Abstract
An efficient protection protocol for the 6-exo-amino group of purine nucleosides with various chloroformates was developed utilizing N-methyl imidazole (NMI). The reaction of an exo-N6-group of adenosine analog 1 with alkyl/ and aryl chloroformates under optimized conditions provided the N6-carbamoyl adenosines (2a-j) in good to excellent yields. The reaction of N6-Cbz-protected nucleosides (5a-c) with phenyl phosphoryl chloride (7) using t-BuMgCl followed by catalytic hydrogenation afforded the corresponding phosphoramidate pronucleotides (8a-c) in excellent yield.
Efficient introduction of protecting groups on polar and/or reactive functional groups is one of the most fundamental and critical aspects of modern organic synthesis.1 For the synthesis of modified nucleosides and nucleotides, the chemoselective protection and subsequent deprotection of polar amino and hydroxyl groups has been integral in the preparation of biology important analogs.2 As part of our nucleoside discovery program we sought a protection/deprotection strategy that would facilitate higher overall yields for the synthesis of phosphoramidate prodrugs.3 Phosphoramidate prodrugs have been shown to enhance biological activity of parent nucleosides by increasing the intracellular nucleoside 5′-triphosphate levels via improved intracellular transport and/or bypassing the rate limiting monophosphorylation step. Typical yields for the formation of phosphoramidate prodrugs with unprotected nucleosides are often single digit and often complicated by difficult isolation. A review of the literature for a potential protective group of exo-amino and/or hydroxyl groups on nucleosides revealed alkyl/aryloxycarbonyl groups as possible candidates for preparing phosphoramidate pronucleotides. Existing possible protecting groups from this class include trichloro-tert-butoxycarbonyl (TcBoc),4 tert- butoxycarbonyl (Boc),5 allyloxycarbonyl (Alloc),6 phenyloxycarbonyl,7 ethyloxycarbonyl,8 β- cyanoethyloxycarbonyl,9 9-fluorenyloxycarbonyl (Fmoc),10 2:(trimethylsilyl)ethoxycarbonyl (Teoc)11 and benzyloxycarbonyl (Cbz)12 groups. While phosphoramidates are known to be somewhat stable to strongly acid conditions13 our internal programs have shown phosphoramidates to have little to no stability to strongly basic and nucleophilic conditions (such as NH3/MeOH, NH4OH, piperazine and NaOMe). The advantages of the mild removal conditions and orthogonally prompted us to consider Cbz as a useful protecting group of purine amino groups to prepare phosphoramidates. Although our previous efforts have shown that phosphoramidates are completely stable to catalytic hydrogenation,14 introduction of a benzyloxycarbonyl (Cbz) group to the N6-amino group of a purine nucleoside has been reported to be difficult, low yielding and often requiring strong base15 or preparation of a powerful Cbz transfer agent.12a
(1-((Benzyloxy)carbonyl)-3-methylimidazolium tetrafluoroborate (Rapoport’s reagent), I (Figure 1) is the most widely utilized reagent for the protection of the exo-N6-group of purines with Cbz.12a However, it is somewhat unstable, not commercially available and must be prepared by a two:step sequence immediately prior to use.16 As a part of our continuing studies, we herein report a more convenient Cbz transfer method for exo-N6-group on purine analogs and its application to an efficient synthetic route to 5′-phosphoramidates utilizing protection/deprotection of a 6-N-Cbz group.
Figure 1.
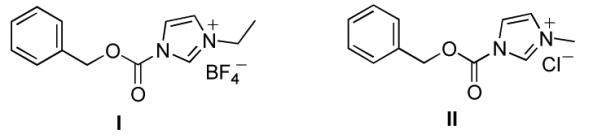
Benzyloxycarbonyl N-alkylimidazolium salts
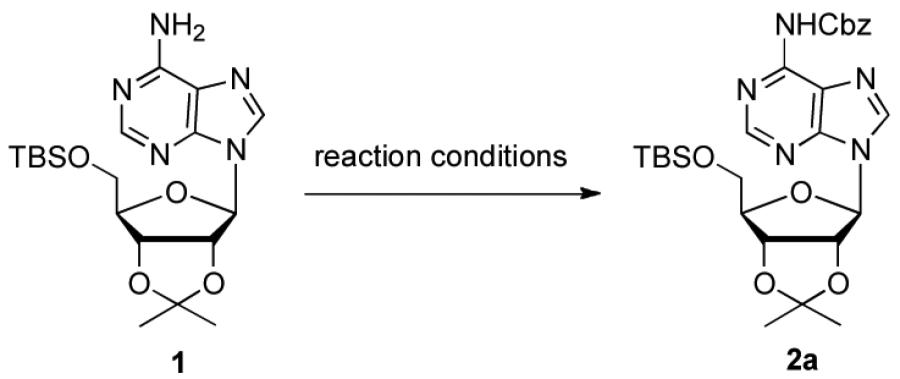
Initially, we chose to investigate the reaction of an exo-N6-group of adenosine derivative (1)17 with CbzCl using previously reported conditions.14,18 The reaction of 1 with CbzCl in the presence of a variety of organic bases such as pyridine, Et3N, DMAP and imidazole in several different solvents gave N6-Cbz-adenosine derivative (2a) in yields that ranged from trace to 12% as summarized in Table 1 (entries 1-9). When N-methyl imidazole (NMI) was used, the yield of 2a was significantly improved to 34-80% depending on the solvent (DMF; 34%, CH3CN; 56%, THF; 45%, CH2Cl2; 72:80%, entries 10-14). The optimal yield of 2a (89%) was obtained with four equivalents of CbzCl in the presence of eight equivalents of NMI in CH2Cl2 for 12 h at room temperature (Table 1, entry 15). We postulate the improvement in yield with NMI is due to transient formation of benzyloxycarbonyl N-methylimidazolium chloride, II, similar to Rapoport’s reagent, I (Figure 1).
Table 1.
Result of protection reaction of N6-Cbz-adenosine (2a)
| |||||
|---|---|---|---|---|---|
| Reaction conditions |
yield of 2a (%)a |
||||
| entry | CbzCl (equiv) |
base (equiv) |
solvent | temp /time |
|
| 1 | 3.0 | Pyridine | Pyridine | 0 °C then rt, 72 h |
--b |
| 2 | 3.0 | Et3N (4.0) | Pyridine | 0 °C then rt, 24 h |
12 |
| 3 | 3.0 | DMAP (4.0) |
Pyridine | 0 °C then rt, 48 h |
-- b |
| 4 | 3.0 | Imc (4.0) | CH2Cl2 | 0 °C then rt, 48 h |
-- b |
| 5 | 3.0 | Et3N/ Im (3.0/6.0) |
CH2Cl2 | 0 °C then rt, 72 h |
-- b |
| 6 | 3.0 | DMAP/ Im (3.0/6.0) |
CH2Cl2 | 0 °C then rt, 48 h |
-- b |
| 7 | 3.0 | DMAP/ Im (3.0/6.0) |
THF | 0 °C then rt, 24 h |
-- b |
| 8 | 3.0 | Et3N (6.0) | CH2Cl2 | 0 °C then rt, 72 h |
-- b |
| 9 | 3.0 | DMAP (6.0) |
CH2Cl2 | 0 °C then rt, 72 h |
-- b |
| 10 | 3.0 | NMI (6.0) | DMF | 0 °C then rt, 24 h |
34 |
| 11 | 3.0 | NMI (6.0) | CH3CN | 0 °C then rt, 24 h |
56 |
| 12 | 3.0 | NMI (6.0) | THF | 0 °C then rt, 24 h |
45 |
| 13 | 3.0 | NMI (6.0) | CH2Cl2 | 0 °C then rt, 24 h |
80 |
| 14 | 2.0 | NMI (4.0) | CH2Cl2 | 0 °C then rt, 48 h |
72 |
| 15 | 4.0 | NMI (8.0) | CH2Cl2 | 0 °C then rt, 12 h |
89 |
Isolated yield from silica gel chromatography.
A trace amount of 2a was detected by LC/MS analysis along with starting material 1.
Im = imidazole.
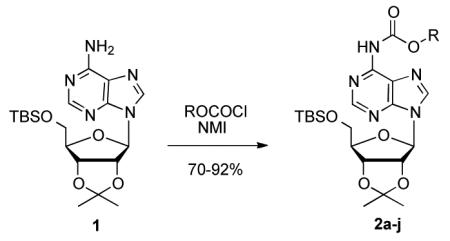
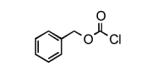
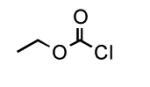
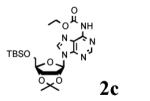
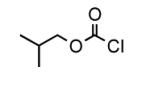
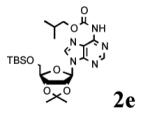
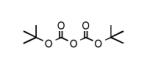
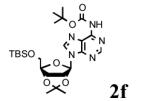
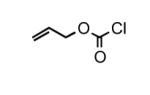
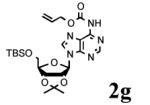
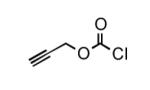
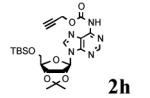
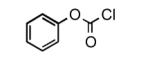
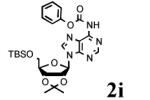
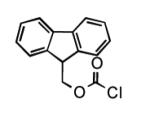
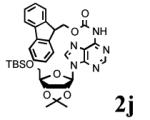
To determine the generality of these conditions, we applied these conditions to the reaction of the exo-N6- group on adenosine derivative 1 with a variety of chloroformate analogs. The reaction of 1 with methyl, ethyl, hexadecyl, and isobutyl chloroformates gave N6- carbamoyl adenosines (2b-e) in high yields as shown in Table 2 (75-92%, entries 2-5). However, the reaction of 1 with t-butoxycarbonyl anhydride ((Boc)2O) gave the corresponding N6-Boc-adennosine 2f in a disappointing 14% yield along with N6,N6-bis:Boc:adenosine (9) in 25% yield19 (Table 2, entry 6). The combination of (Boc)2O with NMI was less efficient than (Boc)2O/DMAP in THF for the protection of exo-amino groups of this nucleoside analog.5a The reaction of 1 with t-butyl chloroformate was not attempted as this reagent has limited stability and therefore not widely available or utilized. The protection of 1 with allyl, propargyl, phenyl and Fmoc chloroformates provided their corresponding carbamoyl derivatives (2g-j) in good yields (70:82%, Table 2, entries 7-10).
Table 2.
Reaction of 1 with various chloroformates

| ||||
|---|---|---|---|---|
| entry | protecting reagent |
time (h) | product (2a-j) | yield (%)a |
| 1 |
|
12 |
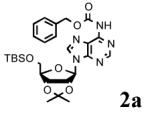
|
89 |
| 2 |
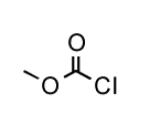
|
12 |
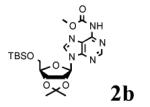
|
75 |
| 3 |
|
12 |
|
86 |
| 4 |
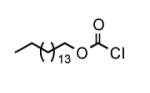
|
12 |
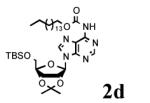
|
92 |
| 5 |
|
24 |
|
88 |
| 6 |
|
24 |
|
14b |
| 7 |
|
12 |
|
82 |
| 8 |
|
24 |
|
76 |
| 9 |
|
12 |
|
70 |
| 10 |
|
12 |
|
78 |
Isolated yield by silica gel column.
Major product was N6,N’6-bis-Boc-adenosine derivative (9) in 25% yield.19
Further, we examined Cbz protected N-/O- groups of purine nucleosides in order to apply this method to prepare aryl phosphoramidates. The synthesis of most phosphoramidates is plagued by low to moderate yields due to both poor chemoselectivity in the phosphoramidate forming reaction and/or poor solubility of the nucleoside in applicable solvents.20 Many of the currently utilized protocols for the synthesis of protected phosphoramidates involve acidic deprotection conditions under which the relative instability of phosphoramidates leads to moderate yields.13,17c
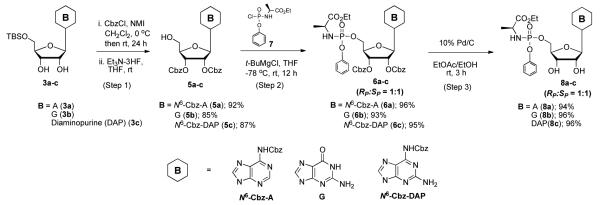
Treatment with Et3N-3HF provided Cbz-protected nucleosides 5a-c in excellent yield (Scheme 1). In the case of DAPR, in addition to the 87% yield of desired N6,2′,3′-O-tris-Cbz-DAPR, 5c, N6,N6,2′,3′-O-tetra-Cbz- DAPR, 10 was obtained in 5% yield.19 Subsequently, the reaction of protected nucleosides 5a-c with the phosphoryl chloride, 7 in the presence of t-BuMgCl in THF gave Cbz-protected phosphoramidates 6a-c as a RP/SP or SP/RP diastereomeric mixture (ranged from 1:1 to 1:1.5 by 1H NMR) in excellent yields without any detectable cleavage of N- or O-Cbz bonds at either chemical step. We also note that the tri-Cbz protected C and di-Cbz protected U previously reported14 when reacted with 7 in the presence of t-BuMgCl in THF gave Cbz-protected phosphoramidates in 94% and 97% respectivly.19 Removal of Cbz groups from the phosphoramidates 6a-c with Pd/C and H2 (1 atm) afforded 8a-c in 94%, 96% and 96% yield respectively.
Scheme 1.
Synthesis of purine phenyl phosphoramidates (8a-c)a
aProduct was a diastereomeric mixture in which RP/SP or SP/RP ratio ranged from 1:1 to 1:1.5.
As an extension of our high yielding approach to phosphoramidates of pyrimidine nucleosides involving Cbz protection of the sugar hydroxyl groups,14 we applied our more forcing Cbz transferring conditions to the preparation of phenyl phosphoramidates of adenosine (A), guanosine (G), and 2,6-diaminopurine ribose (DAPR), as shown in Scheme 1. The Cbz group was introduced in high yield on N6 and 2′,3′-O-positions of 5′-TBS protected A analog 3a and DAPR analog 3c. For the G analog, 3b, only the 2′,3′-O-positions were acylated. Even under the most forcing CbzCl/NMI conditions we could not detect acylation of the 6-O position of the ribo G nucleoside. In addition, although an excess of CbzCl/NMI (as much as 6.0/12.0 equiv) was employed in this protection sequence we did not observe any N2-amino acylation of A, G, or DAPR analogs.
In summary, an efficient CbzCl protection protocol for the N6-amino of adenine and diaminopurine nucleosides which also acylated the hydroxyl groups of the ribose ring was successfully developed by utilizing NMI in CH2Cl2. This robust protocol does not require a two-step synthesis of a Cbz transfer agent and provides good to excellent yields under mild conditions. In addition, we successfully applied this method to a large variety of alkyl and aryl chloroformates to afford their corresponding exo-N6- carbamoyl adenosines in good to excellent yields. The reaction of 6-N, 2′,3′-O protected A (5a), G (5b) and DAPR (5c) with phenyl-(ethoxy-L-alaninyl)- phosphorochloridate (7) provided the Cbz-protected phosphoramidates (6a-c) in excellent yield. Cbz group cleavage was not observed during the phosphoryl chloride coupling conditions of t-BuMgCl or NMI and also not observed during the Et3N:3HF promoted desilylation. Overall, we have developed a highly efficient novel method for the synthesis of purine nucleoside phosphoramidates.
Supplementary Material
Acknowledgment
This work was supported in part by NIH grant 5P30-AI-50409 (CFAR), and by the Department of Veterans Affairs.
Footnotes
Supporting Information Available: Experimental procedures, characterization data, 1H and 13C NMR spectra for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).(a) Wuts PGM, Greene TW. Green’s Protective Groups in Organic Synthesis. 4th edition John Wiley and Sons, Inc.; Hoboken, NJ: 2007. [Google Scholar]; (b) Kocienski PJ. Protecting Groups; Geoug Thieme Verlag Stuttgart. New York: 1994. [Google Scholar]
- (2).(a) Samano V, Miles RW, Robins MJ. J. Am. Chem. Soc. 1994;116:9331–9332. [Google Scholar]; (b) Miles RW, Samano V, Robins MJ. J. Am. Chem. Soc. 1995;117:5951–5957. [Google Scholar]; (c) Cui Z, Zhang L, Zhang B. Tetrahedron Lett. 2001;42:561–563. [Google Scholar]; (d) Nguyen-Trung NQ, Terenzi S, Scherer G, Strazewski P. Org. Lett. 2003;5:2603–2606. doi: 10.1021/ol0346638. [DOI] [PubMed] [Google Scholar]; (e) Nowak I, Robins MJ. Org. Lett. 2003;5:3345–3348. doi: 10.1021/ol035264f. [DOI] [PubMed] [Google Scholar]; (f) Zhong M, Nowak I, Robins MJ. J. Org. Chem. 2006;71:7773–7779. doi: 10.1021/jo061282+. [DOI] [PubMed] [Google Scholar]; (g) Derudas M, Carta D, Brancale A, Vanpouille C, Lisco A, Margolis L, Balzarini J, McGuigan C. J. Med. Chem. 2009;52:5520–5530. doi: 10.1021/jm9007856. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Arico JW, Calhoun AK, Salandria KJ, McLaughlin LW. Org. Lett. 2010;12:120–122. doi: 10.1021/ol9025028. [DOI] [PubMed] [Google Scholar]
- (3).For reviews: Bobeck DR, Coats SJ, Schinazi RF. Antivir. Ther. 2010;15:935–950. doi: 10.3851/IMP1667. Erion MD, Hecker SJ. J. Med. Chem. 2008;51:2328–2345. doi: 10.1021/jm701260b. Schultz C. Bioorg. Med. Chem. 2003;11:885–898. doi: 10.1016/s0968-0896(02)00552-7. Mehellou Y, Balzarini J, McGuigan C. Chem. Med. Chem. 2009;4:1779–1791. doi: 10.1002/cmdc.200900289. Sofia MJ. Antivir. Chem. Chemother. 2011;22:23–49. doi: 10.3851/IMP1797.
- (4).(a) Hotoda H, Saito R, Sekine M, Hata T. Tetrahedron. 1990;46:1181–1190. [Google Scholar]; (b) Strasser M, Ugi I. Acta Chem. Scand. 1993;47:125–130. [Google Scholar]
- (5).(a) Dey S, Garner PJ. Org. Chem. 2000;65:7697–7699. doi: 10.1021/jo000983i. [DOI] [PubMed] [Google Scholar]; (b) Bazzanini R, Gouy M:H, Peyrottes S, Gosselin G, Perigaud C, Manfrendini S. Nucleosides, Nucleotides & Nucleic acids. 2005;24:1635–1649. doi: 10.1080/15257770500267006. [DOI] [PubMed] [Google Scholar]; (c) Wojciechowski F, Hudson RHE. J. Org. Chem. 2008;73:3807–3816. doi: 10.1021/jo800195j. [DOI] [PubMed] [Google Scholar]; (d) Wang R:W, Gold B. Org. Lett. 2009;11:2465–2468. doi: 10.1021/ol9007537. [DOI] [PubMed] [Google Scholar]
- (6).(a) Rigby JH, Moore TL, Rege S. J. Org. Chem. 1986;51:2400–2402. [Google Scholar]; (b) Wu W, Sigmond J, Peters GJ, Borch RF. J. Med. Chem. 2007;50:3743–3746. doi: 10.1021/jm070269u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Anzai K, Hunt JB, Zon G. J. Org. Chem. 1982;47:4281–4285. [Google Scholar]; (b) Anzai K, Uzawa J. J. Org. Chem. 1984;49:5076–5080. [Google Scholar]
- (8).Chheda GB, Hong CI. J. Med. Chem. 1971;14:748–753. doi: 10.1021/jm00290a019. [DOI] [PubMed] [Google Scholar]
- (9).Chen T, Fu J, Greenberg MM. Org. Lett. 2000;2:3691–3694. doi: 10.1021/ol006604p. [DOI] [PubMed] [Google Scholar]
- (10).(a) Kutyanvin IV, Woo J, Lukhtanov EA, Meyer RB, Jr., Gamper HB. WO1997012896. 1997; (b) Alvarado GG. WO2004029297. 2004
- (11).Ferreira F, Morvan F. Nucleosides, Nucleotides & Nucleic acids. 2005;24:1009–1013. doi: 10.1081/ncn-200060342. [DOI] [PubMed] [Google Scholar]
- (12).(a) Watkins BE, Kiely JS, Rapoport H. J. Am. Chem. Soc. 1982;104:5702–5708. [Google Scholar]; (b) Liu F, Austin D. J. Org. Lett. 2001;3:2273–2276. doi: 10.1021/ol015995k. [DOI] [PubMed] [Google Scholar]; (c) Wandzik I, Bieg T, kadela M. Nucleosides, Nucleotides & Nucleic acids. 2008;27:1250–1256. doi: 10.1080/15257770802458303. [DOI] [PubMed] [Google Scholar]
- (13).(a) Perrone P, Daverio F, Valente R, Rajyaguru S, Martin JA, Lévêque V, Pogam SL, Najera I, Klumpp K, Smith DB, McGuigan C. J. Med. Chem. 2007;50:5463–5470. doi: 10.1021/jm070362i. [DOI] [PubMed] [Google Scholar]; (b) Gardelli C, Attenni B, Donghi M, Meppen M, Pacini B, Harper S, Marco AD, Fiore F, Giuliano C, Pucci V, Laufer R, Gennari N, Marcucci I, Leone JF, Olsen DB, MacCoss M, Rowley M, Narjes F. J. Med. Chem. 2009;52:5394–5407. doi: 10.1021/jm900447q. [DOI] [PubMed] [Google Scholar]; (c) Perlíková P, Pohl R, Votruba I, Shih R, Birkuš G, Cihlář T, Hocek M. Bioorg. Med. Chem. 2011;19:229–242. doi: 10.1016/j.bmc.2010.11.029. [DOI] [PubMed] [Google Scholar]
- (14).Cho JH, Amblard F, Coats SJ, Schinazi RF. Tetrahedron. 2011;67:5487–5493. doi: 10.1016/j.tet.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).(a) Azzena U, Dettori G, Pisano L, Pittalis M. Syn. Comm. 2007;37:3623–3634. [Google Scholar]; (b) Rosse G, Sequin U. Helv. Chimica Acta. 1997;80:653–670. [Google Scholar]; (c) Peifer M, De Giacomo F, Schandl M, Vasella A. Helv. Chimica Acta. 2009;92:1134–1166. [Google Scholar]; (d) Sumita Y, Shirato M, Ueno Y, Matsuda A, Shuto S. Nucleosides, Nucleotides & Nucleic Acids. 2000;19:175–187. doi: 10.1080/15257770008033002. [DOI] [PubMed] [Google Scholar]
- (16).For other Cbz transfer reagents applied to N6-purines see: Komiyama M, Aiba Y, Ishizuka T, Sumaoka J. Nature Protocols. 2008;3:646–654. doi: 10.1038/nprot.2008.6. Schlama T, Manfre F. US20060149051. 2006 Rayan A, Falah M. US201000284959. 2010
- (17).(a) Homma H, Watanabe Y, Abiru T, Murayama T, Momura Y, Matsuda A. J. Med. Chem. 1992;35:2881–2890. doi: 10.1021/jm00093a022. [DOI] [PubMed] [Google Scholar]; (b) Cho JH, Bernard DL, Sidwell RW, Kern ER, Chu CK. J. Med. Chem. 2006;49:1140–1148. doi: 10.1021/jm0509750. [DOI] [PubMed] [Google Scholar]; (c) McGuigan C, Gilles A, Madela K, Aljarah M, Holl S, Jones S, Vernachio J, Hutchins J, Bryant KD, Gorovits E, Ganguly B, Hunley D, Hall A, Kolykhalov A, Liu Y, Muhammad J, Raja N, Walters R, Wang J, Chamberlain S, Henson G. J. Med. Chem. 2010;53:4949–4957. doi: 10.1021/jm1003792. [DOI] [PubMed] [Google Scholar]
- (18).Johnson DC, II, Widlanski TS. Org. Lett. 2004;6:4643–4646. doi: 10.1021/ol048426w. [DOI] [PubMed] [Google Scholar]
- (19).Data and experimental conditions for all compounds are available in Supporting Information.
- (20).(a) McGuigan C, Pathirana RN, Mahmood N, Hay AJ. Bioorg. Med. Chem. Lett. 1992;2:701–704. [Google Scholar]; (b) Kumar D, Mamiya BM, Kern JT, Kerwin SM. Tetrahedron Lett. 2001;42:565–567. [Google Scholar]; (c) Sofia MJ, Bao D, Chang W, Du J, Nagarathnam D, Rachakonda S, Reddy PG, Ross BS, Wang P, Zhang H-R, Bansal S, Espiritu C, Keilman M, Lam AM, Steuer HMM, Niu C, Otto MJ, Furman PA. J. Med. Chem. 2010;53:7202–7218. doi: 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]; (d) Chang W, Bao D, Chun B:K, Naduthambi D, Nagarathnam D, Rachakonda S, Reddy PG, Ross BS, Zhang H:R, Bansal S, Espiritu CL, Keilman M, Lam AM, Niu C, Steuer HM, Furman PA, Otto M, Sofia MJ. J. Med. Chem. Lett. 2011;2:130–135. doi: 10.1021/ml100209f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.