Background: The protein kinase Plk1 plays a crucial role in mitotic spindle pole integrity.
Results: Furry binds to Plk1 and Aurora A and promotes Aurora A-mediated Plk1 activation.
Conclusion: Furry specifies the spatiotemporal regulation of Plk1 at centrosomes in early mitosis.
Significance: Our data indicate a new mechanism of the regulation of Plk1 activity and bipolar spindle organization during mitosis.
Keywords: Cyclin-dependent Kinase (CDK), Centrosome, Mitotic Spindle, Protein Kinases, Spindle Pole Body, Aurora A, Furry, Polo-like Kinase 1
Abstract
Bipolar mitotic spindle organization is fundamental to faithful chromosome segregation. Furry (Fry) is an evolutionarily conserved protein implicated in cell division and morphology. In human cells, Fry localizes to centrosomes and spindle microtubules in early mitosis, and depletion of Fry causes multipolar spindle formation. However, it remains unknown how Fry controls bipolar spindle organization. This study demonstrates that Fry binds to polo-like kinase 1 (Plk1) through the polo-box domain of Plk1 in a manner dependent on the cyclin-dependent kinase 1-mediated Fry phosphorylation at Thr-2516. Fry also binds to Aurora A and promotes Plk1 activity by binding to the polo-box domain of Plk1 and by facilitating Aurora A-mediated Plk1 phosphorylation at Thr-210. Depletion of Fry causes centrosome and centriole splitting in mitotic spindles and reduces the kinase activity of Plk1 in mitotic cells and the accumulation of Thr-210-phosphorylated Plk1 at the spindle poles. Our results suggest that Fry plays a crucial role in the structural integrity of mitotic centrosomes and in the maintenance of spindle bipolarity by promoting Plk1 activity at the spindle poles in early mitosis.
Introduction
The bipolarity of mitotic spindles is essential for ensuring accurate chromosome segregation into two daughter cells and genomic stability during mitosis in eukaryotic cells. Centrosomes play a fundamental role in the formation of bipolar spindles. Centrosome abnormalities result in the failure of bipolar spindle organization, which leads to chromosome segregation errors and genomic instability and potentially contributes to the generation of aneuploidy and tumorigenesis (1, 2).
Centrosomes are the principal microtubule (MT)2-organizing centers, consisting of a pair of centrioles surrounded by pericentriolar material in which γ-tubulin ring complexes function as the major MT nucleation cores (3). To generate mitotic spindles, the single centrosome in G1 phase is duplicated in S phase. The duplicate centrosomes subsequently mature by recruiting additional PCMs, separate and move to opposite sites in the cell from G2 phase to prophase. After the onset of nuclear envelope breakdown, MTs emanating from the two diametrically opposed centrosomes capture the paired sister chromosomes via their kinetochores in prometaphase and align them in the center of the bipolar spindles at metaphase. Failure of centrosome duplication, maturation, or separation results in monopolar spindle formation. Because centrosomes at spindle poles are subjected to MT-mediated mechanical forces, they must be strengthened to resist these forces and maintain spindle bipolarity (4). Loss of centrosome structural integrity causes centrosome fragmentation and consequent multipolar spindle formation. Thus, strict control of centrosome structural integrity and function is essential for bipolar spindle organization.
Polo-like kinase 1 (Plk1) is a key regulator of cell division that functions in multiple stages of mitosis, including mitotic entry, centrosome maturation and separation, spindle pole integrity, kinetochore attachment, and cytokinesis (5–7). Plk1 localizes to centrosomes from late G2 to metaphase, and loss of Plk1 function often induces spindle pole fragmentation and multipolar spindle formation, implicating Plk1 in spindle pole integrity (8–11). Previous studies showed that Plk1 contributes to the integrity of centrosomes and centrioles by phosphorylating Kizuna, which stabilizes focused spindle poles (12), and Sgo1, which protects against premature centriole disengagement (13). Plk1 consists of an N-terminal kinase domain and a C-terminal Polo-box domain (PBD). Plk1 is activated by the Aurora A-catalyzed phosphorylation of Thr-210 in the kinase domain (14, 15). The PBD regulates the kinase activity and localization of Plk1 by binding to the specific peptide motif that is phosphorylated by priming kinases, such as cyclin-dependent kinase 1 (Cdk1) (16, 17). However, the exact mechanism that regulates Plk1 activity and localization in each phase of the cell cycle has not yet been elucidated.
Furry (Fry) is an evolutionarily conserved protein in eukaryotes, with a high molecular mass (∼300 kDa) with HEAT/Armadillo-like repeats near the N terminus (18). Fry in Drosophila and it orthologs (Sax-2 in nematode, Tao3p in budding yeast, and Mor2p in fission yeast) genetically interact with members of the NDR family of Ser/Thr kinases (termed Trc in fruit fly, Sax-1 in nematode, Cbk1p in budding yeast, and Orb6p in fission yeast). These proteins are implicated in the regulation of cell division, cell morphogenesis, neurite outgrowth, and dendrite tiling (19, 20). We have shown that mammalian Fry is an MT-binding protein, localizing to centrosomes and spindle MTs in early mitosis, and that Fry binds to and activates NDR1 kinase (21). Depletion of Fry causes chromosome misalignment and spindle deformation in human mitotic cells, suggesting that Fry regulates chromosome alignment and bipolar spindle formation in mitosis (21). However, the mechanisms by which Fry controls these events remain unknown. In this study, we investigated how Fry controls bipolar spindle organization and show evidence that Fry regulates centrosome and centriole integrity by promoting Plk1 activity on centrosomes in early mitosis.
EXPERIMENTAL PROCEDURES
Plasmid Construction
The cDNA coding for mouse Fry was cloned as described previously (21). The cDNAs for Cdk1 and Plk1 were PCR-amplified from a human brain cDNA library. The cDNA for human Aurora A was provided by Y. Terada (Waseda University, Japan). These cDNAs were subcloned into the FPC1-Myc, FPC1-HA, pEGFP-C1, pEYFP-C1, pECFP-C1 (Clontech), pGEX (GE Healthcare), and pcDNA3.1/Myc+His (Invitrogen) expression vectors. The cDNA plasmids expressing the Fry- and Plk1-truncated mutants were constructed by PCR amplification and subcloned into expression vectors. The cDNAs for the point mutants Fry-IV(T2516A), Fry-IV(T2516E), Plk1(D194A), Plk1(S137D/T210D), Cdk1(D146N), and Aurora A(K162R) were constructed using the QuikChange site-directed mutagenesis kit (Stratagene). For protein expression in the baculovirus system, cDNAs coding for GST- or (Myc+His)-tagged proteins were subcloned into a pFastBac1 vector (Invitrogen).
RNA Interference
The Stealth siRNA sequence used for targeting human Fry was 5′-UUUACUUCCCGGAGCAGGAAGUUGG-3′ (Invitrogen). Stealth RNAi negative control (Invitrogen) was used as control siRNA.
Reagents and Antibodies
Nocodazole (Sigma), DAPI (Sigma), MG132 (Sigma), TO-PRO-3 (Molecular Probes), BI-2536 (Axon Medchem), purvalanol A (Calbiochem), staurosporine (Merck), and MLN8237 (Selleck Chemicals) were purchased. Rabbit polyclonal antibodies specific to human Fry were raised against the C-terminal peptide (21). Other antibodies were purchased as follows: Myc (9E10, Roche), HA (3F10, Roche), GFP (Molecular Probes), Plk1 (Invitrogen), Plk1-pT210 and cyclin B1 (BD Biosciences), MPM2 (Upstate), α-tubulin (Sigma), γ-tubulin (Sigma), pericentrin (Covance), and centrin (Sigma).
Cell Culture, Transfection, and Synchronization
HeLa and 293T cells were cultured in DMEM supplemented with 10% FCS. Cells were transfected with expression plasmids using Lipofectamine-2000 (Invitrogen) or FuGENE6 (Promega). The siRNA was transfected with RNAi MAX (Invitrogen) in serum-free medium. To examine the effect of Fry siRNA on spindle formation, HeLa cells transfected with 20 nm siRNA were cultured in DMEM for 12 h and in 2 mm thymidine-containing medium for 24 h, released from thymidine block for 10–12 h, and then fixed and stained. To inhibit Aurora A, 100 nm MNL8237 was added with 10 μm MG132 for 2 h before fixation. To measure Plk1 activity in mitosis, HeLa cells transfected with siRNAs were cultured in DMEM for 12 h and in thymidine-containing medium for 36 h and then synchronized in early mitotic phase by 0.3 μm nocodazole for 12–14 h. Mitotic cells were collected by the mechanical shake-off method (22). To prepare cells in which Plk1 is inactivated during mitosis, HeLa cells transfected with control siRNA were exposed to 300 nm BI-2536 for 2 h before collection. For in vitro kinase assay, 293T cells were synchronized in early mitotic phase by a single thymidine block for 12 h, followed by 0.3 μm nocodazole for 14–16 h, and then harvested by scraping. For purvalanol A or staurosporine exposure, mitotic HeLa cells arrested with 0.3 μm nocodazole were collected by the mechanical shake-off procedure. After removal of nocodazole, mitotic cells were replated and incubated with medium containing 25 μm purvalanol A or 10 μm staurosporine for 2 h in the presence of 10 μm MG132 (23).
Immunofluorescence Microscopy
HeLa cells grown on coverslips were washed with phosphate-buffered saline and fixed with 4% paraformaldehyde at 37 °C for 10 min. This was followed by methanol fixation at −20 °C for 10 min or methanol fixation alone. For centrin or Plk1 pT210 staining or Fry/Plk1 costaining, cells were washed with PHEM buffer (100 mm PIPES (pH 6.8), 20 mm HEPES (pH 6.9), 4 m glycerol, 5 mm EGTA, and 2 mm MgCl2) and permeabilized with 0.1% Triton X-100 in PHEM buffer for 1 min before fixation. Coverslips were blocked with 2% FCS or 2% bovine serum albumin in phosphate-buffered saline for 30 min and incubated with primary antibodies for 1 h at room temperature or overnight at 4 °C and then secondary antibodies for 1 h at room temperature. Fluorescent images were obtained using a LSM510 or LSM710 confocal microscope (Carl Zeiss) equipped with a Plan Apochromat ×63 or ×100 oil immersion objective lens (NA 1.4) using LSM510 software v3.2 or LSM ZEN 2009 software (Carl Zeiss) at room temperature.
Immunoprecipitation and Immunoblotting
Immunoprecipitation and immunoblot analyses were performed as described previously (23).
Protein Purification
GST- or (Myc+His)-tagged proteins were expressed in Sf21 cells by baculovirus (Invitrogen) and purified as described previously (24). GST- and (Myc+His)-tagged proteins were purified with glutathione-Sepharose (GE Healthcare) and nickel-nitrilotriacetic acid-agarose (Qiagen), respectively. Recombinant Fry was purified by cleaving GST-Fry with PreScission protease (GE Healthcare).
GST Pull-down Assay
GST-tagged proteins were expressed in Sf21 cells by baculovirus and purified with glutathione-Sepharose. Cell lysates were precleared with protein A-Sepharose, and supernatants were incubated with GST-tagged proteins bound to glutathione-Sepharose beads. After centrifugation, the beads were washed three times, and samples were subjected to immunoblot analyses.
In Vitro Kinase Assay
Lysates of 293T cells expressing HA-Cdk1, HA- or Myc-Plk1, or Myc-Aurora A were mixed with lysates of 293T cells expressing GFP- or YFP-Fry mutants and immunoprecipitated. Immunoprecipitates were washed twice with lysis buffer containing 500 mm NaCl, three times with lysis buffer containing 150 mm NaCl, and then once with kinase buffer (21). Kinase reactions were conducted in 20 μl of kinase buffer containing 50 mm ATP and 2.5 μCi [γ-32P]ATP for 1 h at 30 °C. The reaction mixture was separated by SDS-PAGE and analyzed by autoradiography to measure 32P-labeled protein. To measure the effect of Cdk1, HA-Cdk1 was immunoprecipitated and mixed with purified Fry and (Myc+His)-Plk1(SD/TD) and subjected to kinase reactions. Similarly, the kinase activity of endogenous Plk1 in mitotic HeLa cells and purified (Myc+His)-Plk1(SD/TD) was measured using 2 μg myelin basic protein as a substrate. To measure the kinase activity of Aurora A, YFP-Aurora A immunoprecipitated from nocodazole-treated 293T cells was incubated with 1 μg of purified GST or GST-Fry-IV and subjected to kinase reactions using 1 μg of purified (Myc+His)-Plk1(DA) or histone H3 as a substrate for 15 min at 30 °C.
Statistical Analysis
Data are presented as the means ± S.E. of triplicate experiments. p values were calculated using an unpaired two-tailed Student's t test in the Microsoft Excel software. p < 0.05 was considered to be significant.
RESULTS
Depletion of Fry Causes Chromosome Misalignment and Multipolar Spindle Formation
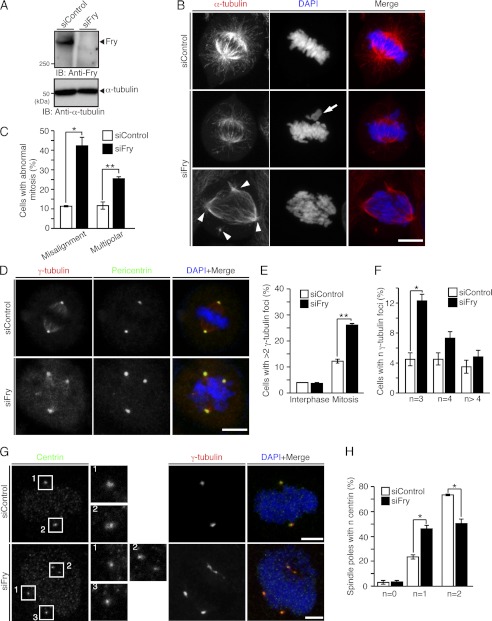
We showed previously that knockdown of Fry by shRNA causes chromosome misalignment and spindle deformation in metaphase (21). To further explore the role of Fry in spindle formation, the effect of Fry depletion by siRNA duplex on mitotic spindle organization was assessed in HeLa cells. Fry siRNA suppressed Fry expression (Fig. 1A). Staining of mitotic HeLa cells with anti-α-tubulin antibody and DAPI revealed that Fry siRNA significantly increased the number of cells displaying chromosome misalignment and multipolar spindles (cells with more than two spindle poles) compared with control siRNA (Fig. 1, B and C). Cell staining with antibodies against γ-tubulin and pericentrin (centrosome marker proteins) revealed that these proteins localized to all spindle poles in multipolar spindles in Fry-depleted cells (Fig. 1D). Fry depletion increased the number of cells possessing more than two γ-tubulin-positive centrosomes in mitosis but not in interphase (Fig. 1E), indicating that Fry is involved in the maintenance of bipolar spindle pole integrity in mitosis and that Fry depletion does not trigger centrosome amplification in interphase. Fry depletion significantly increased the number of cells with three or four γ-tubulin foci, whereas the number of cells with more than four foci increased only slightly (Fig. 1F).
FIGURE 1.
Depletion of Fry causes chromosome misalignment and multipolar spindle formation. A, immunoblot analysis of endogenous Fry. HeLa cells transfected with control or Fry siRNA were cultured for 60 h, and lysates were immunoblotted (IB) with anti-Fry antibody. B, Fry depletion causes chromosome misalignment and multipolar spindle formation. HeLa cells transfected with control or Fry siRNA were cultured for 12 h in growth medium and then for 24 h in thymidine-containing medium. They were then released from thymidine arrest for 12 h before being fixed and stained for α-tubulin (red). DNA was stained with DAPI (blue). Scale bar = 10 μm. The arrow indicates misaligned chromosomes. The arrowheads indicate multiple spindle poles. C, percentages of cells with misaligned chromosomes and multipolar spindles. Data are means ± S.E. of triplicate experiments (more than 100 cells were scored for each experiment). *, p < 0.05; **, p < 0.01. D, Fry depletion causes extra centrosomal foci in mitosis. HeLa cells transfected with control or Fry siRNA were synchronized as in B and stained for γ-tubulin (red) and pericentrin (green). DNA was stained with DAPI (blue). Scale bar = 10 μm. E, percentages of cells with > 2 γ-tubulin-positive foci in control or Fry siRNA-treated HeLa cells in interphase or mitosis. F, percentages of cells with three, four, and more than four γ-tubulin-positive foci in control or Fry siRNA-treated HeLa cells in mitosis. In E and F, data are means ± S.E. of triplicate experiments (more than 100 cells for each experiment). *, p < 0.05; **, p < 0.01. G, Fry depletion causes centriole splitting. HeLa cells transfected with control or Fry siRNA were synchronized as in B. Cells were permeabilized, and then fixed and stained for centrin (green) and γ-tubulin (red). DNA was stained with DAPI (blue). Magnified images of numbered boxes are shown. Scale bars = 5 μm. H, quantitative analysis of spindle poles with no, one, and two centrioles in control or Fry siRNA-treated HeLa cells. Data are the means ± S.E. of triplicate experiments (more than 50 cells were scored for each experiment). *, p < 0.05.
Centriole splitting in early mitosis can cause spindle pole instability and multipolar spindle formation (2). To determine whether centriole splitting is involved in multipolar spindle formation in Fry-depleted cells, the number of centrioles in each spindle pole was counted. Immunostaining of centrin (centriole marker) revealed that Fry depletion caused centriole splitting (Fig. 1G). In control cells, 73% of spindle poles contained two centrioles. In contrast, in Fry-depleted cells, the number of spindle poles containing two centrioles decreased to 51%, whereas the number of spindle poles containing only one centriole increased to 47% (Fig. 1H). Thus, centriole splitting contributes at least in part to Fry depletion-induced multipolar spindle formation.
Fry and Plk1 Colocalize to Spindle Poles in Early Mitosis
Plk1 is known to be a mitotic kinase essential for spindle pole integrity (8–12). To study the role of Fry in spindle organization, the potential colocalization of Fry and Plk1 was assessed by immunofluorescence. As reported previously, (21), Fry was distributed diffusely throughout the cytoplasm in interphase but localized to the separating centrosomes in prophase, to the spindle poles and spindle MTs in prometaphase to metaphase, to spindle MTs in anaphase, and to the distal sections of the midbody in cytokinesis (supplemental Fig. S1). Plk1 was detected during mitosis and localized to centrosomes from prophase to metaphase, to the central spindle in anaphase, and to the midbody in cytokinesis (supplemental Fig. S1), as reported previously (6). Thus, Fry and Plk1 colocalized to separating centrosomes and spindle poles from prophase to metaphase in mitosis but do not colocalize in other stages of the cell cycle.
Fry Binds to the PBD of Plk1
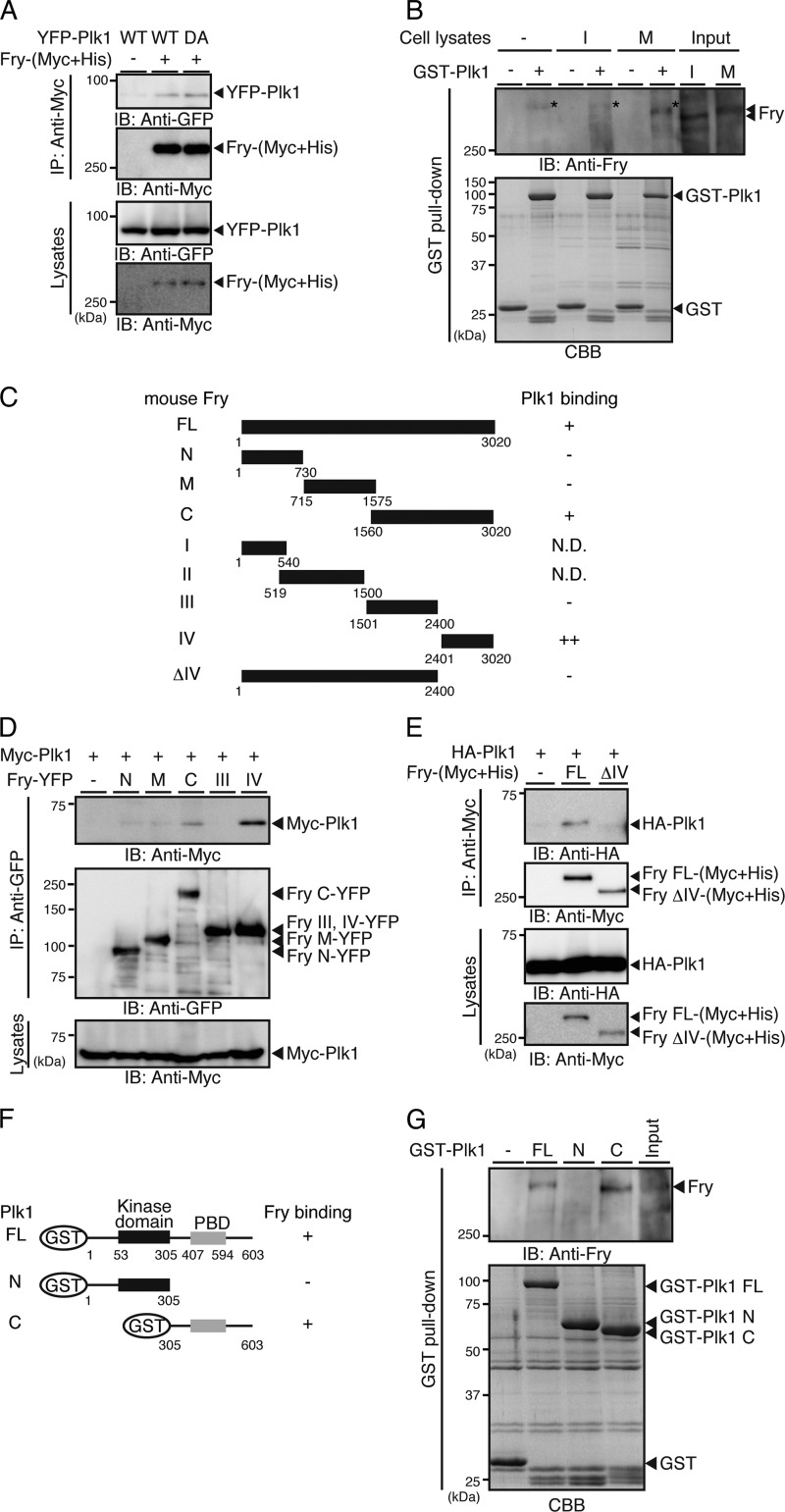
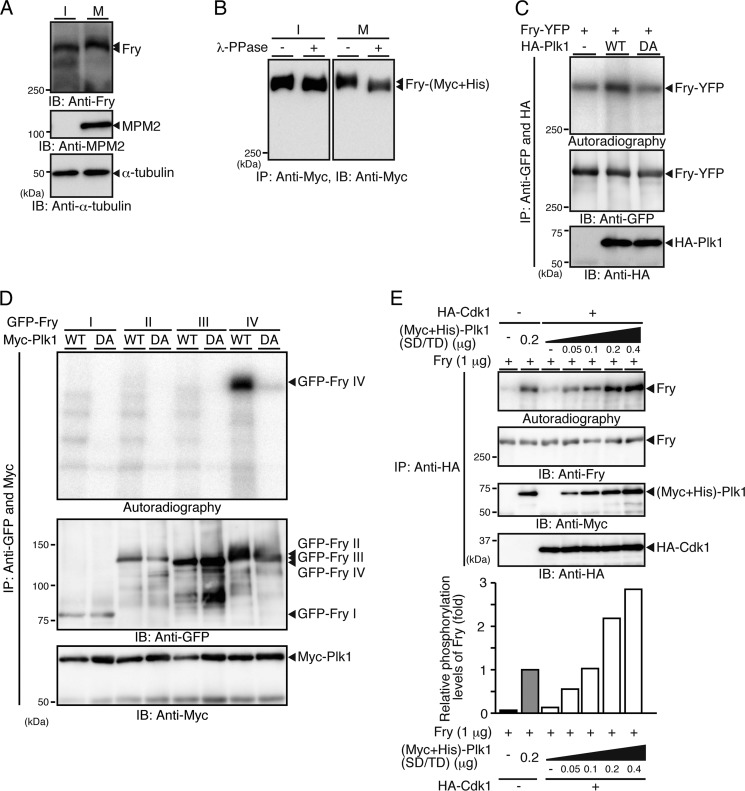
We next examined whether Fry binds to Plk1. When YFP-Plk1 and Fry-(Myc+His) were coexpressed in 293T cells and the cell lysates were immunoprecipitated with anti-Myc antibody, YFP-Plk1 coprecipitated with Fry-(Myc+His) (Fig. 2A). The kinase-dead Plk1(D194A) mutant also coprecipitated with Fry-(Myc+His) (Fig. 2A). The interaction between Fry and Plk1 was also assessed by GST pull-down assay. When lysates of HeLa cells synchronized in interphase by double-thymidine block or in early mitosis by exposure to nocodazole were incubated with GST-Plk1 bound to glutathione-Sepharose beads, endogenous Fry was pulled down with GST-Plk1 in mitotic cells but not in interphase cells (Fig. 2B). Thus, mitotic Fry, but not interphase Fry, has the potential to bind to Plk1. Intriguingly, mitotic Fry showed gel mobility retardation compared with interphase Fry (Fig. 2B, Input; see also Fig. 4A).
FIGURE 2.
Fry binds to the PBD of Plk1. A, Fry binds to Plk1. YFP-Plk1(WT or DA) and Fry-(Myc+His) were coexpressed in 293T cells. Cell lysates were immunoprecipitated (IP) with anti-Myc antibody and analyzed by anti-Myc and anti-GFP immunoblotting (IB). B, mitotic Fry binds to Plk1. HeLa cells were synchronized in interphase (I) by double thymidine block or in early mitotic phase (M) by nocodazole exposure. Lysates were incubated with GST or GST-Plk1-immobilized beads, and the bound proteins were immunoblotted with anti-Fry antibody. The asterisk indicates the nonspecific band that was also detected in the lane of GST-Plk1 beads without cell lysates. C, structure of Fry deletion mutants. D and E, mapping of the Plk1-binding region of Fry. Fry deletion mutants and Plk1 were coexpressed in 293T cells. Lysates were immunoprecipitated with anti-GFP or anti-Myc antibody and analyzed by immunoblotting. F, structure of Plk1 deletion mutants. G, mapping of the Fry-binding region of Plk1. Lysates of nocodazole-arrested HeLa cells were subjected to GST pull-down assays using GST-Plk1 or Plk1 fragments. Precipitates were analyzed by immunoblotting with anti-Fry antibody.
FIGURE 4.
Plk1 phosphorylates the C-terminal region of Fry. A, mobility shift of mitotic Fry. HeLa cell lysates synchronized in early mitosis with nocodazole (M) or lysates from interphase cells (I) were analyzed by immunoblotting (IB). B, mitosis-specific phosphorylation of Fry. 293T cells transfected with Fry-(Myc+His) were arrested by double-thymidine blocks (I) or thymidine-nocodazole (M). Cell lysates were immunoprecipitated (IP) with anti-Myc antibody, exposed to λ-phosphatase and analyzed as in A. C, Plk1 phosphorylates Fry. 293T cells transfected with HA-Plk1 (WT or D194A) were synchronized with thymidine-nocodazole. Cell lysates were mixed with lysates expressing Fry-YFP, coprecipitated with anti-HA and anti-GFP antibodies, and subjected to in vitro kinase assay. D, mapping of Plk1-phosphorylated region of Fry. HA-Plk1 and GFP-Fry fragments were coprecipitated with anti-HA and anti-GFP antibodies and subjected to in vitro kinase assay as in C. E, Cdk1 promotes Fry phosphorylation by Plk1. Recombinant Fry was mixed with purified (Myc+His)-tagged Plk1(S137D/T210D) and subjected to in vitro kinase assay in the presence or absence of HA-Cdk1. Histograms show the relative phosphorylation levels of Fry, with the phosphorylation level of Fry in the absence of Cdk1 set to 1.0.
To map the region of Fry responsible for Plk1 binding, truncated Fry-YFP mutants (Fig. 2C) were coexpressed with Myc-Plk1 in 293T cells, and the cell lysates were immunoprecipitated with anti-GFP antibody. Myc-Plk1 coprecipitated with Fry-C and Fry-IV but not with other Fry fragments (Fig. 2D). In addition, HA-Plk1 coprecipitated with (Myc+His)-tagged full-length Fry but not with Fry-ΔIV (Fig. 2, C and E). Therefore, Fry binds to Plk1 via its C-terminal region (amino acids 2401–3020). Plk1 often interacts with target proteins via its PBD (16, 17). Therefore, binding of Fry to the Plk1 PBD was assessed by GST pull-down. Lysates of mitotic HeLa cells were incubated with GST-Plk1 or Plk1 fragments (Fig. 2F), and precipitates were immunoblotted with anti-Fry antibody. Results showed that Fry coprecipitated with full-length Plk1 and with the Plk1 C-terminal fragment but not with the N-terminal fragment (Fig. 2G). Thus, Fry binds to the C-terminal PBD-containing region of Plk1.
Cdk1-mediated Fry Phosphorylation at Thr-2516 Is Required for Fry Binding to Plk1
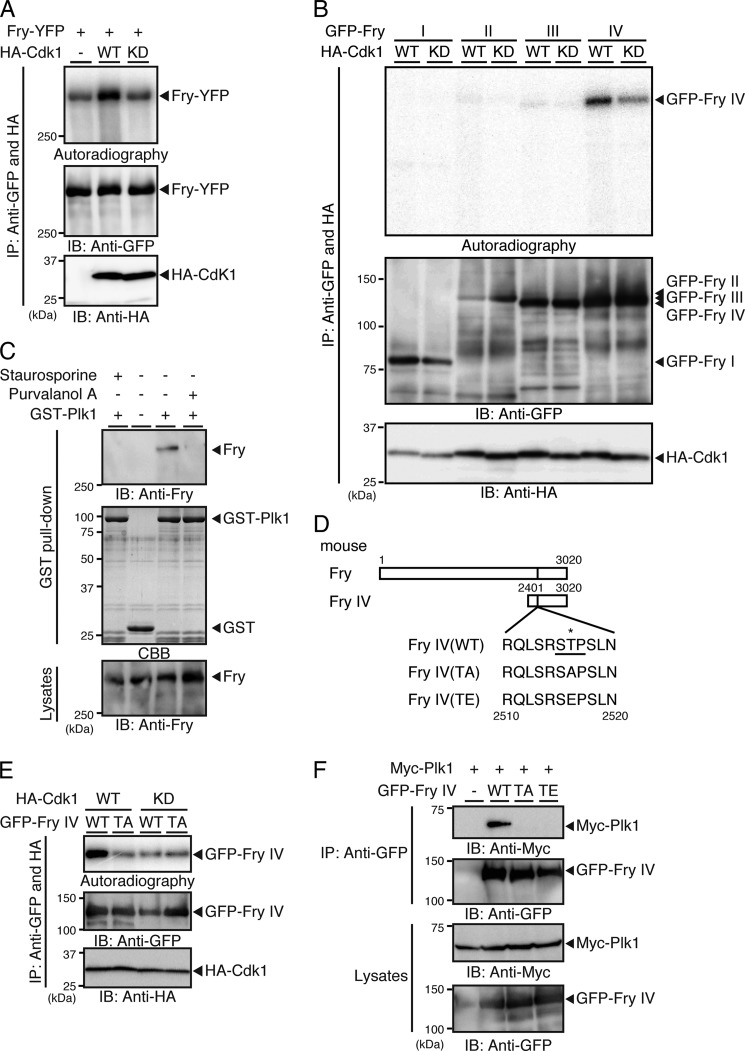
The Plk1 PBD generally binds to the sequence motif Ser-(Ser/Thr)-Pro in target proteins, depending on the phosphorylation status of the middle Ser/Thr (underlined) residue (16, 17). Because Plk1 binds to Fry during mitosis, we tested whether Cdk1 is involved in this interaction by inducing the phosphorylation of Fry in its Plk1-binding motif. In vitro kinase assays showed that Fry was phosphorylated by Cdk1(WT) but not by its kinase-dead mutant (KD; D146N) (Fig. 3A). When Fry fragments were incubated with Cdk1, Cdk1 predominantly phosphorylated the C-terminal Fry-IV fragment (Fig. 3B). Weaker phosphorylation of Fry-YFP or GFP-Fry-IV was also observed upon incubation with kinase-dead Cdk1 or in the absence of Cdk1 (Fig. 3, A and B), which is probably due to phosphorylation by other kinases coprecipitated with Fry or Fry-IV fragment. When mitotic HeLa cells were exposed to purvalanol A (a specific inhibitor of Cdk1) or staurosporine (a general protein kinase inhibitor), Fry lost Plk1-binding ability (Fig. 3C), further indicating that Fry binds to Plk1 in a Cdk1 activity-dependent manner.
FIGURE 3.
Cdk1-mediated phosphorylation of Fry at Thr-2516 is required for Fry binding to Plk1. A, Cdk1 phosphorylates Fry. 293T cells transfected with HA-tagged Cdk1(WT or kinase-dead (KD)) were synchronized in early mitotic phase with nocodazole. Cell lysates expressing HA-Cdk1 were mixed with lysates expressing Fry-YFP, immunoprecipitated (IP) with anti-HA and anti-GFP antibodies, and subjected to in vitro kinase assay. IB, immunoblot. B, mapping of the Cdk1-phosphorylated region of Fry. Cell lysates expressing HA-Cdk1 (WT or KD) were mixed with lysates expressing GFP-tagged Fry fragments, immunoprecipitated, and subjected to in vitro kinase assay as in A. C, Fry binding to Plk1 depends on the kinase activity of Cdk1. Mitotic HeLa cells arrested with nocodazole were collected by the mechanical shake-off method. After removal of nocodazole, cells were exposed to purvalanol A (25 μm) or staurosporine (10 μm) for 2 h in the presence of MG132 (10 μm), and then a GST pull-down assay was performed as in Fig. 2B. D, structures of Fry-IV mutants. The asterisk indicates the potential phosphorylation site. E, Cdk1 phosphorylates Fry-IV at Thr-2516. HA-Cdk1 (WT or KD) and GFP-Fry-IV (WT or TA) were coprecipitated with anti-HA and anti-GFP antibodies and subjected to in vitro kinase assay. F, Fry binding to Plk1 depends on Thr-2516 phosphorylation. Myc-Plk1 and GFP-Fry-IV mutants were coexpressed in 293T cells. Lysates were precipitated with anti-GFP antibody and analyzed by anti-Myc and anti-GFP immunoblotting.
Mouse Fry contains the single Ser-Thr-Pro sequence (amino acids 2515–2517) within fragment IV. To determine whether Cdk1 phosphorylates Fry at Thr-2516 and whether this phosphorylation event is required for binding to Plk1, we constructed the Fry-IV T2516A (TA) and T2516E (TE) mutants, in which Thr-2516 was replaced by Ala and Glu, respectively (Fig. 3D). In vitro kinase assays showed that Cdk1 phosphorylated Fry-IV(WT) but not the TA mutant (Fig. 3E). Immunoprecipitation assays demonstrated that Myc-Plk1 coprecipitated with Fry-IV(WT) but not with the TA or TE mutant (Fig. 3F). Together, these results indicate that the phosphorylation of Fry at Thr-2516 by Cdk1 allows binding of Fry to the Plk1 PBD.
Plk1 Phosphorylates the C-terminal Region of Fry
When endogenous Fry was detected by immunoblotting in HeLa cells during mitosis and interphase, mitotic Fry showed gel mobility retardation compared with interphase Fry (Fig. 4A). Exposure to λ-phosphatase abrogated the mobility shift of mitotic Fry (Fig. 4B), indicating that Fry is phosphorylated in mitosis. Because Plk1 binds to Fry, we examined whether Plk1 phosphorylates Fry. In vitro kinase assays revealed that Plk1(WT) but not kinase-dead Plk1(D194A) phosphorylated Fry (Fig. 4C). Plk1 effectively phosphorylated Fry-IV but not other fragments, indicating that Plk1 predominantly phosphorylates the C-terminal region (amino acids 2401–3020) of Fry (Fig. 4D). We also tested whether Cdk1 affects the ability of Plk1 to phosphorylate Fry. Recombinant Fry and Plk1(S137D/T210D), a constitutively active form of Plk1, were expressed in Sf21 cells and purified (supplemental Fig. S2). Plk1(SD/TD) phosphorylated Fry in the absence of Cdk1, but the phosphorylation of Fry by 0.2 μg of Plk1(SD/TD) was ∼2-fold higher in the presence of Cdk1 than in the absence of Cdk1 (Fig. 4E). Cdk1 alone only modestly phosphorylated Fry (Fig. 4E). These results suggest that Cdk1 promotes the phosphorylation of Fry by Plk1, probably by phosphorylating Fry at Thr-2516 and thereby facilitating the association of Plk1 with Fry.
Fry Depletion Reduces Plk1 Kinase Activity
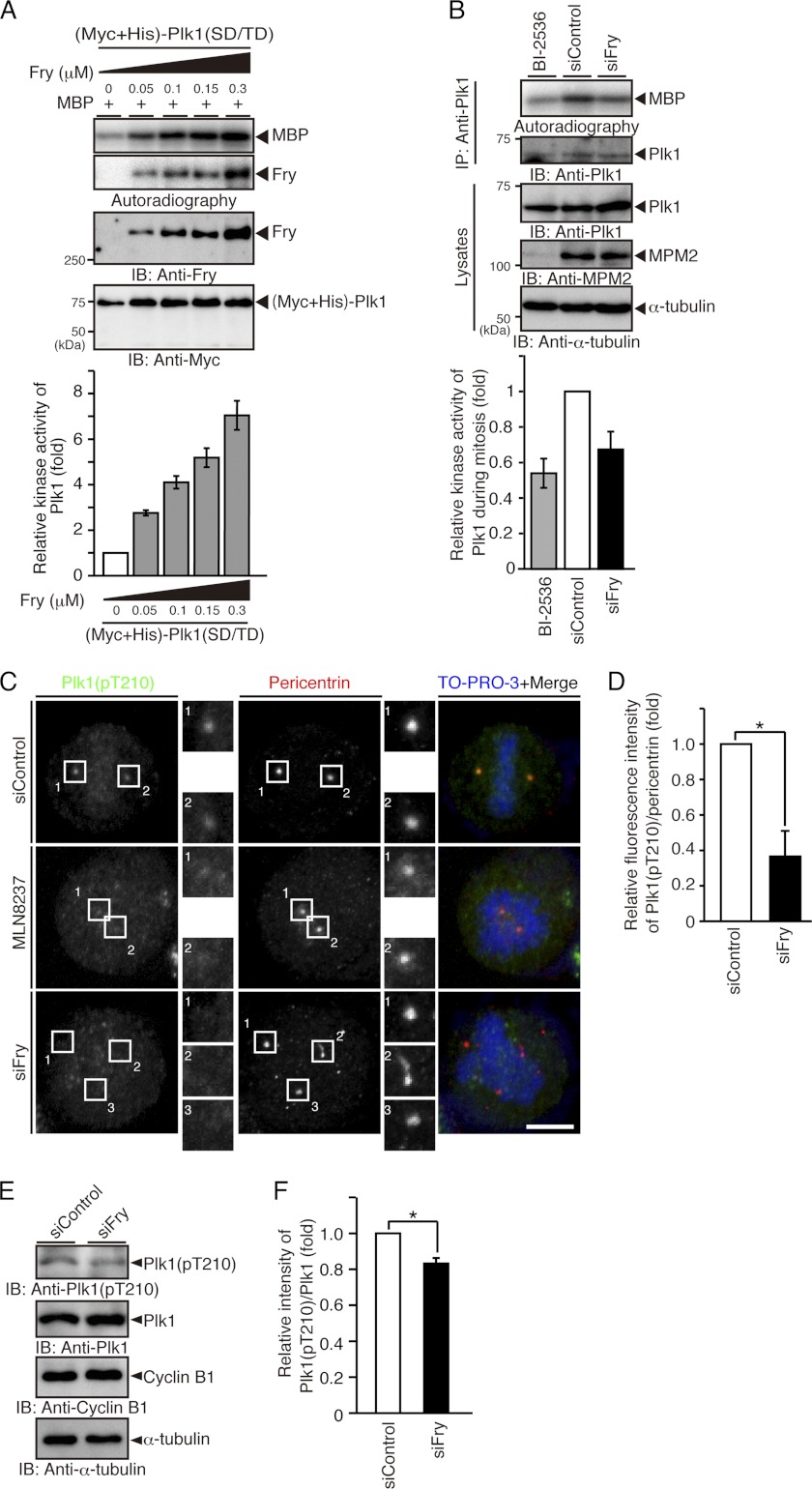
The kinase activity of Plk1 is negatively regulated by the intramolecular binding of the PBD to the kinase domain, which suggests that Plk1 may be activated by the binding of other proteins to the PBD (17). Because Fry binds to the PBD of Plk1, we examined the possibility that Fry promotes the kinase activity of Plk1. The effect of Fry on the kinase activity of Plk1(SD/TD) was assessed using MBP as a substrate: Fry increased the kinase activity of Plk1(SD/TD) in a concentration-dependent manner (Fig. 5A).
FIGURE 5.
Fry depletion decreases the kinase activity and the Thr-210 phosphorylation level of Plk1. A, Fry increases the kinase activity of Plk1 in a concentration-dependent manner. Purified (Myc+His)-Plk1(SD/TD) was mixed with recombinant Fry and subjected to in vitro kinase assay using MBP as a substrate. The relative kinase activity of Plk1 is shown as the means ± S.E. of triplicate experiments. IB, immunoblotting. B, Fry depletion reduces the kinase activity of mitotic Plk1. HeLa cells transfected with siRNAs were synchronized in early mitosis with thymidine-nocodazole. Mitotic HeLa cells were collected by the mechanical shake-off method. Endogenous Plk1 was immunoprecipitated (IP) and subjected to an in vitro kinase assay using MBP as a substrate. Mitotic HeLa cells exposed to BI-2536 (1 μm) were used as a background control. The relative kinase activity of Plk1 is shown as the means ± S.E. of triplicate experiments. C, Fry depletion decreases the level of Thr-210 phosphorylation of Plk1 on spindle poles. HeLa cells transfected with siRNAs were cultured in growth medium for 12 h and in thymidine-containing medium for 36 h. They were then released from thymidine arrest for 12 h before being fixed and stained with anti-Plk1 pT210 (green) and anti-pericentrin (red) antibodies. DNA was stained with TO-PRO-3 (blue). For Aurora A inhibition, after release from thymidine block for 10 h, HeLa cells transfected with control siRNA were incubated for 2 h in medium containing MLN8237 (100 nm) and MG132 (10 μm). Magnified images of the white boxes are also shown. Scale bar, 5 μm. D, relative fluorescence intensity of Plk1(pT210) normalized to the intensity of pericentrin on spindle poles. HeLa cells were treated as in C. The fluorescence intensity in a 2-μm-diameter circular region surrounding the spindle pole was measured. The relative fluorescence intensity is shown as the means ± S.E. of triplicate experiments. *, p < 0.05. E, immunoblot analysis of the level of Thr-210 phosphorylation of Plk1. HeLa cells transfected with siRNAs were synchronized at early mitotic phase with thymidine-nocodazole treatment and collected by the mechanical shake-off procedure. Cell lysates were analyzed by immunoblotting with indicated antibodies. The relative intensity of Plk1(pT210) immunoblot normalized to the intensity of Plk1 is shown as the means ± S.E. of triplicate experiments. *, p < 0.05.
To examine whether endogenous Fry is involved in Plk1 activation in mitotic cells, the effect of Fry depletion on the kinase activity of endogenous Plk1 was assessed. Plk1 was immunoprecipitated from lysates of mitotic HeLa cells previously transfected with control or Fry siRNA. Results showed that the kinase activity of Plk1 was lower in cells transfected with Fry siRNA than in cells transfected with control siRNA (Fig. 5B), indicating that endogenous Fry is involved in the mitotic activation of Plk1. The kinase activity of Plk1 in mitotic HeLa cells exposed to BI-2536, a specific inhibitor of Plk1, was used as a background control.
It is well known that Plk1 is activated by Aurora A-mediated phosphorylation of Thr-210 in the kinase domain (14, 15). Therefore, the level of the Thr-210-phosphorylated form of Plk1 was analyzed by immunostaining in mitotic HeLa cells using anti-Plk1(pT210) antibody. In cells transfected with control siRNA, the pT210 signal was detected in a pair of spindle poles in mitosis (Fig. 5C). In contrast, the fluorescence intensity of the pT210 signal in the spindle poles was markedly decreased in Fry-depleted cells. Exposure to MLN8237, a specific inhibitor of Aurora A, also abrogated the pT210 signal in the spindle poles. Quantitative analysis showed that the relative fluorescence intensity of the pT210 signal, normalized to pericentrin signal, on spindle poles significantly decreased in Fry-depleted cells (Fig. 5D). Furthermore, immunoblot analysis of mitotic HeLa cell lysates with anti-Plk1(pT210) and anti-Plk1 antibodies also showed that the level of Thr-210 phosphorylation of Plk1 was decreased in Fry-depleted cells (Fig. 5E). These results suggest that depletion of Fry suppresses Aurora A-mediated Plk1 phosphorylation on Thr-210 and that endogenous Fry plays a crucial role in the mitotic activation of Plk1 on spindle poles.
Fry Binds to Aurora A and Enhances Aurora A-mediated Plk1 Phosphorylation
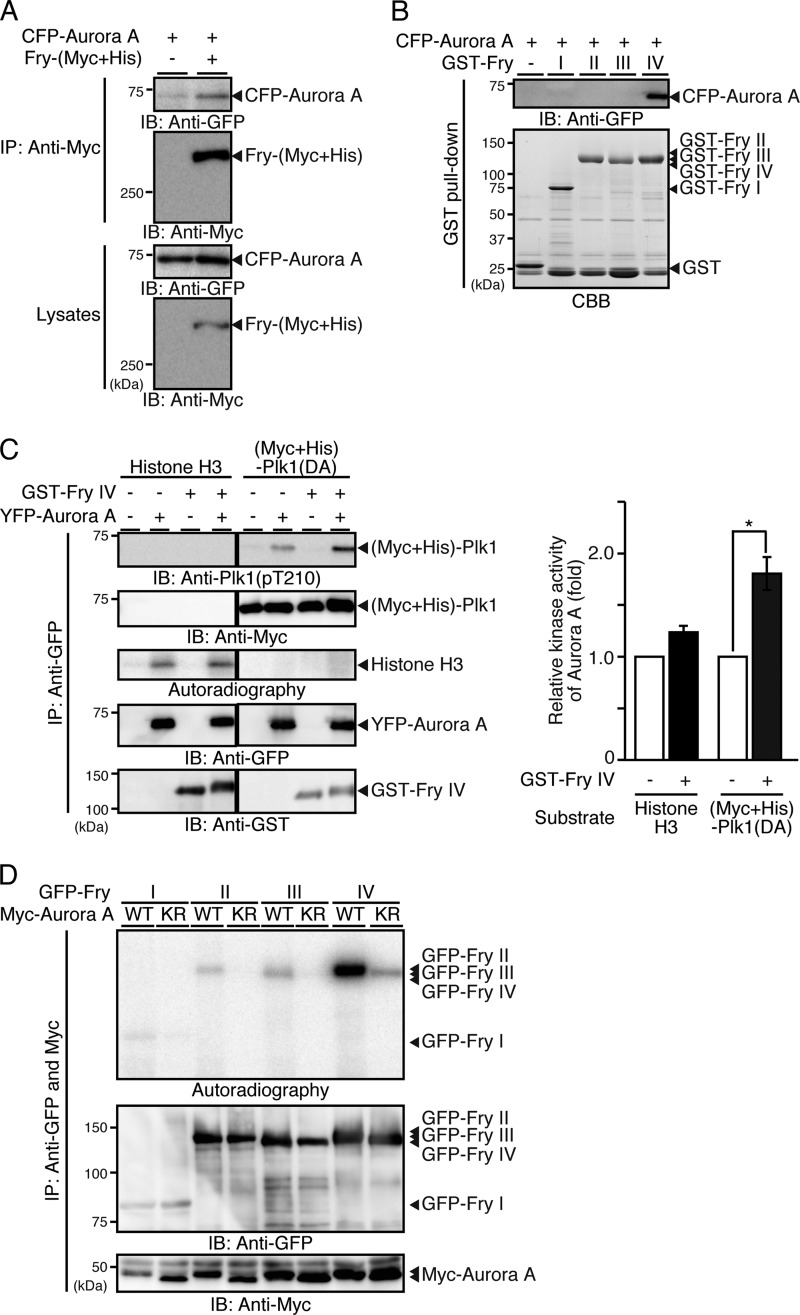
Because Fry appears to be involved in the Aurora A-mediated phosphorylation of Plk1 at Thr-210, the possibility that Fry binds to Aurora A was examined. Lysates of 293T cells cotransfected with Fry-(Myc+His) and CFP-Aurora A and synchronized to early mitosis were immunoprecipitated with anti-Myc antibody. Results showed that CFP-Aurora A coprecipitated with Fry-(Myc+His) (Fig. 6A). GST pull-down assays revealed that Aurora A binds to the Fry-IV fragment but not to other fragments, indicating that Aurora A binds to the C-terminal (2401–3020) region of Fry (Fig. 6B). These results indicate that Fry binds to both Aurora A and Plk1 and may function as a scaffold promoting the interaction between these two mitotic kinases.
FIGURE 6.
Fry binds to Aurora A and promotes Aurora A-mediated Plk1 activation. A, Fry binds to Aurora A. HeLa cells were cotransfected with Fry-(Myc+His) and CFP-Aurora A and synchronized in early mitosis with thymidine-nocodazole. Cell lysates were immunoprecipitated (IP) with anti-Myc antibody and analyzed by immunoblotting (IB). B, Fry binds to Aurora A through the C-terminal region. Cell lysates expressing CFP-Aurora A were subjected to GST pull-down assays using GST-Fry fragments. Precipitates were analyzed by anti-GFP immunoblotting. C, Fry-IV enhances Aurora A-mediated Plk1 phosphorylation. 293T cells were transfected with YFP-Aurora A and synchronized in early mitosis with nocodazole. YFP-Aurora A was immunoprecipitated with anti-GFP antibody and subjected to in vitro kinase assays using an equal amount (1 μg) of purified (Myc+His)-Plk1(DA) or histone H3 as a substrate in the presence or absence of GST-Fry-IV. Histograms indicate the relative kinase activity of Aurora A toward Plk1(DA) or histone H3 with the Aurora A activity in the absence of GST-Fry-IV set to 1.0. Data are the means ± S.E. of triplicate experiments. *, p < 0.05. D, Aurora A phosphorylates the C-terminal region of Fry. 293T cell lysates transfected with Myc-Aurora A (WT or K162R) were mixed with lysates expressing GFP-Fry fragments, coprecipitated with anti-Myc and anti-GFP antibodies, and then subjected to in vitro kinase assay.
The effect of Fry-IV on the kinase activity of Aurora A was also assessed in cell-free assays. In vitro kinase assays revealed that the kinase activity of Aurora A toward Plk1(D194A) was 1.8-fold increased by the addition of Fry-IV, whereas the kinase activity of Aurora A toward a conventional substrate, histone H3, was only barely increased by Fry-IV (Fig. 6C). These data suggest that Fry functions as a scaffold that promotes Aurora A-mediated Plk1 phosphorylation but not as a general activator of Aurora A.
When Fry fragments were subjected to in vitro kinase reactions with Aurora A, Fry-IV was most effectively phosphorylated by Aurora A (Fig. 6D), indicating that Aurora A predominantly phosphorylates the C-terminal (2401–3020) region of Fry, as do Cdk1 and Plk1.
DISCUSSION
This study provides evidence that Fry plays a crucial role in Plk1 activation in early mitosis. Although Fry and Plk1 dynamically change their subcellular localization during the cell cycle, they colocalize to centrosomes in early mitosis. Pull-down assays revealed that mitotic Fry, but not interphase Fry, has the potential to bind to Plk1. Furthermore, Fry binds to the Plk1 PBD, which requires the priming phosphorylation of Fry on Thr-2516 by the mitotic kinase Cdk1. These results suggest that Fry interacts with Plk1 in a cell cycle-dependent manner and that this interaction appears to be confined to the mature centrosomes in the early mitotic phase.
Plk1 is activated by Aurora A-mediated phosphorylation at Thr-210 in the kinase domain (14, 15). Fry depletion reduced the level of Plk1 Thr-210-phosphorylation on spindle poles, as well as the total kinase activity of Plk1 in mitotic cells, indicating that endogenous Fry crucially contributes to Plk1 activation on spindle poles in early mitosis. Because Fry bound to both Plk1 and Aurora A via its C-terminal region, Fry likely functions as a scaffold protein that facilitates the interaction between Plk1 and Aurora A. Additionally, the C-terminal fragment of Fry increased the kinase activity of Aurora A toward Plk1 by ∼2-fold but had only a minor effect on the kinase activity toward a conventional substrate, histone H3, indicating that Fry promotes Aurora A-mediated Plk1 activation primarily by enhancing the accessibility of Aurora A to Plk1, rather than increasing the intrinsic kinase activity of Aurora A.
In addition to Aurora A-mediated phosphorylation, Plk1 activity is also regulated by the intramolecular interaction between the N-terminal kinase domain and the C-terminal PBD. The PBD of Plk1 binds to the catalytic domain to inhibit kinase activity, and full-length Plk1 is activated by relieving the kinase domain from this autoinhibitory interaction (16). Although the phosphopeptide binding site of the PBD is not involved in binding to the kinase domain, phosphopeptide binding to the PBD induces Plk1 activation, probably by inducing a conformational change that disrupts the kinase-PBD interaction (16). Fry dose-dependently increased the kinase activity of Plk1, and Cdk1 enhanced Plk1-mediated Fry phosphorylation, indicating that Cdk1-mediated Fry phosphorylation at Thr-2516 and subsequent Fry binding to the PBD promotes the kinase activity of Plk1. In these experiments, constitutively active Plk1(S137D/T210D) was used because wild-type Plk1 that is not phosphorylated at Thr-210 displayed little kinase activity, even in the presence of Fry. Although the previous study showed that the kinase-PBD interaction was disrupted by Thr-210 phosphorylation (25), our results indicate that Fry binding to the PBD further enhances the kinase activity of Plk1 even after Thr-210 mutation to aspartate. We therefore suggest that Aurora A-mediated Thr-210 phosphorylation is required but not sufficient for full activation of Plk1 and that both Aurora A-mediated phosphorylation and phosphoprotein binding to the PBD synergistically contribute to Plk1 activation. Fry appears to be involved in both of these activation processes.
Plk1 has diverse functions in multiple stages of the cell cycle. One of the important questions to be solved is how Plk1 activity is spatially and temporally regulated during mitosis. Plk1 is initially activated in late G2 and contributes to mitotic entry by phosphorylating cell cycle regulators, including Cdc25 and Wee1. Then, Plk1 controls centrosome maturation by phosphorylating centrosomal proteins, including Nedd1 and pericentrin (26, 27). After centrosome separation, Plk1 contributes to centrosome and centriole integrity by phosphorylating centrosomal proteins, including Kizuna, Sgo1, and ASAP (12, 13, 28). Plk1 also regulates MT-kinetochore attachment and cleavage furrow ingression (6, 29). Loss of Plk1 activity induces both monopolar and multipolar spindles, depending on the levels of deficiency (8–11). In contrast, Fry depletion induced multipolar spindles and chromosome misalignment, but not monopolar spindles. Becauseγ-tubulin was recruited in all centrosome fragments, centrosome maturation normally occurs in Fry-depleted cells. Thus, Fry is principally involved in Plk1 activation after the onset of mitosis, but not in Plk1 activation for centrosome maturation/separation in G2 phase. Because Plk1 is also involved in MT-kinetochore attachment, the defect in Fry-mediated Plk1 activation in early mitosis may also contribute to the chromosome misalignment induced by Fry depletion.
The initial activation of Plk1 in late G2 is induced by the cooperative action of Aurora A and Bora, with Bora binding to the PBD of Plk1 and promoting Aurora A-mediated Plk1 activation. Once Plk1 is activated, Bora is phosphorylated by Cdk1 and Plk1 and thereafter degraded by ubiquitin-assisted proteolysis at the entry into mitosis (30, 31). Thus, an additional mechanism is required to maintain Plk1 activity on centrosomes after mitotic entry. Because Fry binds to and activates Plk1 on spindle poles in early mitosis, Fry appears to play an indispensable role for maintaining Plk1 activity after Bora degradation in early mitosis. Studies on the effects of Fry depletion on the phosphorylation and function of a variety of Plk1 target proteins will further clarify the role of Fry-mediated Plk1 activation in the control of bipolar spindle organization in early mitosis.
We previously showed that Fry is a MT-binding protein localized to centrosomes and spindle MTs in early mitosis but is distributed diffusely in the cytoplasm in interphase (21). However, how Fry localization is controlled during the cell cycle remains unknown. We have data that show that Fry-ΔIV localizes to MTs in both interphase and mitosis (data not shown), suggesting that the N-terminal region of Fry has the potential to bind to MTs but that its MT-binding ability may be masked by intramolecular interactions in interphase. In this respect, we showed that the C-terminal region of Fry is phosphorylated by mitotic kinases Cdk1, Plk1, and Aurora A. Thus, it is conceivable that mitotic kinases-catalyzed phosphorylation of the C-terminal region of Fry may change the conformation of Fry to relieve the MT-binding ability of the N-terminal region and induce mitosis-specific localization of Fry to centrosomes and spindle MTs.
We provide evidence that Fry plays a crucial role in spindle pole integrity by promoting Plk1 activity on centrosomes in early mitosis. Our data propose a new mechanism of cell cycle-dependent regulation of Plk1 activity. Further studies on the molecular mechanism underlying Fry-mediated Plk1 activation and its role in mitotic centrosome and centriole integrity will provide insights into the mechanisms of the establishment of mitotic spindle bipolarity.
Supplementary Material
Acknowledgment
We thank Dr. Y. Terada for providing Aurora A cDNA.
This work was supported by grants for Scientific Research from the Ministry of Education, Culture, Science, Sports, and Technology of Japan.

This article contains supplemental Figs. S1 and S2.
- MT
- microtubule
- PBD
- Polo-box domain.
REFERENCES
- 1. Nigg E. A. (2002) Centrosome aberrations. Cause or consequence of cancer progression? Nat. Rev. Cancer 2, 815–825 [DOI] [PubMed] [Google Scholar]
- 2. Fukasawa K. (2007) Oncogenes and suppressors take on centrosomes. (2012) Nat. Rev. Cancer 7, 911–924 [DOI] [PubMed] [Google Scholar]
- 3. Bettencourt-Dias M., Glover D. M. (2007) Centrosome biogenesis and function. Centrosomics brings new understanding. Nat. Rev. Mol. Cell Biol. 8, 451–463 [DOI] [PubMed] [Google Scholar]
- 4. Dumont S., Mitchison T. J. (2009) Force and length in the mitotic spindle. Curr. Biol. 19, R749-R761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barr F. A., Silljé H. H., Nigg E. A. (2004) Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5, 429–440 [DOI] [PubMed] [Google Scholar]
- 6. Petronczki M., Lénárt P., Peters J. M. (2008) Polo on the rise. From mitotic entry to cytokinesis with Plk1. Dev. Cell 14, 646–659 [DOI] [PubMed] [Google Scholar]
- 7. Archambault V., Glover D. M. (2009) Polo-like kinases. Conservation and divergence in their functions and regulation. Nat. Rev. Mol. Cell Biol. 10, 265–275 [DOI] [PubMed] [Google Scholar]
- 8. Sumara I., Giménez-Abián J. F., Gerlich D., Hirota T., Kraft C., de la Torre C., Ellenberg J., Peters J. M. (2004) Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr. Biol. 14, 1712–1722 [DOI] [PubMed] [Google Scholar]
- 9. De Luca M., Lavia P., Guarguaglini G. (2006) A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles. Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle 5, 296–303 [DOI] [PubMed] [Google Scholar]
- 10. McInnes C., Mazumdar A., Mezna M., Meades C., Midgley C., Scaerou F., Carpenter L., Mackenzie M., Taylor P., Walkinshaw M., Fischer P. M., Glover D. (2006) Inhibitors of polo-like kinase reveal roles in spindle-pole maintenance. Nat. Chem. Biol. 2, 608–617 [DOI] [PubMed] [Google Scholar]
- 11. Lénárt P., Petronczki M., Steegmaier M., Di Fiore B., Lipp J. J., Hoffmann M., Rettig W. J., Kraut N., Peters J. M. (2007) The small-molecular inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 17, 304–315 [DOI] [PubMed] [Google Scholar]
- 12. Oshimori N., Ohsugi M., Yamamoto T. (2006) The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity. Nat. Cell Biol. 8, 1095–1101 [DOI] [PubMed] [Google Scholar]
- 13. Wang X., Yang Y., Duan Q., Jiang N., Huang Y., Darzynkiewicz Z., Dai W. (2008) sSgo1, a major splice valiant of Sgo1, functions in centriole cohesion where it is regulated by Plk1. Dev. Cell 14, 331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seki A., Coppinger J. A., Jang C. Y., Yates J. R., 3rd, Fang G. (2008) Bora and the kinase Aurora A cooperatively activate the kinase Plk1 and control mitotic entry. Science 320, 1655–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macrek L., Lindqvist A., Lim D., Lampson M. A., Klompmaker R., Freire R., Clouin C., Taylor S. S., Yaffe M. B., Medema R. H. (2008) Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 455, 119–123 [DOI] [PubMed] [Google Scholar]
- 16. Elia A. E., Rellos P., Haire L. F., Chao J. W., Ivins F. J., Hoepker K., Mohammad D., Cantley L. C., Smerdon S. J., Yaffe M. B. (2003) The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell 115, 83–95 [DOI] [PubMed] [Google Scholar]
- 17. Lowery D. M., Lim D., Yaffe M. B. (2005) Structure and function of Polo-like kinases. Oncogene 24, 248–259 [DOI] [PubMed] [Google Scholar]
- 18. Gallegos M. E., Bargmann C. I. (2004) Mechanosensory neurite termination and tiling depend on SAX-2 and the SAX-1 kinase. Neuron 44, 239–249 [DOI] [PubMed] [Google Scholar]
- 19. Hergovich A., Stegert M. R., Schmitz D., Hemmings B. A. (2006) NDR kinases regulate essential cell processes from yeast to humans. Nat. Rev. Mol. Cell Biol. 7, 253–264 [DOI] [PubMed] [Google Scholar]
- 20. Emoto K. (2011) The growing role of the Hippo-NDR kinase signaling in Neuronal development and disease. J. Biochem. 150, 133–141 [DOI] [PubMed] [Google Scholar]
- 21. Chiba S., Ikeda M., Katsunuma K., Ohashi K., Mizuno K. (2009) MST2- and Furry-mediated activation of NDR1 kinase is critical for precise alignment of mitotic chromosomes. Curr. Biol. 19, 675–681 [DOI] [PubMed] [Google Scholar]
- 22. Amano T., Kaji N., Ohashi K., Mizuno K. (2002) Mitosis-specific activation of LIM motif-containing protein kinase and roles of cofilin phosphorylation and dephosphorylation in mitosis. J. Biol. Chem. 277, 22093–22102 [DOI] [PubMed] [Google Scholar]
- 23. Skoufias D. A., Indorato R. L., Lacroix F., Panopoulos A., Margolis R. L. (2007) Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed. J. Cell Biol. 179, 671–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurita S., Watanabe Y., Gunji E., Ohashi K., Mizuno K. (2008) Molecular dissection of the mechanisms of substrate recognition and F-actin-mediated activation of cofilin-phosphatase Slingshot-1. J. Biol. Chem. 283, 32542–32552 [DOI] [PubMed] [Google Scholar]
- 25. Jang Y. J., Lin C. Y., Ma S., Erikson R. L. (2002) Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc. Natl. Acad. Sci. U.S.A. 99, 1984–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X., Chen Q., Feng J., Hou J., Yang F., Liu J., Jiang Q., Zhang C. (2009) Sequential phosphorylation of Nedd1 by Cdk1 and Plk1 is required for targeting of the gTuRC to the centrosome. J. Cell Sci. 122, 2240–2251 [DOI] [PubMed] [Google Scholar]
- 27. Lee K., Rhee K. (2011) PLK1 phosphorylation of pericentrin initiates centrosome maturation at the onset of mitosis. J. Cell Biol. 195, 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eot-Houllier G., Venoux M., Vidal-Eychenié S., Hoang M. T., Giorgi D., Rouquier S. (2010) Plk1 regulates both ASAP localization and its role in spindle pole integrity. J. Biol. Chem. 285, 29556–29568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsumura S., Toyoshima F., Nishida E. (2007) Polo-like kinase 1 facilitates chromosome alignment during prometaphase through BubR1. J. Biol. Chem. 282, 15217–15227 [DOI] [PubMed] [Google Scholar]
- 30. Chan E. H., Santamaria A., Silljé H. H., Nigg E. A. (2008) Plk1 regulates mitotic Aurora A function through βTrCP-dependent degradation of hBora. Chromosoma 117, 457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seki A., Coppinger J. A., Du H., Jang C. Y., Yates J. R., 3rd, Fang G. (2008) Plk1- and β-TrCP-dependent degradation of Bora controls mitotic progression. J. Cell Biol. 181, 65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.