens. Thus, the results of the present study suggest that the increase in DR6 expression may be associated with ovarian tumor development and progression in laying hens.
Tumor-associated neoangiogenesis (TAN) and suppression of antitumor immunity are two of the early events required for the survival and progression of the tumor. Increased numbers of immature micro-vessels with disorganized and discontinuous smooth muscle layers are the characteristic features of ovarian TAN in patients as well as in laying hens with OVCA [24]. Compared to hens with normal ovaries, the number of DR6-expressing microvessels was significantly higher in hens with early-stage OVCA and increased further in hens with late-stage OVCA. A recent study has reported increased expression of DR6 by ovarian tumors in patients with advanced-stage OVCA [6]. In this study, the population of DR6-expressing ovarian microvessels increased significantly at an earlier stage even before the tumor became grossly detectable. Recently, DR6 was reported to be required for angiogenesis in the central nervous system [31]. Although precise reason(s) for the increase in the population of DR6-expressing micro-vessels in malignant ovaries is not known, it is possible that DR6 will play a role in the development of ovarian TAN.
Despite the presence of an antitumor immune response [5,32], the rare eradication of ovarian tumors and their progression suggest that multiple mechanisms are used by the tumor to escape immune rejection. The expression of DR6 has been reported to be increased significantly in cell lines and patients with prostate and breast cancers [7,10,33]. In the absence of DR6, ligation of the T-cell receptor results in enhanced T-cell proliferation, activation, and skewed TH2 cytokine production. Similarly, B cells lacking DR6 show increased proliferation, cell division, and cell survival on mitogenic stimulation (anti-CD40 and LPS) or BCR ligation. DR6-/- mice showed increased TH2 immune responses to both T-dependent and -independent antigens. In contrast, it is suggested that increased DR6 expression on tumor cells results in the cleaving of extracellular part of DR6 from the cell surface by matrix metalloproteinase 14. This shed DR6 reported to attenuate the in vitro differentiation of monocytes into immunotolerant instead of immunocompetent dendritic cells, which can contribute to tumor evasion from the immune system [17]. All these reports indicate that DR6 plays important roles in imparting tolerance to local immune response. In the present study, the expression of ovarian DR6 was significantly high in hens with early-stage OVCA than in hens with normal ovaries and increased further in hens with late-stage OVCA. Although the significance of increased DR6 expression by malignant cells is not known, the results of the current study suggest that increased DR6 expression may play important roles in the suppression of immunity against ovarian tumors.
The results of the current study suggest several translational significances. Because the malignant tumors in hens express DR6 and it is also present in the serum, DR6 could be targeted for contrast-enhanced ultrasound imaging to detect the tumor. Thus, use of DR6-expressing epithelium in the ovary as a target may increase the sensitivity of ultrasound scanning to detect OVCA together with serum levels of DR6. Hence, it will bring a significant change in imaging paradigms and improve the specificity of ultrasound scanning. It will also make possible to determine the time between the tumor-associated elevation of DR6 in serum and the earliest detection of tumor by contrast-enhanced ultrasound scanning. This information will enable the detection of OVCA at an early stage and lead to the development of treatment modalities for patients with OVCA. In addition, current findings will also be useful in developing ovarian tumor-associated anti-DR6 therapies, which can be tested in laying hens. Taken together, information on the association of DR6 with the early detection of OVCA and the potential development of anti-DR6-based therapies will establish the foundation for clinical studies. It may thus ultimately lead to the development of an effective diagnostic test and therapies for OVCA at an early stage.
In conclusion, this study showed that the tissue expression of DR6, a potential tumor-associated neoangiogenic and immunosuppressive factor, was significantly higher in hens with early-stage OVCA than in hens with normal ovaries and increased further as the disease progressed to late stages. This information will be useful and contribute to clinical studies to determine the role of DR6 in OVCA development and progression in humans.
Acknowledgments
The authors thank Chet and Pam Utterback and Doug Hilgendorf, staff of the University of Illinois at Urbana-Champaign Poultry Research Farm, for maintenance of the hens. The authors also thank Sergio Abreu Machado and Syed Tahir Abbas Shah, graduate students, Department of Animal Sciences, University of Illinois at Urbana-Champaign, for helping in collecting hen tissue.
Footnotes
This study was supported by the Idea Development Award from the US Department of Defense (OC no. 093303), the National Cancer Institute Pacific Ovarian Cancer Research Consortium Career Development Program (grant P50 CA83636), and the Elmer Sylvia and Sramek Foundation (USA). The authors declare no actual or potential conflict of interest associated with this study.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 3.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-β. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 4.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117:366–372. doi: 10.1016/j.ygyno.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Sasaroli D, Gimotty PA, Pathak HB, Hammond R, Kougioumtzidou E, Katsaros D, Buckanovich R, Devarajan K, Sandaltzopoulos R, Godwin AK, et al. Novel surface targets and serum biomarkers from the ovarian cancer vasculature. Cancer Biol Ther. 2011;12:169–180. doi: 10.4161/cbt.12.3.16260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benschop R, Wei T, Na S. Tumor necrosis factor receptor super-family member 21: TNFR-related death receptor 6, DR6. In: Grewal IS, editor. Therapeutic Targets of the TNF Superfamily. Austin, TX: Landes Bioscience; 2009. Available at: http://www.landesbioscience.com/books/1137/ [Google Scholar]
- 8.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 9.Pan G, Bauer JH, Haridas V, Wang S, Liu D, Yu G, Vincenz C, Aggarwal BB, Ni J, Dixit VM. Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett. 1998;431:351–356. doi: 10.1016/s0014-5793(98)00791-1. [DOI] [PubMed] [Google Scholar]
- 10.Kasof GM, Lu JJ, Liu D, Speer B, Mongan KN, Gomes BC, Lorenzi MV. Tumor necrosis factor-alpha induces the expression of DR6, a member of the TNF receptor family, through activation of NF-κB. Oncogene. 2001;20:7965–7975. doi: 10.1038/sj.onc.1204985. [DOI] [PubMed] [Google Scholar]
- 11.Buckanovich RJ, Sasaroli D, O'Brien-Jenkins A, Botbyl J, Hammond R, Katsaros D, Sandaltzopoulos R, Liotta LA, Gimotty PA, Coukos G. Tumor vascular proteins as biomarkers in ovarian cancer. J Clin Oncol. 2007;25:852–861. doi: 10.1200/JCO.2006.08.8583. [DOI] [PubMed] [Google Scholar]
- 12.Wick M, Dubey P, Koeppen H, Siegel CT, Fields PE, Chen L, Bluestone JA, Schreiber H. Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy. J Exp Med. 1997;186:229–238. doi: 10.1084/jem.186.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29:233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 14.Clarke B, Tinker AV, Lee CH, Subramanian S, van de Rijn M, Turbin D, Kalloger S, Han G, Ceballos K, Cadungog MG, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol. 2009;22:393–402. doi: 10.1038/modpathol.2008.191. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 16.Barua A, Bradaric MJ, Kebede T, Espionosa S, Edassery SL, Bitterman P, Rotmensch J, Luborsky JL. Anti-tumor and anti-ovarian autoantibodies in women with ovarian cancer. Am J Reprod Immunol. 2007;57:243–249. doi: 10.1111/j.1600-0897.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 17.DeRosa DC, Ryan PJ, Okragly A, Witcher DR, Benschop RJ. Tumor-derived death receptor 6 modulates dendritic cell development. Cancer Immunol Immunother. 2008;57:777–787. doi: 10.1007/s00262-007-0413-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barua A, Bitterman P, Abramowicz JS, Dirks AL, Bahr JM, Hales DB, Bradaric MJ, Edassery SL, Rotmensch J, Luborsky JL. Histopathology of ovarian tumors in laying hens: a preclinical model of human ovarian cancer. Int J Gynecol Cancer. 2009;19:531–539. doi: 10.1111/IGC.0b013e3181a41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Burford C, Barnes MN, Berry W, Partridge EE, Grizzle WE. Immunohistochemical expression of molecular markers in an avian model: a potential model for preclinical evaluation of agents for ovarian cancer chemoprevention. Gynecol Oncol. 2001;81:373–379. doi: 10.1006/gyno.2001.6191. [DOI] [PubMed] [Google Scholar]
- 21.Giles JR, Elkin RG, Trevino LS, Urick ME, Ramachandran R, Johnson PA. The restricted ovulator chicken: a unique animal model for investigating the etiology of ovarian cancer. Int J Gynecol Cancer. 2010;20:738–744. doi: 10.1111/igc.0b013e3181da2c49. [DOI] [PubMed] [Google Scholar]
- 22.Bridgham JT, Bobe J, Goetz FW, Johnson AL. Conservation of death receptor-6 in avian and piscine vertebrates. Biochem Biophys Res Commun. 2001;284:1109–1115. doi: 10.1006/bbrc.2001.5093. [DOI] [PubMed] [Google Scholar]
- 23.Barua A, Abramowicz JS, Bahr JM, Bitterman P, Dirks A, Holub KA, Sheiner E, Bradaric MJ, Edassery SL, Luborsky JL. Detection of ovarian tumors in chicken by sonography: a step toward early diagnosis in humans? J Ultrasound Med. 2007;26:909–919. doi: 10.7863/jum.2007.26.7.909. [DOI] [PubMed] [Google Scholar]
- 24.Barua A, Bitterman P, Bahr JM, Bradaric MJ, Hales DB, Luborsky JL, Abramowicz JS. Detection of tumor-associated neoangiogenesis by Doppler ultrasonography during early-stage ovarian cancer in laying hens: a pre-clinical model of human spontaneous ovarian cancer. J Ultrasound Med. 2010;29:173–182. doi: 10.7863/jum.2010.29.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giles JR, Olson LM, Johnson PA. Characterization of ovarian surface epithelial cells from the hen: a unique model for ovarian cancer. Exp Biol Med (Maywood) 2006;231:1718–1725. doi: 10.1177/153537020623101108. [DOI] [PubMed] [Google Scholar]
- 26.Yellapa A, Bahr JM, Bitterman P, Abramowicz JS, Edassery SL, Penumatsa K, Basu S, Rotmensch J, Barua A. Association of interleukin 16 with the development of ovarian tumor and tumor-associated neoangiogenesis in laying hen model of spontaneous ovarian cancer. Int J Gynecol Cancer. 2012;22:199–207. doi: 10.1097/IGC.0b013e318236a27b. [DOI] [PubMed] [Google Scholar]
- 27.Barua A, Edassery SL, Bitterman P, Abramowicz JS, Dirks AL, Bahr JM, Hales DB, Bradaric MJ, Luborsky JL. Prevalence of antitumor antibodies in laying hen model of human ovarian cancer. Int J Gynecol Cancer. 2009;19:500–507. doi: 10.1111/IGC.0b013e3181a39db1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan M, Bahr JM, Yellapa Y, Bitterman P, Abramowicz JS, Edassery SL, Basu S, Rotmensch J, Barua B. Association of immunoglobulin like transcript 3 (ILT3) with the progression of ovarian tumors in laying hens, a preclinical model of spontaneous ovarian cancer. Transl Oncol. 2012;5:85–91. doi: 10.1593/tlo.11328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barua A, Bitterman P, Bahr JM, Basu S, Sheiner E, Bradaric MJ, Hales DB, Luborsky JL, Abramowicz JS. Contrast-enhanced sonography depicts spontaneous ovarian cancer at early stages in a preclinical animal model. J Ultrasound Med. 2011;30:333–345. doi: 10.7863/jum.2011.30.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong YH, Lillehoj HS, Lee SH, Dalloul RA, Lillehoj EP. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet Immunol Immunopathol. 2006;114:209–223. doi: 10.1016/j.vetimm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Tam SJ, Richmond DL, Kaminker JS, Modrusan Z, Martin-McNulty B, Cao TC, Weimer RM, Carano RA, van Bruggen N, Watts RJ. Death receptors DR6 and TROY regulate brain vascular development. Dev Cell. 2012;22:403–417. doi: 10.1016/j.devcel.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Kandalaft LE, Singh N, Liao JB, Facciabene A, Berek JS, Powell DJ, Jr, Coukos G. The emergence of immunomodulation: combinatorial immunochemotherapy opportunities for the next decade. Gynecol Oncol. 2010;116:222–233. doi: 10.1016/j.ygyno.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klima M, Zajedova J, Doubravska L, Andera L. Functional analysis of the posttranslational modifications of the death receptor 6. Biochim Biophys Acta. 2009;1793:1579–1587. doi: 10.1016/j.bbamcr.2009.07.008. [DOI] [PubMed] [Google Scholar]