Abstract
Structural and functional neuroimaging findings suggest that disturbance of the cortico–striato–thalamo–cortical (CSTC) circuits may underlie obsessive-compulsive disorder (OCD). However, some studies with 1H-magnetic resonance spectroscopy (1H-MRS) reported altered level of N-acetylaspartate (NAA), they yielded inconsistency in direction and location of abnormality within CSTC circuits. We conducted a comprehensive literature search and a meta-analysis of 1H-MRS studies in OCD. Seventeen met the inclusion criteria for a meta-analysis. Data were separated by frontal cortex region: medial prefrontal cortex (mPFC), dorsolateral prefrontal cortex, orbitofrontal cortex, basal ganglia and thalamus. The mean and s.d. of the NAA measure were calculated for each region. A random effects model integrating 16 separate datasets with 225 OCD patients and 233 healthy comparison subjects demonstrated that OCD patients exhibit decreased NAA levels in the frontal cortex (P=0.025), but no significant changes in the basal ganglia (P=0.770) or thalamus (P=0.466). Sensitivity analysis in an anatomically specified subgroup consisting of datasets examining the mPFC demonstrated marginally significant reduction of NAA (P=0.061). Meta-regression revealed that NAA reduction in the mPFC was positively correlated with symptom severity measured by Yale–Brown Obsessive Compulsive Scale (P=0.011). The specific reduction of NAA in the mPFC and significant relationship between neurochemical alteration in the mPFC and symptom severity indicate that the mPFC is one of the brain regions that directly related to abnormal behavior in the pathophysiology of OCD. The current meta-analysis indicates that cortices and sub-cortices contribute in different ways to the etiology of OCD.
Keywords: cortico–striato–thalamo–cortical circuity, MRS, N-acetylaspartate (NAA), obsessive-compulsive disorder (OCD), systematic review
Introduction
Frontal–subcortical circuits are effector mechanisms that allow an organism to act in its environment.1 Three cortico–striato–thalamo–cortical (CSTC) circuits originating from three prefrontal regions are particularly important for the manifestation of neuropsychiatric behavior. These circuits include the dorsolateral prefrontal cortex (DLPFC) circuit, which allows the organization of information to facilitate a response, the medial prefrontal cortex (mPFC) circuit, which is required for motivation-related behavior, and the orbitofrontal cortex (OFC) circuit, which allows the integration of limbic and emotional information into behavioral responses.1
Previous structural and functional neuroimaging studies have indicated that dysfunction of CSTC circuits, and imbalance between these circuits may be the neural basis of the symptoms of obsessive-compulsive disorder (OCD).2 Positron emission tomography studies using resting and symptom-provoking designs revealed significantly elevated metabolic rates of glucose in the cerebral cortex and basal ganglia in patients with OCD.3, 4, 5, 6 These studies confirmed abnormal metabolism in the frontal cortex, including the OFC3, 6 and mPFC.3, 4, 5, 6 Recently, functional magnetic resonance imaging (MRI) studies have also reported different pattern of activation of brain regions within CSTC circuits in OCD patients. Although some studies reported altered activation in the frontal cortex including the OFC7, 8, 9 and mPFC,7, 8, 10 others demonstrated abnormalities in the basal ganglia, including the striatum10, 11, 12 and thalamus.4
Evidence from structural MRI studies also indicates volumetric differences between OCD patients and healthy comparison (HC) subjects in CSTC circuits.13 Interestingly, recent meta-analyses of voxel-based morphometry studies and structural MRI studies in OCD patients indicated volume reductions in the frontal cortex, but not in the basal ganglia or thalamus, suggesting that underlying etiologies differ in the frontal cortex and subcortical areas.14, 15 Although functional disturbance and structural abnormality of the CSTC circuit are possible bases for the etiology of OCD, recent studies have suggested that the frontal cortex and subcortical areas have different roles in the symptoms of OCD.14, 15, 16
1H-magnetic resonance spectroscopy (1H-MRS) is a noninvasive neuroimaging technique that estimates specific chemical metabolite measures in vivo.17 N-acetylaspartate (NAA) is a metabolite that can be accurately measured with a 1.5-tesla scanner18 and has been widely studied in the field of neuroscience.19 Although NAA is widely recognized as a marker of neuron density,20 recent experimental studies reported that NAA reflects the functional role of neurochemical alterations.21 Previous studies have used 1H-MRS to examine OCD patients, reporting decreased,22 unchanged,23 or increased24 NAA levels in OCD patients compared with HC subjects.
To our knowledge, neither a systematic review nor a meta-analysis of 1H-MRS studies in OCD patients has been previously reported. The current systematic review and meta-analysis were designed to investigate the neurochemical background of abnormal activity in CSTC circuits, and to identify direction and location of neurochemical alteration that relates to abnormal behavior in OCD patients. In the current study, to examine in which part of CSTC circuit NAA levels alter, at first we conduct three meta-analyses with the datasets from the frontal cortex, thalamus and basal ganglia. Then, to investigate which CSTC circuit among three circuits mentioned above has a key role in the pathophysiology of abnormal behavior of OCD, we separate datasets from the frontal cortex into three subgroups based on anatomical location of volume of interests (VOIs), such as mPFC, DLPFC and OFC, and perform three meta-analyses.
Materials and methods
Data sources
1H-MRS studies that examined metabolite measures in the brains of individuals with OCD and HC subjects were obtained through the digital MEDLINE and EMBASE databases. A comprehensive literature search was performed using the terms ‘obsessive compulsive disorder' combined with ‘magnetic resonance spectroscopy'. The titles and abstracts of the studies were examined to determine whether or not they should be included. The reference lists of the included articles were also examined to search for additional relevant studies to be included.
Selection of studies
Studies were included in our database if (1) they were peer-reviewed brain 1H-MRS studies published between 1980 and December 2011, (2) they examined patients with OCD in comparison with a HC group, and (3) NAA levels were measured. Further, studies were included in the meta-analysis, if (4) they reported sufficient data to estimate their effect sizes, and (5) they located VOIs at least one region among the frontal cortex, basal ganglia or thalamus. The literature search was performed without language restriction. If the study did not provide sufficient data, we emailed the corresponding author to obtain more data. In cases where the author did not respond, we excluded the study. Two of the authors (YA and AA) independently performed the study screening.
Data extraction
To perform the meta-analyses, we defined a standardized mean difference as effect size, which is calculated as the difference between the mean of the experimental group and the mean of the comparison group, divided by the pooled s.d.
In the current meta-analyses, the mean NAA level in individuals with OCD was subtracted from that in the HC group in each VOI, and divided by the pooled s.d. of these VOIs.
Identification of VOIs
The data were separated by the brain region (frontal cortex, thalamus and basal ganglia). The data assigned to frontal cortex were divided into three subgroups, the DLPFC, mPFC and OFC, based on the location of VOI. In addition to an original classification of VOI placement, the classification was evaluated by two independent reviewers who were blind to an original classification (AA and HS). In case of discrepancy, the agreement was achieved by discussion with the third reviewer (YA). All the cases were considered their classification in this process. In the case of a study reporting measures from more than one subregion in one area (for example, left and right anterior cingulate cortex), we calculated the mean of effect sizes from all the VOIs from one area and integrated it into the analysis.25 In the case of studies reporting more than two kinds of measures of metabolites, we adopted the different priority for extraction depending on the method of cerebrospinal fluid (CSF) ratio correction. In studies which conducted tissue segmentation within VOIs, we determined the priority for extraction as the absolute NAA level and then its ratio to creatine (Cre) levels. Whereas in studies which did not implement tissue segmentation within VOIs, we determined the priority for extraction as the ratio of NAA level to Cre and then its absolute levels. In the case of longitudinal or interventional studies, we determined the NAA levels at baseline, that is, before treatment.22 Three independent datasets located in the white matter in the frontal lobe were excluded from the analysis of the frontal cortex.22, 26, 27 VOIs in HC subjects who were compared with more than two OCD groups were identified28 and divided into the appropriate number of comparison subgroups to avoid duplication. Two authors (YA and AA) independently performed all of the data extractions and computations of effect sizes to minimize errors. The Epidemiology guidelines for meta-analyses of observational studies were followed.29
Meta-analysis
All meta-analyses were performed using Comprehensive Meta-analysis version 2.0 from Biostat (Englewood, NJ, USA). A random effects model was adapted for the current meta-analysis to control for potential heterogeneity, such as variations in the location of the VOIs, the implementation of the tissue segmentation within the VOIs, the use of single- vs multi-voxel spectroscopy, the echo time, the volumes of the VOIs and the types of metabolite measure. Meta-analysis was performed only when there were more than three datasets from more than two independent studies. A standardized mean difference was calculated and used as effect sizes. The significance level was set at P<0.05.
Sensitivity analyses
The robustness of significant findings from meta-analysis was further tested using a sensitivity analysis in specified subgroups, excluding studies with potential confounds. These potential confounds included pharmacological status, psychiatric comorbidity, type of metabolite measures, strength of magnetic field and implementation of tissue segmentation within VOIs. The significance level was set at P<0.05.
Meta-regression
To investigate the effect of neurochemical alteration on behavioral abnormality, we conducted meta-regression analyses to examine the relationship between the total, obsession and compulsion scores of the subject on the Yale–Brown Obsessive Compulsive Scale (Y-BOCS) and the effect size for the NAA levels in the mPFC where there was enough number of datasets to perform regression analysis. Further, to explore the effects of various parameters on neurochemical abnormalities, we performed meta-regression analyses to test the relationship between the percentage of OCD patients with diagnosis of depression and anxiety disorder, the mean duration of the illness, the mean age of the subject and size of VOI with the effect size for the NAA levels. Regression was examined using comprehensive meta-analysis and the significance level was set at P<0.05.
Assessing between-study heterogeneity
The current meta-analyses included studies with considerable differences in many factors, such as prescribed medications, types of MRS measures (for example, absolute measures or ratios to Cre), segmentation within the VOI and the volume of the VOI. I 2 statistics was employed to assess between-study heterogeneity. Thresholds for the interpretation of I 2 were based on previous studies suggesting that 0–50% represents mild heterogeneity, 50–75% moderate heterogeneity and 75–100% considerable heterogeneity.30
Publication bias
Publication bias was assessed qualitatively by visual inspection of funnel plots, and quantitatively by linear regression analysis for each group and each brain region. On the basis of previous literature, this calculation was tested with datasets of at least 10 data.30 The significance level was defined as P<0.10 to conclude existence of publication bias.30
Data synthesis
Twenty-seven demographic, clinical and methodological variants were extracted from the included studies, as shown in Table 1. These included the number of participants, the number of male participants, the mean age of the participants, diagnostic tools, the duration of the illness, psychiatric comorbidity, pharmacological status, total, obsessive, and compulsive scores on the Y-BOCS, the sequence used for MRS acquisition, single- vs multi-voxel analysis, whether segmentation within VOIs was used, the strength of magnetic field (Tesla), the echo time, the repetition time, the types of MRS measurements (absolute measure or ratio to Cre), the location of the VOI, and the size of the VOI. The total number of participants, datasets, and mean differences, the effect sizes, P-values, I 2 scores and the significance of the linear regression analysis of the symmetry of the funnel plots calculated from each meta-analysis are shown in Table 2.
Table 1. Summary of included studies.
| Author | Mean age (year) | N of OCDa | N of male OCDa | N of HCa | N of male NHCa | Diagnostic tool | Duration of illness (year) | Psychiatric comorbidity | Medication |
Y-BOCSb |
Magnetic field strength (tesla) | Segmentation | MRS sequence | Single- vs multi- voxel | Types of measure | TE (ms) | TR (ms) | NAA | Cre | Cho | mI | Glx | VOI location | VOI size (ml) | Results | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Obsession | Compulsion | |||||||||||||||||||||||||
| Atmaca et al.51 | 28.1 | 18 | 6 | 18 | 6 | DSM-IV | 5.5 | No | No | 17.2 | NA | NA | 1.5 | Undone | NA | Single | Cre ratio | 15.6 | 2000 | ○ | ○ | ○ | × | × | bl.hippocampus | 4.7 | NAA↓ |
| Bartha et al.50 | 34.9 | 13 | 7 | 13 | 7 | DSM-IV | NA | No | No | 25.9 | 11.6 | 14.3 | 1.5 | Undone | NA | Single | Absolute | 20 | 1500 | ○ | ○ | ○ | ○ | ○ | lt.corpus striatum | 4.5 | NAA↓ |
| Beédard et al.43 | 40.54 | 13 | 4 | 12 | 4 | DSM-IV | 12.77 | No | Medicated | 26.5 | 12.92 | 13.62 | 1.5 | Undone | PROBE | Single | Cre ratio | 102 | 3100 | ○ | × | ○ | ○ | ○ | rt.OFA | 8 | NS |
| ○ | × | ○ | ○ | ○ | lt.OFA | 8 | NS | ||||||||||||||||||||
| ○ | × | ○ | ○ | ○ | rt.MTL | 8 | NS | ||||||||||||||||||||
| ○ | × | ○ | ○ | ○ | lt.MTL | 8 | NS | ||||||||||||||||||||
| ○ | × | ○ | ○ | ○ | rt.thalamus | 9 | NS | ||||||||||||||||||||
| ○ | × | ○ | ○ | ○ | lt.thalamus | 9 | NS | ||||||||||||||||||||
| ○ | × | ○ | ○ | ○ | ACC | 9.6 | NS | ||||||||||||||||||||
| Besiroglu et al.42 | 32.1 | 30 | 12 | 15 | 6 | DSM-IV | 6.35 | No | No | 24.15 | 13.1 | 11.05 | 1.5 | Undone | PRESS | Single | Absolute Cre ratio | 135 | 1500 | ○ | ○ | ○ | × | × | rt.ACC | 8 | NAA↓ |
| ○ | ○ | ○ | × | × | rt.AHC | 4.096 | NAA↓ | ||||||||||||||||||||
| Ebert et al.34 | 27 | 12 | 8 | 6 | 5 | DSM-IV | 11 | No | No | 28 | NA | NA | 2 | Undone | PRESS | Single | Cre ratio | 30 | 1500 | ○ | × | ○ | ○ | ○ | ACC | 8 | NAA↓ |
| ○ | × | ○ | ○ | ○ | rt.striatum | 8 | NAA↓ | ||||||||||||||||||||
| ○ | × | ○ | ○ | ○ | rt.PL | 8 | NS | ||||||||||||||||||||
| Fan et al.24 | 25.62 | 21 | 12 | 19 | 13 | DSM-IV | 4.45 | No | No | 22.52 | NA | NA | 1.5 | Undone | PRESS | Single | Cre ratio | 35 | 1500 | ○ | × | ○ | ○ | × | MPFC | 8 | NAA↓ |
| Jang et al.22 | 27.77 | 13 | 8 | 13 | 8 | DSM-IV | 7.23 | Two: depression | No | 30.23 | 15.31 | 14.92 | 1.5 | Done | PRESS | Multi | Cre ratio | 272 | 2000 | ○ | × | × | × | × | ACC | 1.125 | NAA↓ |
| ○ | × | × | × | × | FWM | 1.125 | NAA↓ | ||||||||||||||||||||
| ○ | × | × | × | × | Prefrontal cortex | 1.125 | NAA↓ | ||||||||||||||||||||
| ○ | × | × | × | × | PC | 1.125 | NS | ||||||||||||||||||||
| ○ | × | × | × | × | PWM | 1.125 | NS | ||||||||||||||||||||
| ○ | × | × | × | × | Posterior cingulate | 1.125 | NS | ||||||||||||||||||||
| Kitamura et al.26 | 22.7 | 12 | 3 | 32 | 10 | DSM-IV | 6.3 | No | Medicated | 20.4 | NA | NA | 3 | Undone | PRESS | Single | Cre ratio | 24 | 6000 | ○ | × | ○ | × | × | ACC | 3 | NS |
| ○ | × | ○ | × | × | lt.basal ganglia | 6.75 | NS | ||||||||||||||||||||
| ○ | × | ○ | × | × | lt.thalamus | 3.375 | NS | ||||||||||||||||||||
| ○ | × | ○ | × | × | lt.FWM | 3.375 | NS | ||||||||||||||||||||
| ○ | × | ○ | × | × | lt.PWM | 3.375 | NS | ||||||||||||||||||||
| Mirza et al.38 | 10.31 | 27 | 13 | 18 | NA | DSM-IV | 2.63 | No | No | 26.8 | NA | NA | 1.5 | Undone | NA | Single | Absolute | 272 | 2300 | ○ | ○ | ○ | × | × | lt.thalamus | 0.8 | NS |
| ○ | ○ | ○ | × | × | rt.thalamus | 0.8 | NS | ||||||||||||||||||||
| Mohamed et al.28 | 36.7 | 10 | 4 | 10 | 4 | DSM-IV | 23.5 | No | Medicated | 14.3 | NA | NA | 1.5 | Undone | NA | Single | Cre ratio | 280 | 2650 | ○ | × | ○ | × | × | lt.thalamus | 1.1 | NS |
| ○ | × | ○ | × | × | rt.thalamus | 1.1 | NAA↓ | ||||||||||||||||||||
| ○ | × | ○ | × | × | lt.basal ganglia | 1.1 | NS | ||||||||||||||||||||
| ○ | × | ○ | × | × | rt. basal ganglia | 1.1 | NS | ||||||||||||||||||||
| Ohara et al.35 | 24.5 | 12 | 10 | 12 | 10 | DSM-IV | 5.6 | No | Medicated | 29.5 | NA | NA | 1.5 | Undone | PROBE | Single | Cre ratio | 40 | 1500 | ○ | × | ○ | × | × | lt.lenticular nucleus | 8 | NS |
| ○ | × | ○ | × | × | rt.lenticular nucleus | 8 | NS | ||||||||||||||||||||
| Rosenberg et al.23 | 11.39 | 20 | 9 | 14 | 5 | DSM-IV | 3.87 | Two: anxiety | No | 25.8 | NA | NA | 1.5 | Undone | PRESS | Single | Absolute | 272 | 2300 | ○ | ○ | ○ | ○ | ○ | ACC | 3 | NS |
| Rosenberg et al. 37 | 11.19 | 11 | 4 | 11 | 4 | DSM-IV | 2.56 | Yes: anxiety | No | 26.55 | NA | NA | 1.5 | Undone | PRESS | Single | Absolute | 272 | 2300 | ○ | ○ | ○ | × | × | lt.medial thalamus | NA | NS |
| ○ | ○ | ○ | × | × | rt.medial thalamus | NA | NS | ||||||||||||||||||||
| ○ | ○ | ○ | × | × | lt.lateral thalamus | NA | NS | ||||||||||||||||||||
| ○ | ○ | ○ | × | × | rt.lateral thalamus | NA | NS | ||||||||||||||||||||
| Rosenberg et al.36 | 11 | 11 | 4 | 11 | 4 | K-SADS-PLc | 1.2 | Yes: anxiety | No | 30.36 | 15.27 | 15.09 | 1.5 | Undone | PRESS | Single | Absolute | 272 | 2300 | ○ | ○ | ○ | ○ | ○ | lt.caudate | 0.7 | NS |
| ○ | ○ | ○ | ○ | ○ | Occipital gray matter | 8 | NS | ||||||||||||||||||||
| Scherk et al.40 | NA | 17 | NA | 16 | NA | DSM-IV | NA | No | Medicated | NA | NA | NA | 1.5 | Undone | NA | Single | Cre ratio | 30 | 1500 | ○ | × | ○ | ○ | ○ | DLPFC | 3.5 | NS |
| ○ | × | ○ | ○ | ○ | PMFC | 3.6 | NS | ||||||||||||||||||||
| ○ | × | ○ | ○ | ○ | Putamen | 3.3 | NS | ||||||||||||||||||||
| Starck et al.41 | 32.6 | 9 | 6 | 15 | 11 | DSM-IV | 19.4 | Yes: depression | Medicated | 22.9 | 11.9 | 11 | 1.5 | Undone | PRESS | Single | Absolute | 30 | 2000 | ○ | ○ | ○ | ○ | ○ | rt.caudate nucleus | 1.5 | NA |
| ○ | ○ | ○ | ○ | ○ | Anterior cingulate gyrus | 3.6 | NA | ||||||||||||||||||||
| ○ | ○ | ○ | ○ | ○ | OC | 4 | NA | ||||||||||||||||||||
| Sumitani et al.27 | 29.99 | 20 | 13 | 26 | 10 | DSM-IV | NA | NA | Medicated | 23.79 | NA | NA | 1.5 | Done | STEAM | Single | Absolute | 18 | 5000 | ○ | ○ | ○ | × | × | ACC | 4.335 | NAA↓ |
| ○ | ○ | ○ | × | × | lt.basal ganglia | 4.335 | NS | ||||||||||||||||||||
| ○ | ○ | ○ | × | × | lt.FL | 4.335 | NS | ||||||||||||||||||||
| Whiteside et al.39 | 41.2 | 15 | 9 | 15 | 9 | DSM-IV | 23.09 | Yes: depression | Medicated | 24.13 | 11.73 | 12.4 | 1.5 | Undone | PROBE | Single | Cre ratio | 35 | 2000 | ○ | × | ○ | ○ | ○ | lt.head of caudate | 4 | NS |
| ○ | × | ○ | ○ | ○ | rt.head of caudate | 4 | NS | ||||||||||||||||||||
| ○ | × | ○ | ○ | ○ | lt.OFWM | 4 | NS | ||||||||||||||||||||
| ○ | × | ○ | ○ | ○ | rt.OFWM | 4 | NAA↓ | ||||||||||||||||||||
| Yucel et al.31 | 34.4 | 20 | 10 | 26 | 13 | DSM-IV | 13.4 | NA | No/stable | 19.55 | 9.35 | 10.2 | 3 | Done | PRESS | Single | Absolute | 30 | 3000 | ○ | ○ | ○ | ○ | ○ | rt.dACC | 6.5 | NS |
| ○ | ○ | ○ | ○ | ○ | rt.rACC | 6.5 | NS | ||||||||||||||||||||
| ○ | ○ | ○ | ○ | ○ | lt.dACC | 6.5 | NS | ||||||||||||||||||||
| ○ | ○ | ○ | ○ | ○ | lt.rACC | 6.5 | NS | ||||||||||||||||||||
Abbreviations: ACC, anterior cingulate cortex; AHC, amygdala hippocampus complex; bl., bilateral; Cre,creatine; dAC, dorsal anterior cingulate; dACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; FL, frontal lobe; FWM, frontal white matter; HC, healthy control; lt., left; MPFC, medial prefrontal cortex; MRS, magnetic resonance spectroscopy; MTL, medial temporal lobe; NA, not applicable; NAA, N-acetylaspartate; NHC, non-healthy control; NS, nonsignificant; OC, occipital cortex; OCD, obsessive-compulsive disorder; OFA, orbitofrontal area; OFWM, orbitofrontal white matter; PC, parietal cortex; PMFC, posterior frontal cortex; PWM, parietal white matter; PL, parietal lobe; rACC, rostal anterior cingulate cortex; rt., right; TE, echo time; TR, repetition time; VOI, volume of interest.
Number.
Yale–Brown Obsessive Compulsive Scale.
The schedule for affective disorders abd schizophrenia for school-age children—present and lifetime versions.
Table 2. Meta-analyses of NAA levels by region.
| Region | Separate dataset | No. of OCD | No. of HC | Z-value | P-value | I 2(%) | Publication bias |
|---|---|---|---|---|---|---|---|
| Frontal cortex | 16 | 225 | 233 | −2.240 | 0.025 | 56.2 | 0.92 |
| mPFC | 15 | 183 | 192 | −1.871 | 0.061 | 63.3 | 0.99 |
| DLPFC | 3 | 30 | 29 | −0.819 | 0.413 | 63.7 | NA |
| Thalamus | 4 | 63 | 73 | −0.729 | 0.466 | 0 | NA |
| Basal ganglia | 10 | 105 | 115 | −0.292 | 0.770 | 42.5 | 0.36 |
Abbreviations: DLPFC, dorsolateral prefrontal cortex; HC, healthy control; mPFC, medial prefrontal cortex; NA, not applicable; NAA, N-acetylaspartate; OCD, obsessive-compulsive disorder.
Results
Study selection
The literature search described above yielded 214 articles, of which 26 were identified as potential candidates for the meta-analysis.22, 23, 24, 26, 27, 28, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 Of these, three studies were excluded because they did not compare metabolites between OCD patients and HC subjects.44, 45, 46 Another study was excluded because it was a review article.47 In addition, three studies were discarded because of overlapping data with other studies from the same research group.32, 48, 49 Thus, 19 studies were included in the database (Table 1). From this database, one study was excluded because they did not provide sufficient data to calculate effect sizes.50 Another study was excluded from the analysis because they located their VOI only in the hippocampus,51 and the hippocampus is not related to the current study. Thus, 17 studies were included in the final meta-analysis.22, 23, 24, 26, 27, 28, 31, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43
Characteristics of included studies
The 17 studies included in the meta-analysis were published between 1997 and 2011, and examined a total of 273 OCD patients and 271 HC subjects. The mean age of OCD patients ranged from 10 to 41. Fifteen studies reported the duration of illness22, 23, 24, 26, 28, 31, 34, 35, 36, 37, 38, 39, 41, 42, 43 with a range from 1.2 years36 to 23.5 years.28 Seven studies involved OCD patients who were not currently treated with medication or had never been treated with medication for OCD.22, 23, 24, 34, 36, 37, 42, 43 Sixteen studies reported the total scores on the Y-BOCS;22, 23, 24, 26, 27, 28, 31, 34, 35, 36, 37, 38, 39, 41, 42, 43 the highest mean score was 30.36 ref.36 and the lowest was 14.3.28 Two studies utilized a 3-tesla MRI scanner,26, 32 one study utilized a 2-tesla MRI scanner,34 and 14 studies utilized a 1.5-Tesla MRI scanner.22, 23, 24, 27, 28, 35, 36, 37, 38, 39, 40, 41, 42, 43 Three studies implemented tissue segmentation within the VOI.22, 27, 31 Seven studies reported absolute NAA levels,23, 27, 31, 36, 37, 38, 42 whereas nine studies reported NAA levels as a ratio of Cre levels.22, 24, 26, 28, 34, 35, 39, 40, 43 Sixteen studies utilized single-voxel MRS.23, 24, 26, 27, 28, 31, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43
Meta-analysis for NAA measures by region
Frontal cortex
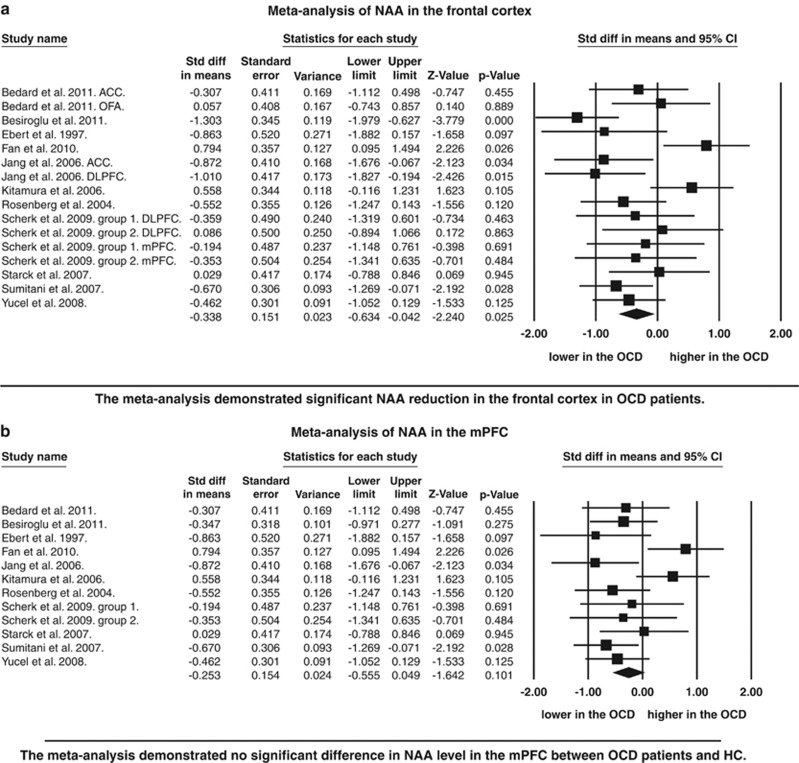
In the frontal cortex, 16 separate datasets from 11 independent studies were included.22, 23, 24, 26, 27, 31, 34, 40, 41, 42, 43 These datasets included 225 individuals with OCD and 233 control subjects. The meta-analysis demonstrated significant reductions in NAA levels in OCD patients compared with HC subjects (P=0.025). Although publication bias was not present, moderate heterogeneity was found (I 2=56.2%) (Figure 1 and Table 2).
Figure 1.
Forest plot of N-acetylaspartate (NAA) measure by regions. (a) Standardized mean differences for NAA levels between obsessive-compulsive disorder patients and healthy comparisons within the frontal cortex, (b) standardized mean differences for NAA levels between obsessive-compulsive disorder patients and healthy comparisons within the medial prefrontal cortex, thalamus. Regarding with P-value for Besiroglu et al, in Figure 1a, P-value was <0.001. ACC, anterior cingulate cortex; CI, confidence interval; DLPFC, dorsolateral prefrontal cortex; OCD, obsessive-compulsive disorder; OFA, orbitofrontal area; VOI, volume of interest.
Thalamus
In the thalamus, four datasets from 63 individuals with OCD and 73 HC subjects were integrated into the meta-analysis, and revealed no significant differences in NAA levels between individuals with OCD compared with HCs (Table 2). Four studies were included in the analysis.23, 26, 38, 42
Basal ganglia
Ten datasets from eight studies were included in the meta-analysis of the basal ganglia.27, 28, 34, 35, 36, 39, 40, 41 The meta-analysis included 105 individuals with OCD and 115 HC subjects, and revealed no significant difference in NAA levels. No significant heterogeneity or significant publication bias were present (Table 2).
Medial prefrontal cortex
Among 16 separate datasets in the frontal lobe, 12 examined the mPFC in 11 independent studies.22, 23, 24, 26, 27, 31, 34, 40, 41, 42, 43 The meta-analysis with 183 OCDs and 192 HCs revealed that NAA was marginally significantly reduced in OCD patients (P=0.061) with moderate heterogeneity (I 2=63.3%) and no publication bias (Figure 1 and Table 2).
Dorsolateral prefrontal cortex
Only three separate datasets from two independent studies investigated NAA levels in the DLPFC.22, 40 These studies enrolled 30 OCD patients and 29 HC subjects, and the meta-analysis reported no significant difference in NAA level between OCD and HC individuals (P=0.413) (Table 2).
Orbitofrontal cortex
Only one study43 reported NAA levels in the OFC. As a sufficient number of studies were not included, we did not conduct a meta-analysis.
Sensitivity analysis
Sensitivity analysis to test the robustness of the significance of NAA decrease in the frontal cortex was conducted. Sensitivity analyses performed in the specified subgroups with studies whose participants were not medicated (P=0.060), that have implemented tissue segmentation (P<0.001), and that utilized 1.5-tesla scanner (P=0.011) preserved the significance, whereas sensitivity analyses with specified-subgroups with studies whose participants do not have comorbid psychiatric disorder and that adopted Cre ratio did not preserve the significance (Table 3).
Table 3. Results from sensitivity analyses in NAA levels in the frontal cortex.
| Specified criteria | No. of OCD | No. of HC | No. of studies | No. of datasets | 95% CI lower | 95% CI upper | Z-value | P-value |
|---|---|---|---|---|---|---|---|---|
| No medication | 106 | 77 | 5 | 6 | −1.268 | 0.026 | −1.881 | 0.060 |
| No psychiatric comorbidity | 130 | 125 | 6 | 10 | −0.613 | 0.273 | −0.753 | 0.452 |
| Cre ratio | 156 | 151 | 7 | 12 | −0.536 | 0.135 | −1.173 | 0.241 |
| 1.5-Tesla scanner | 193 | 175 | 9 | 14 | −0.713 | −0.092 | −2.538 | 0.011 |
| Segmented within VOIs | 66 | 78 | 3 | 4 | −1.035 | −0.357 | −4.023 | <0.001 |
Abbreviations: CI, confidence interval; VOI, volume of interest; mPFC, medial prefrontal cortex; NAA, N-acetylaspartate; OCD, obsessive-compulsive disorder; HC, healthy control.
Meta-regression
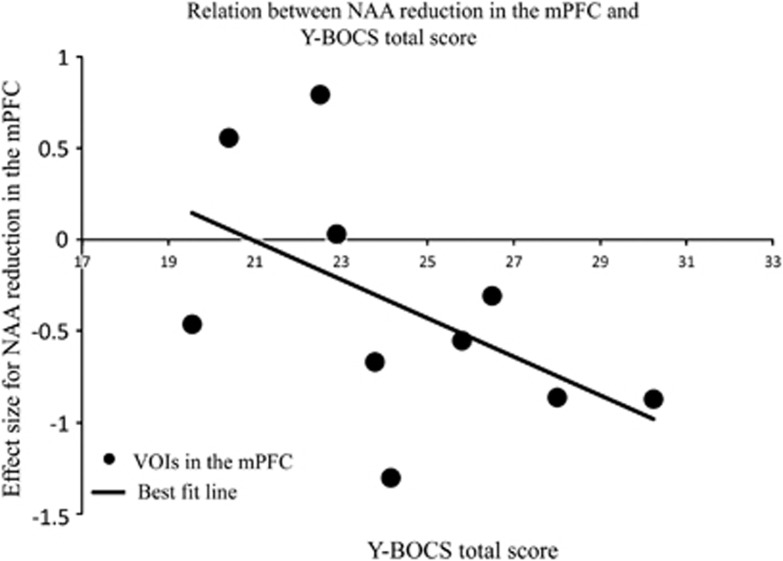
To investigate the effects of NAA reductions in the mPFC on abnormal behavior of OCD, we performed meta-regression analyses with the total, obsession and compulsion of scores of the Y-BOCS. Meta-regression demonstrated significant effect of the reduction of NAA levels in the mPFC on the Y-BOCS total score (P=0.011) but no significant effect on the Y-BOCS obsession score (P=0.245) or compulsion score (P=0.797) (Figure 2 and Table 4). Further meta-regression analyses revealed no significant effects of the percentage of OCD patients with diagnosis of depression (P=0.259), anxiety disorder (P=0.696), the duration of illness of the patients (P=0.742), the age of the OCD patients (P=0.297) and size of VOI (P=0.783) on the reduction of NAA levels in the mPFC (Table 4).
Figure 2.
Meta-regression of N-acetylaspartate (NAA) reduction in the mPFC and Yale–Brown Obsessive Compulsive Scale (Y-BOCS) total score. The relationship between effect sizes for reduced NAA and Y-BOCS total score. Effect sizes from each comparison are plotted by the mean Y-BOCS total scores of participants with obsessive-compulsive disorder of the study. The line of best fit shows a substantial decrease in NAA reduction. mPFC, medial prefrontal cortex; VOI, volume of interest.
Table 4. Meta-regression of NAA levels in the mPFC.
| Modifier | Separate dataset | Intercept | Slope | P-value |
|---|---|---|---|---|
| Y-BOCS | ||||
| Total | 10 | 1.94 | −0.10 | 0.011 |
| Obsession | 5 | −0.51 | −0.09 | 0.245 |
| Compulsion | 5 | −0.33 | −0.02 | 0.797 |
| % Of patients with depression | 10 | −0.20 | −2.00 | 0.259 |
| % Of patients with anxiety | 10 | −0.24 | −1.36 | 0.696 |
| Duration of illness | 9 | −0.22 | −0.01 | 0.742 |
| Age (years) | 10 | 0.11 | −0.02 | 0.297 |
| Size of VOI | 10 | −0.28 | −0.01 | 0.783 |
Abbreviations: mPFC, medial prefrontal cortex; NAA, N-acetylaspartate; VOI, volume of interest;Y-BOCS, Yale–Brown Obsessive Compulsive Scale.
Discussion
Summary
To our knowledge, this is the first systematic review and meta-analysis of 1H-MRS studies in people with OCD. There were three main findings. First, the meta-analysis demonstrated a significant reduction in NAA levels in the frontal cortex of OCD patients compared with HC subjects, but no significant difference in subcortical areas such as the basal ganglia or thalamus. The second finding is that sensitivity analysis in anatomically specified subgroup consisting of datasets examining the mPFC demonstrated marginally significant NAA reductions in OCD patients compared with HC. The third, meta-regression revealed significant positive correlation between NAA reduction and symptom severity of OCD, which indicates that neurochemical alteration in the mPFC is directly related to abnormal behavior of OCDs. The systematic review revealed obvious methodological heterogeneities across studies, including differences in pharmacological status, strength of magnetic field and utilization of segmentation within VOIs. However, the sensitivity analyses, which excluded the potential effects of these confounds, further emphasized the robustness of current findings, regarding the reduction of NAA levels in the frontal cortex in OCD patients.
Methodological considerations
Although we utilized a random effects model to account for between-study heterogeneity, and none of the analyses revealed a severe degree of heterogeneity in the current meta-analyses (Table 2), there is inherent between-study heterogeneity in data acquisition, and subject characteristics between 1H-MRS studies. Thus, the current results should be interpreted cautiously.
Regarding data acquisition, correcting the CSF ratio within VOIs is required in 1H-MRS studies, because there is no metabolite in the CSF. Two methods are commonly used to correct for this problem: The first is to report the level of the metabolite of interest in comparison with the other metabolite. The other is to implement tissue segmentation within VOIs to exclude the CSF component. In the current analysis, some studies reported NAA level to Cre ratio, whereas others reported absolute NAA levels. But as all the studies except two27, 31 included into the meta-analysis reported absolute NAA level without implementing tissue segmentation within VOIs (Table 1), absolute level of NAA does not reflect CSF ratio within VOI and what extent gray and white matter were included. This is the reason why we adopted the different priority for extraction depending on implementation of tissue segmentation within VOIs.
In the current work, the sensitivity analysis, which was conducted by subgrouping studies that implemented tissue segmentation, demonstrated significant NAA reductions in the frontal cortex in OCD patients, whereas those that reported NAA level ratio to Cre did not preserve the significance. The potential explanation for disappearance of significance is decreased number of integrated studies and increased weight of outlier in the meta-analysis. Although supplemental meta-analysis of absolute Cre levels in the frontal cortex demonstrated no significant difference between OCD and HC subjects (data not shown), it should be noted that, for example, decrease of the NAA/Cre could reflect decreased NAA, increased Cre or a combined effect of alterations in the levels of both metabolites.
Several clinical factors may have also affected the current results. Two important clinical characteristics that can affect NAA levels are comorbid psychiatric disorder and treatment. For example, previous studies have demonstrated that other psychiatric disorders, such as schizophrenia and autism, also alter NAA levels.52, 53 Although in the current study, the sensitivity analysis with subgrouping to exclude studies that recruited medicated patients demonstrated marginally significant NAA reduction in the frontal cortex, those that recruited patient with comorbid psychiatric disorder did not reach the significance. However, meta-regression showed no significant relation between percentages of patients with concurrent diagnosis of depression and anxiety disorder and NAA reduction in the mPFC, some studies recruited OCD patients with psychological symptoms, such as subclinical depression and anxiety. Although one meta-analysis demonstrated no significant alteration of NAA in the frontal lobe in patients with depression,54 recent studies have suggested that baseline NAA is decreased among patients with depression19, 55 and recovers after treatment.19 Thus, although meta-regression analysis demonstrated significant correlation between NAA reduction in the mPFC and symptom severity, it should be noted that the NAA reduction in the frontal cortex and mPFC may not only have been caused purely by symptoms of OCD, but also by comorbid depression or anxiety of OCD. Regarding the treatment of OCD, some of the included studies recruited OCD patients with current or prior treatment. Although sensitivity analysis with non-medicated patients showed marginally significant NAA reduction in the mPFC, treatment of OCD may also affect NAA levels measured by 1H-MRS. Two of the studies we included conducted longitudinal assessments of the effects of medication. Jang et al.,22 examined thirteen drug-naive OCD patients before and after treatment with medication and found significant increases in NAA in the prefrontal cortex and anterior cingulate. Besiroglu et al.,42 also found increased NAA in the anterior cingulate cortex. These results suggest that examining OCD patients who have previously undergone treatment with medication does not cause underestimation of NAA levels, which indirectly supports the robustness of NAA reductions in OCD patients.
Context of findings
It is currently unclear exactly what is reflected by the measurement of NAA using 1H-MRS. As NAA is synthesized from aspartate and acetyl-coenzyme A in the mitochondria of neurons, it has been proposed as a marker of neuron density20 and mitochondrial activity.56 Recent studies have revealed that astrocytes and oligodendrocytes were involved in the metabolism of NAA.21 As such, not only increased synthesis of NAA, but also decreased metabolism of NAA may result in increased NAA levels.57 For example, ‘NAA trapping theory' suggests that NAA may increase even in cases of dysfunctional metabolism of the astrocytes or oligodendrocytes.58 For example, patients with Canavan's disease, caused by mutations in the gene that codes for the enzyme of aspartoacylase, which is necessary for NAA metabolism, have reported increased NAA without increased neuron density.57 One experimental study reported that intravenous ethanol infusion enhances the activity of acetylaspartylase, a glial enzyme that degrades NAA, and dynamically decreases cortical NAA levels measured by 1H-MRS.59 These studies suggested that NAA reflects dynamic processes rather than structural effect.60 Thus, some types of pathophysiology may cause NAA reduction by decreasing NAA synthesis (via decreased mitochondrial function in neurons or decreased neuron density), whereas other types may decrease NAA by increasing NAA metabolism (via excessive function of glial cells).
Correlation between NAA reduction in the mPFC and symptom severity
Alteration of function of the mPFC has been recognized in a variety of functional neuroimaging studies in OCD, including resting state studies,6 interference and error processing,61, 62 performance monitoring,63 and symptom provocation studies.9 Further, the importance of the mPFC in individuals with OCD has been highly recognized in the field of neurosurgery. Numerous studies have reported the effectiveness of anterior cingulotomy for OCD64 and the mPFC is recognized as a promising target region for deep brain stimulation for OCD.65
Structural abnormalities of the mPFC have been reported and a meta-analysis of voxel-based morphometry studies of OCD15 and a meta-analysis of the brain volume studies16 demonstrated robust gray matter volume reductions in the mPFC.
No significant difference in NAA level in basal ganglia and thalamus
Although previous studies strongly demonstrated the critical role of basal ganglia and thalamus in the symptoms of OCD and there is an indirect evidence for basal ganglia involvement in OCD from findings that patients who suffer focal lesions often then exhibit striking obsessive-compulsive behaviors,5, 11, 12, 13, 66 the current meta-analyses showed no significant difference in NAA level in these areas between OCD patients and HC. Interestingly, the finding is also concordant with previous meta-analyses of the brain volume studies that reported no reduction of gray matter volume.15, 16 Potential explanation for these negative findings that there were no significant differences in NAA levels in basal ganglia and thalamus between OCD patients and HC may be that the basal ganglia and thalamus were included into other CSTC circuits that may have different role in the etiology of OCD. Recent researches revealed that one potential etiology of OCD is an imbalance of inhibition and disinhibition between CSTC loops.3 As more than two circuits include different part of basal ganglia and thalamus, for example, mPFC circuit includes ventral caudate nucleus, on the other hand DLPFC circuit contains dorsal caudate nucleus, it is assumed that some parts of basal ganglia and thalamus are disinhibited by mPFC circuit and others are inhibited by DLPFC circuit, vice versa.3 Thus, we assume that diversity of location of relatively large VOIs of integrated studies, which may include projections from several circuits, resulted in no significant differences in NAA level between OCD patients and HC. With regard to other CSTC circuits, such as OFC and DLPFC, the current meta-analysis could not provide sufficient data to discuss. Although the current meta-analysis demonstrated significant correlation between NAA reduction in the mPFC and symptom severity, the result does not reduce the importance of other areas in the etiology of OCD.
Limitations
Several limitations of the present study should be considered. First, because of the nature of a meta-analysis, we can only provide statistical analyses at the level of whole studies. The combination of results from different studies into a single analysis might cloud individual differences within studies. Second, because too few studies have examined certain brain areas, we could not assess the role of NAA levels in the OFC, and only two studies were included in the meta-analysis of the DLPFC, even though we recognize that previous neuroimaging studies have reported these areas to be important in OCD.67, 68 Third, although NAA reduction in the mPFC was robustly correlated with scores of symptom severity, and meta-regression showed no significant relation between percentage of comorbid diagnosis of depression and anxiety disorder and NAA reduction, there still remains the possibility that NAA reduction may be due to subclinical depression or anxiety, as sensitivity analysis that excluded patients with comorbid psychiatric disorder did not demonstrate significant NAA reduction and other anxiety disorders also demonstrated NAA reduction in the mPFC.69 Forth, although we focused on three CSTC circuits and demonstrated marginally significant NAA reduction in one of the three, we could not conduct network analysis. Fifth, although we categorized the locations of the VOIs into three brain areas, this classification scheme might be criticized as being over-simplified. Though we recognize the functional variability within subregions of these areas, we could not divide VOIs into subarea such as anterior cingulate cortex accurately and objectively only based on Figures and legend supplied in the included studies. Finally, the current meta-analysis included both pediatric and adult studies. Although we investigated the effect of age, the effect size was calculated from two groups of similar ages. Thus, given the striking developmental changes that occur in these regions, we could not assess the underlying developmental trajectory of NAA levels in OCD.
Conclusion
In conclusion, the current meta-analysis revealed a significant NAA reduction in the frontal cortex in OCD patients, while no significant differences were present in the thalamus and basal ganglia. Sensitivity analysis in an anatomically specified subgroup consisting of datasets examining the mPFC demonstrated marginal significant reduction of NAA. The NAA reduction in the mPFC was correlated to symptom severity of OCD. These findings suggest that neurochemical alteration in the mPFC is one of the abnormal neural bases that directly relate to behavioral abnormality.
Acknowledgments
We thank all of the authors of the included studies and especially Dr Jang and Dr Carlsson and their colleagues for kindly sharing their unpublished data for inclusion in this meta-analysis.
YA and AA performed study screenings independently. In the case of discrepancies, consensus was reached by means of discussion with the third author (HS). YA performed all of the data extractions and computations of effect size twice to avoid mistakes. AA further performed the data extractions and computations of the effect sizes independently. YA wrote the paper.
Author contributions
YA and AA performed study screenings independently. In the case of discrepancies, consensus was reached by means of discussion with the third author (HS). YA performed all of the data extractions and computations of effect size twice to avoid mistakes. AA further performed the data extractions and computations of the effect sizes independently. YA wrote the paper.
The authors declare no conflicts of interest.
References
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinot JL, Allilaire JF, Mazoyer BM, Hantouche E, Huret JD, Legaut-Demare F, et al. Obsessive-compulsive disorder: a clinical, neuropsychological and positron emission tomography study. Acta Psychiatrica Scandinavica. 1990;82:233–242. doi: 10.1111/j.1600-0447.1990.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Mcguire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RS, Dolan RJ. Functional anatomy of obsessive-compulsive phenomena. Br J Psychiatry. 1994;164:459–468. doi: 10.1192/bjp.164.4.459. [DOI] [PubMed] [Google Scholar]
- Perani D, Colombo C, Bressi S, Bonfanti A, Grassi F, Scarone S, et al. [18F]FDG PET study in obsessive-compulsive disorder. A clinical/metabolic correlation study after treatment. Br J Psychiatry. 1995;166:244–250. doi: 10.1192/bjp.166.2.244. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Zamignani DR, Buchpiguel CA, Garrido GE, Glabus MF, Rocha ET, et al. A voxel-based investigation of regional cerebral blood flow abnormalities in obsessive-compulsive disorder using single photon emission computed tomography (SPECT) Psychiatry Res. 2000;99:15–27. doi: 10.1016/s0925-4927(00)00050-0. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:901–910. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, et al. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive-compulsive disorder. Biol Psychiatry. 2007;61:330–336. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. A functional MRI comparison of patients with obsessive-compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Res. 2005;139:101–114. doi: 10.1016/j.pscychresns.2004.12.004. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJLM, van Hartskamp J, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arc Gen psychiatry. 2005;62:301–309. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MMA, van Balkom AJLM, Cath DC, van Oppen P, Uylings HBM, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- Valente AA, Miguel EC, Castro CC, Amaro E, Duran FL, Buchpiguel CA, et al. Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biol Psychiatry. 2005;58:479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Rotge J-Y, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Braber den A, Ent DV, Boomsma DI, Cath DC, Veltman DJ, Thompson PM, et al. White matter differences in monozygotic twins discordant or concordant for obsessive-compulsive symptoms: a combined diffusion tensor imaging/voxel-based morphometry study. Biol Psychiatry. 2011;70:969–977. doi: 10.1016/j.biopsych.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Kato T, Inubushi T, Kato N. Magnetic resonance spectroscopy in affective disorders. J Neuropsychiatry Clin Neurosci. 1998;10:133–147. doi: 10.1176/jnp.10.2.133. [DOI] [PubMed] [Google Scholar]
- De Graaf RA. Principles and Techniques. Wiley-Interscience. John Wiley & Sons Ltd: Chichester, UK; 2008. In vivo NMR spectroscopy. [Google Scholar]
- Merkl A, Schubert F, Quante A, Luborzewski A, Brakemeier E-L, Grimm S, et al. Abnormal cingulate and prefrontal cortical neurochemistry in major depression after electroconvulsive therapy. Biol Psychiatry. 2011;69:772–779. doi: 10.1016/j.biopsych.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Lowry M, Horsman A. Unchanged basal ganglia N-acetylaspartate and glutamate in idiopathic Parkinson's disease measured by proton magnetic resonance spectroscopy. Mov Disord. 1997;12:297–301. doi: 10.1002/mds.870120306. [DOI] [PubMed] [Google Scholar]
- Baslow MH. Evidence that the tri-cellular metabolism of N-acetylaspartate functions as the brain's ‘operating system': how NAA metabolism supports meaningful intercellular frequency-encoded communications. Amino Acids. 2010;39:1139–1145. doi: 10.1007/s00726-010-0656-6. [DOI] [PubMed] [Google Scholar]
- Jang JH, Kwon JS, Jang DP, Moon W-J, Lee J-M, Ha TH, et al. A proton MRSI study of brain N-acetylaspartate level after 12 weeks of citalopram treatment in drug-naive patients with obsessive-compulsive disorder. Am J Psychiatry. 2006;163:1202–1207. doi: 10.1176/ajp.2006.163.7.1202. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, et al. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry. 2004;43:1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Fan Q, Tan L, You C, Wang J, Ross CA, Wang X, et al. Increased N-Acetylaspartate/creatine ratio in the medial prefrontal cortex among unmedicated obsessive-compulsive disorder patients. Psychiatry Clin Neurosci. 2010;64:483–490. doi: 10.1111/j.1440-1819.2010.02128.x. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Inokuchi R, Gunshin M, Yahagi N, Suwa H.Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis J Neurol Neurosurg Psychiatry 2012. PMID: 22797288 (in press). [DOI] [PMC free article] [PubMed]
- Kitamura H, Shioiri T, Kimura T, Ohkubo M, Nakada T, Someya T. Parietal white matter abnormalities in obsessive-compulsive disorder: a magnetic resonance spectroscopy study at 3-tesla. Acta Psychiatr Scand. 2006;114:101–108. doi: 10.1111/j.1600-0447.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- Sumitani S, Harada M, Kubo H, Ohmori T. Proton magnetic resonance spectroscopy reveals an abnormality in the anterior cingulate of a subgroup of obsessive-compulsive disorder patients. Psychiatry Res. 2007;154:85–92. doi: 10.1016/j.pscychresns.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Mohamed MA, Smith MA, Schlund MW, Nestadt G, Barker PB, Hoehn-Saric R. Proton magnetic resonance spectroscopy in obsessive-compulsive disorder: a pilot investigation comparing treatment responders and non-responders. Psychiatry Res. 2007;156:175–179. doi: 10.1016/j.pscychresns.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Wiley: Chichester, UK; 2008. [Google Scholar]
- Yücel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, et al. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust N Z J Psychiatry. 2008;42:467–477. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- Yücel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64:946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yung AR, Wood SJ, Phillips LJ, Nelson B, Cotton S, et al. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol Psychiatry. 2008;64:758–765. doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Ebert D, Speck O, König A, Berger M, Hennig J, Hohagen F. 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: evidence for neuronal loss in the cingulate gyrus and the right striatum. Psychiatry Res. 1997;74:173–176. doi: 10.1016/s0925-4927(97)00016-4. [DOI] [PubMed] [Google Scholar]
- Ohara K, Isoda H, Suzuki Y, Takehara Y, Ochiai M, Takeda H, et al. Proton magnetic resonance spectroscopy of lenticular nuclei in obsessive-compulsive disorder. Psychiatry Res. 1999;92:83–91. doi: 10.1016/s0925-4927(99)00040-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39:1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Amponsah A, Sullivan A, MacMillan S, Moore GJ. Increased medial thalamic choline in pediatric obsessive-compulsive disorder as detected by quantitative in vivo spectroscopic imaging. J Child Neurol. 2001;16:636–641. doi: 10.1177/088307380101600902. [DOI] [PubMed] [Google Scholar]
- Mirza Y, O'Neill J, Smith EA, Russell A, Smith JM, Banerjee SP, et al. Increased medial thalamic creatine-phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus major depression and healthy controls. J Child Neurol. 2006;21:106–111. doi: 10.1177/08830738060210020201. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Port JD, Deacon BJ, Abramowitz JS. A magnetic resonance spectroscopy investigation of obsessive-compulsive disorder and anxiety. Psychiatry Res. 2006;146:137–147. doi: 10.1016/j.pscychresns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Scherk H, Backens M, Schneider-Axmann T, Kraft S, Kemmer C, Usher J, et al. Dopamine transporter genotype influences N-acetyl-aspartate in the left putamen. World J Biol Psychiatry. 2009;10:524–530. doi: 10.1080/15622970701586349. [DOI] [PubMed] [Google Scholar]
- Starck G, Ljungberg M, Nilsson M, Jönsson L, Lundberg S, Ivarsson T, et al. A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: relationship between metabolite concentrations and symptom severity. J Neural Transm. 2008;115:1051–1062. doi: 10.1007/s00702-008-0045-4. [DOI] [PubMed] [Google Scholar]
- Besiroglu L, Sozen M, Ozbebit Ö, Avcu S, Selvi Y, Bora A, et al. The involvement of distinct neural systems in patients with obsessive-compulsive disorder with autogenous and reactive obsessions. Acta Psychiatr Scand. 2011;124:141–151. doi: 10.1111/j.1600-0447.2011.01726.x. [DOI] [PubMed] [Google Scholar]
- Bédard M-J, Chantal S. Brain magnetic resonance spectroscopy in obsessive-compulsive disorder: the importance of considering subclinical symptoms of anxiety and depression. Psychiatry Res. 2011;192:45–54. doi: 10.1016/j.pscychresns.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Benazon NR, Moore GJ, Rosenberg DR. Neurochemical analyses in pediatric obsessive-compulsive disorder in patients treated with cognitive-behavioral therapy. J Am Acad Child Adolesc Psychiatry. 2003;42:1279–1285. doi: 10.1097/01.chi.0000087562.01900.de. [DOI] [PubMed] [Google Scholar]
- Scherk H, Backens M, Zill P, Schneider-Axmann T, Wobrock T, Usher J, et al. SNAP-25 genotype influences NAA/Cho in left hippocampus. J Neural Transm. 2008;115:1513–1518. doi: 10.1007/s00702-008-0103-y. [DOI] [PubMed] [Google Scholar]
- Arnold PD, Macmaster FP, Richter MA, Hanna GL, Sicard T, Burroughs E, et al. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive-compulsive disorder. Psychiatry Res. 2009;172:136–139. doi: 10.1016/j.pscychresns.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzesniak C, Araújo D, Crippa JAS. Magnetic resonance spectroscopy in anxiety disorders. Acta Neuropsychiatr. 2008;20:56–71. doi: 10.1111/j.1601-5215.2008.00270.x. [DOI] [PubMed] [Google Scholar]
- Smith EA, Russell A, Lorch E, Banerjee SP, Rose M, Ivey J, et al. Increased medial thalamic choline found in pediatric patients with obsessive-compulsive disorder versus major depression or healthy control subjects: a magnetic resonance spectroscopy study. Biol Psychiatry. 2003;54:1399–1405. doi: 10.1016/s0006-3223(03)00474-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Moore GJ, Paulson LA, Stewart CM, Rosenberg DR. Proton spectroscopic imaging of the thalamus in treatment-naive pediatric obsessive-compulsive disorder. Biol Psychiatry. 2000;47:174–182. doi: 10.1016/s0006-3223(99)00286-3. [DOI] [PubMed] [Google Scholar]
- Bartha R, Stein MB, Williamson PC, Drost DJ, Neufeld RW, Carr TJ, et al. A short echo 1 H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. Am J Psychiatry. 1998;155:1584–1591. doi: 10.1176/ajp.155.11.1584. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Koc M, Ozler S, Tezcan E. Neurochemistry of the hippocampus in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2009;63:486–490. doi: 10.1111/j.1440-1819.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia--a systematic review and meta-analysis. Biol Psychiatry. 2011;69:495–503. doi: 10.1016/j.biopsych.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Kasai K, Yamasue H. Age-related change in brain metabolite abnormalities in autism: a meta-analysis of proton magnetic resonance spectroscopy studies. Transl Psychiatry. 2012;2:e69. doi: 10.1038/tp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res Neuroimaging. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jia Y, Xu G, Ling X, Liu S, Huang L. Frontal white matter biochemical abnormalities in first-episode, treatment-naive patients with major depressive disorder: a proton magnetic resonance spectroscopy study. J Affect Disord. 2012;136:620–626. doi: 10.1016/j.jad.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson CG, McPhee SWJ, Francis J, Shera D, Assadi M, Freese A, et al. Natural history of Canavan disease revealed by proton magnetic resonance spectroscopy (1H-MRS) and diffusion-weighted MRI. Neuropediatrics. 2006;37:209–221. doi: 10.1055/s-2006-924734. [DOI] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, et al. Intravenous ethanol infusion decreases human cortical γ-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, Fukui T, Fukuda R, Yamada H, Yamasaki S, Kuroki N, et al. 1H-MR spectroscopy and gray matter volume of the anterior cingulate cortex in schizophrenia. Neuroreport. 2002;13:2133–2137. doi: 10.1097/00001756-200211150-00029. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Stern ER, Welsh RC, Fitzgerald KD, Gehring WJ, Lister JJ, Himle JA, et al. Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive-compulsive disorder. Biol Psychiatry. 2011;69:583–591. doi: 10.1016/j.biopsych.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Stern ER, Angstadt M, Nicholson-Muth KC, Maynor MR, Welsh RC, et al. Altered function and connectivity of the medial frontal cortex in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010;68:1039–1047. doi: 10.1016/j.biopsych.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Coghill RC, Gilron I, Sarlani E, Veldhuijzen DS, Lenz FA. Quantitative somatic sensory testing and functional imaging of the response to painful stimuli before and after cingulotomy for obsessive-compulsive disorder (OCD) Eur J Pain. 2008;12:990–999. doi: 10.1016/j.ejpain.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigjes J, de Kwaasteniet BP, de Koning PP, Oudijn MS, van den Munckhof P, Schuurman PR, et al. Surgery for psychiatric disorders World Neurosurg; PMID: 22465369 (in press). [DOI] [PubMed]
- Laplane D, Levasseur M, Pillon B, Dubois B, Baulac M, Mazoyer B, et al. Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study. Brain. 1989;112:699–725. doi: 10.1093/brain/112.3.699. [DOI] [PubMed] [Google Scholar]
- Menzies L, Williams GB, Chamberlain SR, Ooi C, Fineberg N, Suckling J, et al. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. Am J Psychiatry. 2008;165:1308–1315. doi: 10.1176/appi.ajp.2008.07101677. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Nakatani E, Nabeyama M, Sanematsu H, Yoshiura T, et al. Working memory dysfunction in obsessive-compulsive disorder: a neuropsychological and functional MRI study. J Psychiatr Res. 2009;43:784–791. doi: 10.1016/j.jpsychires.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, et al. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res. 2008;162:147–157. doi: 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]