SUMMARY
Resveratrol, a polyphenol in red wine, has been reported as a calorie restriction mimetic with potential antiaging and antidiabetogenic properties. It is widely consumed as a nutritional supplement, but its mechanism of action remains a mystery. Here, we report that the metabolic effects of resveratrol result from competitive inhibition of cAMP-degrading phosphodiesterases, leading to elevated cAMP levels. The resulting activation of Epac1, a cAMP effector protein, increases intracellular Ca2+ levels and activates the CamKKβ-AMPK pathway via phospholipase C and the ryanodine receptor Ca2+-release channel. As a consequence, resveratrol increases NAD+ and the activity of Sirt1. Inhibiting PDE4 with rolipram reproduces all of the metabolic benefits of resveratrol, including prevention of diet-induced obesity and an increase in mitochondrial function, physical stamina, and glucose tolerance in mice. Therefore, administration of PDE4 inhibitors may also protect against and ameliorate the symptoms of metabolic diseases associated with aging.
INTRODUCTION
Calorie restriction (CR) is the most robust intervention demonstrated to extend life span and delay the physiological deterioration associated with aging (McCay et al., 1935). Because CR involves a number of overlapping and interconnected signaling pathways, it is difficult to identify with certainty the mechanism(s) underlying the beneficial effects of CR. Based on studies of the budding yeast Saccharomyces cerevisiae, it was initially proposed that CR extends life span via the activity of Sir2 (Lin et al., 2000), the founding member of the conserved sirtuin family of NAD+-dependent protein deacetylases (Guarente, 2006). Although it remains unclear whether Sir2 plays a direct role in the antiaging effects of CR (e.g., Kaeberlein et al., 2004), overexpression of Sirt1, the mammalian homolog of Sir2, has been reported to protect mice from aging-related phenotypes that are similar to type 2 diabetes (Banks et al., 2008; Bordone et al., 2007; Pfluger et al., 2008), cancer (Herranz et al., 2010), and Alzheimer's disease (Donmez et al., 2010). Suggesting that Sirt1 activity does not protect against aging-related diseases by delaying the aging process, overexpression of Sirt1 does not extend life span in mice (Herranz et al., 2010).
The positive health effects of CR and sirtuin activity in animal models have provoked intense interest in the development of small-molecule activators of Sirt1 to prevent or delay aging-related diseases. An in vitro screen performed using a fluorophore-tagged substrate identified resveratrol as an activator of Sirt1 deacetylase activity (Howitz et al., 2003). Resveratrol is a natural polyphenol produced by plants in response to environmental stress (Signorelli and Ghidoni, 2005) and is present in many plant-based foods, most notably red wine. Subsequent work has shown that resveratrol extends the life spans of lower eukaryotes (Gruber et al., 2007; Viswanathan et al., 2005; Wood et al., 2004). These studies set the stage for testing resveratrol as a CR mimetic in mammals. In mice, long-term administration of resveratrol induced gene expression patterns that resembled those induced by CR and delayed aging-related deterioration, even though it did not extend life span (Pearson et al., 2008). Resveratrol protected against obesity and development of insulin resistance in rodents fed a high-calorie diet (Baur et al., 2006; Lagouge et al., 2006). Resveratrol also decreased insulin resistance in type 2 diabetic patients (Brasnyó et al., 2011), suggesting that the pathway targeted by resveratrol might be important for developing therapies for type 2 diabetes.
An important mediator of the metabolic effects of resveratrol (Lagouge et al., 2006; Um et al., 2010) is peroxisome proliferator-activated receptor γ coactivator, PGC-1α (Puigserver et al., 1998). It is a coactivator that controls mitochondrial biogenesis and respiration and can contribute to fiber-type switching in skeletal muscle (Lin et al., 2002) and increase adaptive thermo-genesis in brown adipose tissue (Puigserver et al., 1998). Consistent with the known ability of Sirt1 to deacetylate and activate PGC-1α (Gerhart-Hines et al., 2007; Rodgers et al., 2005), resveratrol increased Sirt1 and PGC-1α activity in mice fed a high-fat diet (HFD) (Lagouge et al., 2006; Um et al., 2010).
Two findings have raised doubt that resveratrol is a direct Sirt1 activator. First, although resveratrol activates Sirt1 in vivo, it activates Sirt1 to deacetylate fluorophore-tagged substrates but not native substrates in vitro (Beher et al., 2009; Borra et al., 2005; Kaeberlein et al., 2005; Pacholec et al., 2010), suggesting that resveratrol activates Sirt1 indirectly in vivo. Second, resveratrol activates AMP-activated protein kinase (AMPK) in vivo (Baur et al., 2006; Cantó et al., 2010; Dasgupta and Milbrandt, 2007; Park et al., 2007; Um et al., 2010). AMPK is a trimeric complex that senses nutrient deprivation by sensing the AMP/ATP (Carling et al., 1987) and ADP/ATP (Xiao et al., 2011) ratios. AMPK, which is emerging as a key regulator of whole-body metabolism, has been shown to increase NAD+ levels and activate Sirt1 and PGC-1α (Cantó et al., 2009, 2010; Fulco et al., 2008; Um et al., 2010). However, a causal link between the increase in NAD+ and Sirt1 activation has not been established. We and others have shown that AMPK-deficient mice are resistant to the metabolic effects of resveratrol, providing evidence that AMPK is a key mediator of the metabolic benefits produced by resveratrol (Cantó et al., 2010; Um et al., 2010). These findings demonstrated that activation of Sirt1 and PGC-1α by resveratrol is downstream of AMPK activation.
Studies on how resveratrol activates AMPK have led to different and often conflicting mechanisms. Hawley et al. reported that at a high concentration (100–300 μM), resveratrol decreased ATP; and in a cell line expressing a mutated γ subunit of AMPK that made AMPK insensitive to AMP, resveratrol did not activate AMPK (Hawley et al., 2010). This suggested that resveratrol, at high concentrations, activated AMPK by decreasing energy and increasing the AMP/ATP or ADP/ATP ratios. However, resveratrol can activate AMPK at a concentration less than 10 μM (Dasgupta and Milbrandt, 2007; Feige et al., 2008; Park et al., 2007). At low concentrations (≤50 μM), resveratrol appears to activate AMPK without decreasing energy (Dasgupta and Milbrandt, 2007; Suchankova et al., 2009). As the plasma level after oral administration of resveratrol is low (Crowell et al., 2004), the mechanism by which resveratrol activates AMPK at physiologically relevant concentrations most likely does not involve decreasing energy.
For this report, we attempted to find the direct target of resveratrol and to elucidate the biochemical pathway by which it activates AMPK and produces metabolic benefits. We found that resveratrol directly inhibits cAMP-specific phosphodiesterases (PDE) and identified the cAMP effector protein Epac1 as a key mediator of the effects of resveratrol, which leads to the activation of AMPK and Sirt1.
RESULTS
Resveratrol Activates AMPK in an Epac1-Dependent Manner
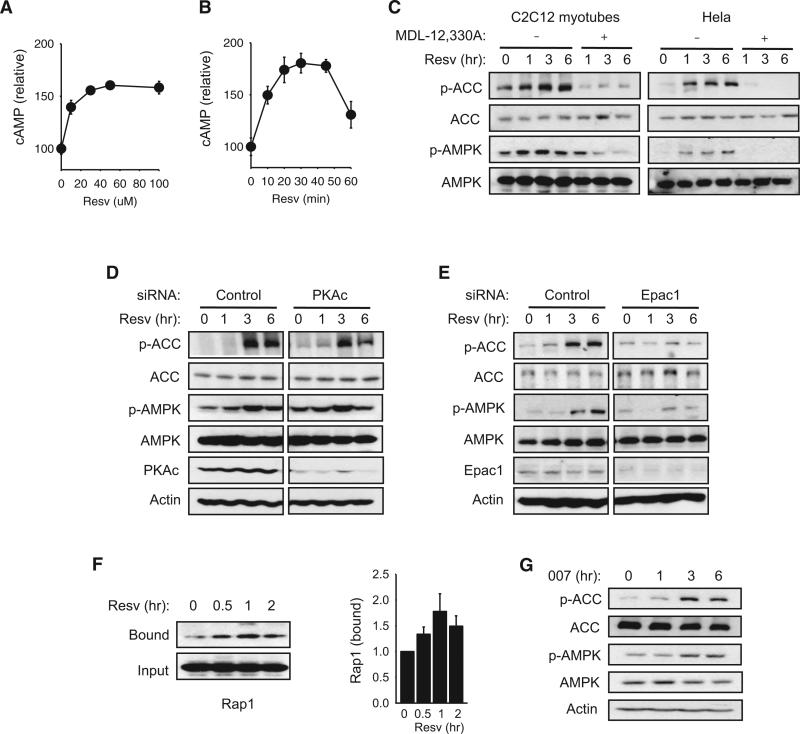
A hint that cAMP may mediate the effects of resveratrol was suggested by a previous study reporting that resveratrol increased cAMP production in breast cancer cells (El-Mowafy and Alkhalaf, 2003). In this study, we found that cAMP levels increased significantly with a low dose (≤50 μM) of resveratrol in C2C12 myotubes (Figures 1A and 1B). To confirm that resveratrol increased cAMP levels in vivo, we administered resveratrol to mice by oral gavage and measured cAMP levels in skeletal muscle and white adipose tissue (WAT) (Figure S1A available online). We found that resveratrol increased cAMP levels in both tissues. In contrast, resveratrol did not significantly increase cGMP levels in myotubes (Figure S1B). The possibility that the increase in cAMP production was responsible for the activation of AMPK by resveratrol was examined by treating myotubes and HeLa cells with resveratrol in the presence of the adenylyl cyclase (AC) inhibitor MDL-12,330A (Figure 1C). In both cell types, MDL-12,330A prevented resveratrol (50 μM) from increasing the phosphorylation of both AMPK (T172) and the AMPK substrate acetyl-CoA carboxylase (ACC) (S79), which are markers of AMPK activity. These findings indicate that cAMP signaling is essential for resveratrol to activate AMPK.
Figure 1. Resveratrol Activates AMPK in an Epac1-Dependent Manner.
(A) Cyclic AMP levels in C2C12 myotubes 30 min after treatment with 0–100 μM resveratrol (Resv).
(B) Cyclic AMP levels in C2C12 myotubes after treatment with resveratrol (50 μM) for the indicated times.
(C) Phosphorylation of AMPK (T172) and AMPK substrate ACC (S79) after treatment with resveratrol (50 μM) in the presence of the AC inhibitor MDL-12,330A in C2C12 myotubes and HeLa cells.
(D) The effect of PKA catalytic subunit α (PKAc) siRNA on resveratrol-induced phosphorylation of ACC and AMPK.
(E) The effect of Epac1 siRNA on resveratrol-induced phosphorylation of ACC and AMPK.
(F) GTP-bound Rap1 was pulled down using the immobilized ras-binding domain (RBD) of RalGDS (left). Quantification of binding is shown on the right (n = 3).
(G) Phosphorylation of ACC and AMPK induced by the Epac agonist 007 (10 μM).
See also Figure S1.
In most cells, intracellular cAMP signaling is mediated by two groups of effectors that bind cAMP: protein kinase A (PKA) and cAMP-regulated guanine nucleotide exchange factors (cAMP-GEFs, Epac1 and Epac2) (de Rooij et al., 1998; Kawasaki et al., 1998). To determine which of these two effector groups activate AMPK in response to resveratrol, we treated cells with siRNA specific for either the PKA catalytic subunit (PKAc) or Epac1, which is more widely expressed than Epac2, prior to resveratrol treatment. For this purpose, we used HeLa cells instead of myotubes because Epac-regulated pathways affect the myogenesis process (Pizon et al., 1999). As shown in Figures 1D and 1E, resveratrol increased the phosphorylation of AMPK and ACC in the presence of PKA siRNA but not in the presence of Epac1 siRNA, suggesting that Epac1 is essential for resveratrol to activate AMPK in HeLa cells.
Epac proteins function as GEFs for Rap1 and Rap2, members of the Ras family of small G proteins that cycle between an inactive GDP-bound state and an active GTP-bound state (de Rooij et al., 1998; Kawasaki et al., 1998). Epac proteins catalyze the exchange of GDP for GTP and thereby activate Rap1 and Rap2. As a result, Epac activity increases the fraction of Rap that is GTP bound, which can be detected by a pull-down assay using the immobilized Ras association (RA) domain of RalGDS (van Triest et al., 2001). We found that Rap1·GTP level in myotubes is increased by resveratrol, indicating that resveratrol increases Epac activity (Figure 1F). Treating myotubes with the Epac-specific agonist 8-(4-chlorophenylthio)-2-O-methyladenosine-3,5-cAMP (also called 007) was sufficient to increase the phosphorylation of AMPK and ACC (Figure 1G). To determine whether resveratrol directly activates Epac, we performed an Epac activity assay in the presence of resveratrol. As shown in Figures S1C and S1D, resveratrol had no direct effect on Epac activity. Together, these findings indicate that resveratrol activates AMPK via Epac1, and resveratrol activates Epac1 indirectly.
Resveratrol Increases NAD+ Levels via Epac1
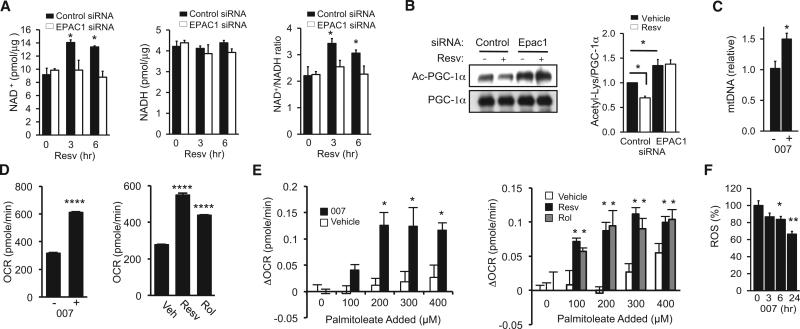
Because AMPK increases NAD+ levels and Sirt1 activity (Cantó et al., 2009, 2010; Fulco et al., 2008; Um et al., 2010), the possibility that the resveratrol-mediated increase in NAD+ levels is also Epac1 dependent was investigated. We found that the resveratrol-mediated increase in NAD+ levels was prevented by Epac1 siRNA but not by control siRNA (Figure 2A). Sirt1-mediated deacetylation of PGC-1α was then examined by immunoprecipitation of PGC-1α and immunoblotting with antibody specific for acetylated Lys. In the presence of Epac1 siRNA, acetylation of PGC-1α was higher and did not decrease after resveratrol treatment (Figure 2B), suggesting that resveratrol activated Sirt1 in an Epac1-dependent manner. To examine whether Epac activity is sufficient to induce mitochondrial biogenesis, myotubes were treated with 007 for 3–4 days before mitochondrial DNA (mtDNA) content was measured. As shown in Figure 2C, 007 increased mitochondrial content by almost 50%. After only 6 hr, 007 significantly increased the basal oxygen consumption rate of myotubes (Figure 2D, left). The measurement of the oxygen consumption rate with increasing concentrations of palmitoleate demonstrated that 007 also increased fat oxidation (Figure 2E, left). Consistent with resveratrol being an Epac activator, resveratrol also increased the oxygen consumption rate (Figure 2D, right) and fat oxidation (Figure 2E, right). Resveratrol, despite increasing metabolic rate, has been shown to reduce the production of reactive oxygen species (ROS) (Um et al., 2010). We found that 007 also decreased ROS levels (Figure 2F). Taken together, our findings provide evidence that resveratrol activates Sirt1 indirectly in an Epac1-dependent manner.
Figure 2. Resveratrol Increases NAD+ Levels and Sirt1 Activity via Epac1.
(A) NAD+, NADH, and NAD+/NADH ratio measured in cells treated with resveratrol and transfected with either control siRNA or Epac1 siRNA (n = 4).
(B) Deacetylation of PGC-1α induced by resveratrol in the presence of either control siRNA or Epac1 siRNA (left). Quantification of PGC-1α acetylation/total PGC-1α is shown in the right panel (n = 3–4).
(C) mtDNA copy number in C2C12 myotubes after 3–4 days of treatment with 007 (10 μM) (n = 4).
(D) Oxygen consumption rate (OCR) in C2C12 myotubes 6 hr after treatment with 007 (10 μM, left), resveratrol (50 μM), rolipram (Rol, 25 μM), or vehicle (Veh) (n = 3).
(E) Change in oxygen consumption rate (DOCR) in C2C12 with palmitoleate injection (100–400 μM) after 6 hr treatment with 007 (10 μM, left), resveratrol (50 μM), rolipram (25 μM), or vehicle (n = 3).
(F) ROS levels in C2C12 myotubes treated with 007 (10 μM) for the indicated times (n = 4). Results are expressed as the mean ± SEM. *p < 0.05; **p < 0.01; ****p < 0.0001 compared to the negative control or vehicle-treated samples.
Resveratrol Activates the CamKKβ-AMPK Pathway via the PLC-Ryr2 Pathway
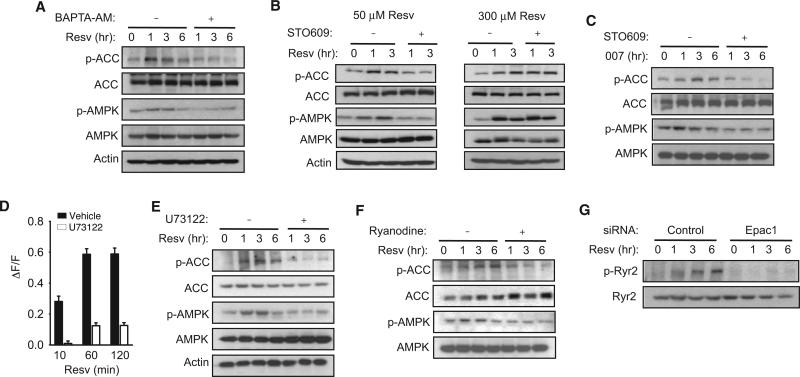
AMPK activation requires phosphorylation by one of two AMPK kinases, LKB1 (Hawley et al., 2003; Woods et al., 2003) or calcium/calmodulin-dependent kinase kinase β (CamKKβ) (Hawley et al., 2005; Hurley et al., 2005; Woods et al., 2005). Activation of AMPK via energy depletion is thought to be dependent on LKB1, and activation of AMPK via increased intracellular Ca2+ is dependent on CamKKβ (Hawley et al., 2005; Hurley et al., 2005; Woods et al., 2005). We asked whether resveratrol and Epac1 activate the CamKKβ-AMPK pathway, based on two observations: First, resveratrol has been shown to increase cytosolic Ca2+ (Campos-Toimil et al., 2007; Sareen et al., 2007; Vingtdeux et al., 2010). Second, in cardiac myocytes, Epac1 has been shown to increase cytosolic Ca2+ in a phospholipase C (PLC)-dependent manner (Oestreich et al., 2007, 2009; Schmidt et al., 2001) via calcium/calmodulin-dependent protein kinase II (CamKII) (Pereira et al., 2007). To examine the potential role of the CamKKβ-AMPK pathway in resveratrol action, we treated myotubes with either the Ca2+ chelator BAPTA-AM or the CamKK inhibitor STO609. We found that both BAPTA-AM (Figure 3A) and STO609 (Figure 3B, left) decreased the phosphorylation of AMPK and ACC after treatment with 50 μM resveratrol, indicating that resveratrol stimulated the phosphorylation of AMPK and ACC in a CamKKβ-dependent manner. Because high concentration of resveratrol was reported to activate AMPK by decreasing energy (Hawley et al., 2010), we examined whether the phosphorylation of AMPK and ACC by high concentration of resveratrol (300 μM) was also sensitive to STO609. As shown in Figure 3B (right), STO609 did not affect the phosphorylation of AMPK and ACC after treatment with 300 μM resveratrol, indicating that the phosphorylation of AMPK and ACC by high concentration of resveratrol did not require CaMKKβ. To demonstrate that the Epac-induced phosphorylation of AMPK and ACC was also CamKKβ dependent, we treated myotubes with 007 in the presence of STO609 (Figure 3C). We found that STO609 prevented 007 from inducing the phosphorylation of AMPK and ACC. Together, these findings indicate that resveratrol at low concentration activates the CamKKβ-AMPK pathway via Epac1.
Figure 3. PLC and Ryr Are Required for Resveratrol to Activate AMPK.
(A) The phosphorylation of ACC and AMPK induced by resveratrol in C2C12 myotubes in the absence (–) or presence (+) of calcium chelator BAPTA-AM (20 μM). We used myotubes derived from low-passage (<10) C2C12 cells.
(B) The phosphorylation of ACC and AMPK induced by resveratrol in C2C12 myotubes in the absence (–) or presence (+) of the CamKK inhibitor STO609 (5 mg/ml) for 50 μM (left) or 300 μM (right) resveratrol. We used myotubes derived from low-passage (<10) C2C12 cells.
(C) The phosphorylation of ACC and AMPK induced by 007 in C2C12 myotubes in the absence (–) or presence (+) of the CamKK inhibitor STO609 (5 μg/ml). C2C12 cells of <10 passages were used.
(D) The increase in intracellular Ca2+ levels after resveratrol treatment (50 μM) in C2C12 myotubes loaded with Ca2+ indicator Fluo-4 AM in the presence of PLC inhibitor U73122 (20 μM). F indicates the fluorescence level and DF indicates the change in fluorescence (n = 3).
(E) The phosphorylation of ACC and AMPK induced by resveratrol (50 μM) in C2C12 myotubes in the absence (–) or presence (+) of the PLC inhibitor U73122 (20 μM).
(F) Phosphorylation of ACC and AMPK induced by resveratrol in C2C12 myotubes in the absence (–) or presence (+) of the Ryr inhibitor ryanodine.
(G) Resveratrol-induced phosphorylation of S2815 in Ryr2 in the presence of siRNA specific for Epac1 or control siRNA.
See also Figure S2.
As Epac1 increases intracellular Ca2+ via PLC, we examined the role of PLC in resveratrol-induced Ca2+ release. The resveratrol-induced increase in intracellular Ca2+ was significantly reduced in the presence of the PLC inhibitor U73122 (Figure 3D), indicating that resveratrol increased intracellular Ca2+ in a PLC-dependent manner. Consistent with this, U73122 prevented resveratrol from stimulating the phosphorylation of AMPK and ACC (Figure 3E). Upon activation of Epac, the ryanodine receptor 2 (Ryr2) is phosphorylated and facilitates the release of Ca2+ from the endoplasmic reticulum/sarcoplasmic reticulum (ER/SR) (Wehrens et al., 2004). We treated myotubes with resveratrol in the presence of the Ryr2 inhibitor ryanodine and found that ryanodine prevented resveratrol from stimulating the phosphorylation of AMPK and ACC (Figure 3F). Another ER/SR Ca2+-release channel, the inositol triphosphate (IP3) receptor, which is activated by IP3, does not appear to be involved in resveratrol action because resveratrol was able to stimulate the phosphorylation of AMPK and ACC in the presence of the IP3 receptor inhibitor 2-aminoethoxy-diphenyl borate (2-APB) (Figure S2). We also used Epac1 siRNA to test the Epac1 dependence of CamKII-mediated phosphorylation of Ryr2 residue S2815 (Figure 3G). In agreement with our hypothesis, resveratrol can only induce Ryr2 phosphorylation when Epac1 is active. These findings indicate that resveratrol activates CamKKβ-AMPK via the Epac1-PLC-Ryr pathway.
Resveratrol Is a Nonselective Phosphodiesterase Inhibitor
The intracellular levels of cAMP are determined by the activities of ACs, which synthesize cAMP from ATP, and cyclic nucleotide PDEs, which hydrolyze cAMP or cGMP to AMP or GMP, respectively. We measured the effect of resveratrol on the activities of representatives of all three major subclasses of mammalian ACs. As shown in Figure S3A, resveratrol had no effect on AC activity, either in the basal state or in the activated state, suggesting that resveratrol increases cAMP levels by inhibiting PDEs.
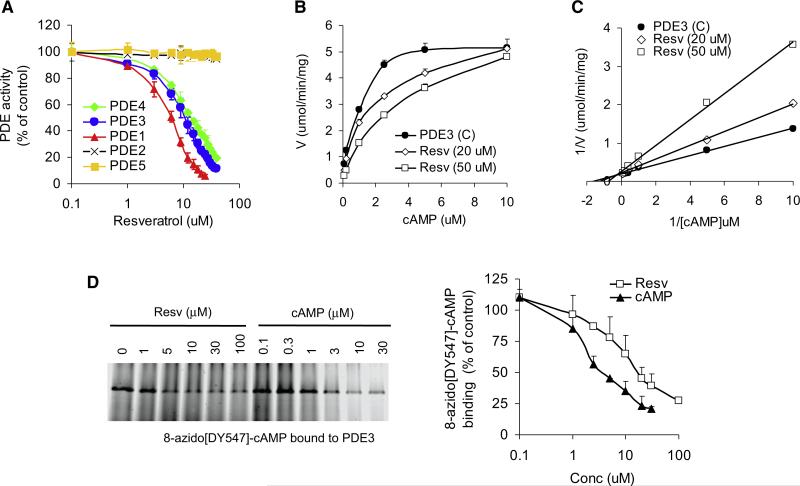
The PDE superfamily is comprised of 11 types of PDEs (PDE1–11) of which multiple isoforms exist. PDEs have different substrate specificities: PDEs 4, 7, and 8 are cAMP-selective hydrolases, PDEs 5, 6, and 9 are cGMP-selective hydrolases, and PDEs 1, 2, 3, 10, and 11 can hydrolyze both cAMP and cGMP to varying degrees. Multiple members of the PDE superfamily are usually expressed in each cell. We measured the activities of recombinant PDEs 1, 2, 3, 4, and 5 in the presence of resveratrol. We found that resveratrol inhibited PDE1 (IC50 ~6 μM), PDE3 (IC50 ~10 μM), and PDE4 (IC50 ~14 μM) but did not affect the activity of PDEs 2 or 5 (Figure 4A).
Figure 4. Resveratrol Is a PDE Inhibitor.
(A) The effect of resveratrol on the activities of recombinant PDEs 1–5.
(B) Velocity of recombinant PDE3 activity as a function of cAMP and resveratrol concentration.
(C) Lineweaver-Burk plot of (B).
(D) Recombinant PDE3 was photoaffinity labeled with the fluorescent cAMP analog 8-azido-[DY-547]-cAMP in the presence of resveratrol or cAMP. 8-azido-[DY-547]-cAMP bound to PDE3 was visualized by fluorescence imaging (left). Quantification of 8-azido-[DY-547]-cAMP binding is shown in the right panel (n = 3).
To determine how resveratrol inhibits PDEs, we measured the effect of cAMP and resveratrol on the kinetics of recombinant PDE3 activity (Figure 4B). At high concentrations of cAMP, the inhibitory effect of resveratrol on PDE3 disappeared, suggesting that resveratrol is competing with cAMP, and a Lineweaver-Burk plot is consistent with a competitive inhibition mechanism (Figure 4C). To directly demonstrate that resveratrol competes with cAMP in its binding site, we incubated PDE3 with the fluorescent cAMP analog 8-azido[DY547]-cAMP, which cross-links to its binding site when stimulated with UV, in the presence of increasing concentrations of either resveratrol or cAMP. Both resveratrol and cAMP competed with 8-azido[DY547]-cAMP for the binding site in PDE3 (Figure 4D). Because the binding of 8-azido[DY547]-cAMP to PDE3 is irreversible, whereas the binding of resveratrol or cAMP is reversible, the data shown in Figure 4D are an underestimation of the actual competitiveness of cAMP or resveratrol. A simulation of the docking of resveratrol into the catalytic pocket of PDE3 suggests that resveratrol may fit into the catalytic pocket in two orientations (Figures S3B–S3E). Taken together, these findings support the conclusion that resveratrol increases cAMP levels by competitively inhibiting PDEs.
Resveratrol Activates AMPK and Increases Mitochondrial Biogenesis by Inhibiting PDEs
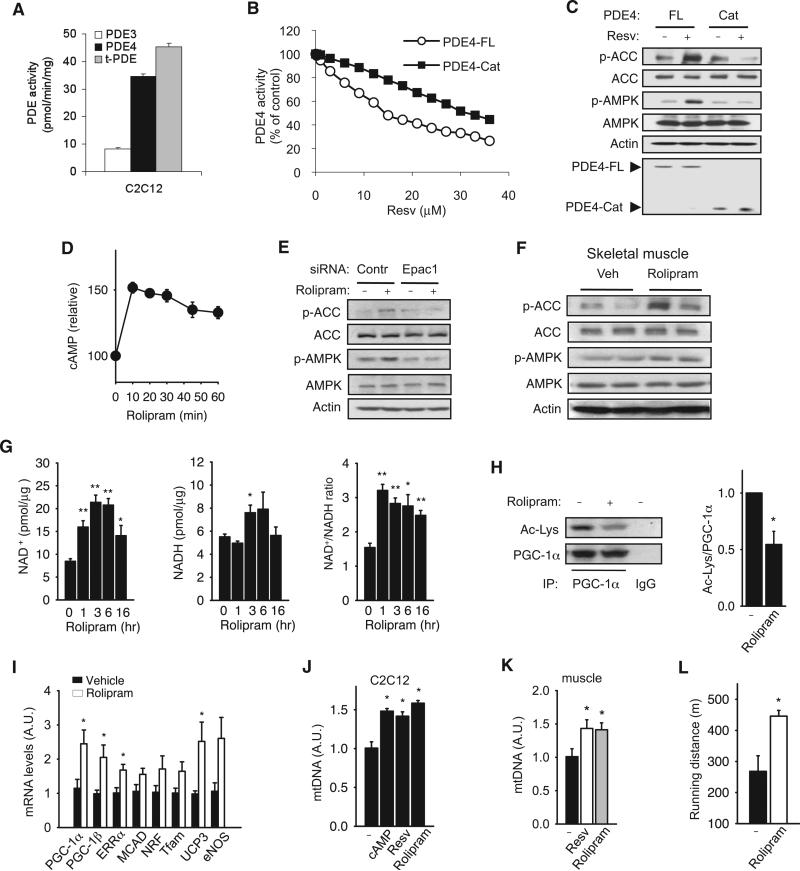
By treating myotubes with specific PDE3 and PDE4 inhibitors, we determined that 76% of the total basal PDE activity was attributable to PDE4 and 18% to PDE3 (Figures 5A and S4). To corroborate our in vitro biochemical findings that resveratrol inhibits PDEs, we sought genetic evidence by using a PDE4 mutation. Previously, it has been shown that the upstream conserved regions (UCRs) of PDE4 increase the sensitivity of PDE4 to competitive inhibitors, whereas the catalytic domain of PDE4, which is missing the UCRs, is more resistant to the known competitive inhibitors (Burgin et al., 2010). We found that the UCRs of PDE4 are also important for inhibition by resveratrol because the resveratrol IC50 of the PDE4 catalytic domain was approximately 2.4-fold higher than that of the full-length PDE4 (~33 μM versus ~14 μM) (Figure 5B). If resveratrol activates AMPK by inhibiting PDE4, resveratrol-induced phosphorylation of ACC and AMPK in myotubes expressing the PDE4 catalytic domain should be reduced compared to those expressing full-length PDE4. As shown in Figure 5C, this was indeed the case.
Figure 5. Resveratrol Activates AMPK and Increases Mitochondrial Biogenesis by Inhibiting PDEs.
(A) The relative contributions of PDE3 and PDE4 to the total PDE (t-PDE) activity in C2C12 myotube lysates were determined by adding either 1 μM cilostamide (PDE3 inhibitor) or 10 μM rolipram (PDE4 inhibitor) to the PDE reaction (n = 3).
(B) The catalytic domain (Cat) of PDE4 is less sensitive to resveratrol than full-length (FL) PDE4. The resveratrol inhibitory curves of recombinant His-tagged PDE4 (FL, Cat) are shown.
(C) The phosphorylation of ACC and AMPK in C2C12 myotubes overexpressing either His-tagged PDE4-FL or PDE4-Cat after they were treated with resveratrol (50 μM) for 3 hr. The levels of PDE4-FL and PDE4-Cat were detected with anti-His antibody.
(D) Cyclic AMP levels in C2C12 myotubes after treatment with rolipram (25 μM) for the indicated times.
(E) The phosphorylation of ACC and AMPK after treatment with rolipram (25 μM) in the presence of control siRNA (Contr) or Epac1 siRNA (Epac1).
(F) The phosphorylation of ACC and AMPK in skeletal muscle (gastrocnemius) after treatment with rolipram (2 mg/kg/day) for 14 weeks.
(G) NAD+, NADH, and the NAD+/NADH ratio in C2C12 myotubes were measured after 1–16 hr of rolipram treatment (25 μM).
(H) Deacetylation of PGC-1α in C2C12 myotubes treated with rolipram (left). Quantification of PGC-1α acetylation/total PGC-1α is shown in the right panel (n = 4).
(I) Expression levels (mRNA) of genes important for mitochondrial biogenesis and function as measured by real-time PCR from skeletal muscle of mice treated with rolipram (n = 4).
(J) mtDNA content in C2C12 myotubes treated with cAMP (100 μM), resveratrol (50 μM), or rolipram (25 μM) for 4 days (n = 5).
(K) mtDNA content in skeletal muscle of mice fed resveratrol (400 mg/kg/day) or rolipram (2 mg/kg/day) for 14 weeks (n = 5).
(L) Mice fed rolipram for 12 weeks were exercised on a treadmill. Running distance prior to exhaustion is shown (n = 5). Results are expressed as the mean ± SEM. *p < 0.05 and **p < 0.01 between the treatment groups.
If the metabolic effects of resveratrol result from inhibiting PDEs, a known PDE inhibitor should produce metabolic effects very similar to those produced by resveratrol. Because PDE4 makes up most of the PDE activity in myotubes, we treated myotubes with the PDE4 inhibitor rolipram and found that rolipram increased cAMP to levels similar to those induced by resveratrol in myotubes (Figure 5D). Like resveratrol, rolipram stimulated the phosphorylation of AMPK and ACC in an Epac1-dependent manner (Figure 5E). To demonstrate that rolipram can activate AMPK in vivo, we treated mice with rolipram and harvested skeletal muscle. As shown in Figure 5F, rolipram-treated mice had higher levels of phosphorylated AMPK and ACC than vehicle-treated mice. Like resveratrol, rolipram increased NAD+ levels (Figure 5G) and increased PGC-1α deacetylation (Figure 5H), suggesting that rolipram increased Sirt1 activity.
To test whether rolipram can reproduce the metabolic effects of resveratrol in vivo, we determined the effect of rolipram (2 mg/ kg/day) on C57BL6/J mice fed with an HFD. After 12–14 weeks of treatment, we isolated skeletal muscle and measured the mRNA levels of genes that are known to be induced by resveratrol and AMPK, such as eNOS, PGC-1α, and others important for mitochondrial biogenesis. We found that rolipram consistently increased the mRNA levels of these genes (Figure 5I). In agreement with this, treatment with resveratrol, rolipram, or cAMP induced mitochondrial biogenesis in myotubes to comparable levels (Figure 5J). Rolipram and resveratrol also increased mitochondrial content to similar levels in mouse skeletal muscle (Figure 5K). To determine whether increased mitochondrial function improved exercise tolerance, we exercised rolipram-treated mice on a treadmill. Rolipram-treated mice ran a significantly greater distance on a treadmill before exhaustion than control mice (445 ± 19 m versus 268 ± 50 m) (Figure 5L). Taken together, these findings indicate that rolipram and resveratrol have very similar effects on mitochondrial biogenesis in skeletal muscle.
PDE Inhibition Protects against Diet-Induced Obesity and Glucose Intolerance
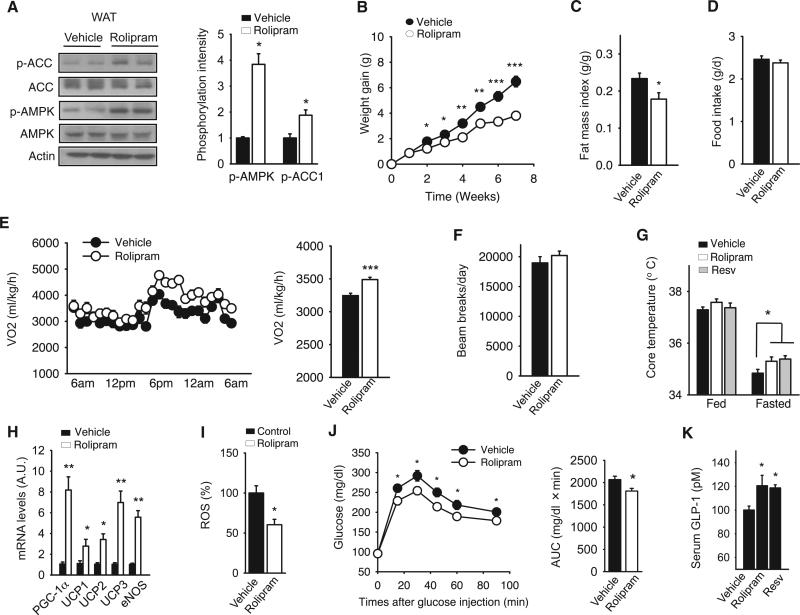
The similarity between the effects of rolipram and resveratrol also extended to WAT. As was the case with resveratrol-treated mice (Baur and Sinclair, 2006; Um et al., 2010), the phosphorylation levels of AMPK and ACC were increased in the WAT of rolipram-treated mice (Figure 6A). C57BL6/J mice treated with rolipram were resistant to weight gain on an HFD (Figure 6B) and had less fat content (Figure 6C) even though their food intake was similar to that of control mice (Figure 6D). Decreased weight gain despite normal food intake suggests that rolipram increased the metabolic rate. Because rolipram, like resveratrol and 007, increased the oxygen consumption rate (Figure 2D, right) and fat oxidation in myotubes (Figure 2E), we measured the oxygen consumption rate in rolipram-treated mice. The oxygen consumption rate was increased in rolipram-treated mice (Figure 6E), but physical activity levels were not affected (Figure 6F), indicating that rolipram increased the basal metabolic rate. Consistent with this, both resveratrol- and rolipram-treated mice had higher body temperatures in the fasting state than control mice (Figure 6G). Rolipram, like resveratrol (Um et al., 2010), increased the expression levels of thermogenic genes such as uncoupling proteins (UCPs) and PGC-1α in the adipose tissue (Figure 6H).
Figure 6. PDE Inhibition Protects against Diet-Induced Obesity and Glucose Intolerance.
(A) Rolipram increases the phosphorylation of ACC and AMPK in WAT. After 14 weeks of rolipram (2 mg/kg/day) treatment, epididymal fat was isolated from mice and analyzed for AMPK and ACC phosphorylation. Quantification of phoshorylation intensity is shown on the right (n = 4).
(B) Rolipram protects against diet-induced obesity. Mice fed HFD were treated with either saline (vehicle) or rolipram (2 mg/kg/day) and the weight gain during treatment is shown (n = 10 per group).
(C) The fat mass index (fat mass/total body weight) of mice from (B) was measured by NMR spectrometry (n = 10).
(D) HFD intake for vehicle- and rolipram-treated mice measured during weeks 1–4 of the treatment (n = 5).
(E) Twenty-four hour oxygen consumption (VO2) of mice treated with either rolipram or vehicle for 12–14 weeks. A 24 hr average of VO2 is shown on the right (n = 6).
(F) Rolipram does not affect locomotor activity. Twenty-four hour locomotor activity level was measured by beam-breaks (n = 5).
(G) Rolipram increases thermogenesis. The rectal temperatures of mice treated with either rolipram or vehicle in the fed or fasting (overnight) state (n = 7–10) are shown.
(H) Rolipram increases the expression of genes important for thermogenesis in WAT. The transcriptional levels of PGC-1α, the uncoupling proteins, and eNOS in WAT after 14 weeks of rolipram treatment were determined by real-time PCR (n = 5).
(I) ROS levels in WAT were measured by relative DCF fluorescence in WAT extract and shown as a percentage of that in control mice (n = 5).
(J) Glucose tolerance test (left) and the area under the curve (AUC) (right) are shown for HFD-fed mice treated with either rolipram or vehicle for 12–14 weeks (n = 7–10).
(K) The serum levels of GLP-1 were measured in mice treated for 7 weeks with resveratrol or rolipram (n = 4–8).
Results are expressed as the mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001 between treatment groups.
PGC-1α increases ROS scavenging capacity (St-Pierre et al., 2003). Consistent with rolipram-treated mice expressing higher levels of PGC-1α, rolipram-treated mice had lower ROS levels (Figure 6I). Considering all of the metabolic changes that rolipram produced, including the reduction of ROS and fat mass and increased mitochondrial function, we expected that rolipram would improve glucose tolerance. Indeed, rolipram-treated mice were more glucose tolerant than were control mice (Figure 6J).
Glucagon-like peptide-1 (GLP-1), which is secreted from the gut, has antidiabetogenic activities, and GLP-1 analogs, as well as drugs that increase the endogenous GLP-1 levels, are part of type 2 diabetes therapy. The expression of GLP-1 is positively regulated by cAMP (Gevrey et al., 2002) and therefore may be induced when either resveratrol and rolipram inhibits PDEs. Supporting this idea, we found that both resveratrol and rolipram increased serum levels of GLP-1 by almost 20% (Figure 6K). Together, these results indicate that both resveratrol and rolipram may protect against type 2 diabetes by diverse mechanisms including hormonal regulation.
DISCUSSION
By demonstrating that resveratrol activates the cAMP-Epac1-AMPK-Sirt1 pathway, this study, in conjunction with previous studies (Beher et al., 2009; Borra et al., 2005; Kaeberlein et al., 2005; Pacholec et al., 2010), explains how resveratrol activates Sirt1 without directly targeting it. Although resveratrol was initially shown to directly activate Sirt1 in an assay that utilized a fluorophore-linked substrate (Howitz et al., 2003), our studies show that resveratrol indirectly activates Sirt1 in vivo due to its effect on cAMP signaling.
Our findings on the mechanism of resveratrol action have implications for other known putative Sirt1 activators such as SRT1720, SRT2183, and SRT1460 (Milne et al., 2007; Pacholec et al., 2010). Like resveratrol, they were discovered as Sirt1 activators by using the fluorophore-tagged substrate and only exhibit Sirt1 activation in the presence of fluorophore-modified substrate. Thus, it is likely that they may also activate Sirt1 via an upstream target in vivo. Because the metabolic effects of SRT1720 are nearly identical to those of resveratrol (Milne et al., 2007; Feige et al., 2008), it is tempting to speculate that these compounds, or at least SRT1720, act via pathways similar to those of resveratrol.
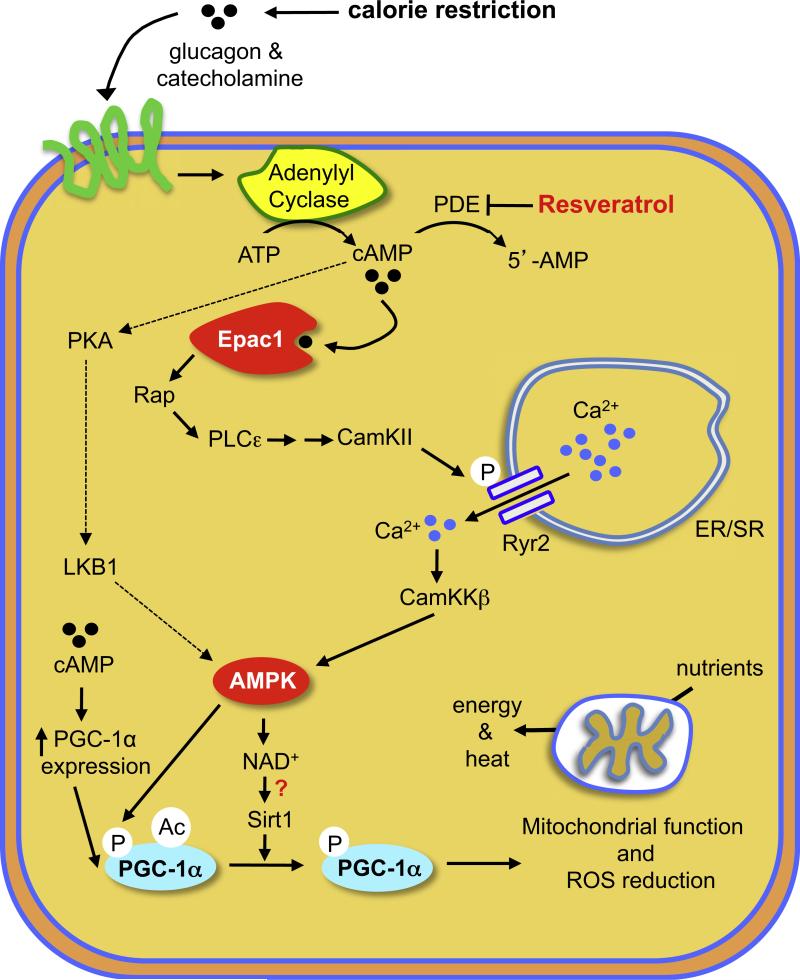
cAMP signaling is highly complex, and its outcomes vary depending on the effector activated by cAMP and on other factors including the cell type, the cellular compartment of cAMP action, and the duration and intensity of cAMP signaling. cAMP is a key mediator of metabolic regulation, and the identification of PDEs as resveratrol targets might explain how resveratrol mimics some aspects of CR. Nutrient deprivation increases cAMP levels as a consequence of increased glucagon and catecholamine signaling and decreased insulin/IGF-1 signaling (Rondinone et al., 2000; Selawry et al., 1973). Resveratrol, by increasing cAMP levels and activating Epac1, may induce some of the pathways that are normally induced during CR (Figure 7). The positive health benefits of PDE4 inhibitors, such as improved memory (Burgin et al., 2010) and protection against aging-related diseases such as Alzheimer's (Smith et al., 2009) and Parkinson's (Yang et al., 2008) diseases, have been demonstrated in animal models. It is therefore possible that PDE4 inhibitors may be useful for treating metabolic diseases and other aging-related diseases in humans.
Figure 7. Proposed Model of How Resveratrol Mimics CR.
Resveratrol inhibits PDE activity and induces cAMP signaling via Epac1, which activates PLCε, resulting in Ca2+ release via the Ryr2 Ca2+ channel and, ultimately, the activation of the CamKKβ-AMPK pathway. CR increases cAMP levels by increasing glucagon and catecholamine levels, which activate AC activity and cAMP production. AMPK increases mitochondrial biogenesis and function by increasing PGC-1α expression, NAD+ levels, and Sirt1 activity. An additional pathway that may contribute to resveratrol action is indicated with dotted lines.
EXPERIMENTAL PROCEDURES
Cell Culture
C2C12 myoblast cells (ATCC) and HeLa cells were maintained in DMEM and 10% fetal bovine serum. To generate C2C12 myotubes, confluent cultures of C2C12 cells were grown in DMEM with 2% horse serum for 3–5 days. We found that C2C12 myotubes generated from early passage (<10 passages after purchase from ATCC) C2C12 myoblast cells yielded most consistent results.
PDE Assay
PDE activity was measured by modification of a previously published method (Ahmad et al., 2009; Manganiello and Vaughan, 1973) by using 10 nM [3H]cAMP (45000 cpm) or [3H]cGMP as substrates. Less than 10%–15% of the substrates were hydrolyzed during the PDE reaction. Portions of solubilized cell lysates were assayed for PDE activity by incubation with resveratrol (0–100 μM) or with specific PDE inhibitors. Recombinant PDE1 (10 ng) activity was assayed by using 4 μg/ml calmodulin and 0.8 μM Ca2+ together with [3H]cAMP as substrate in the reaction mixture. Recombinant PDE2 (15 ng) activity was assayed in the presence of 1 μM cGMP, which activated it by ~3 fold. Activities of recombinant PDE3 (1 ng) and PDE4 (1 ng) were assayed by incubation in a reaction mixture containing 1 mg/ml BSA, with [3H]cAMP. Recombinant PDE5 (150 ng) activity was assayed using [3H]cGMP as substrate. PDE activity is expressed as pmol of cAMP or cGMP hydrolyzed/min/mg protein. The PDE3 inhibitor cilostamide and the PDE4 inhibitor rolipram were used to define PDE specificity. Recombinant full-length PDE4D was purchased from Signalchem, and the recombinant PDE4D catalytic domain was purchased from Enzo Life Sciences. To measure the activities of PDE3 or PDE4 in C2C12 lysates, PDE activity was measured in the presence of 1 μM cilostamide or 10 μM rolipram.
Animal Experiments
All experiments were approved by the NHLBI ACUC (Animal Care and Use Committee). C57BL/6J mice were originally purchased from Jackson Laboratory. Four- to six-week-old male mice were housed with a 12 hr light-dark cycle (light on 6 am–6 pm) with free access to food and water. For all rolipram-related studies, mice were dosed once daily by oral gavage with 2 mg/kg/day rolipram (Enzo Life Sciences) or with saline and were fed a HFD (40% calories from fat, Research Diets) for up to 14 weeks. To measure the effect of resveratrol on cAMP production, C57BL/6J mice were injected (intraperitoneally [i.p.]) with resveratrol (20 mg/kg body weight) or with DMSO (vehicle). For studies involving chronic resveratrol treatment, C57BL/6J mice were fed a HFD containing resveratrol (400 mg/kg/day) for 14 weeks.
Metabolic Measurements
Body weight and caloric intake were monitored weekly. Plasma glucose was measured by using a glucometer (Ascensia). For the glucose tolerance test, mice were fasted for 16 hr, and 1 mg/g glucose was injected i.p. Blood glucose was measured at 0, 15, 30, 45, 60, and 90 min after injection. Prior to the tread-mill endurance tests, the mice were trained for 3 days by running on an Exer-3/6 mouse treadmill (Columbus Instruments) at 10 m/min for 5 min. For the endurance test, the treadmill was set at a 15 incline, and the speed was increased in a stepwise fashion (10 m/min for 10 min followed by 14 m/min for 5 min and then the final speed of 18 m/min). The test was terminated when mice reached exhaustion, which was defined as immobility for more than 30 s. Locomotor activity of mice was measured by photobeam breaks by using the Opto-Varimex-4 (Columbus Instruments). Indirect calorimetry was performed using Oxymax chambers (Columbus Instruments). All mice were acclimatized for 24 hr before measurements. Resting metabolic rate was determined by calculating the average energy expenditure at each 30 min time point during a 24 hr period.
Statistical Analysis
Comparisons between the treatment groups were analyzed by two-tailed Student's t test, and comparisons involving repeated measurements were analyzed by ANOVA repeated measures. Results are expressed as the mean ± standard error of the mean (SEM). Significance was accepted at p < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program, National Heart Lung and Blood Institute, National Institutes of Health. We thank Andrew Marks for the Ryr2 phosphoantibody; Dr. Yan Tang for sharing data regarding inhibition of PDE3 by resveratrol; Marije Rensen-De Leeuw and Yan Tang for technical assistance; Shutong Yang and Dalton Saunders for animal management; and Alexandra Brown for manuscript review and comments. H.R. is supported by the Netherlands Organization for Scientific Research (NWO).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures and four figures and can be found with this article online at doi:10.1016/j.cell.2012.01.017.
REFERENCES
- Ahmad F, Lindh R, Tang Y, Ruishalme I, Ost A, Sahachartsiri B, Strålfors P, Degerman E, Manganiello VC. Differential regulation of adipocyte PDE3B in distinct membrane compartments by insulin and the beta3-adrenergic receptor agonist CL316243: effects of caveolin-1 knockdown on formation/maintenance of macromolecular signalling complexes. Biochem. J. 2009;424:399–410. doi: 10.1042/BJ20090842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem. Biol. Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, Thorsteinsdottir M, Hrafnsdottir S, Hagen T, Kiselyov AS, et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat. Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
- Campos-Toimil M, Elíes J, Alvarez E, Verde I, Orallo F. Effects of trans- and cis-resveratrol on Ca2+ handling in A7r5 vascular myocytes. Eur. J. Pharmacol. 2007;577:91–99. doi: 10.1016/j.ejphar.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol. Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- El-Mowafy AM, Alkhalaf M. Resveratrol activates adenylylcyclase in human breast cancer cells: a novel, estrogen receptor-independent cytostatic mechanism. Carcinogenesis. 2003;24:869–873. doi: 10.1093/carcin/bgg015. [DOI] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevrey JC, Cordier-Bussat M, Némoz-Gaillard E, Chayvialle JA, Abello J. Co-requirement of cyclic AMP- and calcium-dependent protein kinases for transcriptional activation of cholecystokinin gene by protein hydrolysates. J. Biol. Chem. 2002;277:22407–22413. doi: 10.1074/jbc.M201624200. [DOI] [PubMed] [Google Scholar]
- Gruber J, Tang SY, Halliwell B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Ann. N Y Acad. Sci. 2007;1100:530–542. doi: 10.1196/annals.1395.059. [DOI] [PubMed] [Google Scholar]
- Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 2010 doi: 10.1038/ncomms1001. Published online April 12, 2010. 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Manganiello V, Vaughan M. An effect of insulin on cyclic adenosine 3′:5′-monophosphate phosphodiesterase activity in fat cells. J. Biol. Chem. 1973;248:7164–7170. [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 1935;10:63–79. 1935. [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac-mediated activation of phospholipase C(epsilon) plays a critical role in beta-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J. Biol. Chem. 2007;282:5488–5495. doi: 10.1074/jbc.M608495200. [DOI] [PubMed] [Google Scholar]
- Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J. Biol. Chem. 2009;284:1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CE, Kim MJ, Lee JH, Min BI, Bae H, Choe W, Kim SS, Ha J. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp. Mol. Med. 2007;39:222–229. doi: 10.1038/emm.2007.25. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Métrich M, Fernández-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Bénitah JP, et al. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J. Physiol. 2007;583:685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizon V, Méchali F, Baldacci G. RAP1A GTP/GDP cycles determine the intracellular location of the late endocytic compartments and contribute to myogenic differentiation. Exp. Cell Res. 1999;246:56–68. doi: 10.1006/excr.1998.4284. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rondinone CM, Carvalho E, Rahn T, Manganiello VC, Degerman E, Smith UP. Phosphorylation of PDE3B by phosphatidylinositol 3-kinase associated with the insulin receptor. J. Biol. Chem. 2000;275:10093–10098. doi: 10.1074/jbc.275.14.10093. [DOI] [PubMed] [Google Scholar]
- Sareen D, Darjatmoko SR, Albert DM, Polans AS. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol. Pharmacol. 2007;72:1466–1475. doi: 10.1124/mol.107.039040. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat. Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Selawry H, Gutman R, Fink G, Recant L. The effect of starvation on tissue adenosine 3′-5′ monophosphate levels. Biochem. Biophys. Res. Commun. 1973;51:198–204. doi: 10.1016/0006-291x(73)90528-7. [DOI] [PubMed] [Google Scholar]
- Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J. Nutr. Biochem. 2005;16:449–466. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc. Natl. Acad. Sci. USA. 2009;106:16877–16882. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J. Biol. Chem. 2003;278:26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, Ido Y, Puigserver P, Ruderman NB. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem. Biophys. Res. Commun. 2009;378:836–841. doi: 10.1016/j.bbrc.2008.11.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Triest M, de Rooij J, Bos JL. Measurement of GTP-bound Ras-like GTPases by activation-specific probes. Methods Enzymol. 2001;333:343–348. doi: 10.1016/s0076-6879(01)33068-9. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Calingasan NY, Lorenzo BJ, Beal MF. Attenuation of MPTP neurotoxicity by rolipram, a specific inhibitor of phosphodiesterase IV. Exp. Neurol. 2008;211:311–314. doi: 10.1016/j.expneurol.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.