Abstract
Purpose
To assess the prevalence of defective homologous recombination (HR) based DNA repair in sporadic primary breast cancers, examine the clincopathological features that correlate of with defective HR and the relationship with neoadjuvant chemotherapy response.
Experimental Design
We examined a cohort of 68 patients with sporadic primary breast cancer who received neoadjuvant anthracylcine based chemotherapy, with core biopsies taken 24 hours after the first cycle of chemotherapy. We assessed RAD51 focus formation, a marker of HR competence, by immunofluorescence in post chemotherapy biopsies along with geminin as a marker of proliferative cells. We assessed the RAD51 score as the proportion of proliferative cells with RAD51 foci.
Results
A low RAD51 score was present in 26% of cases (15/57, 95% CI, 15-40%). Low RAD51 score correlated with high histological grade (p=0.031) and high baseline Ki67 (p=0.005). Low RAD51 score was more frequent in triple negative breast cancers compared to ER and/or HER2 positive breast cancer (67% vs 19% respectively, p=0.0036). Low RAD51 score was strongly predictive of pathological complete response to chemotherapy, with 33% low RAD51 score cancers achieving pathological complete response compared to 3% of other cancers (p=0.011).
Conclusions
Our results suggest that defective HR, as indicated by low RAD51 score, may be one of the factors that underlie sensitivity to anthracycline based chemotherapy. Defective HR is frequent in triple negative breast cancer, but is also present in a subset of other subtypes, identifying breast cancers that may benefit from therapies that target defective HR, such as PARP inhibitors.
Keywords: RAD51, Homologous recombination, Neoadjuvant chemotherapy, Breast cancer
Introduction
DNA double strand break repair by homologous recombination (HR) is required for the repair of DNA damage arising from many currently used DNA damaging chemotherapy agents (1), as well as DNA single strand breaks that are generated by inhibitors of Poly(ADP ribose) Polymerase 1 (PARP) (2). The tumour suppressor genes BRCA1 and BRCA2 encode proteins that are required for (HR) (3), and tumours arising in carriers of germline mutations in BRCA1/2 are defective in HR and consequently are highly sensitive to DNA damaging chemotherapy (4, 5) and PARP inhibitors (6). Increasing evidence suggests that a proportion of sporadic breast cancers may also have defects in HR (1). Sporadic basal-like breast cancers have low BRCA1 expression (7-9) and BRCA1 promoter methylation is found in approximately 10% of sporadic breast cancers, including non-basal tumours (7). Recently it has also been proposed that loss of PTEN expression may also lead to a defect in HR (3, 10, 11).
In this study we set out to develop a functional assay for HR with the purpose of assessing the prevalence of a functional defect in HR in sporadic breast cancer, and examine whether defective HR correlated with clinicopathological features and sensitivity to chemotherapy. The protein RAD51 is a DNA recombinase and key component of HR (12). In response to DNA double strand breaks RAD51 forms foci at the sites of damage that can be visualised by immunofluorescent microscopy (13). Cancer cell lines with a defect in HR, for example those with loss of BRCA1 or BRCA2, are unable to induce RAD51 foci after DNA damage (3). The inability to form RAD51 after damage represents a functional readout of a defect in HR.
Chemotherapy for primary breast cancer is frequently given neoadjuvantly (prior to surgery) to reduce tumour size with the aim of avoiding mastectomy (14, 15). Although the majority of patients receiving neoadjuvant therapy achieve a clinical response to chemotherapy, only a minority achieve a pathological complete response – absence of invasive tumour cells in the breast and axillary lymph nodes. Achieving a pathological complete response (pathCR) is a strong predictor of good outcome, with substantially improved outcomes in comparison with patients who do not enter a pathCR (16, 17)
In this study, we examined a cohort of patients with sporadic primary breast cancer who had tumour biopsy 24 hours after the first cycle of neoadjuvant anthracycline based chemotherapy (18, 19). RAD51 focus formation was assessed in the post chemotherapy biopsy to determine the prevalence of defective HR in sporadic primary breast cancer, investigate whether defective HR predicts for pathCR to chemotherapy, and identify clinicopathological features that correlate with defective HR.
Materials and Methods
Clinical material
Patients and tumour material have been described previously (18, 19). Briefly, we identified all patients who, between 1995-2002, received neoadjuvant chemotherapy for primary breast cancer at Royal Marsden Hospital and had a tumour biopsy 24 hours after the first cycle chemotherapy. All patients with formalin-fixed and paraffin embedded material available were included in this study. Pathological complete response (pathCR) to chemotherapy was defined as the absence of invasive tumour cells in the breast and axillary lymph nodes in the surgical resection specimen. Clinicopathological characteristics of patients included in the study are listed in Table 1. No patients were known to carry germline mutations in BRCA1 or BRCA2. Oestrogen receptor (ER), and Ki67 (assessed with MIB1) were assessed as reported previously (18, 19). To assign tumour subtype, progesterone receptor (PR) expression was assessed on ER negative/HER2 negative cancers (Dako clone PgR 636). A single ER negative/HER2 negative cancer expressed PR, and was assigned as ER positive/HER negative subtype, with the remainder being triple negative (12 cases). Patients gave informed consent for collection of biopsies, and research was approved by the Royal Marsden Hospital Research Ethics Committee.
Table 1.
Clinicopathological characteristics of patients and cancers included in study
| All patients | Patients assessable for RAD51 |
|
|---|---|---|
|
| ||
| n | 68 | 57 |
| Median Age | 47 (29-67) | 48 (29-67) |
| Pathology | ||
| IDC | 53 | 45 |
| ILC | 11 | 9 |
| Other | 2 | 1 |
| Median tumour size (range mm) | 51 (20-110) | 53 (21-110) |
| Grade | ||
| 1 | 1 | 0 |
| 2 | 29 | 25 |
| 3 | 32 | 29 |
| Axillary node positive | 54% (30/52) | 55% (25/45) |
| ER positive | 64% (41/64) | 63% (35/55) |
| HER2 positive | 19% (10/52) | 18% (9/49) |
| Menopausal status | ||
| Pre | 38 | 36 |
| Peri | 2 | 2 |
| Post | 16 | 14 |
| Chemotherapy | ||
| AC | 36 | 28 |
| FEC | 5 | 4 |
| ECisF | 14 | 14 |
| ECycloF | 5 | 4 |
| NE | 8 | 7 |
| Tamoxifen | 82% (48/58) | 79% (43/54) |
| Clinical response | ||
| CR | 12 | 10 |
| PR | 38 | 35 |
| SD | 12 | 7 |
| PD | 3 | 3 |
| path CR | 10% (6/60) | 10% (5/49) |
Eleven tumours had no proliferation in the post chemotherapy biopsy and were not assessable for RAD51. IDC – Invasive ductal carcinoma; ILC – Invasive lobular carcinoma. Chemotherapy – AC (Doxorubicin, Cyclophosphamide), FEC (Fluorouracil, Epirubicin, Cyclophosphamide), ECisF (Epirubicin, Cisplatin, infusional Fluorouracil), ECycloF (Epirubicin, Cyclophosphamide, infusional Fluorouracil), NE (Navelbine, Epirubuicin) (35, 36). Clinical response; CR – complete response; PR – partial response; SD – stable disease; PD – progressive disease.
Cell lines, materials and antibodies
CAPAN1 cells, and derived PIR2 PARP inhibitor resistant cell line, were described previously (20). CAL51 cells were obtained from ATCC and maintained in phenol red free DMEM or RPMI with 10% FBS (PAA gold) and 2mM L-glutamine (Sigma-Aldrich, Dorset, UK). Antibodies RAD51 (Clone 14B4, GeneTex), γH2AX (phospho-Histone H2AX Ser129 clone 20E3, Cell signaling), and geminin (10802-1-AP, ProteinTech Group, Chicago, IL).
Screening of RAD51 focus antibodies
CAPAN1 (BRCA2 mutant HR deficient) and derived PIR2 (20) (BRCA2 revertant HR competent) cell lines were irradiated in vitro with 10Gy, incubated for 6hrs post irradiation, pelleted and fixed in formalin overnight prior to standard paraffin embedding. RAD51 antibodies were screened by immunofluorescence and the best performing antibody was selected for further study (Supplementary table 1).
Xenograft irradiation
CAPAN1 and PIR cells (6×106 cells) were injected, admixed 1:1 with matrigel, into the lateral flanks of 18 Ncr-nude female mice and for the CAL51 cells 2×106 cells were injected into both flanks of 11 Ncr-nudes. When tumours were visible the mice were divided into 2 groups, one group was irradiated with 8 Gy IR, and the other not, the tumours were excised 6 hours later and formalin fixed.
RAD51 immunofluorescence assay
Immunofluorescence analysis was performed on 3μm sections of formalin fixed paraffin embedded tumour material (FFPE). Following antigen retrieval by microwaving at pH 9 (DAKO pH 9 buffer, Copenhagen, Denmark) for 18 minutes followed by 20 minutes cooling in buffer, sections were treated with Triton 0.2% for permeabilisation for 20min, washed in PBS, treated with 100μl of DNAse I (Roche) for 1h at 37°C and blocked with IFF (1% bovine serum albumin, 2% fetal bovine serum in PBS) for 30min at room temperature. Sections were stained with geminin antibody 1:400 in IFF for 1h at RT, washed with PBS, followed by anti-rabbit Alexa488 conjugate 1:1000 in IFF for 1h at RT, washed with PBS, fixed with 4% paraformaldehyde (PFA) solution for 15min, stained with RAD51 antibody 1:100 in IFF for 1h at RT, washed with PBS, followed by anti-mouse Alexa555 conjugate 1:1000 in IFF for 1h at RT, washed in PBS with DAPI (1:10000) for 15 mins, and sections were fixed again with 4% PFA. The protocol for γH2AX staining was similar, with primary antibody 1:200 in IFF for 1hr at room temperature.
Scoring RAD51 focus assay
Images were captured on a Leica TCS confocal microscope. We scored the nuclear foci staining as follows. Between 100 to 500 invasive tumour cells were counted at representative areas across the section. Cores with only DCIS were deemed not assessable. A cell was counted as being geminin positive with any nuclear staining. A cell was counted as being RAD51 positive if there was at least 1 distinct focus per nucleus. This threshold was selected after examining baseline, pre-chemotherapy, cores where >99.9% of cells lacked any RAD51 foci. Proliferative fraction in the section was taken as the number of geminin positive cells (S and G2 phase marker) divided by total number of cells. The raw RAD51 count was taken as the number of RAD51 positive cells divided by the total number of cells expressed as a percentage. The RAD51 score was assessed as the percentage of geminin positive cells that were also positive for RAD51. The γH2AX staining was performed on consecutive slide to that assessed for RAD51, with a cell considered positive with at least 1 foci.
Assessment of all immunofluorescence was performed blinded to clinicopathological and chemotherapy response data. The cut-off to define low RAD51 score was finalised by examining RAD51 and geminin before unblinding to clinicopathological and response data. A tumour was defined as being deficient in HR with a RAD51 score <10% (ie less than 10% of geminin positive cells had RAD51 foci).
Statistical analysis
All statistical analysis was two sided and performed with GraphPad Prism version 5.0.
Results
Establishment and validation of RAD51 focus formation assay
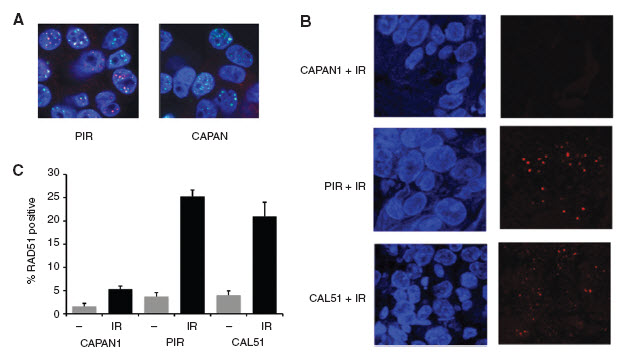
We set out to identify a RAD51 antibody for use in formalin-fixed paraffin-embedded (FFPE) tissues. We screened eight RAD51 antibodies and identified GeneTex Clone 14B4 to be the optimal antibody for use in FFPE material (Supplementary table 1 and Figure 1A). To validate the RAD51 antibody we generated xenografts with the CAPAN1 BRCA2 mutant cell line (HR deficient), the CAPAN1 derived ‘PIR’ (HR competent) cell line that carries a revertant mutation in BRCA2 (20), and the CAL51 breast cancer cell line (HR competent). Established xenografts were irradiated, and 6hrs post irradiation tumours fixed in formalin. FFPE sections were subject to RAD51 focus assay. Both PIR and CAL51 xenografts demonstrated a substantial induction of RAD51 foci that was not seen in CAPAN1 (Figure 1B).
Figure 1. Development of RAD51 focus assay.
A. HR competent PIR (Left) and HR incompetent BRCA2 mutant CAPAN1 cells (Right) were irradiated in vitro with 10Gy, and 6 hours post irradiation examined by immunofluorescent confocal microscopy. Red – RAD51, Green - γH2AX, Blue – DAPI nuclear stain.
B. and C. Xenografts of CAPAN1, PIR, or CAL51 breast cancer cells were irradiated with 6Gy, or not, and 8 hours post irradiation tumour excised and fixed in formalin. FFPE sections were examined by immunofluorescent confocal microscopy. Red – RAD51, Blue – DAPI nuclear stain. B. Representative images post irradiation. C. Quantification of percentage of tumour cells with RAD51 foci in sections from five xenografts per condition. Post irradiation raw RAD51 count CAPAN1 5.3%, PIR 25.3%, CAL51 30.0% (p<0.0001 CAL51 vs CAPAN1 and p<0.0001 PIR vs CAPAN1, Student's T test).
Patient and tumours included in the study
To examine the ability of the RAD51 focus assay to predict response to neoadjuvant chemotherapy and assess the prevalence of HR defects in primary breast cancer, we examined a series of 68 women who had received neoadjuvant chemotherapy for primary breast cancer and had core biopsies taken at 24 hours after the first cycle of anthracycline based chemotherapy. The clinicopathological characteristics are described in Table 1.
Assessment of RAD51 focus formation in baseline biopsies
We initially examined RAD51 focus formation in the baseline biopsies of 35 patients. RAD51 foci were observed in baseline biopsies in only two of the 35 cancers, and in these cancers only 2% of cells had RAD51 foci (Figure 2). Therefore, although low levels of RAD51 foci may form during the repair of endogenous DNA damage (21), these were below the level of sensitivity of this assay. This was advantageous, as it implied that assessment of RAD51 foci was only required in the post-chemotherapy biopsy, as RAD51 foci present in post chemotherapy biopsies would reflect induction of RAD51 foci by damage. We restricted all further assessment to biopsies taken 24hrs after the first cycle of neoadjuvant chemotherapy.
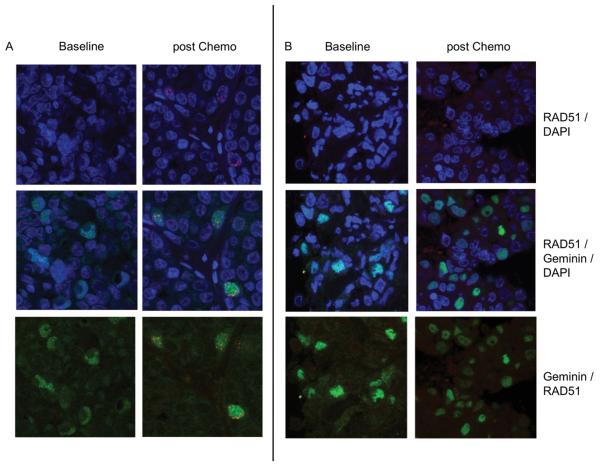
Figure 2. RAD51 and geminin immunofluorescence in core biopsies of primary breast cancer.
Confocal images of an HR competent and deficient primary breast cancers, with immunofluorescence staining of RAD51 (red) with DAPI counter stain (Blue) (Top panels), RAD51 and geminin (green) overlay with DAPI counter stain (Middle panels), and RAD51 - geminin overlay (bottom panels).
A. Images from baseline biopsy (left) and 24 hrs post chemotherapy (right) from a tumour demonstrating induction of RAD51 foci in geminin positive cells following chemotherapy.
B. Images from baseline biopsy (left) and 24 hrs post chemotherapy (right) from a tumour demonstrating no induction of RAD51 foci despite high geminin expression.
Assessment of RAD51 focus induction at 24 hours post chemotherapy
Both the expression of RAD51, and the ability to perform HR, are cell cycle regulated; HR is suppressed in G0/1 and M phases of the cell cycle, and RAD51 foci form only in S and G2 phases of the cell cycle (12). To control for differences in proliferation between tumours we co-stained each section for geminin; Geminin is expressed only in S and G2 phases of the cell cycle (22), and is involved in the inhibition of replication licensing (23). This correction was important as proliferation is decreased in many tumours at 24hrs post chemotherapy (18, 19), and without correction a tumour may be falsely attributed as HR deficient if proliferation is low or absent. Tumour sections from 11 women (16%, 11/68) taken 24 hours after chemotherapy did not score for geminin, and these tumours were excluded from further analysis (Supplementary figure 1). There was a significant correlation between geminin and Ki67 assessed on the same biopsy 24hrs after chemotherapy (ρ=0.68, 95% CI 0.49-0.81, p<0.0001 Spearman's Correlation Coefficient, Data not shown).
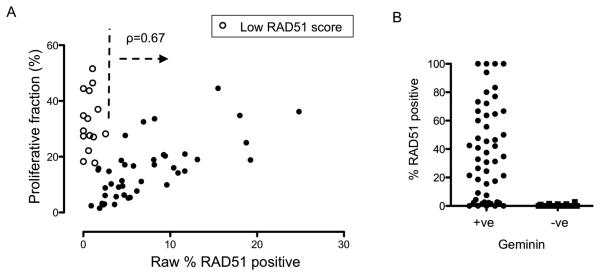
We initially examined the raw RAD51 count, the proportion of all tumour cells positive for RAD51 foci. The relationship between raw RAD51 count and proliferative fraction, assessed in the same section as the proportion of tumour cells geminin positive, was biphasic (Figure 3A). In general, raw RAD51 count and proliferative fraction were highly significantly positively correlated in tumours with >2% raw RAD51 count (ρ=0.67, 95% CI 0.45-0.82, p<0.0001 Spearman's correlation coefficient) (Figure 3A). However, for tumours with low raw RAD51 count (<2%) the relationship between raw RAD51 count and proliferation was lost (ρ=−0.2, P=NS), with these cancers having substantially raised proliferative fraction (Figure 3A). Although a post-hoc analysis, this data suggested that the majority of tumours were HR competent, with appropriate cell cycle regulation of HR. In contrast, a minority of tumours displayed potential evidence of deficient HR (Figure 3A).
Figure 3. Raw RAD51 count shows a biphasic relationship with proliferation in 24 hour post chemotherapy biopsies.
A. Plot of raw RAD51 counts (x axis, percentage of tumour cells that have RAD51 foci) against proliferative fraction assessed on same section (y axis, percentage of tumour cells with nuclear geminin expression) in core biopsies from 57 tumours taken at 24 hours post chemotherapy. In tumours with a raw RAD51 count >2% there is a positive correlation between RAD51 count and proliferation (ρ=0.67, 95% CI 0.45-0.82, p<0.0001, Spearman's correlation coefficient), but not in tumours with raw RAD51 count <2% (r=−0.21, p=NS). Open circles indicate tumours subsequently assessed as having a low RAD51 score (Figure 4).
B. Assessment of the relationship between geminin positive and RAD51 positive cells. For each tumour the percentage of geminin positive cells that had RAD51 foci, and the percentage geminin negative cells that had RAD51 foci was assessed. Displayed are the results from all 57 tumours, with the percentage of geminin positive cancer cells that have RAD51 foci (median 40%, left), versus the percentage of geminin negative cancer cells that have RAD51 foci (median 0%, right). RAD51 foci are almost exclusively expressed in geminin positive cells (p<0.0001 Mann-Whitney U Test).
We investigated whether there was a pharmacokinetic explanation for lack of RAD51 foci, ie that biopsied tumour had not been exposed to sufficient concentrations of chemotherapy to induce DNA damage. To do this we measured DNA damage by examining for induction of phosphorylated H2AX (γH2AX), a marker of DNA double strand breaks (24), in 3 tumours with RAD51 foci and in 4 tumours without RAD51 foci post chemotherapy (Supplementary figures 2 and 3). No difference in the induction of γH2AX was found between tumours with and without RAD51 foci (median increase in γH2AX 18% vs 38% respectively, p=0.09 Student's T test), with γH2AX induction actually being numerically greater in tumours without RAD51 foci (Supplementary figure 2). This strongly suggested that the observed differences reflected tumour biology, as opposed to poor drug exposure.
Cell cycle adjusted RAD51 score
To control for the effect of proliferation on RAD51, we assessed RAD51 foci only in geminin positive cells, therefore assessing RAD51 foci only in tumour cells in the correct cell cycle phase. We termed this the RAD51 score (the percentage of geminin positive cells also positive for RAD51). In all tumours analysed RAD51 foci were almost exclusively observed in geminin positive cells (Median of 40% geminin positive cells were RAD51 positive compared to 0% geminin negative cells, p<0.0001 Mann-Whitney U Test) (Figure 3B). The strength of this anticipated relationship provided further validation of the immunofluorescent staining.
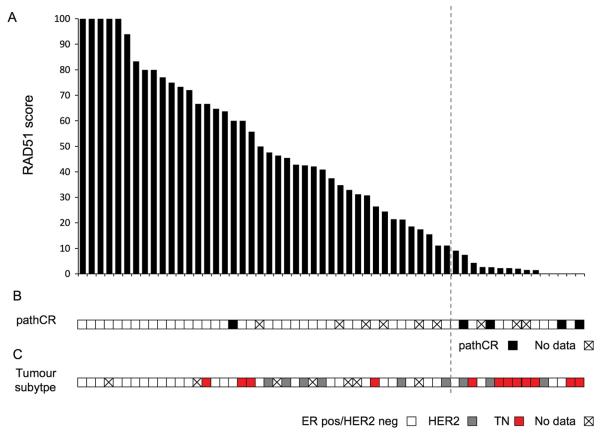
There was a wide range of RAD51 score at 24hrs post chemotherapy, from 0-100% (Figure 4). We determined the optimal cut-off to assign low RAD51 score by examining the point at which the association between raw RAD51 count and proliferation broke down (Figure 3A), suggesting that a RAD51 score of <10% was the most appropriate to reflect deficient HR, potentially identifying two distinct populations (Figure 3A). Overall, 26% of primary breast cancers had a RAD51 score <10% (15/57, 95% CI 15-40%) (Figure 4).
Figure 4. Assessment of RAD51 score in primary breast cancer and relationship with pathological complete response to chemotherapy and tumour subtypes.
A. RAD51 score (Percentage of geminin postive cells that have RAD51 foci) in biopsies taken at 24 hours post chemotherapy from 57 primary breast cancers, displayed in order RAD51 score. Tumours with 100% of geminin positive cells having RAD51 foci are displayed on the left, and tumours were no RAD51 foci in geminin positive cells on the right. A tumour was defined as being HR defective with a RAD51 score <10% (indicated with a vertical dotted line). RAD51 score was assessed blinded to clinicopathological and chemotherapy response data.
B. Data on pathological response to chemotherapy in post chemotherapy surgical specimen. Tumours that achieved a pathological complete response (pathCR) to neoadjuvant chemotherapy had lower RAD51 scores than those that did not achieve pathCR (Median RAD51 score; pathCR 2.6% vs non-pathCR 44%, p=0.028 Mann-Whitney U Test). Pathological response data was not available on 9 cases as discussed in Supplementary figure 1.
C. Data on tumour subtype according to ER and HER2 status. Tumours negative for ER and HER2 (TN breast cancers) had lower low RAD51 scores than other breast cancer subtypes (Median RAD51 score; TN cancers 2.3% vs other subtypes 42.5%, p=0.0077 Mann-Whitney U Test).
Correlations of RAD51 score with clinical features
We examined the relationship between RAD51 score and clinicopathological features (Table 2 and Figure 4). Low RAD51 score was associated with high histological grade, ER negative tumours, and high proliferation in baseline core biopsy and 24 hour biopsy, potentially reflecting an association between loss of HR and tumour proliferation (Table 2). We examined the prevalence of defective HR according to tumour subtype. Triple negative cancers had a lower RAD51 score than other cancers (Median RAD51 score; TN cancers 2.3% vs other subtypes 42.5%, p=0.0077 Mann-Whitney U Test) (Figure 4), and a low RAD51 score was observed in 67% (8/12) TN breast cancers compared to 19% non-TN (7/37, p=0.0036 Fisher's exact test). We conducted multivariate analysis of the features associated with RAD51 score. In a model incorporating tumour subtype and baseline Ki67, TN subtype was a significant predictor of low RAD51 score (Supplementary table 3).
Table 2.
Clinicopathological features according to RAD51 score
| Low RAD51 score (HR deficient) |
Others (HR Competent) |
p value | |
|---|---|---|---|
|
| |||
| n | 15 | 42 | |
| Median RAD51 score | 2% | 49% | |
| Median age | 47 | 48 | 0.42+ |
| Pathology | |||
| IDC | 14 | 31 | 0.42 |
| ILC | 1 | 8 | |
| Medain tumour size (mm) | 60 | 50 | 0.09+ |
| Histological grade | |||
| 2 | 3 | 22 | 0.031 |
| 3 | 12 | 17 | |
| Axillary node positive | 36% (4/11) | 62% (21/34) | 0.17 |
| ER positive | 43% (6/14) | 88% (29/33) | 0.003 |
| HER2 positive | 21% (3/14) | 22% (6/27) | 1 |
| Tumour subtype | |||
| ER positive/HER2 negative | 4 | 24 | 0.0043 * |
| HER2 positive | 3 | 6 | |
| Triple negative | 8 | 4 | |
| Median geminin 24 hr biopsy | 29.3% | 14.8% | <0.0001 + |
| Median Ki67 | |||
| Baseline | 44.2% | 21.3% | 0.005 + |
| 24 hr biopsy | 29.7% | 15.4% | 0.0012 + |
| Clinical response | |||
| CR | 3 | 7 | 0.2* |
| PR | 11 | 24 | |
| SD | 0 | 7 | |
| PD | 0 | 3 | |
| Pathological response | |||
| Complete | 4 | 1 | 0.011 |
| Not complete | 8 | 35 | |
IDC- Invasive ductal carcinoma; ILC – Invasive lobular carcinoma. Statistical analysis was with Fisher's exact test, unless indicated + Mann-Whitney U Test or * Chi Squared test. Clinical response; CR – complete response; PR – partial response; SD – stable disease; PD – progressive disease. Pathological response data was not available on 9 cases as discussed in Supplementary figure 1.
Mann-Whitney U Test
Chi Squared test
Inability to form RAD51 foci correlates with pathological CR
Overall there was no association between clinical response to chemotherapy and low RAD51 score (Table 2), although all tumours with a low RAD51 score achieved a clinical response compared to 76% without a low score (14/14 vs 31/41, p=0.051, Fishers' Exact test). Clinical response to chemotherapy is a poor surrogate for outcome, and we therefore examined pathological complete response to chemotherapy.
Tumours that achieved a pathological complete response (pathCR) with neoadjuvant chemotherapy had lower RAD51 scores than those that did not achieve pathCR (Median RAD51 score; pathCR 2.6% vs non-pathCR 44%, p=0.028 Mann-Whitney U Test) (Figure 4). Of the tumours with low RAD51 score 33% (4/12) achieved pathCR, compared to 3% (1/36) of tumours with RAD51 foci (p=0.011, Fisher's exact test), corresponding to a sensitivity of 80% and specificity of 81% for low RAD51 score as a predictive marker of pathCR.
Discussion
In this study we have used a functional RAD51 assay to provide a comprehensive assessment of HR in sporadic breast cancer. We provide evidence that a functional defect in HR is common in TN breast cancer, but also provide evidence for defective HR in a subset of high grade ER and/or HER2 positive breast cancer. Other recent studies have used RAD51 to examine breast and ovarian cancer samples (25-27). One study examined RAD51 foci in breast cancers irradiated ex-vivo (25), and a further study examined RAD51 in primary breast cancers without considering the confounding factor of proliferation in the biopsy, identifying only moderate predictive power for clinical response to chemotherapy (26). Clinical response to chemotherapy is a poor surrogate for outcome, and here we have investigated for predictors of pathological complete response, which is strongly predictive of good outcome (16, 17).
Our study illustrates the importance of considering proliferation when assessing a cell cycle regulated marker such as RAD51 foci. Without making any assessment of proliferation RAD51 would have less predictive power for response to chemotherapy (p=0.07, Mann-Whitney U test, data not shown) and no association with TN phenotype (p=0.13, Mann-Whitney U Test, data not shown), primarily as the eleven cases with no geminin expression would all have been, potentially falsely, assigned as HR deficient. Lack of geminin expression following chemotherapy is likely to reflect lack of proliferation resulting from the engagement of cell cycle checkpoints. However, an alternative explanation would be failure of immunofluoresecnce due to poor fixation of the tissue core. Assessment of geminin may therefore improve RAD51 assessment by both screening out cores where proliferation is absent, as well as providing an internal control.
Predicting the responsiveness of an individual tumour to chemotherapy has been a major challenge of breast cancer research (28). A number of prior studies have examined pathological markers in biopsies 24-48hrs post chemotherapy. Assessment of apoptosis at 48hrs post chemotherapy may predict for benefit to chemotherapy (29, 30), but another studies has not confirmed this at 24hrs post chemotherapy (18). Similarly, assessment of Ki67 post chemotherapy has only weak predictive power (18, 19, 31), and these studies have not identified a consistent predictor of response to chemotherapy. Here, we have shown that the inability to form RAD51 foci is strongly associated with pathological complete response to neoadjuvant chemotherapy, suggesting that defective HR may be a mechanism underlying pathological complete response to anthracycline based chemotherapy. Our data suggests that it may be possible to identify patients who are less likely to benefit from anthracycline based chemotherapy as soon as 24 hours following the first cycle of chemotherapy. In this study, high RAD51 score (induction of RAD51 by chemotherapy) had a 97% negative predictive value for failure to achieve pathCR. Potentially, after further validation of this test, tumours with a high RAD51 score may be better treated with taxane-based chemotherapy or switched to hormonal therapy. However, it is important to emphasize that path CR is a surrogate endpoint, and only a very substantially larger validation study could assess the negative predictive value for outcomes such recurrence free survival. In future clinical trials it may also be possible to assess HR competency by treating with a single agent PARP inhibitor prior to assessing RAD51 foci.
Inhibitors of Poly-ADP-Ribose polymerase 1 (PARP) are showing great promise in the treatment of hereditary BRCA1/BRCA2 related breast and ovarian cancer (6). Similarly, a PARP inhibitor has demonstrated substantial activity in TN breast cancers in combination with carboplatin/gemcitabine chemotherapy (32). We have previously shown that basal-like cancers have suppressed BRCA1 expression (7). Here, we extend this observation to suggest that a substantial proportion of TN breast cancers have evidence for defective HR, and this potentially explains the benefit seen with PARP inhibitors in this subtype of cancers. In addition, certain chemotherapy drugs, such as inter-strand cross linking drugs including platinums, are highly toxic to HR deficient cells and defective HR may also explain the high sensitivity of platinum chemotherapy seen in TN breast cancer (9, 33). However, our data suggests that approximately 30-40% of TN breast cancers do not have defective HR, providing further evidence for the heterogeneity of TN breast cancer, and raising the possibility that these 30-40% TN cancers would not benefit from PARP inhibitors. However, mechanisms of sensitivity to PARP inhibitors do exist other than loss of HR. For example, cells with loss of Fanconi anaemia genes are sensitive to PARP inhibitors in vitro (13), and PARP inhibitors have the potential to improve outcomes via chemopotentiation (34). Nevertheless, our data emphasises the importance of assessing biomarkers in ongoing PARP inhibitor trials to identify the subset of patients who benefit.
Our data suggests that approximately 20% of non-TN breast cancers have defective HR and might be expected to benefit from PARP inhibitors. Although all low RAD51 score cancers responded clinically to chemotherapy, the majority did not acheive a pathCR (Table 2). Anthracycline/cyclophosphamide based chemotherapy generates multiple cytotoxic lesions, of which only a small fraction result in DNA damage that would specifically target HR deficient tumours. It is likely that HR deficient tumours would benefit from DNA damage that is targeted more specifically at defective HR, to increase the therapeutic window. This observation potentially suggests that a combination of chemotherapy and PARP inhibitor might increase the fraction achieving pathCR and improve the long term outcome of these patients.
Statement of Translational Relevance.
Here we show that a substantial proportion of sporadic breast cancers have evidence of defective homologous recombination (HR). Our findings have potential significance for the development of therapies that target defective HR, such as PARP inhibitors, identifying new tumour subtypes that might benefit from therapy.
Supplementary Material
Acknowledgements
This work was supported by grants from Dr. Mildred Scheel foundation for Cancer Research grant number: 109027 Breakthrough Breast Cancer Research and Cancer Research UK. We acknowledge NHS funding to the NIHR Biomedical Research Centre, and funding from the Stand Up To Cancer Initiative.
References
- 1.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–9. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 2.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 3.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–82. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 4.Byrski T, Gronwald J, Huzarski T, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 28:375–9. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 5.Tan DS, Rothermundt C, Thomas K, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26:5530–6. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 6.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 7.Turner NC, Reis-Filho JS, Russell AM, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–32. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 8.Lambie H, Miremadi A, Pinder SE, et al. Prognostic significance of BRCA1 expression in sporadic breast carcinomas. J Pathol. 2003;200:207–13. doi: 10.1002/path.1348. [DOI] [PubMed] [Google Scholar]
- 9.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol. 28:1145–53. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen WH, Balajee AS, Wang J, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–70. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1:315–22. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 13.McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–15. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann M, Hortobagyi GN, Goldhirsch A, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24:1940–9. doi: 10.1200/JCO.2005.02.6187. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Angulo AM, McGuire SE, Buchholz TA, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol. 2005;23:7098–104. doi: 10.1200/JCO.2005.11.124. [DOI] [PubMed] [Google Scholar]
- 17.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–9. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 18.Archer CD, Parton M, Smith IE, et al. Early changes in apoptosis and proliferation following primary chemotherapy for breast cancer. Br J Cancer. 2003;89:1035–41. doi: 10.1038/sj.bjc.6601173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parton M, Krajewski S, Smith I, et al. Coordinate expression of apoptosis-associated proteins in human breast cancer before and during chemotherapy. Clin Cancer Res. 2002;8:2100–8. [PubMed] [Google Scholar]
- 20.Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–5. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 21.Tarsounas M, Davies D, West SC. BRCA2-dependent and independent formation of RAD51 nuclear foci. Oncogene. 2003;22:1115–23. doi: 10.1038/sj.onc.1206263. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez MA, Tachibana KE, Chin SF, et al. Geminin predicts adverse clinical outcome in breast cancer by reflecting cell-cycle progression. J Pathol. 2004;204:121–30. doi: 10.1002/path.1625. [DOI] [PubMed] [Google Scholar]
- 23.Tada S, Li A, Maiorano D, Mechali M, Blow JJ. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat Cell Biol. 2001;3:107–13. doi: 10.1038/35055000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartkova J, Horejsi Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 25.Willers H, Taghian AG, Luo CM, Treszezamsky A, Sgroi DC, Powell SN. Utility of DNA repair protein foci for the detection of putative BRCA1 pathway defects in breast cancer biopsies. Mol Cancer Res. 2009;7:1304–9. doi: 10.1158/1541-7786.MCR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asakawa H, Koizumi H, Koike A, et al. Prediction of breast cancer sensitivity to neoadjuvant chemotherapy based on status of DNA damage repair proteins. Breast Cancer Res. 12:R17. doi: 10.1186/bcr2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay A, Elattar A, Cerbinskaite A, et al. Development of a Functional Assay for Homologous Recombination Status in Primary Cultures of Epithelial Ovarian Tumor and Correlation with Sensitivity to Poly(ADP-Ribose) Polymerase Inhibitors. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-09-2758. [DOI] [PubMed] [Google Scholar]
- 28.Lee JK, Coutant C, Kim YC, et al. Prospective comparison of clinical and genomic multivariate predictors of response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 16:711–8. doi: 10.1158/1078-0432.CCR-09-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis DW, Buchholz TA, Hess KR, Sahin AA, Valero V, McConkey DJ. Automated quantification of apoptosis after neoadjuvant chemotherapy for breast cancer: early assessment predicts clinical response. Clin Cancer Res. 2003;9:955–60. [PubMed] [Google Scholar]
- 30.Buchholz TA, Davis DW, McConkey DJ, et al. Chemotherapy-induced apoptosis and Bcl-2 levels correlate with breast cancer response to chemotherapy. Cancer J. 2003;9:33–41. doi: 10.1097/00130404-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Chang J, Powles TJ, Allred DC, et al. Biologic markers as predictors of clinical outcome from systemic therapy for primary operable breast cancer. J Clin Oncol. 1999;17:3058–63. doi: 10.1200/JCO.1999.17.10.3058. [DOI] [PubMed] [Google Scholar]
- 32.O'Shaughnessy J. Efficacy of BSI-201, a PARP Inhibitor, in Combination with Gemcitabine/Carboplatin in Triple Negative Metastic Breast Cancer: Results of a Phase II Study ASCO annual meeting; 2009. Abstract 3. [Google Scholar]
- 33.Sikov WM, Dizon DS, Strenger R, et al. Frequent pathologic complete responses in aggressive stages II to III breast cancers with every-4-week carboplatin and weekly paclitaxel with or without trastuzumab: a Brown University Oncology Group Study. J Clin Oncol. 2009;27:4693–700. doi: 10.1200/JCO.2008.21.4163. [DOI] [PubMed] [Google Scholar]
- 34.Delaney CA, Wang LZ, Kyle S, et al. Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines. Clin Cancer Res. 2000;6:2860–7. [PubMed] [Google Scholar]
- 35.Chua S, Smith IE, A'Hern RP, et al. Neoadjuvant vinorelbine/epirubicin (VE) versus standard adriamycin/cyclophosphamide (AC) in operable breast cancer: analysis of response and tolerability in a randomised phase III trial (TOPIC 2) Ann Oncol. 2005;16:1435–41. doi: 10.1093/annonc/mdi276. [DOI] [PubMed] [Google Scholar]
- 36.Smith IE, A'Hern RP, Coombes GA, et al. A novel continuous infusional 5-fluorouracil-based chemotherapy regimen compared with conventional chemotherapy in the neo-adjuvant treatment of early breast cancer: 5 year results of the TOPIC trial. Ann Oncol. 2004;15:751–8. doi: 10.1093/annonc/mdh175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.