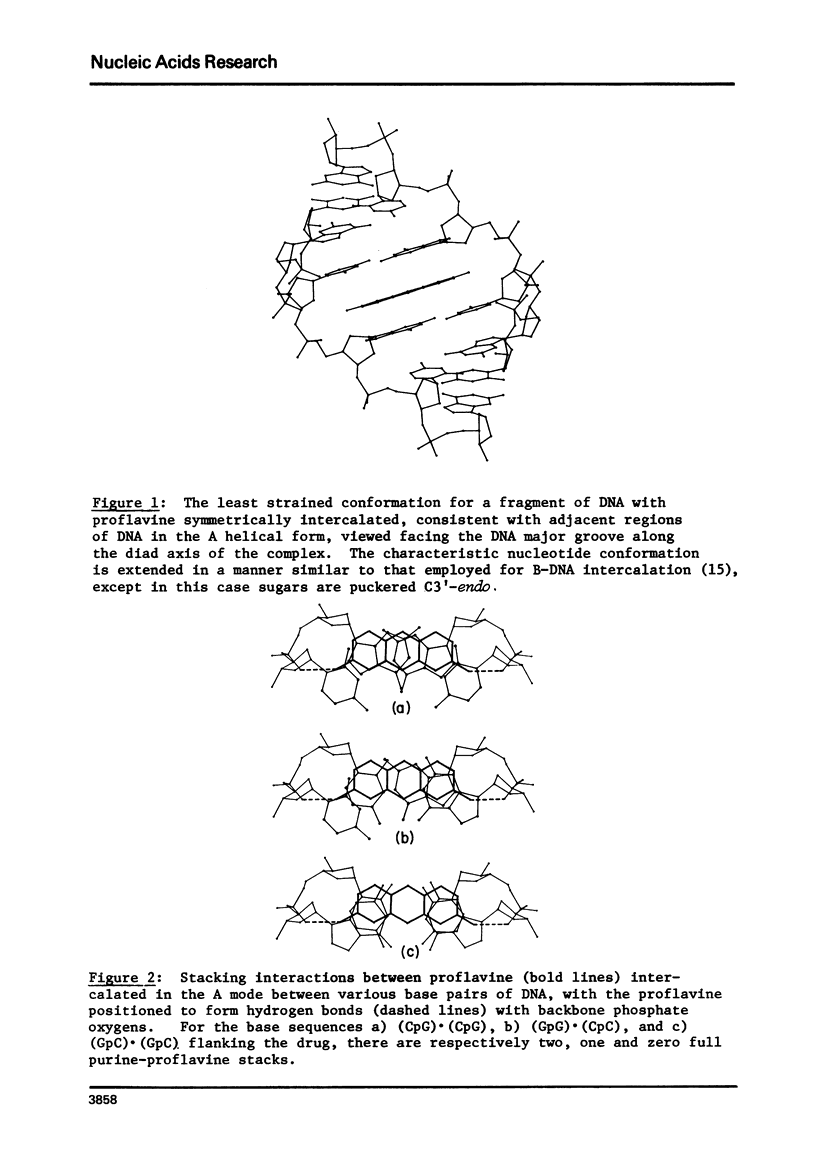
Abstract
Linked-atom molecular modelling was employed to determine the steric and torsional requirements for intercalation of proflavine into a double-stranded region of DNA compatible with adjacent regions of cohelical A-DNA. The optimum intercalation conformation is characterized by the dihedral angles xi and psi becoming trans, with all sugars retaining the characteristics C3'-endo pucker. This extended conformation results in virtually no helical unwinding, suggesting it may be an appropriate model for an intercalative intermediary in mutagenesis by virtue of its similarity to standard helical DNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alden C. J., Arnott S. Visualization of planar drug intercalations in B-DNA. Nucleic Acids Res. 1975 Oct;2(10):1701–1717. doi: 10.1093/nar/2.10.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291x(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Waring M. J. Supercoiling of polyoma virus DNA measured by its interaction with ethidium bromide. J Mol Biol. 1967 Apr 14;25(1):23–30. doi: 10.1016/0022-2836(67)90276-8. [DOI] [PubMed] [Google Scholar]
- Dimicoli J. L., Hélène C. Interactions of aromatic residues of proteins with nucleic acids. I. Proton magnetic resonance studies of the binding of tryptophan-containing peptides to poly(adenylic acid) and deoxyribonucleic acid. Biochemistry. 1974 Feb 12;13(4):714–723. doi: 10.1021/bi00701a013. [DOI] [PubMed] [Google Scholar]
- Jain S. C., Sobell H. M. Stereochemistry of actinomycin binding to DNA. I. Refinement and further structural details of the actinomycin-deoxyguanosine crystalline complex. J Mol Biol. 1972 Jul 14;68(1):1–20. doi: 10.1016/0022-2836(72)90258-6. [DOI] [PubMed] [Google Scholar]
- Krugh T. R., Wittlin F. N., Cramer S. P. Ethidium bromide-dinucleotide complexes. Evidence for intercalation and sequence preferences in binding to double-stranded nucleic acids. Biopolymers. 1975 Jan;14(1):197–210. doi: 10.1002/bip.1975.360140114. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Bünemann H., Dattagupta N. Interactions of heteroaromatic compounds with nucleic acids. 2. Influence of substituents on the base and sequence specificity of intercalating ligands. Eur J Biochem. 1975 May;54(1):279–291. doi: 10.1111/j.1432-1033.1975.tb04138.x. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Interactions of heteroaromatic compounds with nucleic acids. 1. The influence of heteroatoms and polarizability on the base specificity of intercalating ligands. Eur J Biochem. 1975 May;54(1):267–277. doi: 10.1111/j.1432-1033.1975.tb04137.x. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L. Sequence specificity of mutagen-nucleic acid complexes in solution: intercalation and mutagen-base pair overlap geometries for proflavine binding to dC-dC-dG-dG and dG-dG-dC-dC self-complementary duplexes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2624–2628. doi: 10.1073/pnas.74.7.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman N. C., Day R. O., Rich A. Nucleic acid-mutagen interactions: crystal structure of adenylyl-3',5'-uridine plus 9-aminoacridine. Nature. 1975 Jan 31;253(5490):324–327. doi: 10.1038/253324a0. [DOI] [PubMed] [Google Scholar]
- Tichadou J. L., Genest D., Wahl P., Aubel-Sabron G. The use of fluorescence anisotropy decay of poly d(A-T) ethidium bromide complex to estimate the unwinding angle of the double helix. Biophys Chem. 1975 Apr;3(2):142–146. doi: 10.1016/0301-4622(75)80003-2. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Jain S. C., Sobell H. M. X-ray crystallographic visualization of drug-nucleic acid intercalative binding: structure of an ethidium-dinucleoside monophosphate crystalline complex, Ethidium: 5-iodouridylyl (3'-5') adenosine. Proc Natl Acad Sci U S A. 1975 Feb;72(2):628–632. doi: 10.1073/pnas.72.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Drugs which affect the structure and function of DNA. Nature. 1968 Sep 28;219(5161):1320–1325. doi: 10.1038/2191320a0. [DOI] [PubMed] [Google Scholar]
- Zama M., Ichimura S. Induced circular dichroism of acridine orange bound to double-stranded RNA and transfer RNA. Biopolymers. 1976 Sep;15(9):1693–1699. doi: 10.1002/bip.1976.360150907. [DOI] [PubMed] [Google Scholar]


