Abstract
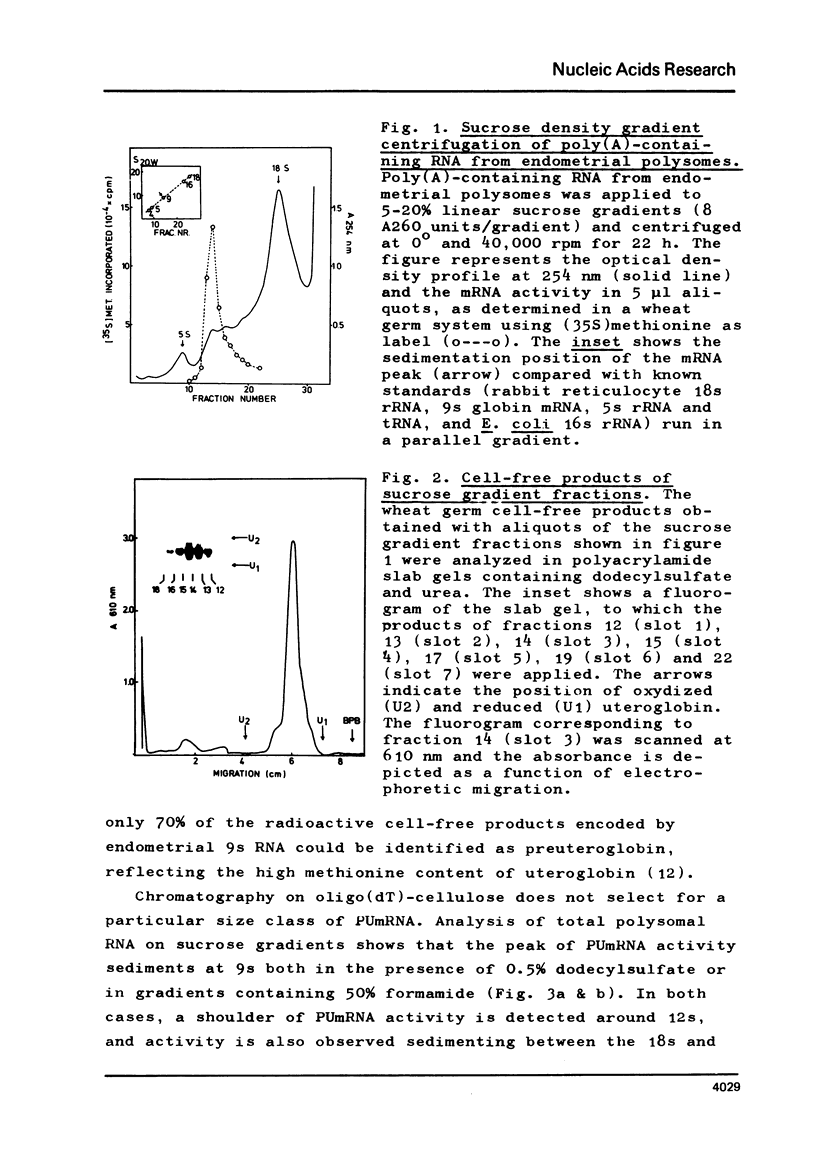
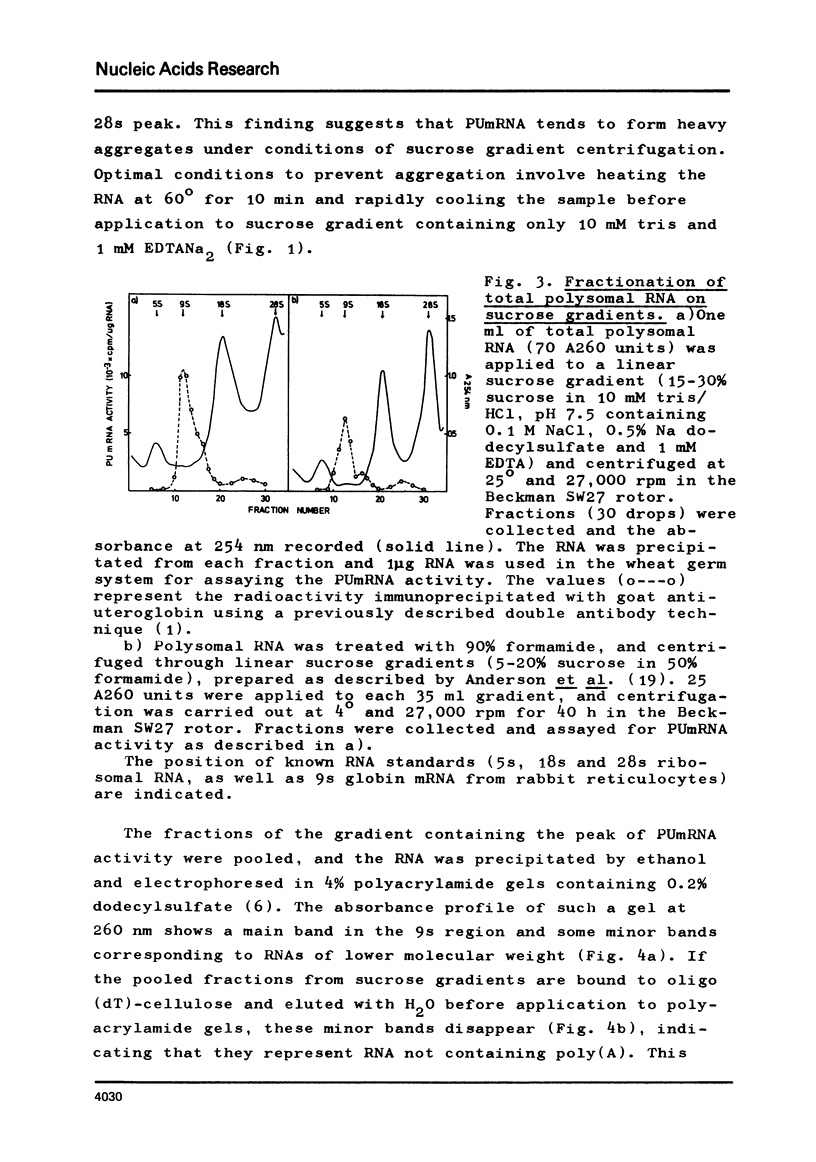
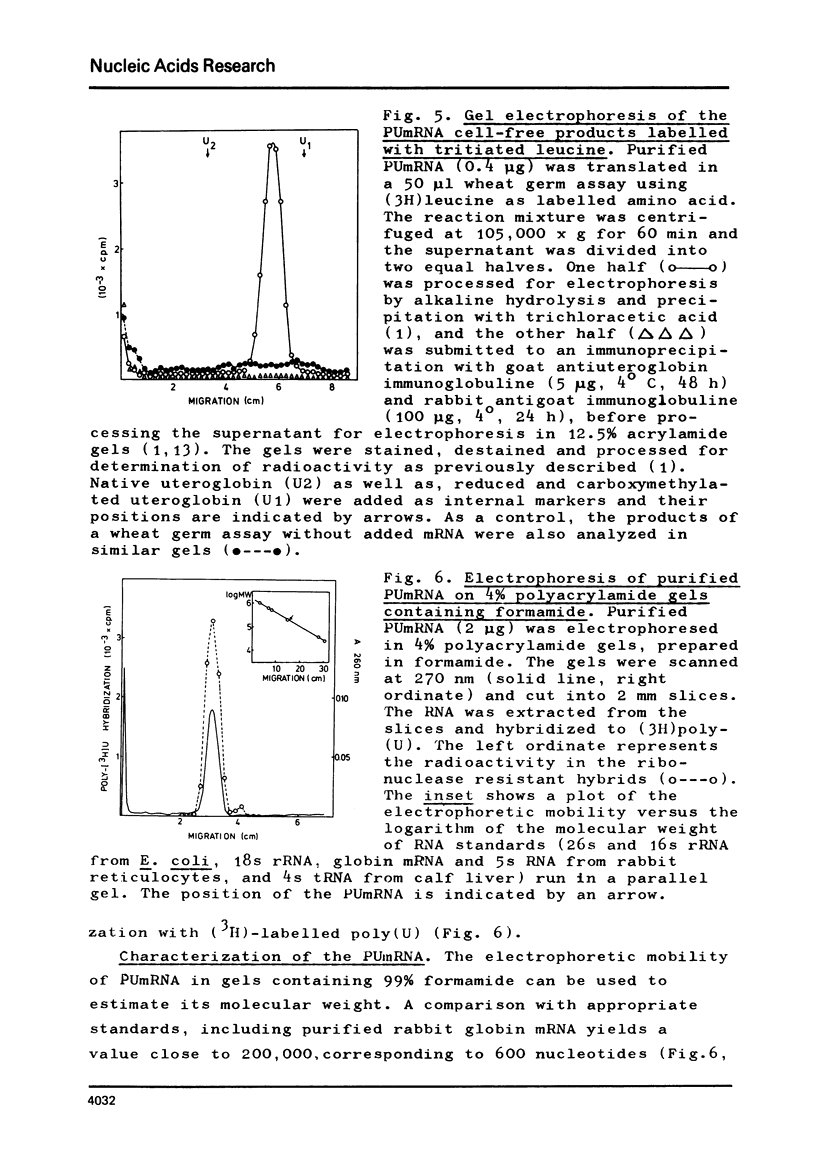
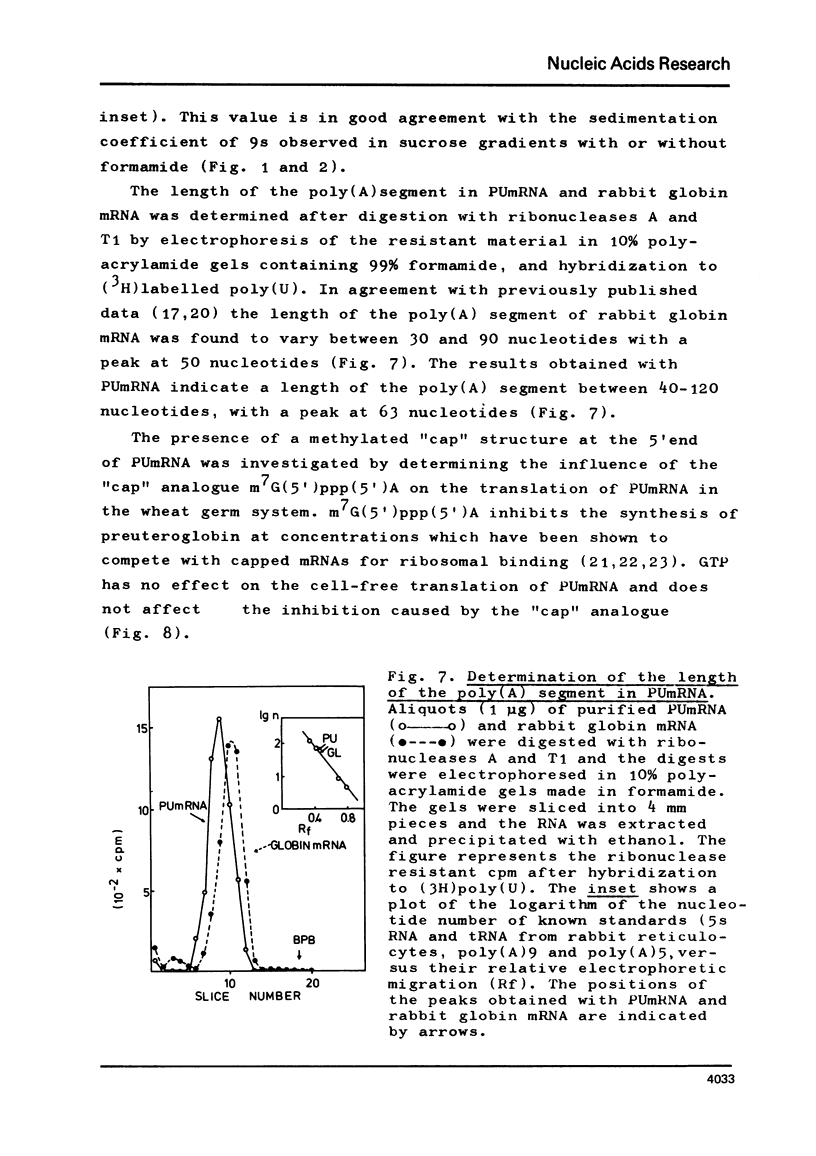
The mRNA for preuteroglobin, a precursor of the hormonally induced protein uteroglobin, has been partially purified from the endometrium of progesterone treated rabbits. The purification procedure starts with total endometrial polysomes and involves treatment with proteinase K and dodecylsulfate, chromatography on oligo(dT)-cellulose, sucrose density gradient centrifugation, electrophoresis in polyacrylamide gels containing dodecylsulfate, and a second absorption to oligo(dT)-cellulose. The final mRNA preparation codes exclusively for preuteroglobin in a wheat germ cell-free system and migrates as a single band in polyacrylamide gels containing 99% formanide. The average length of the poly(A) segment is 60 nucleotides and the translation of the preuteroglobin mRNA is inhibited by m7G(5')ppp(5')A, indicating that it contains a "capped" 5'-terminus. Comparison with known standards yields a molecular weight of 200,000 (600 nucleotides) for preuteroglobin mRNA, approximately twice as many nucleotides as required for encoding the 90 aminoacids of its cell-free product.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atger M., Milgrom E. Progesterone-induced messenger RNA. Translation, purification, and preliminary characterization of uteroglobin mRNA. J Biol Chem. 1977 Aug 10;252(15):5412–5418. [PubMed] [Google Scholar]
- Beato M., Arnemann J. Hormone-dependent synthesis and secretion of uteroglobin in isolated rabbit uterus. FEBS Lett. 1975 Oct 15;58(1):126–129. doi: 10.1016/0014-5793(75)80240-7. [DOI] [PubMed] [Google Scholar]
- Beato M., Baier R. Binding of progesterone to the proteins of the uterine luminal fluid. Identification of uteroglobin as the binding protein. Biochim Biophys Acta. 1975 Jun 12;392(2):346–356. doi: 10.1016/0304-4165(75)90016-1. [DOI] [PubMed] [Google Scholar]
- Beato M., Nieto A. Translation of the mRNA for rabbit uteroglobin in cell-free systems. Evidence for a precursor protein. Eur J Biochem. 1976 Apr 15;64(1):15–25. doi: 10.1111/j.1432-1033.1976.tb10270.x. [DOI] [PubMed] [Google Scholar]
- Beato M., Rungger D. Translation of the messenger RNA for rabbit uteroglobin in Xenopus oocytes. FEBS Lett. 1975 Nov 15;59(2):305–309. doi: 10.1016/0014-5793(75)80398-x. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Brawerman G. The isolation of messenger RNA from mammalian cells. Methods Enzymol. 1974;30:605–612. doi: 10.1016/0076-6879(74)30058-4. [DOI] [PubMed] [Google Scholar]
- Bullock D. W., Woo S. L., O'Malley B. W. Uteroglobin messenger RNA: translation in vitro. Biol Reprod. 1976 Nov;15(4):435–443. doi: 10.1095/biolreprod15.4.435. [DOI] [PubMed] [Google Scholar]
- Gaskill P., Kabat D. Unexpectedly large size of globin messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1971 Jan;68(1):72–75. doi: 10.1073/pnas.68.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner Y., Grosfeld H., Littauer U. Z. 5'-Capping structures of Artemia salina mRNA and the translational inhibition by cap analogs. Eur J Biochem. 1976 Dec;71(1):281–293. doi: 10.1111/j.1432-1033.1976.tb11114.x. [DOI] [PubMed] [Google Scholar]
- Hunt J. A. Terminal sequence studies of high-molecular-weight ribonucleic acid. The 3' termini of rabbit globin messenger ribonucleic acid. Biochem J. 1973 Feb;131(2):315–325. doi: 10.1042/bj1310315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayol R. F., Longenecker D. E. Development of a radioimmunoassay for blastokinin. Endocrinology. 1974 Dec;95(6):1534–1542. doi: 10.1210/endo-95-6-1534. [DOI] [PubMed] [Google Scholar]
- Modak M. J., Marcus S. L. Purification and properties of Rauscher leukemia virus DNA polymerase and selective inhibition of mammalian viral reverse transcriptase by inorganic phosphate. J Biol Chem. 1977 Jan 10;252(1):11–19. [PubMed] [Google Scholar]
- Nieto A., Ponstingl H., Beato M. Purification and quaternary structure of the hormonally induced protein uteroglobin. Arch Biochem Biophys. 1977 Apr 15;180(1):82–92. doi: 10.1016/0003-9861(77)90011-x. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Staynov D. Z., Gratzer W. B. Electrophoresis of RNA in formamide. Biochemistry. 1974 Dec 17;13(26):5373–5378. doi: 10.1021/bi00723a019. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg H., Wehrli W., Staehelin M. Isolation of mammalian transfer RNA. Biochim Biophys Acta. 1969 Nov 19;195(1):13–15. doi: 10.1016/0005-2787(69)90597-8. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Palacios R., Sullivan D., Kiely M. L., Gonzales C., Taylor J. M. Immunoadsorption of ovalbumin synthesizing polysosmes and partial purification of ovalbumin messenger RNA. Methods Enzymol. 1974;30:631–648. doi: 10.1016/0076-6879(74)30061-4. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Vournakis J. N., Gelinas R. E., Kafatos F. C. Short polyadenylic acid sequences in insect chorion messenger RNA. Cell. 1974 Nov;3(3):265–273. doi: 10.1016/0092-8674(74)90141-x. [DOI] [PubMed] [Google Scholar]
- Weber L. A., Feman E. R., Hickey E. D., Williams M. C., Baglioni C. Inhibition of HeLa cell messenger RNA translation by 7-methylguanosine 5'-monophosphate. J Biol Chem. 1976 Sep 25;251(18):5657–5662. [PubMed] [Google Scholar]


