Abstract
N -methyl-D-aspartate (NMDA) receptors play an important role in excitatory neurotransmission and mediate synaptic plasticity associated with learning and memory. NMDA receptors are composed of two NR1 and two NR2 subunits and the identity of the NR2 subunit confers unique electrophysiologic and pharmacologic properties to the receptor. The precise role of NR2C-containing receptors in vivo is poorly understood. We have performed a battery of behavioral tests on NR2C knockout/nβ-galactosidase knock-in mice and found no difference in spontaneous activity, basal anxiety, forced-swim immobility, novel object recognition, pain sensitivity and reference memory in comparison to wildtype counterparts. However, NR2C knockout mice were found to exhibit deficits in fear acquisition and working memory compared to wildtype mice. Deficit in fear acquisition correlated with lack of fear conditioning-induced plasticity at the thalamo-amygdala synapse. These findings suggest a unique role of NR2C-containing receptors in associative and executive learning representing a novel therapeutic target for cognitive deficits and mental disorders such as post-traumatic stress disorder.
Keywords: NMDA, NR2C, knockout, fear acquisition, working memory
Introduction
Glutamate mediates majority of the excitatory neurotransmission in the mammalian central nervous system. There are three major classes of ionotropic glutamate receptors classified on the basis of sequence similarity and pharmacology (Dingledine, Borges, Bowie, & Traynelis, 1999; Erreger, Chen, Wyllie, & Traynelis, 2004). One such class of ionotropic glutamate receptors is the N-methyl-D-aspartate (NMDA) receptor, which is involved in key physiologic events such as synaptic plasticity and neural development. The NMDA receptor is a tetrameric receptor composed of two NR1 and two NR2 subunits (Monyer et al., 1992). The identity of the NR2 subunit (NR2A-D) confers unique electrophysiologic and pharmacologic properties to the NMDA receptor. NR2A- and NR2B-containing receptors are expressed widely throughout the central nervous system and are essential for integration of environmental stimuli and synaptic processes of learning and memory. In contrast, the role of NR2C- and NR2D-containing receptors is poorly understood. The NR2C subunit is abundantly expressed in the cerebellar granule neurons first appearing at postnatal day 10 in rodents (Monyer, Burnashev, Laurie, Sakmann, & Seeburg, 1994; Farrant, Feldmeyer, Takahashi, & Cull-Candy, 1994; Wenzel, Fritschy, Mohler, & Benke, 1997; Cathala, Misra, & Cull-Candy, 2000; Karavanova, Vasudevan, Cheng, & Buonanno, 2007). NR2C mRNA is also expressed in the olfactory bulb, thalamus, retrosplenial cortex, pontine and vestibular nuclei (Wenzel et al., 1997; Karavanova et al., 2007) and certain interneurons in the cerebral cortex and hippocampus (Monyer et al., 1994).
Activation of the NMDA receptor is both ligand- and voltage-dependent. NMDA receptors are inactive under resting membrane potential due to Mg2+-block (Mayer, Westbrook, & Guthrie, 1984). However, NR2C-containing receptors exhibit relatively low sensitivity to Mg2+-block compared to NR2A- and NR2B-containing receptors (Monyer et al., 1994; Cull-Candy, Brickley, & Farrant, 2001). This property allows the NR2C-containing receptors to be activated by ambient glutamate without the requirement for prior depolarization as seen in layer 4 spiny stellate cells in barrel cortex and reticular thalamic nuclei (Binshtok, Fleidervish, Sprengel, & Gutnick, 2006; Zhang, Llinas, & Lisman, 2009). Deletion of NR2C gene leads to higher charge transfer in cerebellar granule cells in agreement with lower open probability of NR2C-containing receptors (Ebralidze, Rossi, Tonegawa, & Slater, 1996; Dravid, Prakash, & Traynelis, 2008) but does not affect general motor coordination or motor learning (Kadotani et al., 1996; Ikeda et al., 1995).
NMDA receptors have been identified as playing an important role in a variety of learning and memory functions. NMDA receptor antagonists block the acquisition but not expression of associative learning in a number of tasks, such as spatial learning (Handelman, Contreras, & O’Donohue 1987; Heale & Harley, 1990; Morris, Anderson, Lynch, & Baudry 1986) and working memory function in the prefrontal cortex (Honey et al., 2003). Indicating that ability to maintain information over short periods is dependent on NMDA receptor activity (Robbins & Murphy, 2006). Pharmacological and knockout studies have identified a crucial role of NR2A- and NR2B-containing NMDA receptors in behavioral learning including fear conditioning and long-term potentiation (Sakimura et al., 1995; Zhang et al., 2008; Gao et al., 2009; von Engelhardt et al., 2008; Zhao & Constantine-Paton, 2007). The neural circuits involved in acquisition, consolidation and extinction of fear memory consist of the hippocampus, the amygdala and the medial prefrontal cortex, with the amygdala thought to be the central region of fear memory formation and expression (Ledoux, Iwata, Cicchetti, & Reis, 1988). Intra-amygdala infusion of NMDA receptor antagonists block the acquisition of conditioned fear (Miserendino, Sananes, Melia, & Davis, 1990; Gewirzt & Davis, 1997; Fendt, 2001) and NMDA-dependent long-term potentiation has been reported in the amygdala (Maren & Fanselow, 1995; Huang & Kandel, 1998). Further studies indicate that NR1/NR2B receptors play a predominant role in the acquisition of fear whereas NR1/NR2A receptors have a generalized effect on synaptic plasticity (Walker & Davis, 2008). The role of NR2C-containing receptors in fear conditioning and other memory processes is poorly understood due to lack of a subunit-specific antagonist.
Previously reported behavioral assessment of NR2C knockout mice has focused on cerebellar function (Kadotani et al., 1996; Ikeda et al., 1995) due to predominant expression of NR2C-containing receptors in mature cerebellar granule cells. However, given the unique biophysical properties (Monyer et al., 1994; Cull-Candy et al., 2001) and selective expression pattern of NR2C receptors (Wenzel et al., 1997; Karavanova et al., 2007), a detailed behavioral analysis examining the role of NR2C-containing receptors in non-cerebellar functions is warranted. Using an NR2C knock-out/β-galactosidase knock-in mouse strain (Karavanova et al., 2007) we find that mice lacking the NR2C subunit exhibit deficiency in fear acquisition and working memory. Additionally, we propose that NR2C knockout mice may model some aspects of the negative symptoms of schizophrenia such as emotional blunting and cognitive deficits in agreement with the NMDA receptor hypofunction hypothesis.
Methods
General Behavior Protocol
Experimental procedures were performed on male NR2C +/+, NR2C +/− and NR2C −/− littermate mice (Karavanova et al., 2007) at 1–2 months of age. Animals were group housed on a 12:12 light-dark cycle with ad libitum access to food and water. Prior to all behavioral procedures, animals were handled for 3 days at the approximate time of the day the procedures were to occur. All procedures took place in the light phase of the light-dark cycle unless indicated otherwise. Unless indicated otherwise, all experimental surfaces were thoroughly cleaned with 70% ethanol between trials. Behavior procedures were video-recorded and scored by an individual blind to the genotype of the animal via a random coding system of the video files. All behavioral procedures were approved by the Creighton University Institutional Animal Care and Use Committee and conformed to the 1996 NIH Guide for the Care and Use of Laboratory Animals.
Fear Conditioning/Extinction/Testing Apparatus (tests 1–5)
For fear conditioning, mice were placed in a Plexiglas rodent conditioning chamber (chamber A; model 2325-0241 San Diego Instruments, San Diego, CA) with a metal grid floor that was enclosed in a sound-attenuating chamber. The chamber was illuminated with either red or white light depending on the type of conditioned stimulus (CS, tone or light) associated with the unconditioned stimulus (US, foot-shock), indicated below. Chamber A was cleaned with a 19.5% ethanol, 1% vanilla solution to give the chamber a distinct scent. For extinction training and CS testing; mice were placed in a novel Plexiglas chamber (chamber B; model 2325-0241 San Diego Instruments, San Diego, CA) with different visual cues and a solid Plexiglas floor to minimize generalization to the conditioning chamber. Chamber B was cleaned with a 70% ethanol solution, scented with linen-scented air freshener and illuminated with a 40-watt white light unless indicated otherwise. White noise was provided in each isolation cabinet with a fan. A web-camera (Logitech QuickCam) was mounted at the top of each isolation chamber to videotape all sessions.
3CS-US Conditioning (test 1)
Prior to conditioning (day 0) animals were acclimated to chamber A for 30 minutes. On the day of conditioning (day 1) mice were placed in chamber A for 3 minutes followed by three CS-US pairings. The CS was an 85 dB, 3 kHz tone delivered for 30 seconds with a 1 minute inter-trial interval (ITI). The US was a 0.8 mA foot-shock delivered for 2 seconds that coterminated with the CS. Mice were removed from chamber A 1 minute after the final CS-US pairing. On testing day (day 2), the mice were placed in chamber B and after a two-minute delay exposed to the CS for two minutes and removed from the chamber two minutes later. The procedure for long-term memory testing (day 7) was the same as that on day 2 testing. Tests 2–5 were variations of the fear conditioning procedure used in test 1.
Context Conditioning (test 2)
To determine contextual influence on fear conditioning in NR2C −/− mice, context conditioning and testing were performed. Animals were not pre-exposed to the conditioning chamber (chamber A) prior to conditioning. Conditioning proceeded as described in test 1. On testing day (day 2) instead of being placed into chamber B the mice were placed in chamber A for 4 minutes, freezing behavior was scored during the entire duration of context exposure.
Light-Cue Conditioning (test 3)
The CS was changed from a tone to light cue to assure that the observed deficit in fear conditioning was independent of cue perception. Acclimation, conditioning and testing procedures were the same as described test 1, except that instead of a tone, the CS for conditioning and testing was a white-light. Also, the houselight in chambers A and B were provided by a 25-watt red light.
Conditioning/Testing in Dark Phase of Light/Dark Cycle (test 4)
To assess the role of fear conditioning in the light versus dark phase of the light/dark cycle and reproduce previously published fear-conditioning results in NR2C −/ − mice, fear conditioning and testing was conducted as described in Moriya et al. (2000). Animals were acclimated, conditioned and tested in the dark phase of the light-dark cycle. On day 1 of fear conditioning animals were placed in chamber A for 17 minutes prior to 3 CS-US pairings. The CS was an 85 dB, 3 kHz tone delivered for 10 seconds and the US was a 0.2 mA foot-shock delivered for 1 second that co-terminated with the CS. The isolation cabinet of chamber A and B was illuminated with a 25-watt red light. On testing day (day 2) the animal was placed in chamber B and after a nine-minute delay exposed to the CS for four minutes.
5 CS-US Conditioning/Extinction Training (test 5)
To determine if number of CS-US pairings would affect level of fear conditioning in NR2C −/− mice, the number of CS-US pairings was increased from three to five. Additionally, extinction training was conducted to evaluate any deficit in fear extinction learning. Acclimation was performed as described in test 1. On the day of conditioning (day 1), mice were placed in chamber A and exposed to 5 CS-US pairings. Duration and ITI of the CS-US pairings was the same as described in test 1. Extinction training (day 2) occurred in chamber B and consisted of ten CS presentations for 2 minutes each with a 1-minute ITI for a total extinction training duration of 30 minutes. On extinction-recall testing day (day 3) the animal was placed in chamber B and after a 2-minute delay, exposed to the CS for 2 minutes.
Scoring of Fear Conditioning
Behavioral freezing was measured visually as the absence of all non-respiratory movements every five seconds. Scores of 0 for immobility and 1 for movement were averaged and divided by the total number of readings to derive a percent freezing. Behavioral freezing was also analyzed with the Freeze Monitor System (San Diego Instruments) software to verify visual scores.
Non-Fear Conditioning Procedures
Open Field Test (test 6)
Potential difference in spontaneous locomotor activity between NR2C −/− and NR2C +/+ mice was assessed with an open field test. Activity was recorded in a 25.4 × 25.4 cm open field divided into 16, 6.4 × 6.4 cm sections. Mice were placed in the open field for 15 minutes and allowed to explore. Locomotor activity was recorded using an elevated video camera during the session. Line crosses were tallied and expressed as cumulative line crosses during the entire session as well as line crosses per minute.
Elevated Plus Maze (test 7)
Basal anxiety level was assessed using a custom-made Plexiglas elevated plus maze (elevated 84.6 cm, 27.9 × 5.1 cm arms with closed arms having 27.9 cm wall enclosure). Mice were placed on the elevated plus maze for 15 minutes. Arm location was recorded with an elevated video camera. Duration and number of entries into each arm were recorded with the experimenter blind to the genotype.
Pain Sensitivity (test 8)
Pain sensitivity to footshock was assessed in the fear conditioning apparatus. Following two minutes of habituation, a series of 2-second footshocks were delivered ranging from 0.1–0.8 mA at 0.1 mA ascending increments with a 20 second ITI. All sessions were videotaped and the current intensity required to elicit flinching, vocalization, and jumping was scored.
Novel Object Recognition (test 9)
Recognition memory was assessed to determine potential short-term and long-term memory deficits in NR2C −/− mice. The novel object recognition chamber was a square open field (25.4 × 25.4 × 17.8 cm).
The novel object recognition task was performed in three phases; environmental acclimation, training and testing. During acclimation all animals were handled 1–2 minutes a day for 3 days. On days 2 and 3 of handling, animals were placed in the experimental apparatus for 10 minutes to acclimate animals to the environment. On days 4 and 5 of training mice were placed in the chamber with two identical objects and allowed to explore for 10 minutes. Thirty minutes after training on day 5 short-term memory was assessed by exchanging one familiar object with a novel object and mice were allowed to explore the experimental apparatus for 10 minutes. Time spent inspecting both the novel and familiar objects within a 1 cm radius were recorded. Location of the novel object was counterbalanced with half of animals in each group exposed to the novel object on the left side of the chamber and the other half exposed on the right side of the chamber. A second novel object was presented 24 hours after training to test for long-term memory, again location of the novel object was counterbalanced in the chamber. Relative exploration time was recorded and expressed as a discrimination index [D.I. = (tnovel − tfamiliar)/(tnovel + tfamiliar) × 100%]. All objects were cleaned with 70% ethanol between trials to eliminate potential olfactory cues or preference for each object.
Forced Swim Test (test 10)
Forced swim-induced despair was monitored for NR2C +/+ and NR2C −/− mice to determine potential difference in depression-like behavior. Animals were placed in a beaker containing 600 mL of water (25±1°C) for five minutes. Behavior of immobility or mobility was scored every five seconds to derive a percent immobility for each group.
8-Arm Radial Maze (test 11)
Working and reference memory were tested with an 8-arm radial maze (Coulbourn Inst.) and took place over an 11-day session broken into three phases; acclimation, training and testing. During all phases of the procedure animals had 23 hours of food deprivation to increase saliency of food pellets (45 mg pellets; BioServ, Frenchtown, NJ) located at the end of each bated arm.
During acclimation (days 1–3) animals were allowed to explore the 8-arm radial maze with randomly placed food pellets throughout the maze. During training (days 4–6) animals were placed in the maze facing arm 1, arms 1, 2, 4 and 7 were baited with a food pellet at the end of each arm, arms 3, 5, 6 and 8 were closed. Internal maze visual cues (black tape) were present at the entry and end of each baited arm. External visual cues such as wall-paper, location of door and experimenter provided additional spatial reference cues. Four training sessions occurred for each animal on days 4–6, each lasting until all food pellets had been retrieved or five minutes had elapsed. The testing phase occurred over days 7–11. During testing all arms of the radial maze were open. At the beginning of each testing session the animal was placed in the maze facing arm 1. Four testing sessions occurred each day for every animal and lasted until all food reward was retrieved or five minutes had elapsed.
Entries into unbaited arms were counted as reference memory errors and re-entry into previously baited arms were counted as working memory errors. Errors were compiled daily for each animal to derive average errors per day. Experimenters were blind to genotype of each animal.
Identification of NR2C Localization by β-Gal Staining
Immunofluorescence labeling was performed to examine the localization of β-Gal in the thalamus and amygdala of NR2C +/− mice. Mice were anesthetized using isoflurane and perfused intracardially with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were then isolated and stored in 4% paraformaldehyde solution overnight at 4°C. Storage solution was subsequently changed to sucrose solutions (10%, 20% and 30%, respectively) in 0.1 M phosphate buffer for 24 hours each at 4°C. Following treatment in 30% sucrose/0.1 M PB solution, brains were frozen in 2-methylbutane solution maintained at −35°C and stored at −80°C.
Coronal brain sections (20 μm) prepared using a cryostat (Leica CM 1900, IL) were blocked with normal goat serum (NGS) followed by incubation with anti-β-Gal antibody (Promega Corporation, WI) [1:200 dilution in 3% NGS in PBST (PGT)] overnight at room temperature. Following washing, sections were incubated with goat anti-mouse IgG conjugated to Alexafluor 594 (Invitrogen, CA) [1:300 dilution in PGT] for 2 hours at room temperature in the dark. For double-immunofluorescence labeling to determine co-localization of β-Gal with neuronal NeuN or astrocytic GFAP, the sections were washed and blocked with 10% normal horse serum (NHS) followed by 1% BSA in PBST for 30 minutes each. Sections were incubated with anti-NeuN antibody conjugated with Alexafluor 488 (Chemicon Intl.) or anti-mouse GFAP antibody (Sigma) overnight followed by 2 hour incubation in secondary antibody conjugated to Alexafluor 488. Sections were washed, mounted on slides, air-dried and cover slipped using Fluoromount G. Sections were visualized using IX74 Olympus inverted microscope and Image Pro Plus 6.2 image acquisition software.
Amygdala Slice Electrophysiology
Acute amygdala slices were obtained from conditioned (as in test 1) and naïve NR2C wildtype and knockout mice (P20 to P25) in accordance with the approved protocols of Creighton University Institutional Animal Care and Use Committee. Briefly, after isoflurane anesthesia mice were decapitated and brains were removed rapidly and placed in ice-cold artificial cerebrospinal fluid (ACSF) of the following composition (in mM): 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 3 MgCl2 and 10 glucose saturated with 95% O2/5% CO2. 300–350 μm thick coronal sections were prepared using vibrating microtome (Vibratome series 1000, Ted Pella, Redding, CA).
Whole-cell patch recordings were obtained from lateral amygdala (LA) principal neurons in voltage-clamp configuration with an Axopatch 200B (Molecular Devices, CA) and a pipette resistance of 5–10 mOhm. EPSCs were evoked by stimulation of thalamic afferents emerging from the internal capsule using a platinum electrode (FHC, ME). The internal solution consisted 2 of (in mM) 110 cesium gluconate, 30 CsCl2, 5 HEPES, 4 NaCl, 0.5 CaCl2, 2 MgCl2, 5 BAPTA, Na2ATP, and 0.3 Na2GTP (pH 7.35). 20mM QX314 was added to block voltage-gated sodium channels. The standard recording solution was composed of (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 1.5 CaCl2, 1.5 MgCl2 and 10 glucose saturated with 95% O2/5% CO2, pH 7.4. Whole-cell recordings with a pipette access resistance less than 20 mOhm and that changed less than 20% during the duration of recording were included. Signal was filtered at 5 kHz and digitized at 10 kHz using an Axon Digidata 1440A analog-to-digital board (Molecular Devices, CA). Recordings were performed in the presence of 100 μM picrotoxin or 20 μM bicuculline. AMPA receptor-mediated EPSC were recorded by evoking currents at a holding potential of −70 mV, and NMDA receptor-mediated EPSC were determined as the current amplitude at 50 ms after the peak EPSC amplitude at a holding potential of +40 mV. At least 10–15 sweeps were averaged for each condition.
Results
A battery of behavioral tests including tests for learning and memory (fear conditioning, novel object recognition, and 8-arm radial maze), despair (forced-swim test), pain sensitivity, locomotor activity (open field test) and anxiety (elevated plus maze) were performed on NR2C wildtype and knockout mice. Fear-conditioning studies were performed under a variety of conditions to address potential non-associative sensory-motor deficits in NR2C knockout mouse. Additionally, fear conditioning-induced synaptic strengthening at the thalamo-amygdala synapse was assessed by performing whole-cell voltage-clamp recordings from lateral amgydala pyramidal neurons in NR2C wildtype and knockout mice.
NR2C Heterozygous and Knockout Mice Exhibit Deficit in Fear Acquisition
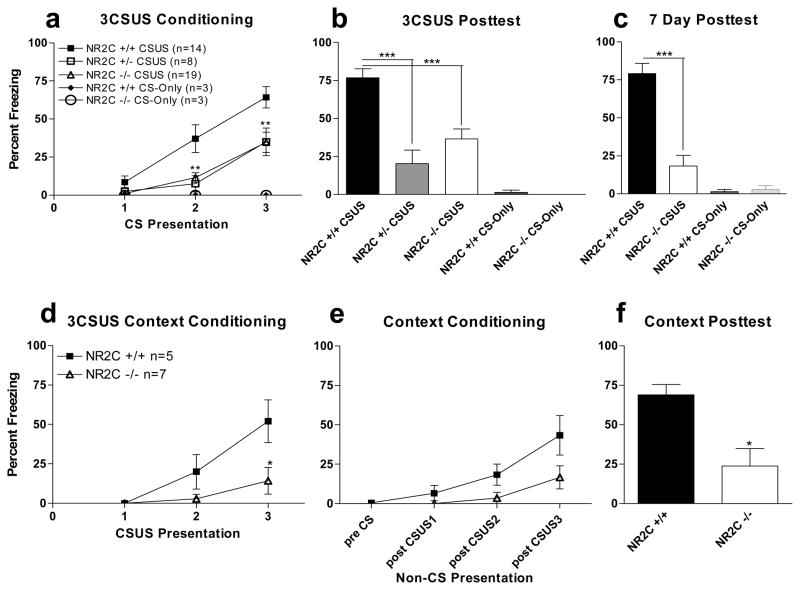
Fear conditioning was performed in NR2C +/+, NR2C +/− and NR2C −/− mice as described in the test 1 protocol that consisted of three pairings of a tone (CS) co-terminating with foot-shock (US) (Figure 1a). Freezing activity was recorded during the duration of tone delivery. Freezing activity was lower for NR2C +/− and NR2C −/− mice relative to NR2C +/+ control during the second and third tone delivery during conditioning. A mixed factor ANOVA revealed a significant difference in genotype [F(4,84)=8.3, P<0.0001], CS-US presentation [F(2,84)=13.3, P<0.0001] and interaction (CS-US pairing x genotype) [F(8,84)=2.9, P=0.0063]. Twenty-four hours after conditioning the CS-elicited fear was assessed in a novel environment. Freezing activity in response to the CS was significantly lower in NR2C +/− and NR2C −/− relative to NR2C +/+ mice (Figure 1b, one-way ANOVA, P<0.0001, F=14.08). Further analysis using Tukey/Kramer post-hoc test revealed significant difference in NR2C +/− [P<0.001] and NR2C −/− [P<0.001] versus NR2C +/+. No significant difference was observed between NR2C +/− and NR2C −/−. To address potential deficit in the time required for consolidation of memory in NR2C −/− mice we tested the fear response after seven days. Similar to 24 hours after fear conditioning a significantly lower level of freezing was observed in NR2C −/− mice compared to NR2C +/+ 7 days after conditioning (Figure 1c, one-way ANOVA, P<0.0001, F=33.0).
Figure 1. NR2C +/− and NR2C −/− mice exhibit deficit in cued and contextual fear acquisition.
(a) NR2C +/− and NR2C −/− mice exhibit deficit in fear acquisition compared to NR2C +/+ mice (two-way ANOVA [F(4,84)=8.3, P<0.0001]). (b) 24 hours after conditioning NR2C +/− and NR2C −/− mice displayed significantly lower freezing compared to NR2C +/+ (one-way ANOVA [F=13.8, P<0.0001]). (c) 7 days after conditioning NR2C −/− show significantly lower CS-dependent freezing compared to NR2C +/+ (one-way ANOVA [F=33.0, P<0.0001]). NR2C +/+ and NR2C −/− mice not exposed to foot-shock (a–b) display no difference in response to CS presentation. (d) NR2C −/− show deficit in freezing response to the CS at the 3rd presentation of the CS-US pairing. (e) No significant difference is observed in freezing while in the conditioning context during fear conditioning. (f) 24 hours after fear conditioning NR2C −/− mice exhibit deficit in fear response versus +/+ when placed back into the conditioning context (unpaired t-test, p=0.01, F=4.0). * and *** represent P< 0.05 and 0.001 respectively.
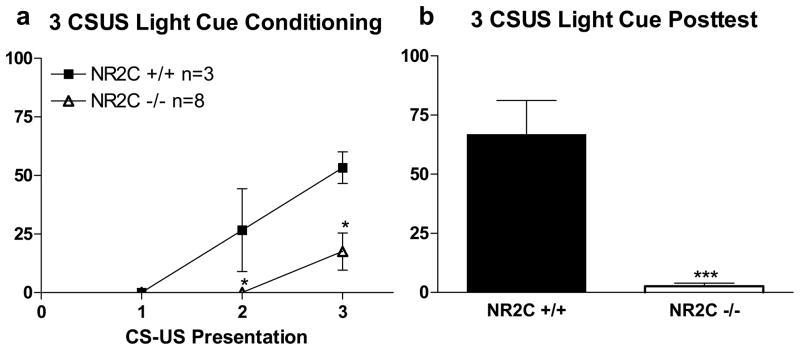
We further tested whether contextual fear conditioning was also abnormal in NR2C −/− mice. Conditioning protocol (test 2) was conducted and freezing activity during inter-trial interval was recorded (Figure 1e). Although not statistically significant a lower within-session contextual fear was observed in NR2C −/− compared to NR2C +/+ mice. Twenty-four hours after conditioning when animals were placed back into the conditioning environment, a significantly lower freezing was observed in NR2C −/− mice in comparison to NR2C +/+ mice (Figure 1f, unpaired t-test, P=0.01, F=3.9). To determine if the deficits observed in fear conditioning and testing were due to the type of CS used (tone) we changed the CS from a tone to a light cue (test 3, Figure 2). Again, significant deficit in fear acquisition in NR2C −/− versus NR2C +/+ during conditioning (Figure 2a, two-way ANOVA; genotype [F(1,18)=11.4, P=0.0082], CS-US presentation [F(2,18)=14.6, P=0.0002] and interaction [F(2,18)=4.0, P=0.0375]) and in CS-testing 24 hours after conditioning (Figure 2b, unpaired t-test [p<0.0001, F=42.8]).
Figure 2. NR2C −/− mice exhibit deficit in fear acquisition when the CS is a Light Cue.
(a) Significant deficit in fear acquisition is observed in NR2C −/− mice during the 2nd and 3rd pairing of the CS and US when the CS is a light cue (two-way ANOVA [F(1,18)=11.4, P=0.008]). (b) 24 hours after fear conditioning with a light cue the freezing response to the CS is significantly reduced in NR2C −/− mice versus +/+ (unpaired t-test, p<0.0001, F=42.9). Data presented as mean ± SEM.
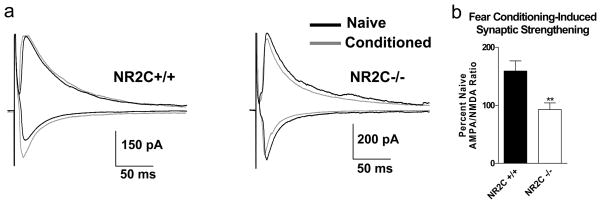
Fear conditioning leads to facilitation of thalamo-amygdala synapses (Radley et al., 2007; Rumpel et al., 2005; LeDoux, 2000; Maren, 2001). Thus, we examined fear conditioning-induced change in AMPA/NMDA ratio at thalamo-amygdala synapses. Similar to previous studies we found that fear conditioning lead to an increase in AMPA/NMDA ratio in NR2C +/+ mice (159.1±17.4% of naïve, n=4, unpaired t-test, P=0.002, F=2.9). However, no significant increase in AMPA/NMDA ratio was observed in NR2C −/− mice (92.8±11.4% of naïve, n=7, unpaired t-test, P=0.9, F=3.8). Thus conditioning-induced % increase in AMPA/NMDA ratio was significantly different between NR2C +/+ and NR2C −/− mice (figure 1d–e; P=0.008, F=1.030, unpaired t-test).
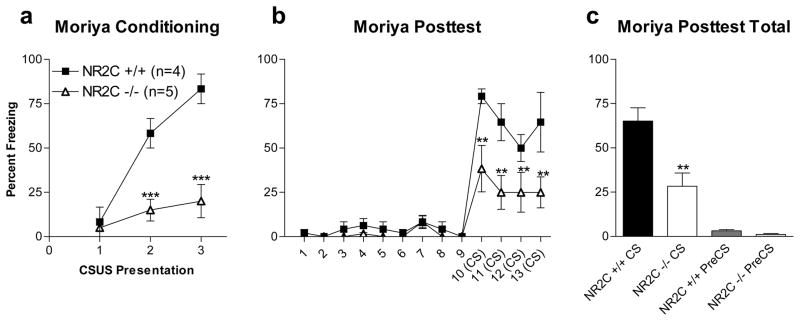
A previous study found cued fear conditioning to be normal in a NR2C−/− mice (Moriya et al., 2000). It should be noted that the mice used in this study were generated by a different group (Karavanova et al., 2007) and factors such as mice strain and method of generation of knockout may lead to these contrasting results. Nonetheless to eliminate any difference in experimental design that may account for these contrasting results we replicated the protocol used in Moriya et al. (test 4). Replicating the Moriya protocol produced results much like those in test 1 where the amount of freezing in response to the CS was significantly lower in NR2C −/− compared to NR2C +/+ control (Figure 4a–c, two-way ANOVA; genotype [F(1,84)=12.7, P=0.009], time [F(12,84)=26.0, P<0.0001] and interaction (genotype x time) [F(12,84)=3.9, P=0.0001]. No difference in freezing was observed between groups prior to CS-exposure.
Figure 4. Deficit in fear acquisition in NR2C −/− mice is independent of the light-dark cycle.
(a) NR2C −/− display significant difference from NR2C +/+ in freezing response to the CS during fear conditioning at the 2nd and 3rd presentation of the CS-US pairing during the dark phase of the light-dark cycle (two-way ANOVA [F(1,12)=41.9, P=0.0006]). (b–c) 24 hours after conditioning NR2C −/− display significant difference from NR2C +/+ in freezing response to the CS (two-way ANOVA [F(1,84)=12.7, P=0.009]). Data are presented as mean ± SEM. ** and *** represent P<0.01 and 0.001 respectively.
Fear Extinction is Intact in NR2C −/− Mice
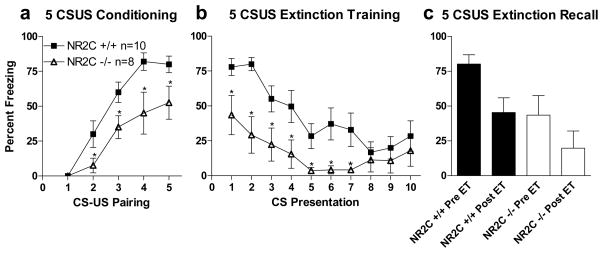
NMDA receptors have also been shown to be necessary for consolidation of fear extinction in both the amygdala (Sotres-Bayon, Bush, & LeDoux, 2007) and the ventromedial prefrontal cortex (Burgos-Robles, Gonzalez, Santini, & Quirk, 2007). Additionally D-cycloserine, a NMDA receptor glycine site agonist, that is 2-fold more efficacious than glycine at NR1/NR2C receptors (Sheinin, Shavit, & Benveniste, 2001; Dravid et al., 2010), has been shown to facilitate fear extinction (Ledgerwood, Richardson, & Cranney, 2003; Walker, Ressler, Lu, & Davis, 2002; Ressler et al., 2004). Thus we tested whether fear extinction was abnormal in NR2C −/− mice (test 5). Since fear acquisition was lower in NR2C −/− mice we used a more robust 5 CS-US pairing protocol inasmuch as this may allow us to increase % freezing in NR2C −/− mice to levels sufficient to perform extinction training. Freezing was monitored within-session during both fear conditioning and extinction training. In agreement with previous experiments showing lower fear acquisition in the 5 CS-US pairing protocol, NR2C +/+ and NR2C −/− mice (Figure 5a–c) showed significant difference in both sessions; within-session conditioning (two-way ANOVA; genotype [F(1,64)=9.7, P=0.007], number of CS-US pairings [F(4,64)=34.9, P<0.0001]) and within-session extinction (two-way ANOVA; genotype [F(1,144)=6.8, P=0.01] and CS-presentation [F(9,144)=11.8, P<0.0001] but no significant difference in interaction [F(9,144)=2.1, P=0.2]). In terms of fear extinction these results further indicate that NR2C −/− mice displayed normal fear extinction since a reduction in freezing with multiple CS presentations was observed. Moreover the in-session extinction was not different between NR2C −/− and NR2C +/+ mice. Twenty-four hours after extinction training using two-way repeated measures ANOVA analyzing extinction recall versus the percent freezing prior to extinction training (1st CS-presentation of extinction training) shows significant effect of genotype [F(1,11)=5.7, P=0.04] and time [F(1,11)=6.3, P=0.03] but not in interaction (genotype x time) [F(1,11)=0.1, P=0.8]. This suggests that the retention of fear extinction is also normal in NR2C −/− mice. Thus both within-session extinction and retention of extinction appears to be unaffected in NR2C−/− mice.
Figure 5. NR2C−/− mice exhibit deficit in fear acquisition when the number of CS-US pairing is increased and display within-session extinction.
(a) Increasing the number of CS-US pairings to 5 shows significant difference between NR2C +/+ and −/− mice at the 2nd –5th pairing of the CS and US (two-way ANOVA [F(1,64)=9.7, P=0.007]). (b) Extinction training shows significant difference between NR2C +/+ and NR2C −/− (two-way ANOVA [F(1,135)=6.8, P=0.02]). (c) Extinction recall versus percent freezing prior to extinction training shows that fear extinction is intact in NR2C deficient mice. Extinction recall compared to the amount of freezing during the first presentation of the CS in extinction training using two-way repeated measures ANOVA show significant effect of genotype [F(1,11)=5.7, P=0.4] and time point (pre- versus post-extinction training) [F(1,11)=6.3, P=0.3] but not interaction (genotype x time) [F(1,11)=0.1, P=0.8].
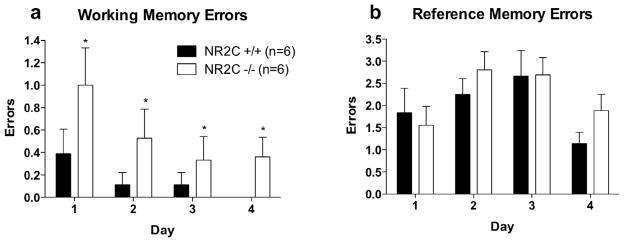
NR2C Knockout Mice Exhibit Deficit in Working Memory But Not in Reference Memory or Anxiety-like Behavior
Previous studies on NR2C knockout have mainly focused on the behaviors related to cerebellum since NR2C subunit is predominantly expressed by cerebellar granule neurons (Farrant et al., 1994; Karavanova et al., 2007). However recent knock-in mice with nβ-galactosidase inserted into the translation initiation site of the NR2C gene has revealed novel expression patterns of NR2C subunit in many regions of the forebrain (Karavanova et al., 2007). We therefore tested whether NR2C deletion may lead to other deficits in learning. Assessment of working and reference memory (Figure 6a–b) using 8-arm radial maze revealed a significantly higher working memory errors in NR2C −/− mice compared to NR2C +/+ mice (Figure 6a). Using repeated measures ANOVA and Bonferroni post-hoc test, significant difference was observed in working memory for genotype [F(1,30)=5.6, P=0.04] and days of testing [F(3,30)=3.1, P=0.04] but not for interaction. In contrast, no significant difference was observed in reference memory between NR2C +/+ and NR2C −/− mice (Figure 6b, repeated measures ANOVA, [F(1,30)=0.5, P=0.49].
Figure 6. NR2C −/− mice exhibit deficit in working memory versus +/+ but not in reference memory.
(a) Analysis of working memory errors committed during the four days of testing in the 8-arm radial maze shows significant difference between NR2C −/− and +/+ mice (two-way repeated measures ANOVA [F(1,30)=5.6, P=0.04]). (b) Observation of reference memory errors shows no significant difference between NR2C +/+ and −/− (two-way repeated measures ANOVA [F(1,30)=0.5, P=0.5]).
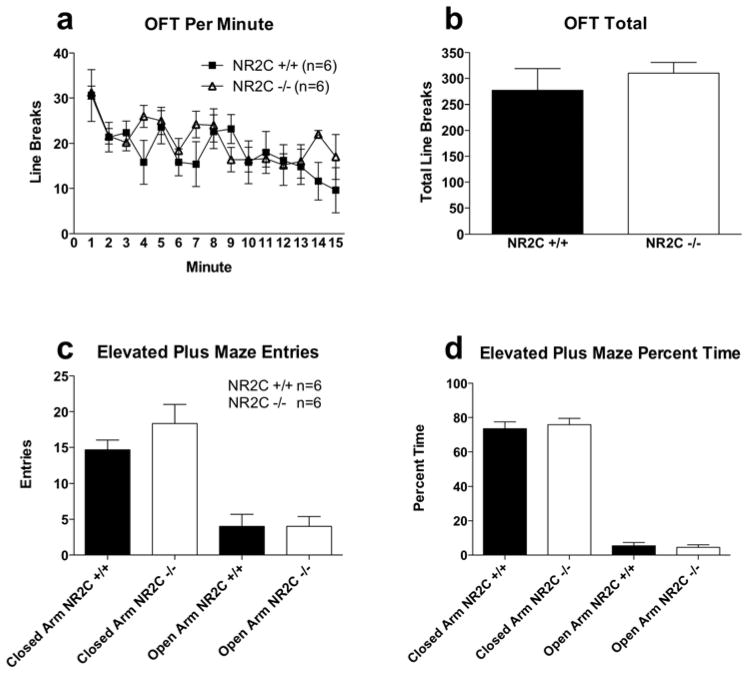
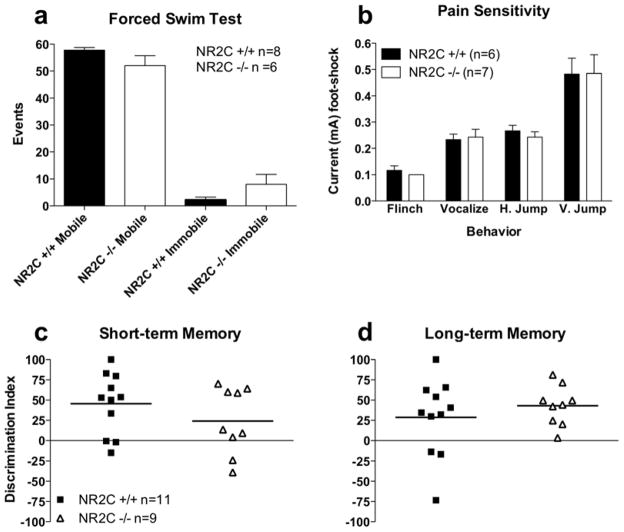
Due to the high level of expression of NR2C subunit in the adult cerebellum one might expect deletion of NR2C may result in locomotor deficits. However, consistent with previous reports (Kadotani et al., 1996; Ikeda et al., 1995) we observed no deficiency in spontaneous locomotor activity or basal anxiety in NR2C −/− mice (Figure 7). Locomotor activity measured in the open field test was not significantly different between NR2C +/+ and NR2C −/− mice in activity per minute (Figure 7a, two-way ANOVA; genotype [F(1,112)=0.5, P=0.5]) or total activity during the test (Figure 7b, unpaired t-test [P=0.5, F=4.1]). Basal anxiety level as assessed with the elevated plus maze showed no difference between groups in the number of entries into open arms (Figure 7c, unpaired t-test [P=1.0, F=1.4]) or the percent time spent in open arms (Figure 7d, unpaired t-test [P=0.7, F=1.7]). Additionally, we found no deficit in pain sensitivity (Figure 8b, two-way ANOVA; genotype [F(1,33)=0.08, P=0.8]), forced-swim induced immobility (Figure 8a, unpaired t-test [P=0.3, F=6.0]) or short-term (Figure 8c, unpaired t-test [P=0.2, F=1.1]) and long-term (Figure 8d, unpaired t-test [P=0.4, F=3.7]) memory assessed by novel object recognition test in the NR2C −/− mice.
Figure 7. NR2C +/+ and −/− mice exhibit similar performance in the open field test and elevated plus maze.
Activity of NR2C +/+ and −/− reveals no significant difference (a) per minute in the open field test (two-way ANOVA [F(1,112)=0.5, P=0.5]) or (b) in cumulative activity during 15 minute session (unpaired t-test, p=0.5, F=4.1). Elevated plus maze behavior shows no difference between NR2C +/+ and −/− the (c) number of entries (unpaired t-test, p=0.7, F=1.7) and (d) percent time (unpaired t-test, p=0.7, F=1.7) in open arms. Data presented as mean ± SEM.
Figure 8. NR2C +/+ and −/− mice display no difference in forced swim test, pain sensitivity and short- and long-term novel object recognition.
Age matched male NR2C +/+ and −/− mice exhibit no difference in (a) forced swim immobility (unpaired t-test, p=0.3, F=6.0), (b) pain sensitivity (two-way ANOVA [F(1,33)=0.08, P=0.8]) and discrimination index (DI) in (c) short-term (unpaired t-test, p=0.2, F=1.1) and (d) long-term (unpaired t-test, p=0.4, F=3.7) novel object recognition. Data presented in a–b as mean ± SEM, and in c–d as DI and mean.
Discussion
Our results indicate that NR2C-containing receptors play an important role in the acquisition of conditioned fear and working memory. Moreover, the deficits in fear acquisition in NR2C −/− mice were independent of the type of conditioned stimulus (tone versus light), the number of CS-US pairings, conditioning in the light or dark phase and the intensity of the US (data not shown). Acquisition of contextual conditioning was also significantly lower in NR2C −/− mice. In CS-only presentations we detected no difference between NR2C +/+ and NR2C −/−, indicating that presentation of the CS alone was not an aversive event for either group. Additionally, we observed no difference between NR2C +/+ and NR2C −/− in the novel/testing environment (chamber B) prior to CS presentation on testing day, indicating difference in freezing behavior was not due to difference in baseline generalized fear expression associated with performing the experiment. No significant difference was observed between the NR2C +/+ and NR2C −/− mice in spontaneous locomotor activity and basal anxiety levels as assessed with the open field test and elevated plus maze respectively, as well as pain sensitivity. These results indicate that the deficit in the acquisition of fear was not due to motor deficits or due to differences in anxiety levels or pain sensitivity. Additionally, NR2C knockout mice exhibit no significant differences compared to their wildtype counterpart in novel object recognition and reference memory tests indicating general learning is not compromised in NR2C knockout mice. Based on previous findings that consolidation of conditioned fear is accompanied with synaptic strengthening at thalamo-amygdala synapses (Rumpel, LeDoux, Zanod, & Malinow, 2005; LeDoux, 2000; Maren, 2001), we predicted that synaptic facilitation would be absent in NR2C knockout mice. Indeed the AMPA/NMDA ratio, an indicator of synaptic plasticity (Perkel & Nicoll, 1993; Nicoll & Malenka, 1999; Pape & Paré 2010; Rumpel et al., 2005; Kessels & Malinow, 2009; Lin, Mao, Su, & Gean, 2010), increased 24 hours after conditioning in wildtype but not in NR2C knockout mice (Figure 3b). These results are in agreement with the deficit in fear acquisition and further eliminate a role of general impairment of motor function in NR2C knockout.
Figure 3. Evoked thalamo-amygdala whole cell recordings show difference in fear-conditioning induced synaptic strengthening between NR2C +/+ and −/− mice.
(a) Representative traces of evoked EPSC recordings from naïve and conditioned NR2C +/+ and NR2C −/− mice. EPSCs were recorded at −70 mV and +40 mV holding potential for assessing AMPA and NMDA receptor component respectively (b) Percent Naïve AMPA/NMDA ratio of conditioned NR2C +/+ and NR2C−/− mice. Fear conditioning-induced facilitation of thalamic to lateral amygdala synapse increased AMPA/NMDA ratio in NR2C +/+ but not in NR2C −/− mice (unpaired t-test, p=0.008, F=1.0). Data are expressed as mean ± SEM. ** represents p<0.01
Unlike NR2A- and NR2B-containing receptors, the NR2C subunit has a more localized expression pattern in the rodent brain. NR2C-containing receptors are primarily expressed in granule neurons of the cerebellum (Karavanova et al., 2007). Although the cerebellum is involved in the acquisition of conditioned eye-blink (Fanselow & Poulos 2005), and in the regulation of certain autonomic and motor responses associated with fear (Sacchetti, Scelfo, & Strata, 2009), it has not been implicated in the acquisition of conditioned fear. The acquisition of fear primarily occurs through integration of thalamic and cortical excitatory glutamatergic inputs to the basolateral amygdala (LeDoux et al., 1988; Goosens & Maren, 2004; LeDoux, 2000) with afferents from the thalamus to the amygdala being the site of plasticity during fear conditioning (Radley et al., 2007; Rumpel et al., 2005; LeDoux, 2000; Maren, 2001). We did not observe strong nβ-gal staining in the amygdala (Supplemental Figure 1) and what was observed did not co-localize with the neuronal marker NeuN, however there is evidence suggesting possible expression of NR2C-containing receptors in LA neurons (Weisskopf & LeDoux, 1999; Lin, Bovetto, Carver, & Giordano, 1996). In contrast, NR2C subunit is abundant in the thalamus including mediodorsal, posterior, reticular and ventral nuclei (Monyer et al., 1994; Karavanova et al., 2007; Supplemental Figure 1). Thus the NR2C-containing receptors in the thalamus may be required for fear acquisition. Indeed thalamus forms a site of plasticity during fear conditioning. Auditory fear conditioning requires synthesis of mRNA and protein in the thalamus for fear memory formation (Apergis-Schoute, Debiec, Doyere, LeDoux, & Schafe, 2005; Parsons, Riedner, Gafford, & Helmstetter, 2006). Moreover, in addition to the primary role of hippocampus in contextual conditioning (Kim & Fanselow, 1992; Philips & LeDoux, 1992; Maren, 2001), a role of mediodorsal thalamic nuclei has also been identified (Li, Inoue, Nakagawa, & Koyama, 2004). Thus, based on the expression pattern and deficit in both contextual and cued fear conditioning in NR2C-knockout, we hypothesize that NR2C-containing receptors in the thalamus may be crucial for the associative learning involved in fear conditioning.
Our results also indicate that NR2C-containing receptors play a role in working memory. Working memory as assessed with the 8-arm radial maze was significantly impaired in NR2C knockout mice. Although prefrontal cortex is primarily involved in working memory processing in both primate (Friedman & Goldman-Rakic, 1994; Castner, Goldman-Rakic, & Williams, 2004) and rodent models (Dunnett, Wareham, & Torres, 1990; Dalley, Cardinal, & Robbins, 2004), work by Floresco et al. (1999) also points to an important role of the medial dorsal nuclei of the thalamus in the performance of working memory. As mentioned previously, NR2C expression is high in the thalamus including mediodorsal nuclei (Karavanova et al., 2007; Supplemental Figure 1). Thus it is possible that the deficit in working memory in NR2C-knockout may be due to lack of NR2C-containing receptors in thalamic and other cortical regions associated with short-term memory execution deficit.
The findings in this study identify a heretofore unknown role of NR2C-containing receptors in fear acquisition and working memory. The significance of this finding applies not only to our understanding of fear acquisition and working memory individually, but may also point to a potential role of NR2C receptors in the negative symptoms and cognitive deficits observed in schizophrenia. Deficit in fear acquisition has been suggested to represent a negative symptom referred to as emotional blunting in schizophrenia (Mandal, Pandey, & Prasad, 1998; Pietersen et al., 2007). Moreover, cognitive deficits observed in schizophrenia, such as deficit in working memory (Gray & Roth 2007; Paradiso et al., 2003) have been linked to NMDA receptor hypofunction (Goff & Coyle 2001; Javitt, 2001). Interestingly, a study in humans has identified lower expression of the NR2C subunit in the thalamus of schizophrenic patients (Ibrahim et al., 2000). Additionally, Neuregulin-1 (NRG1), a strong candidate gene in schizophrenia (Stefansson et al., 2002; Harrison & Weinberger, 2005), is intimately involved in regulation and expression of NR2C (Ozaki, Sasner, Yano, Lu, & Buonanno, 1997; Schmitt et al., 2010). Finally, a recent study has proposed that NR2C-containing receptors play an important role in delta frequency bursting originating from the nucleus reticularis of the thalamus (Zhang et al., 2009), an experimental model correlated with the awake-state delta oscillations observed in schizophrenic patients. Thus, results from the current study suggest that NR2C-containing receptors may be a promising therapeutic target for mental disorders including PTSD and schizophrenia.
Supplementary Material
Acknowledgments
This work was supported by Health Future Foundation (SMD), National Science Foundation/Nebraska EPSCoR (SMD) and National Institute of Mental Health MH085327 (SMD). A. Buonanno received support from the Eunice Shriver Kennedy National Institute of Child Health and Human Development Intramural Research Program.
The project was also supported by G20RR024001 from National Center for Research Resources. The content is solely the responsibility of authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- Apergis-Schoute AM, Debiec J, Doyere V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system. J Neurosci. 2005;25(24):5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage- gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22 (12):5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, Fleidervish IA, Sprengel R, Gutnick MJ. NMDA receptors in layer 4 spiny stellate cells of the mouse barrel cortex contain the NR2C subunit. J Neurosci. 2006;26 (2):708–715. doi: 10.1523/JNEUROSCI.4409-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of Fear Extinction Requires NMDA Receptor-Dependent Bursting in the Ventromedial Prefrontal Cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cathala L, Misra C, Cull-Candy S. Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci. 2000;20(16):5899–5905. doi: 10.1523/JNEUROSCI.20-16-05899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS, Williams GV. Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology. 2004;174:111–125. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Cur Opinion Neurobio. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis S. The Glutamate Receptor Ion Channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal cognitive and executive function in rodents: neural and neurochemical substrates. Neurosci and Behav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, Vellano K, Snyder JP, Traynelis SF. Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci. 2010;30(7):2741–2754. doi: 10.1523/JNEUROSCI.5390-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J Physiol. 2008;586(18):4425–4439. doi: 10.1113/jphysiol.2008.158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Wareham AT, Torres EM. Cholinergic blockade in prefrontal cortex and hippocampus disrupts short-term memory in rats. Neuroreport. 1990;1(1):61–64. doi: 10.1097/00001756-199009000-00017. [DOI] [PubMed] [Google Scholar]
- Ebralidze AK, Rossi DJ, Tonegawa S, Slater NT. Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene. J Neurosci. 1996;16(16):5014–5025. doi: 10.1523/JNEUROSCI.16-16-05014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Chen PE, Wyllie DJ, Traynelis S. Glutamate receptor gating. Crit Rev Neurobiol. 2004;16:187–224. doi: 10.1615/critrevneurobiol.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–34. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994;368:335–339. doi: 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- Fendt M. Injections of the NMDA Receptor Antagonist Aminophosphonopentanoic Acid in the Lateral Nucleus of the Amygdala Block the Expression of Fear-Potentiated Startle and Freezing. J Neurosci. 2001;21(11):4111–4115. doi: 10.1523/JNEUROSCI.21-11-04111.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Braaksma DN, Phillips AG. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci. 1999;19 (24):11061–11071. doi: 10.1523/JNEUROSCI.19-24-11061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HR, Goldman-Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci. 1994;14 (5):2775–2788. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Gill M, Tronson NC, Guedea AL, Guzman YF, Huh KH, Corcoran KA, Swanson GT, Radulovic J. Hippocampal NMDA Receptor Subunits Differentially Regulate Memory Formation and Neuronal Signal Propagation. Hippocampus. 2010 doi: 10.1002/hipo.20705. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature. 1997;388:471–474. doi: 10.1038/41325. [DOI] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psych. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. NMDA receptors are essential for the acquisition, but not expression, of conditioned fear and associative spike firing in the lateral amygdala. E J Neurosci. 2004;20:537–548. doi: 10.1111/j.1460-9568.2004.03513.x. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33(5):1100–1119. doi: 10.1093/schbul/sbm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelman GE, Contreras PC, O’Donohue TL. Selective memory impairment by phencyclidine in rats. E J Pharm. 1987;140:69–73. doi: 10.1016/0014-2999(87)90635-2. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression and neuropathology: on the matter of their convergence. Mol Psych. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Heale V, Harley C. MK-801 and AP5 impair acquisition, but not retention, of the Morris milk maze. Pharm Biochem and Behav. 1990;36:145–149. doi: 10.1016/0091-3057(90)90140-d. [DOI] [PubMed] [Google Scholar]
- Honey RA, Turner DC, Honey GD, Sharar SR, Kumaran D, Pomarol-Clotet E, McKenna P, Sahakian BJ, Robbins TW, Fletcher PC. Subdissociative dose ketamine produces a deficit in manipulation but not maintenance of the contents of working memory. Neuropsychopharm. 2003;28(11):2037–2044. doi: 10.1038/sj.npp.1300272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Kandel ER. Postsynaptic Induction and PKA-dependent expression of LTP in the Lateral Amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Ibrahim HM, Hogg AJ, Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psych. 2000;157 (11):1811–1823. doi: 10.1176/appi.ajp.157.11.1811. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Araki K, Takayama C, Inoue Y, Yagi T, Aizawa S, Mishina M. Spontaneous activity of mice defective in the ε4 subunit of the NMDA receptor channel. Mol Brain Res. 1995;33:61–71. doi: 10.1016/0169-328x(95)00107-4. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Management of negative symptoms of schizophrenia. Cur Psych Rep. 2001;3:413–417. doi: 10.1007/s11920-996-0036-9. [DOI] [PubMed] [Google Scholar]
- Karavanova I, Vasudevan K, Cheng J, Buonanno A. Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C-subunit-β-galactosidase knock-in mice. Mol Cell Neurosci. 2007;34:468–480. doi: 10.1016/j.mcn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadotani H, Hirano T, Masugi M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Motor Discoordination Results from Combined Gene Disruption of the NMDA Receptor NR2A and NR2C Subunits, But Not from Single Disruption of the NR2A or NR2C Subunit. J Neurosci. 1996;16:7859–7869. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8(7):2517–29. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117(2):341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Li XB, Inoue T, Nakagawa S, Koyama T. Effect of mediodorsal thalamic nucleus lesion on contextual fear conditioning in rats. Brain Res. 2004;1008:261–272. doi: 10.1016/j.brainres.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. Alterations of excitatory transmission in the lateral amygdala during expression and extinction of fear memory. Int J of Neuropsychopharm. 2010;13:335–345. doi: 10.1017/S1461145709990678. [DOI] [PubMed] [Google Scholar]
- Lin JY, Bovetto S, Carver JM, Giordano T. Cloning of the human NMDA receptor NR2C subunit and its expression in the central nervous system and periphery. Mol Brain Res. 1996;43:57–64. doi: 10.1016/s0169-328x(96)00146-5. [DOI] [PubMed] [Google Scholar]
- Mandal MK, Pandey R, Prasad AB. Facial expressions of emotions and schizophrenia: a review. Schizophr Bull. 1998;24(1):399–412. doi: 10.1093/oxfordjournals.schbul.a033335. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic Plasticity in the Basolateral Amygdala Induced by Hippocampal Formation Stimulation in vivo. J Neurosci. 1995;15(11):7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage dependent block by Mg2+ of NMDA responses in spinal cord neurons. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345(6277):716–8. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA Receptors: Molecular and Functional Distinction of Subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moriya T, Kouzu Y, Shibata S, Kadotani H, Fukunaga K, Miyamoto E, Yoshioka T. Close linkage between calcium/calmodulin kinase II α/β and NMDA-2A receptors in the lateral amygdala and significance for retrieval of auditory fear conditioning. Eur J Neurosci. 2000;12:3307–3314. doi: 10.1046/j.1460-9568.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann N Y Acad Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-β induces expression of an NMDA-receptor subunit. Nature. 1997;390:691–694. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso S, Andreasen NC, Crespo-Facorro B, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psych. 2003;160(10):1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Riedner BA, Gafford GM, Helmstetter FJ. The formation of auditory fear memory requires synthesis of protein and mRNA in the auditory thalamus. Neurosci. 2006;141(3):1163–1170. doi: 10.1016/j.neuroscience.2006.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel DJ, Nicoll RA. Evidence for all-or-none regulation of neurotransmitter release: implications for long-term potentiation. J Physiol. 1993;471:481–500. doi: 10.1113/jphysiol.1993.sp019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pietersen CY, Bosker FJ, Doorduin J, Jongsma ME, Postema F, Haas JV, Johnson MP, Koch T, Vladusich T, den Boer JA. An animal model of emotional blunting in schizophrenia. PLoS One. 2007;2(12):e1360. doi: 10.1371/journal.pone.0001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Farb C, He Y, Janssen WGM, Rodrigues SM, Johnson LR, Hof PR, LeDoux JE, Morrison JH. The distribution of NMDA and AMPA receptor subunits at thalamo-amygdala dendritic spines. Brain Res. 2007;1134(1):87–94. doi: 10.1016/j.brainres.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy; use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psych. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci. 2006;27(3):141–148. doi: 10.1016/j.tips.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux JE, Zanod A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Scelfo B, Strata P. Cerebellum and emotional behavior. Neurosci. 2009;162(3):756–62. doi: 10.1016/j.neuroscience.2009.01.064. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, Mishina M. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor ε1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Koschel J, Zink M, Bauer M, Sommer C, Frank J, Treutlein J, Schulze T, Schneider-Axmann T, Parlapani E, Rietschel M, Falkai P, Henn FA. Gene expression of NMDA receptor subunits in the cerebellum of elderly patients with schizophrenia. Eur Arch Psych Clin Neurosci. 2010;260:101–111. doi: 10.1007/s00406-009-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharm. 2001;41:151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DEA, LeDoux JE. Acquisition of Fear Extinction Requires Activation of NR2B-Containing NMDA Receptors in the Lateral Amygdala. Neuropsychopharm. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Doganci B, Jensen V, Hvalby O, Gongrich C, Taylor A, Barkus C, Sanderson DJ, Rawlins JNP, Seeburg PH, Bannerman DM, Monyer H. Contribution of Hippocampal and Extra-Hippocampal NR2B-Containing NMDA Receptors to Performance on Spatial Learning Tasks. Neuron. 2008;60:846–860. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Amygdala infusions of an NR2B-selective or an NR2A-preferring NMDA receptor antagonist differentially influence fear conditioning and expression in the fear-potentiated startle test. Learn Mem. 2008;15(2):67–74. doi: 10.1101/lm.798908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22(6):2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, LeDoux JE. Distinct populations of NMDA receptors at subcortical and cortical inputs to principle cells of the lateral amygdala. J Neurophysiol. 1999;81:930–934. doi: 10.1152/jn.1999.81.2.930. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B and NR2C subunit proteins. J Neurochem. 1997;68(2):469–78. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Liu F, Chen Q, Zhang CL, Zhuo M, Xiong ZQ, Li BM. Conditioning-strength dependent involvement of NMDA NR2B subtype receptor in the basolateral amygdala in acquisition of auditory fear memory. Neuropharm. 2008;55:238–246. doi: 10.1016/j.neuropharm.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Llinas RR, Lisman JE. Inhibition of NMDARs in the nucleus reticularis of the thalamus produces delta frequency bursting. Front Neural Circuits. 2009;3:20. doi: 10.3389/neuro.04.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JP, Constantine-Paton M. NR2A−/− Mice Lack Long-Term Potentiation But Retain NMDA Receptor and L-Type Ca2+ Channel-Dependent Long-Term Depression in the Juvenile Superior Colliculus. J Neurosci. 2007;27(50):13649–13654. doi: 10.1523/JNEUROSCI.3153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.