Abstract
Poly(ADP)-ribose polymerase (PARP) is an abundant nuclear protein that is activated by DNA damage; once active, it modifies nuclear proteins through attachment of poly(ADP)-ribose units derived from β-nicotinamide adenine dinucleotide (NAD+). In mice, the deletion of PARP-1 attenuates tissue injury in a number of animal models of human disease, including streptozotocin-induced diabetes. Also, inflammatory cell signaling and inflammatory gene expression are attenuated in macrophages isolated from endotoxin-treated PARP-1-deficient mice. In this study, the effects of PARP-1 deletion on cytokine-mediated β-cell damage and macrophage activation were evaluated. There are no defects in inflammatory mediator signaling or inflammatory gene expression in macrophages and islets isolated from PARP-1-deficient mice. While PARP-1 deficiency protects islets against cytokine-induced islet cell death as measured by biochemical assays of membrane polarization, the genetic absence of PARP-1 does not effect cytokine-induced inhibition of insulin secretion or cytokine-induced DNA damage in islets. While PARP-1 deficiency appears to provide protection from cell death, it fails to provide protection against the inhibitory actions of cytokines on insulin secretion or the damaging actions on islet DNA integrity.
Keywords: insulin, nitric oxide, poly(ADP)-ribose polymerase
poly(adp)-ribose polymerase (PARP) is a highly abundant nuclear protein that is activated by DNA nicks and breaks. PARP uses β-nicotinamide adenine dinucleotide (NAD) as a substrate for the addition of poly(ADP)-ribose to acceptor proteins (for review see Refs. 55 and 61). PARP scans DNA, identifies DNA rupture, and participates in base-excision repair by metabolizing NAD+ into branched polymers of ADP ribose that are then transferred to nuclear binding proteins such as DNA polymerase I and II, Ca2+-Mg2+ endonuclease, histones, chromatin binding proteins, and PARP-1 itself (55, 61). It has been hypothesized that poly(ADP) ribosylation modifies genomic proteins proximal to DNA breaks and facilitates the opening of chromatin, which is required for the recruitment of repair enzymes (67). While this mechanism of action has been challenged by evidence that DNA repair can occur in PARP-1-deficient (PARP-1−/−) mice (64), the functions performed by PARP do not exist in a solitary enzyme. The PARP family of enzymes is encoded at several gene loci, all of which contain the ADP ribosylation function at the COOH end of the protein molecule. There are a number of isoforms of PARP: PARP-1 (26), short PARP (56), tankyrase (60), PARP-2 (1, 4), vault PARP (33), and PARP-10 (29) (70). With the discovery of multiple isoforms of PARP has come an increase in the variety of nuclear processes catalyzed by the enzyme family, including the regulation of genomic stability, gene expression, gene transcription and amplification, cellular differentiation, malignant transformation, and DNA replication, in addition to the participation of these enzymes in many physiological and developmental pathways (45, 55, 59, 61).
Despite the theoretically beneficial effects of the activation of the PARP family of enzymes in the repair of DNA nicks and breaks, PARP-1 may also mediate cell death through the depletion of cellular energy stores (NAD+ and the subsequent depletion of ATP), as proposed in the Okamoto model >25 years ago (46, 68, 69). Consistent with the Okamoto model, mice lacking PARP-1 are resistant to diabetes induced by a single bolus injection of streptozotocin (STZ) (9, 43, 50). STZ is a β-cell toxin that induces DNA damage through alkylation (38, 65). While inhibition of PARP-1 (49) protects islets against free radical- and cytokine-mediated islet damage (2, 52, 57), these inhibitors, as well as PARP-1 deficiency, have also been associated with attenuation of cytokine and endotoxin signaling, as evidenced by reduced NF-κB activation and subsequent expression of inflammatory gene targets, such as inducible nitric oxide (NO) synthase (iNOS) (3, 18, 37, 48).
Glucose stimulates insulin secretion by β-cells through its oxidation to CO2, with the subsequent accumulation of ATP to levels sufficient to stimulate Ca2+-dependent exocytosis (28). Treatment of rat islets with the macrophage-derived cytokine IL-1 or mouse and human islets with IL-1 and IFN-γ results in an inhibition of glucose-stimulated insulin secretion that is mediated by the expression of iNOS and the subsequent production of micromolar levels of NO (11, 12, 14, 19). NO inhibits insulin secretion by attenuating the oxidation of glucose to CO2 and, thereby, reducing cellular levels of ATP. NO also induces DNA damage through direct strand breaks and base modification and has been shown to inhibit the activity of enzymes that participate in the repair of damaged DNA (15, 17, 27, 30, 31).
The purpose of this study was to determine if PARP-1 participates in the destructive effects of cytokines on islet function and viability. To address this question, the actions of IL-1 + IFN-γ on iNOS expression, NO production, insulin secretion, DNA damage, and cell viability were examined in islets isolated from wild-type and PARP-1−/− mice. Since NF-κB activation and iNOS expression are impaired in macrophages isolated from PARP-1−/− mice treated with endotoxin (47, 48), the effects of endotoxin and double-stranded (ds) RNA on macrophage iNOS expression were also evaluated. We show that PARP-1 deficiency does not modify iNOS expression, NO production, or inflammatory effector signaling in peritoneal macrophages and β-cells isolated from PARP-1−/− mice. While PARP-1 deficiency appears to provide protection from cytokine-mediated islet cell death, as determined by biochemical assays of membrane polarization, under conditions of iNOS expression and NO production, the absence of PARP-1 does not prevent the inhibitory actions of cytokines on insulin secretion or the induction of DNA damage.
MATERIALS AND METHODS
Materials and animals.
Wild-type (PARP-1+/+) and PARP-1−/− mice were obtained from Wang and colleagues and are described elsewhere (64). Collagenase type XI, polyinosinic:polycytidylic acid [poly(IC)], and LPS were purchased from Sigma Chemical (St. Louis, MO); CMRL-1066 and DMEM tissue culture medium, l-glutamine, penicillin, streptomycin, and rat recombinant IFN-γ from Life Technologies (Grand Island, NY); fetal calf serum from Hyclone Laboratories (Logan, UT); human recombinant IL-1 from Cistron Biotechnology (Pine Brook, NJ); Tris-glycine gels (8–16%, 1 mm × 10 wells) from Invitrogen (Carlsbad, CA); Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane from Amersham Biosciences (Buckinghamshire, UK); ECL reagent from Amersham (Piscataway, NJ); horseradish peroxide-conjugated donkey anti-rabbit and donkey anti-mouse IgG from Jackson ImmunoResearch Laboratories (West Grove, PA); rabbit anti-iNOS from Cayman Chemical (Ann Arbor, MI); rabbit anti-Stat-1 and anti-IκB from Santa Cruz Biotechnology (Santa Cruz, CA); PhosphoSafe extraction buffer from Novagen; and NG-monomethyl-l-arginine (NMMA) from Alexis Biochemicals (San Diego, CA).
Islet isolation and culture.
Pancreatic islets were isolated from male and female PARP-1+/+ and PARP-1−/− mice by collagenase digestion, as described previously (32, 36). After isolation, islets were cultured overnight in complete CMRL (CMRL-1066 containing 2 mM l-glutamine, 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin) under an atmosphere of 95% air-5% CO2 at 37°C. For each experiment, islets were washed with complete CMRL, counted, and then cultured for an additional 3 h. Experiments were initiated by the addition of cytokines [IL-1 (15 U/ml) and IFN-γ (150 U/ml)], and the islets were cultured as indicated.
Peritoneal macrophage isolation and culture.
Peritoneal exudate cells were isolated from PARP-1+/+ and PARP-1−/− mice by lavage, as previously described (41). After isolation, 4 × 105 cells in 400 μl of complete CMRL were incubated at 37°C under an atmosphere of 95% air-5% CO2 for 3 h. Cells were washed with complete CMRL to remove nonadherent cells before treatment with LPS (10 μg/ml), poly(IC) (50 μg/ml), and 150 U/ml IFN-γ.
Insulin secretion.
Mouse islets (120 islets/ml complete CMRL) were cultured in the presence or absence IL-1 and IFN-γ and NMMA for 24 h. The islets were washed three times in Krebs-Ringer bicarbonate buffer (25 mM HEPES, 115 mM NaCl, 24 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, and 0.1% bovine serum albumin, pH 7.4) containing 3 mM d-glucose. Groups of 15 islets were counted into 10 × 75-mm borosilicate test tubes and preincubated for 30 min at 37°C with shaking in 200 μl of the same buffer. The preincubation buffer was removed, and glucose-stimulated insulin secretion was initiated by the addition of 200 μl of Krebs-Ringer bicarbonate buffer containing 3 or 20 mM d-glucose. Islets were then incubated at 37°C for 45 min, the incubation buffer was removed, and insulin content was determined by radioimmunoassay at the Diabetes Research and Training Center at Washington University School of Medicine (St. Louis, MO).
Animal authorization.
The experiments were performed in accordance with the provisions of the Public Health Service Policy on the Humane Care and Use of Laboratory Animals and as described in the animal use protocols approved by the Saint Louis University and the University of Alabama at Birmingham Institutional Animal Care and Use Committees.
Nitrite determination.
Nitrite production was determined by mixing 50 μl of culture medium with 50 μl of the Griess reagent, as previously described (16). Absorbance was measured at 540 nm, and nitrite concentrations were calculated from a sodium nitrite standard curve.
Electrophoresis and Western blot analysis.
SDS-gel electrophoresis (8–16% gradient gels) was performed using lysates prepared from islets or peritoneal macrophages, as previously described (21). Protein was transferred to Hybond nitrocellulose membranes, and antigens were detected by chemiluminescence (ECL; Amersham), as previously described (21). In cases where the target antigen is phosphorylated, cell lysates were prepared using PhosphoSafe extraction buffer as recommended by the manufacturer (Novagen). Antibody dilutions were 1:1,000 for primary antibodies and 1:7,000 for horseradish peroxide-conjugated secondary antibodies.
Cell viability.
Cell viability was evaluated using a modified version of the neutral red dye uptake assay (7, 62). Briefly, after cytokine exposure of islets, the culture supernatant was removed, and the islets were isolated by centrifugation. The islets were then transferred to 1.5-ml microfuge tubes and incubated in fresh medium containing 50 μg/ml neutral red. After 2 h of incubation at 37°C, the supernatant was removed, the islets were washed with a 1% formaldehyde-1% CaCl2 solution, and the neutral red dye was extracted in 100 μl of a 50% ethanol-1% acetic acid lysing solution. The accumulation of neutral red dye in the lysing solution was measured at wavelength of 540 nm (62).
DNA damage.
Islet cells (4 × 105 cells/400 μl of complete CMRL), treated as indicated, were centrifuged onto glass slides, and DNA damage was quantified by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining according to the manufacturer's instructions using the In Situ Cell Death Detection Kit, Fluorescein (Roche, Indianapolis, IN). Cells containing DNA damage were colocalized with insulin-containing cells, and nuclei were identified using 4′,6-diaminido-2-phenylindole (DAPI), as outlined previously (58).
Quantification and statistical analysis.
Western blots were quantified using ImageJ (version 1.34, National Institutes of Health, Bethesda, MD). Statistical comparisons were made between groups using a one-way ANOVA. Significant differences between groups (P < 0.01) were determined using Bonferroni's post hoc analysis.
RESULTS
The presence of functional PARP-1 is not required for iNOS expression and NO production.
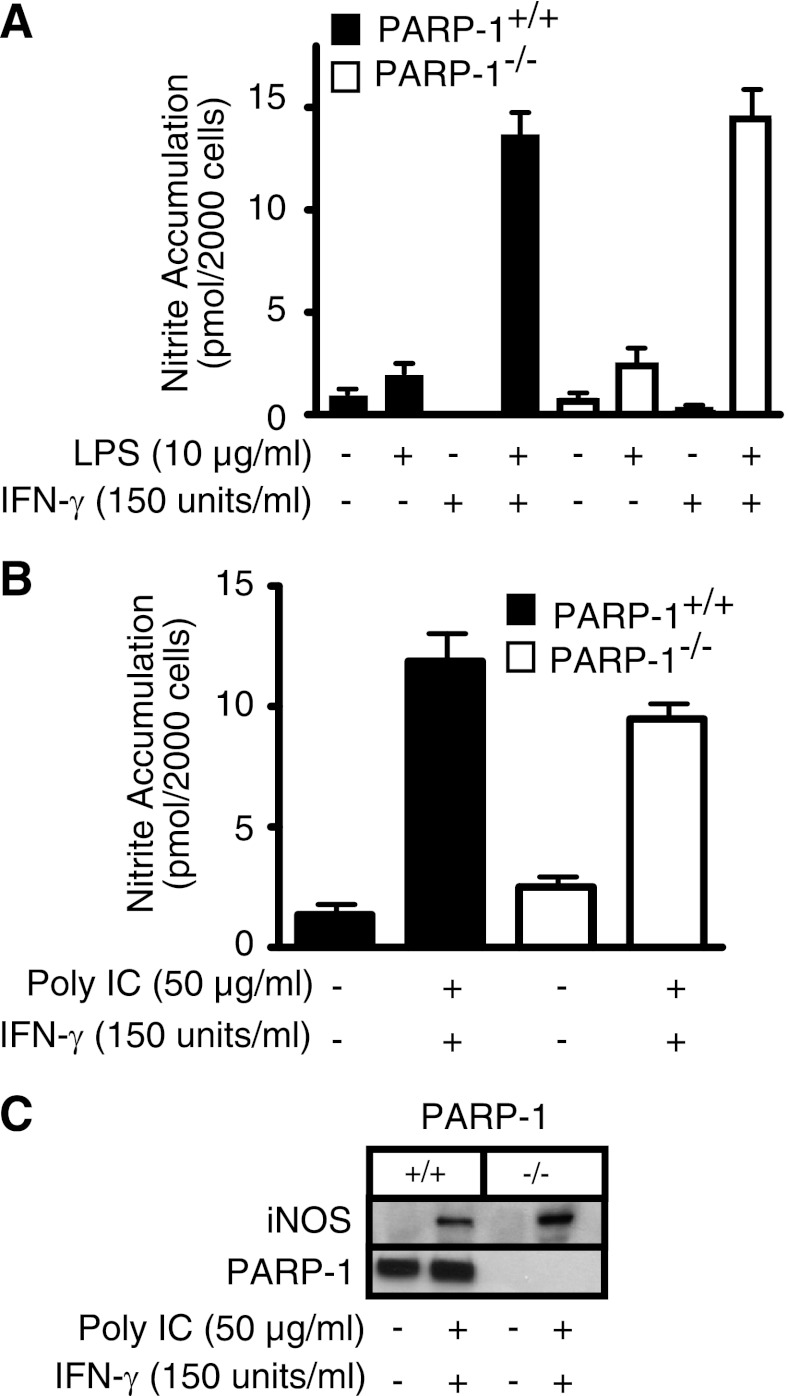
Since NO is a primary mediator of β-cell damage in response to cytokines (5, 14, 19) and previous studies suggest that iNOS expression and NO production are attenuated in PARP-1−/− cells (39, 47), the effects of cytokine and endotoxin treatment on macrophage (Fig. 1) and islet (Fig. 2) iNOS expression and NO production were examined. Peritoneal macrophages derived from PARP-1+/+ and PARP-1−/− mice respond in a similar manner to LPS + IFN-γ treatment, with an increase in the production of nitrite (Fig. 1A) and the expression of iNOS (data not shown). This is consistent with a requirement for two proinflammatory signals to activate macrophages, as LPS or IFN-γ alone does not stimulate nitrite production by mouse macrophages (Fig. 1A). In addition to the bacterial product LPS, the viral dsRNA mimetic poly(IC), in the presence of IFN-γ, also stimulates iNOS expression and nitrite formation by macrophages (20). Similar to the findings using LPS, there is no difference in the induction of iNOS or production of NO in response to poly(IC) and IFN-γ by macrophages derived from PARP-1+/+ and PARP-1−/− mice (Fig. 1, B and C). These findings indicate that macrophage expression of iNOS and production of NO are not dependent on the presence of PARP-1. Much like the response of murine peritoneal macrophages, responses to proinflammatory cytokines in islets from PARP-1−/− mice are not altered. Like macrophages, mouse islets require two inflammatory signals, IL-1 and IFN-γ, to stimulate iNOS expression (22), and incubation for 24 h with IL-1 + IFN-γ results in the production of NO and the expression of iNOS to similar levels in islets isolated from PARP-1+/+ and PARP-1−/− mice (Fig. 2).
Fig. 1.
Induction of inducible nitric oxide (NO) synthase (iNOS) in macrophages isolated from wild-type and poly(ADP)-ribose polymerase (PARP)-deficient (PARP-1+/+ and PARP-1−/−) mice. Peritoneal macrophages harvested from PARP-1+/+ and PARP-1−/− mice were treated for 24 h with LPS, synthetic double-stranded RNA {polyinosinic:polycytidylic acid [poly(IC)]}, and IFN-γ. Supernatants were harvested, and nitrite accumulation was determined by the Griess assay (A and B) and iNOS expression was determined by Western blot analysis of the cells (C). PARP-1 levels were determined by Western blot analysis and are shown as a control for PARP-1 deficiency. Results are representative of 3 independent experiments.
Fig. 2.
iNOS induction and NO production by islets isolated from PARP-1+/+ and PARP-1−/− mice. Mouse islets (120 per 400 μl of complete CMRL) were treated for 24 h with IL-1 and murine IFN-γ. Supernatants were harvested, and nitrite production was determined by the Griess assay (A) and iNOS expression was evaluated by Western blot analysis of the islets (B). PARP-1 levels were determined by Western blot analysis and are shown as a control for PARP-1 deficiency. Results are representative of 3 independent experiments.
Effects of PARP-1 deficiency on inflammatory cell signaling cascade activation in islets and macrophages.
The transcription factor NF-κB plays a primary role in the regulation of inflammatory gene expression, including iNOS, and NF-κB activation in response to inflammatory stimuli has been reported to be impaired in cells from PARP-1−/− mice (39, 47). NF-κB is held in the cytoplasm of cells in an inactive complex with inhibitory protein κB (IκB). In response to proinflammatory agonists, IκB is phosphorylated and targeted for proteasome-mediated degradation. NF-κB is then released and translocates from the cytoplasm to the nucleus, where it stimulates the transcriptional activation of inflammatory genes. NF-κB activation is required for LPS-induced iNOS expression by macrophages and cytokine-induced iNOS expression by β-cells, and we have shown that IκB degradation is a reliable indicator of NF-κB nuclear localization, DNA binding, and transcriptional activation in both cell types (35, 40). Therefore, the effects of LPS, poly(IC), and cytokines on IκB degradation in macrophages (Fig. 3A) and islets (Fig. 3B) isolated from PARP-1+/+ and PARP-1−/− mice were examined. Treatment for 30 min with LPS or poly(IC) results in the degradation of IκB to similar levels in macrophages isolated from PARP-1+/+ and PARP-1−/− mice (Fig. 3A). Like macrophages, the presence or absence of PARP-1 in islets does not influence the degradation of IκB in response to IL-1 + IFN-γ (following 30- and 60-min incubations; Fig. 3B). Furthermore, PARP-1 does not modify IFN-γ signaling in islets, as IL-1 + IFN-γ stimulates Stat-1 phosphorylation to similar levels in islets from PARP-1+/+ and PARP-1−/− mice (Fig. 3B). IFN-γ signaling is mediated by the activation of JAK kinases, followed by the phosphorylation of Stat transcription factor, such as Stat-1 (Fig. 3), and the translocation of these factors to the nucleus, where they bind to DNA to stimulate transcription. This activation is tightly regulated by phosphorylation/dephosphorylation, as the pathway is inactivated by phosphatase activity. The activation observed at 30 min (phosphorylation of Stat-1; Fig. 3) is followed by inactivation of this signaling cascade through loss of this phosphorylation. The results presented in Figs. 1–3 indicate that the presence of PARP-1 is not required for the activation of signaling cascades that are responsible for controlling the expression of iNOS by macrophages and islets of Langerhans.
Fig. 3.
Effects of cytokines and endotoxin on cellular signaling in macrophages and islets from PARP-1+/+ and PARP-1−/− mice. Macrophages (200,000 per 400 μl of complete CMRL) isolated from PARP-1+/+ and PARP-1−/− mice were treated for 30 min with LPS or poly(IC), cells were harvested, and IκB degradation was examined as an index of NF-κB activation by Western blot analysis (A). Islets (120 per 400 μl of complete CMRL), isolated from PARP-1+/+ and PARP-1−/− mice, were treated for 30 min with IL-1 and IFN-γ. Islets were harvested, and IκB degradation and Stat-1 phosphorylation (Stat-1-P) were determined by Western blot analysis (B). GAPDH and Stat-1 levels are shown as loading controls. Results are representative of 3 independent experiments.
PARP-1 deficiency and islet cell viability.
PARP-1−/− mice are protected from the development of diabetes induced by a single bolus injection of STZ, and this protection is associated with the preservation of β-cell mass (9, 43, 50). In mice, diabetes induced by a single bolus injection of STZ is mediated by the selective uptake of STZ in β-cells, followed by the induction of DNA alkylation and strand breaks (38, 66). STZ also liberates NO during its decomposition, resulting in an impairment of β-cell oxidative metabolism (63). Since cytokine-induced DNA damage and β-cell death are mediated by β-cell production of NO (10, 62) and PARP-1 deficiency protects β-cells from STZ-mediated death (9, 43, 50), the effects of PARP-1 deficiency on cytokine-mediated β-cell damage were examined. Consistent with a number of previous studies (10, 42, 62), treatment of islets isolated from PARP-1+/+ mice for 24 h with IL-1 + IFN-γ results in a 25% loss of islet viability (Fig. 4A), as determined using neutral red dye assay. The loss of viability occurs under conditions in which the cytokines stimulate ∼10-fold increase in NO production (Fig. 4B). Islets from PARP-1−/− mice produce NO to levels that are comparable to those produced by islets from PARP-1+/+ mice in response to IL-1 + IFN-γ (Fig. 4B); however, the islets from PARP-1−/− mice appear to be protected against the toxic actions of the cytokine combination (Fig. 4A). This protection from cell death is not universal, as islets from PARP-1−/− and PARP-1+/+ mice are equally sensitive to the effects of staurosporine, a classical inducer of apoptosis (Fig. 4A).
Fig. 4.
Effects of cytokines on islet cell viability. Islets (120 per 400 μl of complete CMRL), isolated from PARP-1+/+ and PARP-1−/− mice, were treated for 24 h with IL-1 and IFN-γ prior to assessment of cell viability using the neutral red assay (A). Supernatants from the treatments were harvested, and nitrite accumulation was determined (B). Results are averages ± SE of 3 independent experiments. *Significantly different from control (P < 0.05).
Insulin secretion and PARP-1 deficiency.
While islets from PARP-1−/− mice appear to be protected from the toxic actions of cytokines, even under conditions where they produce micromolar levels of NO, the inhibitory actions of these cytokines on glucose-stimulated insulin secretion are unchanged. Treatment of islets isolated from PARP-1−/− mice for 24 h with IL-1 + IFN-γ results in an inhibition of insulin secretion to levels similar in magnitude to the inhibitory actions of these cytokines on insulin secretion by islets isolated from PARP-1+/+ mice (Fig. 5). The cytokine treatment inhibits the secretion of insulin from islets isolated from PARP-1+/+ and PARP-1−/− mice by 70%, and the NOS inhibitor NMMA prevents this inhibition.
Fig. 5.
Effects of cytokines on glucose-stimulated insulin secretion. Islets (120 per 400 μl of complete CMRL), isolated from PARP-1+/+ and PARP-1−/− mice, were treated for 24 h with IL-1, IFN-γ, and NG-monomethyl-l-arginine (NMMA). Islets were harvested, and insulin secretion in response to 45 min of incubation with 3 or 20 mM glucose was determined. Results are averages ± SE of 3 independent experiments. *Significantly different from control (P < 0.05). There are no statistically significant differences between PARP-1+/+ and PARP-1−/− mice.
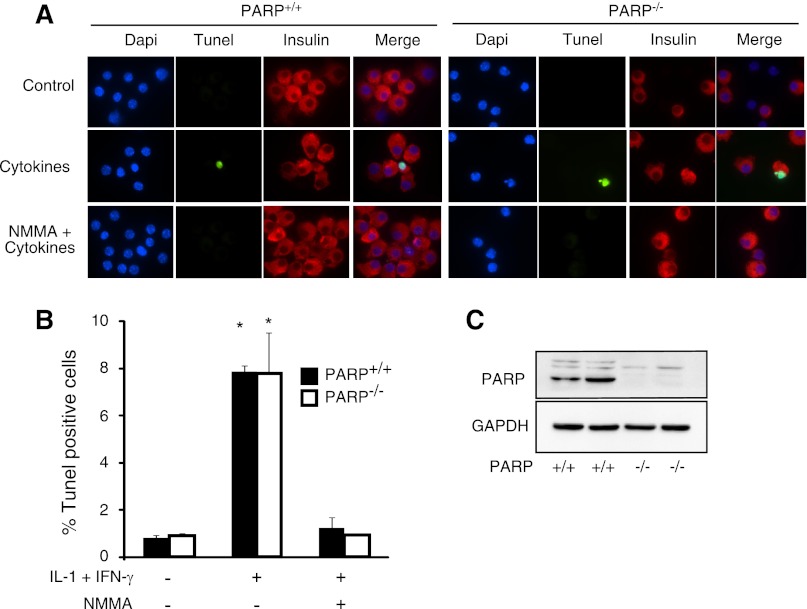
Cytokines induce DNA damage in islet cells isolated from PARP-1−/− mice.
Since islets from PARP-1−/− mice appear to be protected from cytokine-induced death (Fig. 4), yet they are sensitive to cytokine-mediated inhibition of insulin secretion (Fig. 5), we examined effects of cytokine treatment on islet cell viability by a second assay, TUNEL (Fig. 6). While commonly used as an assay of apoptosis, TUNEL detects DNA strand breaks. For these experiments, isolated islets were treated with IL-1 + IFN-γ for 24 h and then dispersed into individual cells. The islet cells were centrifuged onto slides, and DNA integrity was evaluated by TUNEL (green), β-cells were identified by immunohistochemical staining for insulin (red), and nuclei were identified by DAPI staining (blue). As shown in Fig. 6A, cytokine treatment induces DNA damage in insulin-containing cells from PARP-1−/− and PARP-1+/+ islets. The damaging actions of IL-1 + IFN-γ treatment on islet cell DNA integrity are prevented by NMMA, indicating that DNA damage is mediated by NO. Quantification of these data reveals no differences in the levels of DNA damage observed in insulin-containing islet cells from PARP-1+/+ and PARP-1−/− mice, as IL-1 + IFN-γ stimulates a nearly eightfold increase in the number of TUNEL-positive cells, in the presence or absence of PARP-1 (Fig. 6B). Western blot analysis was used to confirm the absence of PARP-1 in islets isolated from PARP-1−/− mice (Fig. 6C).
Fig. 6.
Effects of cytokines on islet cell DNA damage. Islets were isolated from PARP-1+/+ and PARP-1−/− mice and treated for 24 with a combination of cytokines [IL-1 (15 U/ml) + IFN-γ (150 U/ml)] with or without NMMA. Islets were dispersed into individual cells, and integrity of islet cell DNA was examined by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining (green), insulin-containing β-cells by immunocytochemistry (red), and nuclei by 4′,6-diaminido-2-phenylindole (DAPI) staining (blue) (A). Number of insulin-containing cells with DNA damage was quantified by microscopy (B). As a control, PARP-1 levels were examined by Western blot analysis (C). Results are averages ± SE of 3 independent experiments. *Significantly different from control (P < 0.05). There are no statistically significant differences between PARP-1+/+ and PARP-1−/− mice.
DISCUSSION
In the early 1980s, the Okamoto model was proposed to explain damage and death of pancreatic β-cells during the development of diabetes. This model, updated in recent reviews (46), posits that extensive DNA damage leads to the consumption of NAD+ and ATP, which results in the depletion of energy stores and necrotic cell death. Central to this model is the hyperactivation of PARP-1. In response to high levels of DNA damage, PARP-1 is recruited to DNA, where its catalytic activity is dramatically enhanced, resulting in NAD+-dependent polymerization of ADP-ribose conjugated to proteins. These poly(ADP)-ribose units are then rapidly degraded by enzymatic cleavage in an ATP-dependent manner, causing the depletion of cellular NAD+ and ATP and cell death by necrosis (71, 72). Chemotherapeutic agents and chemical toxins, such as the diabetogenic agent STZ, are known to induce DNA damage to levels sufficient to hyperactivate PARP-1 and cause PARP-1-dependent cell death (68, 69). While these studies provide evidence in support of the Okamoto model of β-cell death in response to chemical toxins and DNA-damaging agents (46), the role of PARP-1 in cytokine-mediated β-cell damage has yet to be fully explored.
NO, produced following iNOS expression, is the primary mediator of cytokine-induced β-cell damage. Produced in micromolar levels by β-cells following cytokine treatment, NO inhibits the mitochondrial oxidation of glucose to CO2, glucose-induced insulin secretion, and protein synthesis and induces DNA damage (14, 19). When supplied exogenously using chemical donors such as 3-morpholinosydnonimine, NO induces PARP-1-dependent DNA damage and death of islet cells and insulinoma cells (23, 34). While the effects of PARP-1 inhibition/deletion on NO donor-mediated damage have been examined, the role of PARP-1 in cytokine-mediated β-cell damage has not been explored. In this study, the role of PARP-1 in cytokine-induced β-cell damage was examined using islets and macrophages isolated from PARP-1+/+ and PARP-1−/− mice. Because previous studies suggest that inflammatory mediator signaling is attenuated in macrophages isolated from PARP-1−/− mice (3, 39, 48), we initiated these studies by examining the effects of cytokines, endotoxin, and dsRNA on the activation of signaling cascades responsible for inflammatory gene expression (iNOS) in macrophages and islets. The stimulatory actions of LPS, poly(IC), and IFN-γ on transcription factor (NF-κB or Stat-1 activation), iNOS expression, and NO production by macrophages or islets were not modified in macrophages or islets isolated from PARP-1−/− mice. Thus cytokine- and pathogen-activated molecular pattern signaling [LPS and poly(IC)] is not defective in macrophages or islets isolated from PARP-1−/− mice.
Since PARP-1 is activated in response to DNA damage (e.g., single-strand breaks, double-strand breaks, and DNA deamination) and NO is known to induce islet cell DNA damage (23, 31), the effects of the endogenous production of NO on islet cell viability were examined using islets isolated from PARP-1+/+ and PARP-1−/− mice. Consistent with previous studies (10, 14, 62), 24-h incubation with IL-1 + IFN-γ results in ∼10-fold increase in NO production by islets isolated from PARP-1+/+ mice, and this correlates with a 25% reduction in islet cell viability. In contrast, islets isolated from PARP-1−/− mice appear to be resistant to the loss of viability in response to IL-1 + IFN-γ treatment, even though they produce NO to levels equivalent to those produced by islets from PARP-1+/+ mice. While previous studies show that NO is the primary mediator of cytokine-induced islet damage (10, 14, 62), these findings could suggest that NO is not responsible for the loss of islet cell viability under these conditions.
The protective effect of PARP-1 deficiency on islet cell viability is not congruent with the lack of protection against the inhibitory actions of cytokines on insulin secretion or the damaging actions of cytokines on DNA integrity. This somewhat paradoxical finding may be due to the relative levels of PARP-1 activation (physiological or hyperactivation), which are proportional to the level of DNA damage. In response to IL-1 + IFN-γ-induced NO production, there is a physiological activation of PARP-1 that occurs in response to NO-mediated DNA damage. This level of activation is in contrast to the hyperactivation of PARP-1 that is commonly observed in response to high levels of DNA damage, such as that induced by STZ (8, 46, 71). The result of this physiological PARP-1 activation in response to cytokine treatment is a reduction in NAD+ and ATP levels that prevents the uptake of the neutral red dye into islet cells (7). Consistent with this interpretation, Bolaffi et al. (6) showed that IL-1 reduces rat islet NAD+ levels by 50% and that this effect is prevented by NMMA. Furthermore, the neutral red dye assay is based on the accumulation of this dye in acidic cellular compartments (such as lysosomes), and acidification is based on the ATPase activity; thus the neutral red dye assay is an indirect assay of cellular ATP levels (54). In its absence, this physiological activation of PARP-1 does not take place, thus maintaining ATP at levels sufficient to support the acidification of cellular compartments, allowing for the sequestration of neutral red dye. While these results are suggestive of viable cells that may be resistant to the destructive effects of cytokines, the absence of PARP-1 does not provide protection against the inhibitory actions of IL-1 + IFN-γ on glucose-stimulated insulin secretion or the induction of DNA damage. In contrast, iNOS inhibition prevents the loss of cell viability, as well as the damaging actions of cytokine treatment on insulin secretion and DNA integrity (Figs. 5 and 6).
In contrast to the effects of cytokine-induced NO production, STZ or prolonged incubations with high (millimolar) concentrations of NO donors cause hyperactivation of PARP-1, large (severalfold) reductions in cellular NAD+ and ATP levels, and PARP-1-dependent islet cell death (23, 69). Importantly, a number of studies support different modes of cell death under these conditions. We have shown that short exposure (24- and 48-h incubation) of islets to IL-1 results in low levels of islet cell death that are associated with the NO-dependent release of high-mobility group box-1 protein (62) and necrotic cell death. Studies have shown that PARP-1 overactivation is also associated with the translocation of high-mobility group box-1 protein from the nucleus to the cytoplasm, where it can be released in response to necrotic cell death (13). Recently, we identified the forkhead transcription factor FoxO1 and the NAD+-dependent deacetylase Sirt-1 as central players controlling β-cell fate in response to cytokine treatment (25). In an NO-dependent manner, cytokines stimulate FoxO1 nuclear localization, and when Sirt-1 is active, FoxO1 is deacetylated and stimulates the expression of protective molecules such as GADD45α (25). Under conditions in which Sirt-1 is less active, FoxO1-dependent expression of GADD45α is attenuated, and the expression of proapoptotic genes, such as the Bcl-2 scavenger Puma, is enhanced (25). There also appears to be a regulatory association between PARP-1 and Sirt-1. In response to hyperactivation of PARP-1, the activity of Sirt-1 is attenuated due to NAD+ depletion (51). PARP-1 activity can also be enhanced by acetylation, and Sirt-1 can physically associate with and deacetylate PARP-1, resulting in an attenuation of PARP-1 activity (53). A physiological activation of PARP-1 by NO, produced following short (18–24 h) exposures to cytokines, would be consistent with previous findings (51, 53), as this activation would take place under conditions in which Sirt-1 is also active, and through its deacetylase activity Sirt-1 would further limit the extent of PARP-1 activation.
Overall, these findings suggest that the amount of NO, length of exposure, and extent of DNA damage determine whether PARP-1 participates in cytokine- and NO-dependent β-cell damage. When supplied exogenously for long periods (24 h) and at high (millimolar) concentrations, there is extensive DNA damage, hyperactivation of PARP-1, and PARP-1-dependent necrosis (23, 55, 72). Furthermore, hyperactivation of PARP-1 causes the depletion of cellular levels of ATP, leading to PARP-1-dependent activation of AMP kinase (24, 55). NO, produced exogenously following short (0.5–3 h) exposures to chemical donors or endogenously following cytokine treatment, activates AMP kinase in β-cells; however, this activation is not PARP-1-dependent. NO activates AMP kinase by a pathway that requires the unfolded protein response transducer inositol-requiring enzyme-1α (44). Additional evidence supporting the PARP-1-independent nature of AMP kinase activation by NO includes the absence of ADP-ribose polymer formation in cytokine-treated (24 h) or NO-treated (0.5–3 h) islets. In contrast, hydrogen peroxide, which activates AMP kinase in a PARP-1-dependent manner, stimulates the formation of ADP-ribose polymers (unpublished observations). These findings provide further evidence that cytokines and cytokine-induced NO production do not stimulate hyperactivation of PARP-1, unlike the effects of STZ or other β-cell toxins, which induce high levels of DNA damage in β-cells.
The Okamoto model is an attractive hypothesis to explain β-cell death in response to agents that cause severe DNA damage. This model is consistent with a role for PARP-1 in the loss of β-cell mass and the subsequent development of diabetes in mice treated with a bolus of STZ (46, 69). The model has also been extremely useful in identifying potential pathways that may mediate the loss of β-cell viability and function; however, it seems to fall short of explaining the mechanisms responsible for the loss of β-cell viability and function in response to cytokine treatment. While PARP-1 is likely activated in response to cytokines, this is physiological activation associated with NO-mediated DNA damage. This activation causes loss of cellular NAD+ and ATP to levels that result in what appears to be a loss of viability using biochemical assays of cell death. While cytokines do not appear to reduce the viability of PARP-1−/− islets when examined using this biochemical assay, PARP-1 deficiency does not protect against the induction of DNA damage or the inhibition of glucose-induced insulin secretion in cytokine-treated islets. Unlike the role of PARP-1 in STZ-induced diabetes, these findings suggest that cytokine-induced, NO-dependent β-cell damage is not mediated by the hyperactivation of PARP-1. In contrast, β-cell function and fate in response to cytokines appear to be regulated by NO-dependent activation of FoxO1 and the control of FoxO1 transcriptional activity by Sirt1 (25).
GRANTS
This work was supported by National Institutes of Health Grants DK-52194 and AI-44458 (J. A. Corbett).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.A., K.J.H., P.A.H., and J.A.C. are responsible for conception and design of the research; T.A., G.P.M., and P.A.H. performed the experiments; T.A., G.P.M., K.J.H., P.A.H., and J.A.C. analyzed the data; T.A., G.P.M., K.J.H., P.A.H., and J.A.C. interpreted the results of the experiments; T.A., G.P.M., and J.A.C. prepared the figures; T.A. and J.A.C. drafted the manuscript; T.A., G.P.M., K.J.H., P.A.H., and J.A.C. edited and revised the manuscript; T.A., G.P.M., K.J.H., P.A.H., and J.A.C. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Colleen Kelly Bratcher for expert technical assistance and Drs. Anna Scarim and Michael Moxley for helpful discussions and suggestions.
Present address for K. J. Hughes: Buck Institute for Research on Aging, 8001 Redwood Blvd., Novato, CA 94945.
REFERENCES
- 1. Ame JC, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, Muller S, Hoger T, Menissier-de Murcia J, de Murcia G. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J Biol Chem 274: 17860–17868, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Andersen HU, Jorgensen KH, Egeberg J, Mandrup-Poulsen T, Nerup J. Nicotinamide prevents interleukin-1 effects on accumulated insulin release and nitric oxide production in rat islets of Langerhans. Diabetes 43: 770–777, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Andreone TL, O'Connor M, Denenberg A, Hake PW, Zingarelli B. Poly(ADP-ribose) polymerase-1 regulates activation of activator protein-1 in murine fibroblasts. J Immunol 170: 2113–2120, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Berghammer H, Ebner M, Marksteiner R, Auer B. pADPRT-2: a novel mammalian polymerizing(ADP-ribosyl)transferase gene related to truncated pADPRT homologues in plants and Caenorhabditis elegans. FEBS Lett 449: 259–263, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Bergholdt R, Heding P, Nielsen K, Nolsoe R, Sparre T, Storling J, Nerup J, Pociot F, Mandrup-Poulsen T. Type 1 database mellitus: an inflammatory disease of the islet. Adv Exp Med Biol 552: 129–153, 2004 [PubMed] [Google Scholar]
- 6. Bolaffi JL, Rodd GG, Wang J, Grodsky GM. Interrelationship of changes in islet nicotine adenine dinucleotide, insulin secretion, and cell viability induced by interleukin-1β. Endocrinology 134: 537–542, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24: 119–124, 1985 [DOI] [PubMed] [Google Scholar]
- 8. Burkart V, Blaeser K, Kolb H. Potent beta-cell protection in vitro by an isoquinolinone-derived PARP inhibitor. Horm Metab Res 31: 641–644, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Burkart V, Wang ZQ, Radons J, Heller B, Herceg Z, Stingl L, Wagner EF, Kolb H. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozocin. Nat Med 5: 314–319, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Collier JJ, Fueger PT, Hohmeier HE, Newgard CB. Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes 55: 1398–1406, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci USA 90: 1731–1735, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunningham JM, Green IC. Cytokines, nitric oxide and insulin secreting cells. Growth Regul 4: 173–180, 1994 [PubMed] [Google Scholar]
- 13. Ditsworth D, Zong WX, Thompson CB. Activation of poly(ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol Chem 282: 17845–17854, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eizirik DL, Flodstrom M, Karlsen AE, Welsh N. The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic beta cells. Diabetologia 39: 875–890, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Fehsel K, Jalowy A, Qi S, Burkart V, Hartmann B, Kolb H. Islet cell DNA is a target of inflammatory attack by nitric oxide. Diabetes 42: 496–500, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126: 131–138, 1982 [DOI] [PubMed] [Google Scholar]
- 17. Grishko VI, Druzhyna N, LeDoux SP, Wilson GL. Nitric oxide-induced damage to mtDNA and its subsequent repair. Nucleic Acids Res 27: 4510–4516, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hassa PO, Buerki C, Lombardi C, Imhof R, Hottiger MO. Transcriptional coactivation of nuclear factor-κB-dependent gene expression by p300 is regulated by poly(ADP)-ribose polymerase-1. J Biol Chem 278: 45145–45153, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Heitmeier MR, Corbett JA. Cytotoxic role of nitric oxide in diabetes. In: Nitric Oxide Biology and Pathobiology, edited by Ignarro LJ. San Diego, CA: Academic, 2000, p. 785–810 [Google Scholar]
- 20. Heitmeier MR, Scarim AL, Corbett JA. Double-stranded RNA-induced inducible nitric-oxide synthase expression and interleukin-1 release by murine macrophages requires NF-κB activation. J Biol Chem 273: 15301–15307, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Heitmeier MR, Scarim AL, Corbett JA. Interferon-γ increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem 272: 13697–13704, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Heitmeier MR, Scarim AL, Corbett JA. Prolonged STAT1 activation is associated with interferon-γ priming for interleukin-1-induced inducible nitric-oxide synthase expression by islets of Langerhans. J Biol Chem 274: 29266–29273, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Heller B, Wang ZQ, Wagner EF, Radons J, Burkle A, Fehsel K, Burkart V, Kolb H. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J Biol Chem 270: 11176–11180, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Huang Q, Wu YT, Tan HL, Ong CN, Shen HM. A novel function of poly(ADP-ribose) polymerase-1 in modulation of autophagy and necrosis under oxidative stress. Cell Death Differ 16: 264–277, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Hughes KJ, Meares GP, Hansen PA, Corbett JA. FoxO1 and SIRT1 regulate beta-cell responses to nitric oxide. J Biol Chem 286: 8338–8348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobson MK, Jacobson EL. Discovering new ADP-ribose polymer cycles: protecting the genome and more. Trends Biochem Sci 24: 415–417, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology 120: 190–199, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 53: 1019–1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johansson M. A human poly(ADP-ribose) polymerase gene family (ADPRTL): cDNA cloning of two novel poly(ADP-ribose) polymerase homologues. Genomics 57: 442–445, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Kallmann B, Burkart V, Kroncke KD, Kolb-Bachofen V, Kolb H. Toxicity of chemically generated nitric oxide towards pancreatic islet cells can be prevented by nicotinamide. Life Sci 51: 671–678, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Keefer LK, Wink DA. DNA damage and nitric oxide. Adv Exp Med Biol 387: 177–185, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Kelly CB, Blair LA, Corbett JA, Scarim AL. Isolation of islets of Langerhans from rodent pancreas. Methods Mol Med 83: 3–14, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Kickhoefer VA, Siva AC, Kedersha NL, Inman EM, Ruland C, Streuli M, Rome LH. The 193-kD vault protein, VPARP, is a novel poly(ADP-ribose) polymerase. J Cell Biol 146: 917–928, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kolb H, Burkart V. Nicotinamide in type 1 diabetes. Mechanism of action revisited. Diabetes Care 22 Suppl 2: B16–B20, 1999 [PubMed] [Google Scholar]
- 35. Kwon G, Corbett JA, Hauser S, Hill JR, Turk J, McDaniel ML. Evidence for involvement of the proteasome complex (26S) and NFκB in IL-1β-induced nitric oxide and prostaglandin production by rat islets and RINm5F cells. Diabetes 47: 583–591, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16: 35–39, 1967 [DOI] [PubMed] [Google Scholar]
- 37. Le Page C, Sanceau J, Drapier JC, Wietzerbin J. Inhibitors of ADP-ribosylation impair inducible nitric oxide synthase gene transcription through inhibition of NF-κB activation. Biochem Biophys Res Commun 243: 451–457, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51: 216–226, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Liaudet L, Pacher P, Mabley JG, Virag L, Soriano FG, Hasko G, Szabo C. Activation of poly(ADP-ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med 165: 372–377, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Maggi LB, Jr, Heitmeier MR, Scheuner D, Kaufman RJ, Buller RM, Corbett JA. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO J 19: 3630–3638, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maggi LB, Jr, Moran JM, Buller RM, Corbett JA. ERK activation is required for double-stranded RNA- and virus-induced interleukin-1 expression by macrophages. J Biol Chem 278: 16683–16689, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol 66: 1433–1440, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Masutani M, Suzuki H, Kamada N, Watanabe M, Ueda O, Nozaki T, Jishage K, Watanabe T, Sugimoto T, Nakagama H, Ochiya T, Sugimura T. Poly(ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin-induced diabetes. Proc Natl Acad Sci USA 96: 2301–2304, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meares GP, Hughes KJ, Naatz A, Papa FR, Urano F, Hansen PA, Benveniste EN, Corbett JA. IRE1-dependent activation of AMPK in response to nitric oxide. Mol Cell Biol 31: 4286–4297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nguewa PA, Fuertes MA, Valladares B, Alonso C, Perez JM. Poly(ADP-ribose) polymerases: homology, structural domains and functions. Novel therapeutical applications. Prog Biophys Mol Biol 88: 143–172, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Okamoto H, Takasawa S. Recent advances in the Okamoto model: the CD38-cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in beta-cells. Diabetes 51 Suppl 3: S462–S473, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Oliver FJ, Menissier-de Murcia J, de Murcia G. Poly(ADP-ribose) polymerase in the cellular response to DNA damage, apoptosis, and disease. Am J Hum Genet 64: 1282–1288, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oliver FJ, Menissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, de la Rubia G, Stoclet JC, de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly(ADP-ribose) polymerase-1 deficient mice. EMBO J 18: 4446–4454, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pero RW, Axelsson B, Siemann D, Chaplin D, Dougherty G. Newly discovered anti-inflammatory properties of the benzamides and nicotinamides. Mol Cell Biochem 193: 119–125, 1999 [PubMed] [Google Scholar]
- 50. Pieper AA, Brat DJ, Krug DK, Watkins CC, Gupta A, Blackshaw S, Verma A, Wang ZQ, Snyder SH. Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes. Proc Natl Acad Sci USA 96: 3059–3064, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2α deacetylase activity. J Biol Chem 280: 43121–43130, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Rabinovitch A, Suarez-Pinzon WL, Strynadka K, Schulz R, Lakey JR, Warnock GL, Rajotte RV. Human pancreatic islet beta-cell destruction by cytokines is independent of nitric oxide production. J Clin Endocrinol Metab 79: 1058–1062, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Rajamohan SB, Pillai VB, Gupta M, Sundaresan NR, Birukov KG, Samant S, Hottiger MO, Gupta MP. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol 29: 4116–4129, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protocols 3: 1125–1131, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer 10: 293–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sallmann FR, Vodenicharov MD, Wang ZQ, Poirier GG. Characterization of sPARP-1. An alternative product of PARP-1 gene with poly(ADP-ribose) polymerase activity independent of DNA strand breaks. J Biol Chem 275: 15504–15511, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Sandler S, Bendtzen K, Borg LA, Eizirik DL, Strandell E, Welsh N. Studies on the mechanisms causing inhibition of insulin secretion in rat pancreatic islets exposed to human interleukin-1β indicate a perturbation in the mitochondrial function. Endocrinology 124: 1492–1501, 1989 [DOI] [PubMed] [Google Scholar]
- 58. Scarim AL, Arnush M, Blair LA, Concepcion J, Heitmeier MR, Scheuner D, Kaufman RJ, Ryerse J, Buller RM, Corbett JA. Mechanisms of beta-cell death in response to double-stranded (ds) RNA and interferon-γ: dsRNA-dependent protein kinase apoptosis and nitric oxide-dependent necrosis. Am J Pathol 159: 273–283, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 7: 517–528, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282: 1484–1487, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Sodhi RK, Singh N, Jaggi AS. Poly(ADP-ribose) polymerase-1 (PARP-1) and its therapeutic implications. Vascul Pharmacol 53: 77–87, 2010 [DOI] [PubMed] [Google Scholar]
- 62. Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med 3: e17, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Turk J, Corbett JA, Ramanadham S, Bohrer A, McDaniel ML. Biochemical evidence for nitric oxide formation from streptozotocin in isolated pancreatic islets. Biochem Biophys Res Commun 197: 1458–1464, 1993 [DOI] [PubMed] [Google Scholar]
- 64. Wang ZQ, Auer B, Stingl L, Berghammer H, Haidacher D, Schweiger M, Wagner EF. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev 9: 509–520, 1995 [DOI] [PubMed] [Google Scholar]
- 65. Wilson GL, Hartig PC, Patton NJ, LeDoux SP. Mechanisms of nitrosourea-induced beta-cell damage. Activation of poly(ADP-ribose) synthetase and cellular distribution. Diabetes 37: 213–216, 1988 [DOI] [PubMed] [Google Scholar]
- 66. Wilson GL, Leiter EH. Streptozotocin interactions with pancreatic beta cells and the induction of insulin-dependent diabetes. Curr Top Microbiol Immunol 156: 27–54, 1990 [DOI] [PubMed] [Google Scholar]
- 67. Woodhouse BC, Dianov GL. Poly ADP-ribose polymerase-1: an international molecule of mystery. DNA Repair (Amst) 7: 1077–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 68. Yamamoto H, Uchigata Y, Okamoto H. DNA strand breaks in pancreatic islets by in vivo administration of alloxan or streptozotocin. Biochem Biophys Res Commun 103: 1014–1020, 1981 [DOI] [PubMed] [Google Scholar]
- 69. Yamamoto H, Uchigata Y, Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature 294: 284–286, 1981 [DOI] [PubMed] [Google Scholar]
- 70. Yu M, Schreek S, Cerni C, Schamberger C, Lesniewicz K, Poreba E, Vervoorts J, Walsemann G, Grotzinger J, Kremmer E, Mehraein Y, Mertsching J, Kraft R, Austen M, Luscher-Firzlaff J, Luscher B. PARP-10, a novel Myc-interacting protein with poly(ADP-ribose) polymerase activity, inhibits transformation. Oncogene 24: 1982–1993, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev 18: 1272–1282, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev 20: 1–15, 2006 [DOI] [PubMed] [Google Scholar]