Abstract
The control of viral infections, especially those caused by influenza viruses, is of great interest in Public Health. Bio prospection has shown the presence of active principles in the hemolymph of arthropods, and in the salivary gland of ticks, and some of these are of interest for the development of new pharmacological drugs. Ticks lay their eggs in the environment, and to protect them from desiccation and microbial attack they involve the eggs in a waxy layer produced by an organ known as Gené’s Organ. In this study, the eggs wax from tick Amblyomma cajennense (Fabricius) was extracted using ice cold phosphate buffer. The antiviral activity was evaluated with picornavirus and influenza virus. In both cases egg wax was able to inhibit virus replication. For influenza virus, an amount as small as 12 μg/mL of crude egg wax suspension neutralized 128 UHA (hemaglutinant unit) of H1N1 influenza virus. With picornavirus, egg wax led to a 256-fold reduction in virus production by L929 cells. Egg wax was not cytotoxic to VERO, MDCK and L929 cell, being observed that the cell morphology was preserved with concentration as high as 2 mg/mL. In addition no genotoxic effect was observed for Vero cells, suggesting a very interesting potential antiviral activity.
Keywords: Antiviral activity, Tick egg wax, Amblyomma cajennense, EMC virus, Influenza virus
Introduction
The search for antimicrobial substances from animal species has improved significantly in the last years (Bulet et al. 1999; Bulet 2002; Xu et al. 2006; Ravichandran et al. 2009). Arthropods are a rich field for this search, because they represent 80% of the animal species (Marchini et al. 1993; Lai et al. 2004). In special, the control of viral infections is of great interest in Public Health, and for this reason the development of new and better antiviral compounds is vital and desirable. The presence of a potent antiviral activity against measles, influenza and polioviruses in the hemolymph of caterpillar Lonomia obliqua has been previously shown (Greco et al. 2009). Experiments with the purified protein led to a 157-fold reduction in measles virus production and a 61-fold reduction in polio virus production. We have also observed the presence of antiviral protein in the snake Crotalus durissus terrificus venom (Petricevich and Mendonça 2003). The medical and economic importance of ticks has long been recognized due to their ability to transmit microorganisms to humans and animals (Castro 1997; Jongejan and Uilenberg 2004). However, besides the potential harm that they can cause, blood sucking parasites like ticks have been exhaustively studied because of the richness of their genome. Bioprospection has shown the presence of active principles in the hemolymph of arthropods as well as in the salivary gland of ticks. Some of these are of interest for the development of new pharmacological drugs. It has been demonstrated that some insects produce antimicrobial substances in glands, like the female fruit fly Ceratitis capitata (Diptera) (Marchini et al. 1993). According to these authors, this species produces two antibacterial peptides on the reproductive accessory glands to cover their eggs.
Tick females lay their eggs in the environment, on the ground where they are exposed to soil micro flora. They cover the eggs in a waxy layer produced by an organ known as Gené’s Organ to protect them from desiccation and microbial attack. Arrieta et al. (2006) studied the wax covering the eggs of the African tick Amblyomma hebreum Koch and successfully demonstrated antimicrobial activity in this wax. The antimicrobial activity of the egg wax from the neotropical tick Amblyomma cajennense (Fabricius) was observed for some microorganism including virus (Lima-Netto et al. 2011). In this study the antiviral activity of the eggs wax crude extract of A. cajennense were confirmed for influenza virus and for picornavirus.
Materials and methods
Tick eggs wax extraction
Specimens of Amblyomma cajennense were obtained from a colony maintained for the Laboratory of Parasitology in Instituto Butantan. Adults were allowed to mate and feed on rabbits following the Protocol on Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation (COBEA). Infested rabbits were kept in individual wired cages in a ventilated room under natural light regimen, fed with commercial food pellets and water ad libitum. The infestation was carried out inside muslin sleeves glued with non-toxic glue on the rabbit’s shaved back according to Pinter et al. (2002). Engorged females were left in Petri dishes and kept in an incubator at (27 ± 1) °C and (90 ± 5)% of humidity, in order to obtain ovipositions. By the end of the ovipositions the wax was extracted from egg masses according to the technique proposed by Esteves et al. (2009) as follows: egg masses were washed with ice-cold phosphate buffer pH 6.8, the extract lyophilized, resuspended in purified water, filtered through 0.22 μm and kept at −18 °C to the moment of use.
VERO, MDCK and L929 cell cultures
VERO, MDCK and L929 cells were grown in plastic T-flasks or in multiwell plates using Leibovitz-15 (L15) medium containing 0.9 g L−1 of d-galactose, 0.3 g L−1 of l-glutamine and supplemented with 5% fetal bovine serum (FBS). Viable cell counts were performed in Neubauer chamber using Trypan blue (0.05%) exclusion.
Virus and cell infection
Influenza (H1N1) and picornavirus (EMC-encephalomyocardite virus) were used to determine the antiviral activity of the tick eggs wax. MDCK cells were infected with influenza viruses and L929 cells were infected with picornavirus (EMC). The cell infection was performed on the 3rd day post cell culture and started with 100 TCID50 of picornavirus or with 128 UHA (hemaglutinant unit) of influenza virus. To determine the antiviral effect on picornavirus, L929 cell culture was treated or not with 0.8 mg/mL of the extract of egg wax of A. cajennense 1 h prior infection. Cell culture were maintained for 48 h (EMC) at 37 °C. The cultures were observed daily in an inverted microscope for cytopathic detection. The egg wax was maintained in culture during all time of infection.
For influenza virus, culture of MDCK cells, performed in microplate 96 wells, was treated with 800, 400, 200, 100, 50, 25 and 12.5 μg/mL of wax eggs crude extracts of A. cajennense, 1 h before infection. After 72 h post infection cytopathic effect induced by the virus was observed, the culture medium was removed and the cells in the plate were stained with crystal violet (0.2% in 20% methanol). The egg wax was maintained in culture during the time of infection.
Determination of the virus infectious dose
In order to determine the amount of virus produced in cultures infected with picornavirus, in treated or not treated culture, L929 cell culture, seeded on 96-well plates, were infected, with a serial dilution (r:2) of picornavirus (initial titer of 1 × 104 TCID50). The micro well plates were then incubated at 37 °C for 2 days as described by Griffiths and Thornton (1982). Virus titers were determined by monitoring the cytopathic effect (CPE) in an endpoint dilution assay. They were expressed as TCID50 (the highest dilution of virus able to induce CPE in 50% of cells).
Cytotoxicity activity
The cytotoxic effects of the eggs wax was assessed by using a standard VERO cell assay. Briefly, exponential phase VERO cells (day 3) were exposed to different amounts of egg wax (0.125, 0.25, 0.5, 1 and 2 mg/mL). Daily, the cultures were observed for cytopathic effect. After cell count, determining the % of cells with cytotoxicity effect, the supernatant of culture were removed and the remaining cells were stained with crystal violet (0.2% in 20% methanol). The percentage values were calculated by the formula: (A control – A sample)/(A control) × 100.
Genotoxic activity
Comet assay
The general procedures of the alkaline comet assay utilized were based on Singh et al. (1988), with slight modifications according to Savi et al. (2006). The experiment was performed in the dark to minimize any ambient light DNA damage due to ambient light. Each microscope slide (three slides/egg wax concentrations) was covered with 1.5% NMP agarose (Sigma) dissolved in Ca+2 and Mg+2 free PBS and maintained overnight at room temperature. Once a suspension of cells is obtained, 60 μL of 0.5% LMP agarose (Sigma) similarly melted in PBS (37 °C) was mixed with 40 μL of cell suspension in Eppendorf tubes. This suspension was added to each slide containing a layer of NMP agarose and spread using a coverslip (26 × 76 mm). After solidification at 4 °C for 5 min, the coverslip was removed and slides were immersed in a cold, freshly prepared lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris with pH 10, 1% Triton-100 and 10% DMSO added freshly prior to use) for 1 h at 4 °C in the dark. After cell lysis, slides were gently placed in a horizontal gel electrophoresis chamber filled with freshly prepared chilled alkaline electrophoresis buffer (1 mM EDTA, 300 mM NaOH, pH > 13.0) for 30 min to allow the expression of DNA damage. Electrophoresis was conducted at 4 °C for 30 min at 0.74 V/cm, 300 mA. Slides were then washed (three times for 5 min) in a neutralization buffer (0.4 M Tris–HCl, pH 7.5) and fixed for 10 min in absolute ethanol. Finally, slides were stained with 50 μL ethidium bromide (20 μg/mL) and examined by fluorescence microscopy (Olympus BX51, equipped with Olympus XM10 camera) at 400× magnification with an exciting filter of 510–550 nm and an emission filter of LP 590 nm.
Data analysis
All slides were codified and examined by a single investigator to minimize scoring variation. A total of 100 comets/slide was randomly selected and analyzed by the visual classification based on the extent of DNA migration. The classification was carried out according to the tail size into 4 classes: 0 = undamaged; 1 = low damage; 2 = medium damage and 3 = high damage. Comets with small or nonexistent head and large diffuse tails were not included in the analysis. The total number of comets for each class was multiplied by the value of its class, thus creating an arbitrary unit (score). For each concentration, the score for 300 comets can range from 0 (all undamaged) to 900 (all totally damaged). Means were compared by the one-way analysis of variance (ANOVA) followed by the Tukey–Kramer test for multiple comparison of means. Result was considered statistically significant at P < 0.005.
Results
Effect of the egg wax extract on cell growth
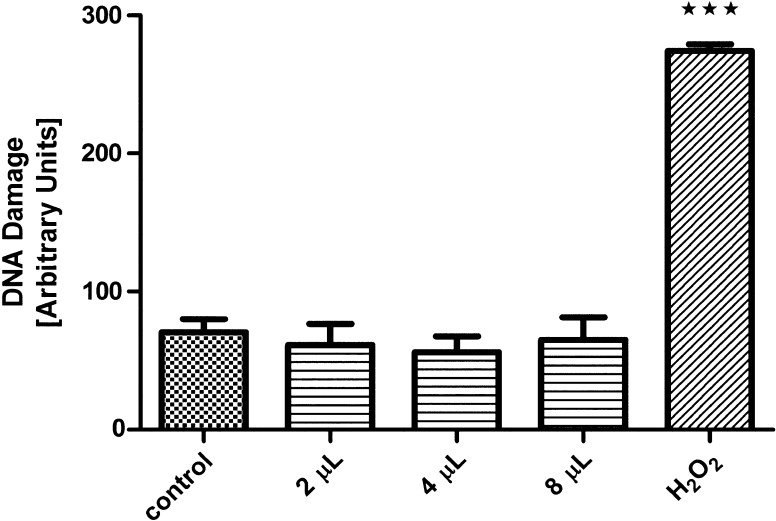
The first issue to be addressed was the examination of possible cytotoxicity and genotoxicity of the crude eggs wax extract, and for that purpose VERO cell lines were used. Synchronized VERO cells were exposed at various concentrations of crude egg wax suspension as described at “Materials and methods” and the cytotoxicity of cells was quantified. Concentrations equal or lower than 2 mg/mL were not toxic to VERO cells, and high percentages of viable cells were observed throughout the three experiments. The same result was found in the genotoxicity test, when no alteration in the cells was observed too, as shown in Fig. 1. As L929 and MDCK cells were used to determine the antiviral effect of egg wax, the cytotoxicity of the egg wax on L929 cell growth also was performed. Similarly to that observed to VERO cells, no adverse effect in terms of viability or morphological alteration was observed when concentration of 2 mg/mL of egg wax was used.
Fig. 1.
Comet assay: DNA damage in Vero cells exposed to the A. cajennense eggs wax; H2O2 was used as positive control
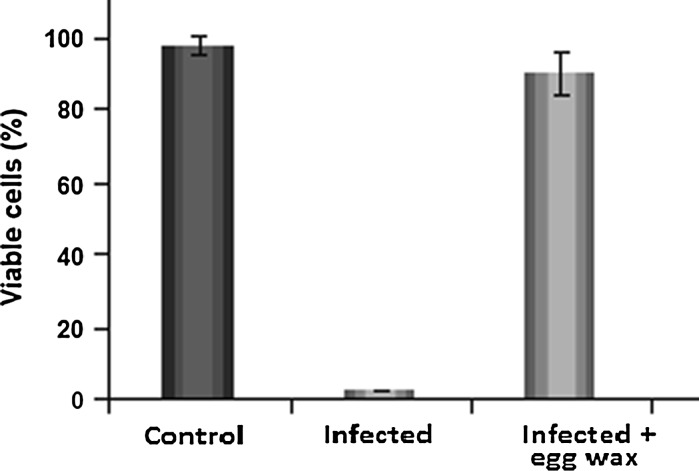
In Fig. 2 is shown the antiviral effect of eggs wax in L-929 cells, after cell infection with 100 TCID50 of picornavirus (EMC). An intensive cytopathic effect was observed in infected culture (without eggs wax suspension) after 48 h. Nevertheless, when the infected culture was pre-treated with egg wax extract (0.8 mg/mL), no adverse effect in the cell culture was observed, and it remained highly viable (Fig. 3).
Fig. 2.
Cytopathic effect in L929 cells culture infected with 100 TCID50 of picorna virus (EMC) and treated or not with 0.8 mg/mL of crude extract of egg wax. Cell cultures were observed after 48 h and the cytopathic effect determined with an inverted microscope
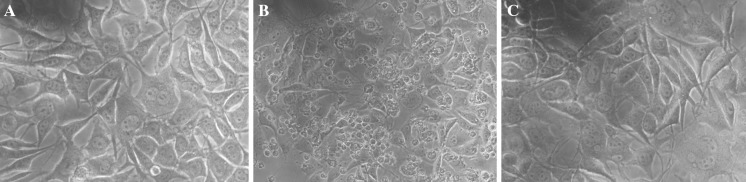
Fig. 3.
L929 cell culture (48 h), infected with picornaviruses and treated or not with crude extract of egg wax. The cultures were observed under an inverted microscope (×40). a Control; b infected culture (100 TCID50 of picornavirus); c infected culture treated 1 h before infection with 0.8 mg/mL crude extract of egg wax
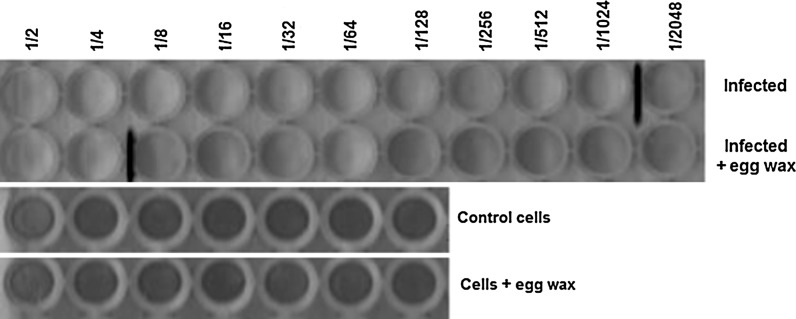
To verify the effect of eggs wax on virus replication, cultures of L929, treated or not with 0.8 mg/mL of egg wax, were infected with serial dilution (r:2) of picornavirus (initial titer of 1 × 104 TCID50). As can be observed in Fig. 4, virus infection was observed up to dilution 1/1,024, either for cell culture infected or not treated. This result is in accordance with the initial virus titer used to cell infection. Nevertheless, when the same procedure was performed in culture treated with egg wax, cytopathic effect (CPE) was observed only until dilution 1:4, showing that egg wax was able to inhibit the virus replication by 256-fold.
Fig. 4.
Effect of egg wax on virus replication. Cultures of L929, treated or not with 0.8 mg/mL of egg wax, were infected with serial picornavirus dilution (r:2) (initial titer of 1 × 104 TCID 50). Cell cultures were observed after 48 h and the cytopathic effect determined on an inverted microscope. Cells without cytophatic effect are stained in blue. The last virus dilution seen as positive is marked with a red line
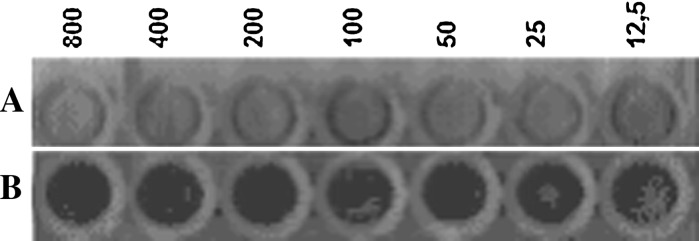
To determine the antiviral effect of eggs wax, MDCK cell cultures, were treated or not treated with a suspension of crude eggs wax extract of A. cajennense, (at concentrations of 800, 400, 200, 100, 50, 25 and 12.5 μg/mL) 1 h before infection. Then, the cultures were infected with a fixed amount of 128 UHA of influenza virus (H1N1) (Fig. 5). After 72 h post infection cytopathic effect induced by the produced Influenza virus was observed, the culture medium was removed and the cells on the plate were stained with crystal violet. As can be observed in Fig. 5, amount as small as 12.5 μg/mL of crude egg wax was able to inhibit the replication of 128 UHA of with influenza virus.
Fig. 5.
Effect of egg wax in influenza virus (H1N1) replication. Culture of MDCK cells, performed in 96 wells microplate, were infected with 128 UHA of influenza virus (A and B). In B, crude A. cajennense eggs wax extract with different concentrations (800, 400, 200, 100, 50, 25 and 12.5 μg/mL) was added 1 h before. After 72 h post infection cytopathic effect induced by the virus was observed. The medium of culture was removed and the cells in the plates were stained with crystal violet. Cells without cytopathic effect stained in blue
Discussion
Many studies have shown that tick salivary gland extract can act in immune response, promoting virus growth (Hajnická et al. 1998; Kokáková et al. 1999), enhancement of virus transmission, (Labuda et al. 1993), affect the natural killer cell activity (Kubes et al. 1994), modulate immune suppressive effects on innate and acquired immune response (Mejri et al. 2002) and modulation of human lymphocyte proliferation (Rolníková et al. 2003). Despite this large range of effects in immune response, few studies are addressed to the study of the potential antiviral effect from other products obtained from ticks besides its saliva. In insect, the antimicrobial protection is due to the presence of many peptides, including defensins. Defensins are a major group of antimicrobial peptides and are found widely in vertebrates, invertebrates and plants. Invertebrate defensins have been identified from insects, scorpions, mussels and ticks. Nakajima et al. (2003) and Johns et al. (1998) have shown the control of bacterial infections in the hard tick Dermacentor variabilis (Acari: Ixodidae) in tick hemolymph by demonstrating the presence of a protein with M(r) 14.5 kDa, that comigrated with human lysozyme, as the protein with potential antimicrobial activity. Recently, Arrieta et al. (2006) have shown that egg wax of the African cattle tick Amblyomma hebraeum contained antibacterial activity inhibiting the growth of Escherichia coli and Serratia marcescens (Gram-negative bacteria) in solid culture. They suggest that Gené’s organ, (the egg-waxing organ in ticks) is the major source of the antibacterial substance in the egg wax. In this study we tested eggs wax of A. cajennense for potential antiviral activity against influenza virus and picornavirus. The result shows that the eggs wax extract is highly effective against both viruses. The EC50 value against influenza A virus (H1N1), as tested by the plaque reduction assay on MDCK cells, was about 12.5 μg/mL (Fig. 5). This amount is according to that obtained in literature by other source. Earlier, Shin et al. (2010), have shown that some Korean medicinal plant extracts have potential antiviral activity against influenza viruses. Using an extract of Agrimonia pilosa they have shown that the EC50 value against influenza A virus is around 14–23 μg/mL. On the other hand, extracts of egg wax of A cajennense led to a 256-fold reduction in picornavirus production by L929 cells. These results are in conformity to those obtained with hemolymph of Lonomia obliqua (Greco et al. 2009). The present data suggest that the eggs wax of A. cajennense can be an important source to obtain products with antiviral or antimicrobial activity. The broad-spectrum antiviral activity of eggs wax of A. Cajennense on various viruses, as well as its mechanism of action deserve further investigation.
Acknowledgments
The authors acknowledge the financial support of FAPESP (2008/57263-5) and CNPq for academic career scholarship to DMBB and RZM.
References
- Arrieta MC, Leskin BK, Kaufman WR. Antimicrobial activity in the egg wax of the African cattle tick Amblyomma hebraeum (Acari: Ixodidae) Exp Appl Acarol. 2006;39:297–313. doi: 10.1007/s10493-006-9014-5. [DOI] [PubMed] [Google Scholar]
- Bulet P (2002) Antiviral and antitumor peptides from insects. Proc Natl Acad Sci USA 99:12628–12632 [DOI] [PMC free article] [PubMed]
- Bulet P, Hetru C, Dimarcq J, Hoffmann D. Antimicrobial peptides in insects: structure and function. Dev Comp Immunol. 1999;23:329–334. doi: 10.1016/S0145-305X(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Castro JJ. Sustainable tick and tickborne disease control in livestock improvement in developing countries. Vet Parasitol. 1997;71:77–97. doi: 10.1016/S0304-4017(97)00033-2. [DOI] [PubMed] [Google Scholar]
- Esteves E, Fogaça AC, Maldonado R, Silva FD, Manso PPA, Pelajo-Machado M, Valle DS, Daffre S. Antimicrobial activity in the tick Rhipicephalus (Boophilus) microplus eggs: cellular localization and temporal expression of microplusin during oogenesis and embryogenesis. Dev Comp Immunol. 2009;33:913–919. doi: 10.1016/j.dci.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Greco KN, Mendonça RMZ, Moraes RHP, Mancini DAP, Mendonça RZ. Antiviral activity of the hemolymph of Lonomia obliqua (Lepidoptera: Saturniidae) Antiviral Res. 2009;84:84–90. doi: 10.1016/j.antiviral.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Griffiths B, Thornton B. Use of microcarrier culture for the production of herpes simplex virus (type 2) in MRC-5 cells. J Chem Technol Biotechnol. 1982;32:324–329. doi: 10.1002/jctb.5030320137. [DOI] [Google Scholar]
- Hajnická V, Fuchsberger N, Slovák M, Kokáková P, Labuda M, Nuttall PA. Ticks salivary gland extract promotes virus growth in vitro. Parasitology. 1998;116:533–538. doi: 10.1017/S0031182098002686. [DOI] [PubMed] [Google Scholar]
- Johns R, Sonenshine DE, Hynes WL. Control of bacterial infections in the hard tick Dermacentor variabilis (Acari: Ixodidae): evidence for the existence of antimicrobial proteins in tick haemolymph. J Med Entomol. 1998;35:458–464. doi: 10.1093/jmedent/35.4.458. [DOI] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kokáková P, Hajnická V, Slovák M, Nuttall PA, Fuchsberger N. Promotion of vesicular stomatitis virus nucleocapsin protein production by arthropod saliva. Acta Virol. 1999;43:251–254. [PubMed] [Google Scholar]
- Kubes M, Fuchsberger N, Labuda M, Zuffová M, Nuttall PA. Salivary gland extracts of partially fed Dermacentor reticulatus ticks decrease natural killer cell activity in vitro. Immunology. 1994;82:113–116. [PMC free article] [PubMed] [Google Scholar]
- Labuda M, Jones LD, Williams T, Nuttall PA. Enhancement of tick-borne encephalitis virus transmission by tick salivary gland extract. Med Vet Entomol. 1993;7:193–196. doi: 10.1111/j.1365-2915.1993.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Lai R, Lomas LO, Jonczy J, Turner PC, Rees HH. Two novel non-cationic defensin-like antimicrobial peptides from haemolymph of the female tick, Amblyomma hebraeum. Biochem J. 2004;379:681–685. doi: 10.1042/BJ20031429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Netto S, Mendonça RZ, Franzolin MR, Utescher CL, Orozco S, Máximo-Espindola C, Labruna M, Barros-Battesti DM. An interesting antimicrobial activity of egg wax from Amblyomma cajennense (Acari: Ixodidae) Syst Appl Acarol. 2011;16:3–6. [Google Scholar]
- Marchini D, Giordano PC, Amons R, Bernini LF, Dallai R. Purification and primary structure of ceratotoxin A and B, two antibacterial peptides from the female reproductive accessory glands of the medfly Ceratitis capitata (Insecta:Diptera) Insect Biochem Mol Biol. 1993;23:591–598. doi: 10.1016/0965-1748(93)90032-N. [DOI] [PubMed] [Google Scholar]
- Mejri N, Rutti B, Brossard M. Immunosuppressive effects of Ixodes ricinus tick saliva or salivary gland extracts on innate and acquired immune response of BALB/c mice. Parasitol Res. 2002;88:192–197. doi: 10.1007/s00436-001-0515-1. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Ishibashi J, Yukuhiro F, Asaoka A, Taylor D, Yamakawa M. Antibacterial activity and mechanism of action of tick defensin against Gram-positive bacteria. Biochim Biophys Acta. 2003;1624:125–130. doi: 10.1016/j.bbagen.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Petricevich VL, Mendonça RZ. Effect of Crotalus venom on growth of measles virus. Toxicon. 2003;42:01–11. doi: 10.1016/S0041-0101(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Pinter A, Labruna MB, Faccini JLH. The sex ratio of Amblyomma cajennense (Acari: Ixodidae) with notes on the male feeding period in the laboratory. Vet Parasitol. 2002;105:79–88. doi: 10.1016/S0304-4017(01)00650-1. [DOI] [PubMed] [Google Scholar]
- Ravichandran S, Wahidulla S, D’Souza L, Rameshkumar G (2009) Antimicrobial lipids from the hemolymph of brachyuran crabs. Appl Biochem Biotechnol 162(4):1039–1051 [DOI] [PubMed]
- Rolníková T, Kazimírová M, Buc M. Modulation of human lymphocyte proliferation by salivary gland extracts of ixodid ticks (Acari: Ixodidae): effect of feeding stage and sex. Folia Parasitol. 2003;50:305–312. [PubMed] [Google Scholar]
- Savi LA, Barardi CRM, Simões CMO. Evaluation of antiherpetic activity and genotoxic effects of tea catechin derivatives. J Agric Food Chem. 2006;54:2552–2557. doi: 10.1021/jf052940e. [DOI] [PubMed] [Google Scholar]
- Shin WJ, Lee KH, Park MH, Seong BL. Broad-spectrum antiviral effect of Agrimonia pilosa extract on influenza viruses. Microbiol Immunol. 2010;54:11–19. doi: 10.1111/j.1348-0421.2009.00173.x. [DOI] [PubMed] [Google Scholar]
- Singh NP, Mc Coy MT, Tice RR, Schnider EL. A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Xu X, Li J, Lu Q, Yang H, Zhang Y, Lai R. Two families of antimicrobial peptides from wasp (Vespa magnifica) venom. Toxicon. 2006;47:249–253. doi: 10.1016/j.toxicon.2005.10.015. [DOI] [PubMed] [Google Scholar]