Extracellular adenosine acts as a neuromodulator through two subtypes of membrane-bound receptors. Agonist activation of A1- or A2- adenosine receptors is generally linked to an inhibitory or stimulatory effect, respectively, on the enzyme adenylate cyclase, which converts intracellular 5′-ATP to cyclic 3′,5′-AMP. Many N6-alkyl- and N6-aryl-substituted adenosine analogs have been synthesized as potent agonists having nanomolar Ki values at the high-affinity A1 site.1 At each receptor subtype, xanthines act as competitive antagonists. Nonselective antagonists caffeine and theophylline have Ki values in the 10 μM range, and at A1 receptors some synthetic 1,3-dialkyl-8-substituted analogs have Ki values close to 1 nM.2 Adenosine antagonism is the principal mechamsm by which caffeine acts as a stimulant in vivo.
The avidin–biotin complex has been utilized in studies directed toward the isolation, histochemical localization, and microscopic structural probing of the receptor protein.3 Both adenosine and xanthine analogs have been coupled to biotin,4,5 forming bifunctional probes to serve as noncovalent cross-linkers between adenosine receptors and avidin. Structure–activity relationships for adenosine agonists and antagonists were studied initially to identify potential sites for derivatization. Since neither adenosine nor theophylline contains a readily derivatized functional group that is nonessential for biological activity, a “functionalized congener” methodology1,2 was used. In this approach, a chemically functionalized chain (terminating in an amine or carboxylic acid) is incorporated in the molecular structure. Insensitive structural sites for attachment of chains were located, and the effect on activity of substituents present in a sequentially extended chain were investigated. In brief, an N6- p-(carboxymethyl)phenyl substituent on adenosine or an 8-p-(car-boxymethyloxy)phenyl substituent on 1,3-dipropylxanthine provided sites for functionalization tolerated at the receptor. Furthermore, the enhanced affinity of certain related amide derivatives for adenosine receptors indicated accessory binding sites at or near the receptor.
A range of chain lengths separating biotin and adenosine pharmacophore moieties were synthesized (Table 1). Spacer groups consisting of ε-aminocaproic acid and oligoglycine were utilized. The affinity-enhancing effect noted previously1,2 of an amino group present on the chains of congeners in the series of ADAC and XAC suggested substitution of an additional amino group on the ε-aminocaproyl spacer, as in the d-Iysyl residue5 of compound 3. The ε-biotinyl-d-amino acid isomer (equivalenl to d-biocytin) was included to minimize proteolytic cleavage, a potential problem in biological assay systems. Nα-(9-Fluorenemethyloxycarbonyl)-d-biocytin was prepared as a synthetic intermediate. The base-sensitive FMOC protecting group, removable in 10% diethylamine in dimethylformamide, was used because of the acid lability of purine nucleosides.
TABLE I.
Biotinyl Conjugates of Adenosine Receptor Ligandsa
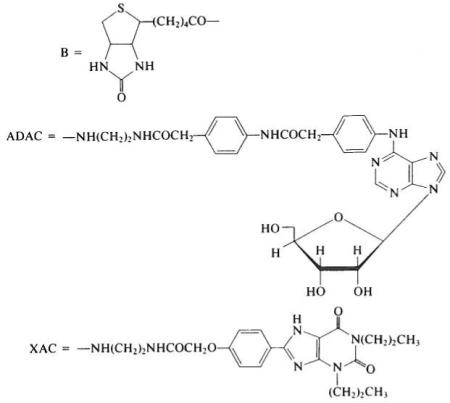
| Biotinyl (B) derivative |
Ki (no avidin) |
Ki (+ avidin) |
|---|---|---|
| (1) B–ADAC | 11.4 | 36 |
| (2) B–NH(CH2)5CO-ADAC | 18 | 35 |
| (3) B–NH(CH2)4CHNH2CO–ADAC (d-configuration) | 8.9 | n.d.b |
| (4) B–XAC | 54 | >500 |
| (5) B–NH(CH2)5CO–XAV | 50 | >500 |
| (6) B–NH(CH2)5CO-Gly3–XAC | 50 | 260 |
Synthetic Methods
ADAC and XAC (amine congeners, see Table 1) are available from Research Biochemicals, Inc. (Natick, MA).
Nα-(9-Fluorenemethyloxycarbonyl)-d-biocytin.5 Nα(9-fluorenemethyloxycarbonly)-N-ε-benzyloxycarbonyl-d-lysine5 (0.70 g, 1.4 mmol) is treated with 30% HBr–acetic acid for 1 hr. Most of the solvent is removed under a nitrogen stream, and the residue is purified by preparative thin-layer chromatography (silica, chloroform–methanol–acetic acid, 70: 25: 5, v/v) to give Nα(9-fluorenemethyloxycarbonyl)-d-lysine as an amorphous glass (0.14 g, 27% yield).
FMOC-d-lysine (83 mg, 0.23 mmol) is combined with succinimidyl-biotin (88 mg, 0.23 mmol) and diisopropylethylamine (39 μl, 0.23 mmol) in 5 ml of dimethylformamide-tetrahydrofuran (2: 1) and stirred overnight. Ethyl acetate and 0.1 M HCl are added, and the phases are mixed. The upper phase is washed with water and evaporated, and the residue is triturated with ethyl acetate and petroleum ether. The solid product, Nα(9-fluorenemethyloxycarbonyl)-d-biocytin, is collected. Yield, 63 mg (43%). mp 139–143°. [α]D at 23° in dimethylformamide, 36.5°. The product is then coupled to ADAC5 and deprotected to give compound 3.
Biotinyl-ε-aminocaproyl-ADAC (2). ADAC5 (an adenosine amine congener, 17 mg. 29 μmol) is suspended in 0.5 ml of dimethylformamide and treated with succinimidyl 6-(biotinamido)hexanoate (Pierce Chemical Co., Rockford, IL; 17 mg, 36 μmol). Solubilization of thc reactants is followed by gradual precipitation of the product. Methanol (1 ml) and dry ether (2 ml) are added. The product is obtained by filtration. Yield, 25 mg (93%), mp 195–198°.
Other biotin conjugates, 1 and 4–6, are prepared similarly by acylation of a xanthine or adenosine amine congener1,2,5 using biotinyl-N-hydroxysuccinimide ester. The pure biotin conjugates are recrystallized from dimethylformamide–ether and characterized by thin-layer chromatography [chloroform–methanol–acetic acid, 85: 10 : 5, v/v; biotin derivatives turn pink after spraying with a fresh 0.1% solution of 4-(dimethylamino)cinnamaldehyde in ethanolic sulfuric acid, 1%, then heating], elemental analysis, NMR, and mass spectroscopy. The low volatility precludes using standard chemical ionization mass spectrometry. Instead, californium plasma desorption mass spectrometry6 can be used and generally gives intense positive ion peaks corresponding to (M + H)+ and (M + Na)+.
Biochemical and Physiological Characterization
Affinity at A1-adenosine receptors is determined in a competitive binding assay using a tritiated agonist radioligand (see Table 1).4,5 The potencies of both free ligand and that which is precomplexed to avidin4,7 are measured. All of the adenosine conjugates (1–3) bind simultaneously to A1-adenosine receptors and to avidin. Thus, based on estimates of the extended chain lengths of the conjugates and the depth of the biotin binding site, the adenosine agonist binding site must be located within 12 Å from the receptor protein surface. In the form of avidin complexes, the xanthine conjugates lose their affinity for the receptor, which suggests conformational or topographical differences betwecn agonist- and antagonist-occupied receptors.
Adenosine acts as a potent vasodilator through vascular A2 receptors. When infused directly into canine coronary arteries,8 compounds 1 and 2 are 3.0 ± 0.2-fold and 10.2 ± 7.3-fold, respectively, more potent than adenosine. In the form of avidin complexes (purified by gel filtration), only the longer chain derivative 2 is active (relative potency 2.1 ± 0.4), with a slow onset of action and fast washout. Compound 1 is active in stimulating adenylate cyclase9 through A2-adenosine receptors in human platelets (EC50 2.43 μM).
References
- 1.Jacobson KA, Kirk KL, Padgett WL, Daly JW. J. Med. Chem. 1985;28:134. doi: 10.1021/jm00147a039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson KA, Kirk KL, Padgett WL, DOly JW. J. Med. Chem. 1985;28:1334. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stiles GL. Trends Pharmacol. Sci. 1986;7:486. [Google Scholar]
- 4.Jacobson KA, Kirk KL, Padgett W, Daly JW. FEBS Lett. 1985;184:30. doi: 10.1016/0014-5793(85)80646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson KA, Ukena D, Padgett W, Kirk KL, Daly JW. Biochem. Pharmacol. 1987;36:1697. doi: 10.1016/0006-2952(87)90056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson KA, Pannell LK, Kirk KL, Fales HM, Sokoloski EA. J. Chem. Soc., Perkin Trans. 1986;1:2143. [Google Scholar]
- 7.Kohanski RA, Lane MD. J. Biol. Chem. 1985;260:5014. [PubMed] [Google Scholar]
- 8.Jacobson KA, Yamada N, Kirk KL, Padgett WL, Daly JW, Olsso RA. Biochem. Biophys. Res. Commun. 1986;136:1097. doi: 10.1016/0006-291x(86)90446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jacobson KA, Yamada N, Kirk KL, Padgett WL, Daly JW, Olsson RA. Biochem. Biophys. Res. Commun. 1986;139:375. doi: 10.1016/0006-291x(86)90446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ukena D, Olsson RA, Daly JW. Can. J. Physiol. Pharmacol. 1985;65:365. doi: 10.1139/y87-063. [DOI] [PubMed] [Google Scholar]


