Abstract
Rheumatoid arthritis (RA) and osteoarthritis (OA) are prevalent chronic health conditions. However, despite recent advances in medical therapeutics, their treatment still represents an unmet medical need because of safety and efficacy concerns with currently prescribed drugs. Accordingly, there is an urgent need to develop and test new drugs for RA and OA that selectively target inflamed joints thereby mitigating damage to healthy tissues.
Conceivably, biocompatible, biodegradable, disease-modifying antirheumatic nanomedicines (DMARNs) could represent a promising therapeutic approach for RA and OA. To this end, the unique physicochemical properties of drug-loaded nanocarriers coupled with pathophysiological characteristics of inflamed joints amplify bioavailability and bioactivity of DMARNs and promote their selective targeting to inflamed joints. This, in turn, minimizes the amount of drug required to control articular inflammation and circumvents collateral damage to healthy tissues. Thus, nanomedicine could provide selective control both in space and time of the inflammatory process in affected joints.
However, bringing safe and efficacious DMARNs for RA and OA to the marketplace is challenging because regulatory agencies have no official definition of nanotechnology, and rules and definitions for nanomedicines are still being developed. Although existing toxicology tests may be adequate for most DMARNs, as new toxicity risks and adverse health effects derived from novel nanomaterials with intended use in humans are identified, additional toxicology tests would be required. Hence, we propose that detailed pre-clinical in vivo safety assessment of promising DMARNs leads for RA and OA, including risks to the general population, must be conducted before clinical trials begin.
Keywords: Drug delivery, Nanoparticles, Lipid, Polymer, Plasma exudation, Osteoarthritis, Rheumatoid arthritis
1. Purpose
The goal of this overview is to provide the practicing physician a sensible insight on the role nanomedicine could play in improving the long-term outcome of patients with rheumatoid arthritis (RA) and osteoarthritis (OA), two prevalent chronic health conditions that still represent unmet medical needs. Given the large volume of published experimental data on drug-loaded nanoparticles for various indications, target-seeking nanobiotherapeutics in particular, the focus of this presentation is on systemically administered, safe and efficacious disease-modifying antirheumatic nanomedicines (DMARNs) that were investigated in animal models of RA and OA and, thus, could proceed to clinical trials.
1.1. Chronic, non-infectious arthritis
Chronic, non-infectious arthritis encompasses a plethora of inflammatory disorders affecting the joints. Evidence of arthritis in humans dates back to 4500 BC [1–4]. The most common forms of chronic, non-infectious arthritis are RA, an autoimmune inflammatory disease, and OA, a ‘wear and tear’ form of arthritis [1–4]. The cause(s) of RA and OA is still unknown and there is no cure.
Current therapeutic approaches, including non-steroidal anti-inflammatory drugs (NSAIDs) and disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX), monoclonal anti-TNF-α antibodies and other biopharmaceuticals, address predominantly symptoms and physical disability by reducing articular inflammation and pain [1–5]. By decreasing local inflammation, progressive joint damage may be slowed as well. Unfortunately, these treatment modalities are not restorative and often end in total joint replacement [6,7].
Rheumatoid arthritis and OA are serious national health problems in the USA with more than 20 million patients having severe limitations in function on a daily basis [1–4]. Consequently, absenteeism and frequent visits to the physician are common among these patients. To this end, the total annual cost of arthritis to society is estimated at $100 billion of which almost 50% accounts from lost earnings. Each year, arthritis results in nearly 1 million hospitalizations and approximately 45 million outpatient visits. Importantly, over 1 million joint replacements are performed each year in the USA and European Union combined [1–4].
Despite recent advances in medical therapeutics, treatment of RA and OA still represents an unmet medical need because of safety and efficacy concerns with currently prescribed drugs [8–13]. Withdrawal of certain cyclooxygenase-2 inhibitors from the market due to serious adverse cardiovascular events illustrates this point [8,9]. Importantly, a large proportion of patients with RA and OA fail to respond adequately or become refractory to drug therapy [11,14]. Among patients who respond to DMARDs, long-term use of these drugs to sustain remission of RA may be associated with serious adverse events, such as development of tuberculosis, life-threatening fungal infections, lymphomas, liver injury, myelosuppression and heart failure [1,2,12]. Accordingly, there is an urgent need to develop and test new DMARDs for RA and OA that target the inflamed joints yet avoid collateral damage to healthy tissues [6,7,15].
2. Nanomedicine
Nanomedicine is a nascent branch of nanobiotechnology that exploits and assimilates recent scientific breakthroughs in nanobiotechnology to prevent, diagnose, treat and cure human disease [16]. The past two decades have witnessed a dramatic increase in the number of engineered drug-loaded nanoparticles propelled by the booming nanobiotechnology industry. Although significant progress has been made, several important shortcomings of passive and active targeted delivery of nanomedicines to injured tissues have emerged, including irrational nanoformulation design using complex and potentially toxic nanomaterials and ligands, absence of concomitant safety and efficacy studies in appropriate animal models relevant to the clinical indication being sought, administration of novel nanomedicines as monotherapy rather than incorporating them into existing therapeutic regimens and insufficient application of theranostics as personalized, nanomedicine-based therapeutic intervention. Clearly, these issues should be addressed and resolved before novel nanomedicines are tested in clinical trials.
Accordingly, to streamline a safe and cost-effective bench-to-bedside transition of novel nanomedicines for RA and OA, we posit that investigators and other stakeholders in the arthritis arena should conduct animal studies early in the pre-clinical drug development stage. In these studies, both efficacy and toxicity of lead anti-rheumatic drugs loaded onto U.S. Food and Drug Administration (FDA)-approved injectable nanocarriers, such as liposomes, micelles, nanocrystals, nanoparticles, nanotubes and supermagnetic iron oxide will be determined simultaneously in the same animal groups. Only safe and efficacious DMARNs thus discovered would then proceed to clinical trials. This approach should circumvent the need for an exhaustive and costly safety testing of novel nanocarriers that have not yet been approved for human use by regulatory agencies.
Conceivably, biocompatible, biodegradable, DMARNs, which are comprised of a nanocarrier/delivery system loaded with therapeutic agent, targeting agent and/or imaging agent, could represent a promising therapeutic approach to address this challenge [16]. The unique and versatile physicochemical properties of drug-loaded nanocarriers that work as a system and are critical to achieve desired therapeutic effect coupled with pathophysiological characteristics of inflamed joints, such as hyperpermeable (‘leaky’) post-capillary venules and upregulation of membrane receptors in key local effector cells, amplify bioavailability and bioactivity of DMARNs and promote their selective targeting to inflamed joints [16–23]. This, in turn, minimizes the amount of drug required to abate inflammation thereby mitigating collateral damage to healthy tissues. Although DMARNs must still be injected, their sustained bioavailability and bioactivity enable long time intervals (months) between injections. Thus, nanomedicine could provide selective control both in space and time of the inflammatory process in affected joints.
However, bringing novel, safe and efficacious DMARNs to the marketplace is challenging because governmental drug regulatory agencies, including U.S. FDA, have no official definition of nanotechnology, and rules and definitions for nanomedicines are still being developed [24]. Although existing toxicology tests may be adequate for most DMARNs, as new toxicity risks derived from nanomaterials with intended use in humans are identified, such as dendrimers, aptamers and water-soluble fullerene (C60), additional toxicology and immunological tests would be required in vivo [22,24–29].
This notion is important because, unlike nanomedicines presently approved by U.S. FDA, injectable DMARNs will be administered repeatedly for a prolonged period of time (years) in patients with RA and OA [1–3,16,25]. Hence, detailed pre-clinical in vivo safety assessment of promising DMARNs leads, including risks to the general population, must be conducted before clinical trials begin.
3. Nanomedicines in animal models of RA and OA
Several biocompatible DMARNs whose components have been approved by various regulatory agencies worldwide have been developed and tested in animal models of RA but less so in OA. To this end, the concept of liposomes as drug nanocarriers to improve the therapeutic index of anti-rheumatic drugs is very attractive [16,19,23,29].
Liposomes possess great flexibility in terms of composition, physicochemical properties and ability to accommodate a wide spectrum of drugs. They encapsulate drugs at high loading efficiency and shield them from external conditions. Surface modifications of liposomes with biocompatible and biodegradable polymers, such as poly(ethylene glycol) (PEG), can also be accomplished to improve their target localization by prolonging the circulation time (passive targeting) and/or by selective ligands, such as RGD peptide and folate, that interact with their cognate receptors on target cells of interest (active targeting) [16,21,23]. Long-circulating liposomes evade uptake by the reticuloendothelial system, such as the liver, spleen and bone marrow, and passively extravasate from hyperpermeable (‘leaky’) post-capillary venules into the interstitial space of inflamed joints. The encapsulated anti-rheumatic drug is then released from liposomes by various mechanisms and exert anti-inflammatory and immunomodulatory effects. The size of these liposomes pre-empt extravasation through the intact microvacular wall in healthy organs thereby mitigating adverse events.
Liposomal formulations, including long-circulating (see below), of MTX, glucocorticoids, NSAIDs and superoxide dismutase, have been tested in experimental models of RA and have shown improved efficacy and reduced toxicity at lower doses when administered systemically in comparison to the free drug [16,19,23]. Accordingly, these novel nanomedicines may be suitable for further testing in patients with RA and OA either alone or in combination with biologics. However, long-term (years) administration of drug-loaded liposomes has not been tested so far in humans. Accordingly, their prolonged use in patients with RA and OA could be challenging because of a complex and expensive manufacturing process and potentially serious allergic reactions ascribed, in part, to complement activation [16].
To begin to address these apparent limitations, Koo et al. [17] devised a distinct therapeutic approach to actively and selectively target a DMARNs to effector cells in inflamed joints of mice with collagen-induced arthritis (CIA), a well-established animal model of rheumatoid arthritis. They developed sterically stabilized phospholipid micelles (SSM; hydrodynamic diameter, ~13 nm) composed of negatively charged 1,2-distearoylsn-glycero-3-phosphoethanolamine-(polyethylene glycol)2000 (DSPE-PEG2000), a component of U.S. FDA-approved Doxil® (PEGylated liposomal doxorubicin) [30], to which camptothecin (CPT), a U.S. FDA-approved selective topoisomerase I inhibitor for cancer chemotherapy, self-associates (CPT-SSM; [31]). The synthetic polymer provides electrostatic and steric stabilizations and a longer circulation half-life to the drug in vivo as well as functional-end groups for the attachment of targeting ligands, such as antibodies, peptides and aptamers.
Koo et al. [17] found that self-association of CPT with SSM increased drug solubility and stability by 25- and 3-fold, respectively, in aqueous solution in comparison to CPT alone. Importantly, because vasoactive intestinal peptide (VIP) receptors are overexpressed in activated T-lymphocytes, macrophages and proliferating synoviocytes in inflamed joints of patients with RA, Koo et al. [17] surface-modified CPT-SSM with covalently conjugated VIP (CPT-SSM-VIP), a potent, pleiotropic 28-amino acid mammalian immunomodulator belonging to the glucagon-secretin superfamily of peptides [32], to actively target VIP receptors overexpressed on these effector cells receptors in mice with CIA thereby further promoting CPT efficacy and reducing its systemic toxicity. Unlike other targeting receptors in the microcirculation, such as αvβ3 integrins, VIP receptors are not expressed on endothelial cells. This, in turn, circumvents interactions of circulating CPT-SSM-VIP with VIP receptors in the microcirculation of extra-articular organs and tissues thereby prolonging circulation time and bioavailability of these nanoconstructs. Injectable VIP (aviptadil) is U.S. FDA-approved for treatment of acute food impaction in the esophagus, and by other non-U.S. regulatory agencies for erectile dysfunction when combined with phentolamine [33].
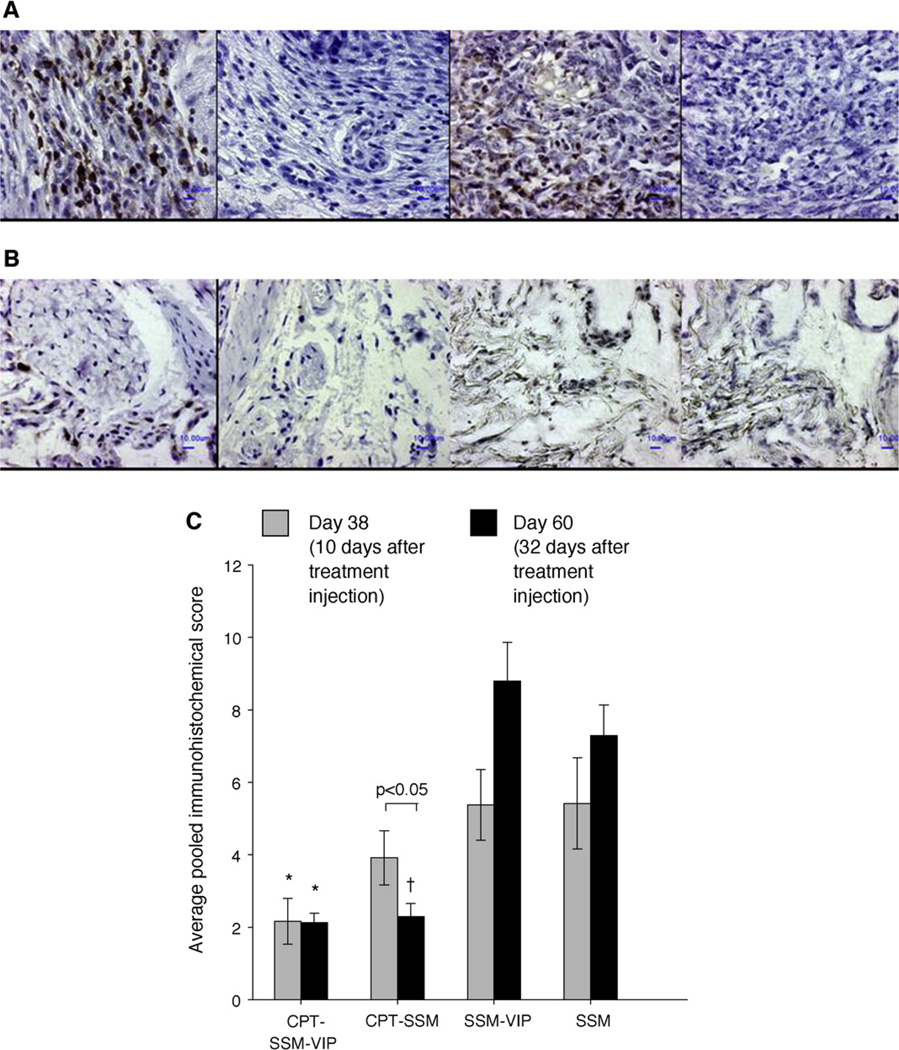
Koo et al. [17] showed that a single, subcutaneous injection of low-dose CPT-SSM-VIP, but neither irinotecan, a CPT analog, nor MTX, abrogated CIA in mice for 32 days without demonstrable systemic toxicity (Fig. 1). Injection of empty SSM had no significant effects of CIA. The dose of CPT-SSM-VIP used in these studies was 10-folder lower than that used for CPT alone and ~100-fold lower than that used in cancer therapy [31]. Unlike liposomes, CPT-SSM-VIP is prepared and stored in a lyophilized form and reconstituted in water for injection or saline just before use thereby simplifying storage and prolonging shelf life at point-of-care [16,17]. Active targeting of CPT-SSM-VIP can also be accomplished by grafting all D-VIP, the inactive enantiomer of naturally occurring L-VIP, to functional-end groups of PEG molecule thereby circumventing possible L-VIP-induced activation of intracellular signal transduction pathways in targeted cells. Thus, CPT-SSM-VIP represents a promising, safe and efficacious DMARN for RA.
Fig. 1.
CPT in micelles reduces cellular content within the CIA joints. Representative joint sections of (A) empty SSM-VIP-injected mice, (B) CPT-SSM-VIP treated mice; (left to right) CD3+, CD3−, lysozyme+, lysozyme− staining. Bars represent 10 µm. (C) Average pooled immunohistochemical scores of cellular infiltration and distribution in joints of CIA mice on ( ) Day 38 and (■) Day 60. Results are expressed as mean ± S.E.M. (6 mice/group). *, †p< 0.05 versus SSM-VIP and SSM, respectively.
) Day 38 and (■) Day 60. Results are expressed as mean ± S.E.M. (6 mice/group). *, †p< 0.05 versus SSM-VIP and SSM, respectively.
Sethi et al. [34] passively loaded VIP onto SSM (VIP-SSM) to generate a novel, safe, long-acting immunomodulatory nanomedicine for the treatment of RA (Fig. 2). They showed that, unlike VIP alone or empty SSM, a single subcutaneous or intravenous injection of low-dose VIP passively loaded onto SSM (VIP-SSM) preferentially localizes in the joints of mice with CIA and significantly ameliorates arthritis by modulating key cytokines, chemokines and proteinases involved in the disease process without affecting systemic arterial pressure or inducing diarrhea. Self-association of VIP with SSM confers stability to the peptide molecule and prevents its hydrolysis and inactivation in the circulation thereby prolonging its circulation time [32–34]. This sterically stabilized nanoconstruct then extravasates from the hyperpermeable (‘leaky’) post-capillary venules in inflamed joints where VIP exerts its anti-inflammatory effects. Similar to CPT-SSM-VIP, VIP-SSM is prepared and stored in a lyophilized form and reconstituted just before use. Thus, VIP-SSM may also represent a novel DMARN for the treatment of RA [32–34].
Fig. 2.
Freeze-dried cake of vasoactive intestinal peptide (VIP; 67 µM) self-associated with sterically stabilized phospholipid nanomicelles (SSM; 10 mM).
Long-circulating, biocompatible and biodegradable polymeric nanoparticles are attractive nanocarriers for vascular drug delivery because of relatively simple manufacturing process, loading efficiency, stability in biological fluids and low toxicity [16]. To this end, Ishihara et al. [35] developed a series of long-circulating (PEGylated) nanoformulations of betamethasone, a synthetic glucocorticosteroid, encapsulated in U.S. FDA-approved, biocompatible and biodegradable poly(d,l-lactic/glycolic acid (PLGA) and poly(d,l-lactic acid) (PLA) polymers (size, 45–115 nm). They showed potent and prolonged anti-inflammatory activity of these nanocorticosteroids in rats and mice with experimentally induced arthritis. This was due to prolonged circulation time, passive targeting to inflamed joints and local sustained release of betamethasone. Conceivably, these passively targeted novel nanomedicines could improve the therapeutic index of corticosteroids in comparison to that of pulse therapy, intra-articular injection and liposome administration.
Chrysotherapy has been used extensively as a DMARD in patients with RA before the advent of biologics [1,2]. However, long-term use of these drugs was associated with serious nephrotoxicity. To this end, subcutaneous injection of nanosized (particle size, 27 nm) colloidal metallic gold suppressed collagen-, mycobacterial- and pristane-induced arthritis in rats and was 1000-fold more potent than sodium aurothiomalate (I) [36]. Unfortunately, the effects of these non-sterically stabilized nanoparticles on renal function and other organs were not determined illustrating the need for parallel toxicology studies before embarking on clinical trials [36,37].
Cyclodextrins are a family of naturally occurring compounds composed of glucose monomers ranging from six to eight units in a cone-shaped ring configuration [20]. They are produced from starch by enzymatic degradation. Chemically modified β-cyclodextrins are generally recognized as safe (GRAS) excipients by U.S. FDA and are used as such in several marketed drugs in the U.S. and worldwide. To this end, a linear cyclodextrin polymer composed of β-cyclodextrin and PEG was conjugated to a derivative of methylprednisolone through an ester linker that undergoes pH-dependent or enzyme-induced cleavage in the inflamed joint [20]. The water-soluble, long-circulating, biocompatible, nontoxic and nonimmunogenic construct self-assembles into nanoparticles of 27 nm in size. This novel nanomedicine has been shown to reduce collagen-induced arthritis in mice at doses up to 100-fold lower using weekly injections [20]. Whether long-term administration of the nanomedicine is also associated with substantial reduction in methylprednisolone-related toxicity remains to be determined. Conceivably, this novel nanomedicine could improve efficacy of methylprednisolone while decreasing frequency of administration in patients with RA.
In the future, we envision that personalized regenerative medicine therapy of RA and OA would involve long-term administration of nanomedicines composed of anti-rheumatic drugs, genes, siRNAs and microRNAs as well as nanomedicines that recruit, attract and stimulate local stem cells to promote joint repair [26–28]. Nanomedicines could also be grafted onto stem cells and humanized microparticles that once administered and attracted into inflamed joints would modulate the local immune response, downregulate synovial inflammation and promote articular repair.
4. Conclusion
We highlighted the role nanomedicine could play in improving long-term outcome of patients with RA and OA, two unmet medical needs. To streamline and expedite bench-to-bedside transition of novel nanomedicines for RA and OA in safe, cost-effective fashion, we propose that investigators and other stakeholders in the arthritis arena should conduct animal studies early on in the pre-clinical drug development stage to test U.S. FDA-approved injectable nanocarriers, such as liposomes, micelles, nanocrystals, nanoparticles, nanotubes and supermagnetic iron oxide, loaded with promising anti-rheumatic drugs. In these studies, both efficacy and toxicity of lead nanomedicines will be determined simultaneously in the same animal groups. Only safe and efficacious DMARNs thus discovered would then be tested in clinical trials. This approach would circumvent the need for a lengthy and costly safety testing of novel nanocarriers that have not been previously administered to human subjects.
Acknowledgment
This study was supported, in part, by VA Merit Review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Both co-authors have read and approved the manuscript as submitted. The manuscript has not been submitted nor is being reviewed by another journal. Both contributed equally to the writing of the manuscript.
Competing interest
Dr. Rubinstein is co-inventor of lipid-based formulations of VIP and has assigned certain issued and pending U.S. and international patents on this subject matter to the University of Illinois at Chicago. He was/is funded by grants from the National Institutes of Health and the Department of Veteran Affairs, USA. Dr. Weinberg has no disclosures to make related to the subject matter outlined in this manuscript.
Provenance and peer review
Commissioned and externally peer reviewed.
References
- 1.McInnes IB, O’Dell JR. State-of-the-art: rheumatoid arthritis. Ann Rheum Dis. 2010;69:1898–1906. doi: 10.1136/ard.2010.134684. [DOI] [PubMed] [Google Scholar]
- 2.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 3.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 4.Patra D, Sandell LJ. Recent advances in biomarker in osteoarthritis. Curr Opin Rheumatol. 2011;23:470–485. doi: 10.1097/BOR.0b013e328349a32b. [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Christensen R, Wells GA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2009;4 doi: 10.1002/14651858.CD007848.pub2. CD007848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraus VB, Burnett B, Coindreau J, et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage. 2011;19:515–542. doi: 10.1016/j.joca.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buch MH, Emery P. New therapies in the management of rheumatoid arthritis. Curr Opin Rheumatol. 2011;23:245–251. doi: 10.1097/BOR.0b013e3283454124. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 9.Amer M, Bead VR, Bathon J, Blumenthal RS, Edwards DN. Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease: a cautionary tale. Cardiol Rev. 2010;18:204–212. doi: 10.1097/CRD.0b013e3181ce1521. [DOI] [PubMed] [Google Scholar]
- 10.Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2 doi: 10.1002/14651858.CD008794.pub2. CD008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salliot C, Finckh A, Katchamart W, et al. Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysis. Ann Rheum Dis. 2011;70:266–271. doi: 10.1136/ard.2010.132134. [DOI] [PubMed] [Google Scholar]
- 12.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 13.Hoes JN, Jacobs JW, Buttgereit F, Bijlsma JW. Current view of glucocorticoid co-therapy with DMARDs in rheumatoid arthritis. Nat Rev Rheumatol. 2010;6:693–702. doi: 10.1038/nrrheum.2010.179. [DOI] [PubMed] [Google Scholar]
- 14.Blum MA, Koo D, Doshi JA. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther. 2011;33:901–913. doi: 10.1016/j.clinthera.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Davila L, Ranganathan P. Pharmacogenetics: implications for therapy in rheumatic diseases. Nat Rev Rheumatol. 2011;7:537–550. doi: 10.1038/nrrheum.2011.117. [DOI] [PubMed] [Google Scholar]
- 16.Pham CTN. Nanotherapeutic approaches for the treatment of rheumatoid arthritis. WIREs Nanomed Nanobiotechnol. 2011 doi: 10.1002/wnan.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo OM, Rubinstein I, Önyüksel H. Actively targeted low-dose camptothecin as a safe, long-acting, disease-modifying nanomedicine for rheumatoid arthritis. Pharm Res. 2011;28:776–787. doi: 10.1007/s11095-010-0330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernardi A, Zilberstein AC, Jager E, et al. Effects of indomethacin-loaded nanocapsules in experimental models of inflammation in rats. Br J Pharmacol. 2009;158:1104–1111. doi: 10.1111/j.1476-5381.2009.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Hoven JM, Van Tomme SR, Metselaar JM, Nuijen B, Beijnen JH, Storm G. Liposomal drug formulations in the treatment of rheumatoid arthritis. Mol Pharm. 2011;8:1002–1015. doi: 10.1021/mp2000742. [DOI] [PubMed] [Google Scholar]
- 20.Hwang J, Rodgers K, Oliver JC, Schluep T. α-Methylprednisolone conjugated cyclodextrin polymer-based nanoparticles for rheumatoid arthritis therapy. Int J Nanomed. 2008;3:359–371. doi: 10.2147/ijn.s3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas TP, Goonewardena SN, Majoros IJ, et al. Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis Rheum. 2011;63:2671–2680. doi: 10.1002/art.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosch X. Dendrimers to treat rheumatoid arthritis. ACS Nano. 2011 Aug; doi: 10.1021/nn203190x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23.Koning GA, Schiffelers RM, Wauben MH, et al. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate-containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006;54:1198–1208. doi: 10.1002/art.21719. [DOI] [PubMed] [Google Scholar]
- 24.Bawa R. Regulating nanomedicine: can the FDA handle it? Curr Drug Deliv. 2011;8:227–234. doi: 10.2174/156720111795256156. [DOI] [PubMed] [Google Scholar]
- 25.Chang C. The immune effects of naturally occurring and synthetic nanoparticles. J Autoimmun. 2010;34:j234–j246. doi: 10.1016/j.jaut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Schiffelers RM, Xu J, Storm G, Woodle MC, Scaria PV. Effects of treatment with small interfering RNA on joint inflammation in mice with collagen-induced arthritis. Arthritis Rheum. 2005;52:1314–1318. doi: 10.1002/art.20975. [DOI] [PubMed] [Google Scholar]
- 27.Wittmann J, Jack HM. MicroRNAs in rheumatoid arthritis: midget RNAs with a giant impact. Ann Rheum Dis. 2011;70(Suppl. 1):pi92–pi96. doi: 10.1136/ard.2010.140152. [DOI] [PubMed] [Google Scholar]
- 28.Nakasa T, Nagata Y, Yamasaki K, Ochi M. A mini-review: microRNAs in arthritis. Physiol Genomics. 2011;43:566–570. doi: 10.1152/physiolgenomics.00142.2010. [DOI] [PubMed] [Google Scholar]
- 29.Luisa Corvo M, Jorge JC, van’t Hof R, Cruz ME, Crommelin DJ, Storm G. Superoxide dismutase entrapped in long-circulating liposomes: formulation design and therapeutic activity in rat adjuvant arthritis. Biochim Biophys Acta. 2002;1564:227–236. doi: 10.1016/s0005-2736(02)00457-1. [DOI] [PubMed] [Google Scholar]
- 30.Perez AT, Domenech GH, Frankel C, Vogel CL. Pegylated liposomal doxorubicin (Doxil) for metastatic breast cancer: the Cancer Research Network, Inc., experience. Cancer Invest. 2002;20(Suppl. 2):22–29. doi: 10.1081/cnv-120014883. [DOI] [PubMed] [Google Scholar]
- 31.Jackson JK, Higo T, Hunter WL, Burt HM. Topoisomerase inhibitors as antiarthritic agents. Inflamm Res. 2008;57:126–134. doi: 10.1007/s00011-007-7163-6. [DOI] [PubMed] [Google Scholar]
- 32.Moody TW, Ito T, Osefo N, Jensen RT. VIP and PACAP: recent insights into their functions/roles in physiology and disease from molecular and genetic studies. Curr Opin Endocrinol Diabetes Obes. 2011;18:61–67. doi: 10.1097/MED.0b013e328342568a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keijzers GB. Aviptadil (Senatek) Curr Opin Investig Drugs. 2001;2:545–549. [PubMed] [Google Scholar]
- 34.Sethi V, Rubinstein I, Onyuksel H. Vasoactive intestinal peptide (VIP) loaded sterically stabilized micelles (SSM) for improved therapy of collagen induced arthritis (CIA) in mice. PharmSci. 2002;4(Suppl.):T2036. [Google Scholar]
- 35.Ishihara T, Kubota T, Choi T, Higaki M. Treatment of experimental arthritis with stealth-type polymeric nanoparticles encapsulating betamethasone phosphate. J Pharmacol Exp Ther. 2009;329:412–417. doi: 10.1124/jpet.108.150276. [DOI] [PubMed] [Google Scholar]
- 36.Brown CL, Whitehouse MW, Tiekink ER, Bushell GR. Colloidal metallic gold is not bio-inert. Inflammopharmacology. 2008;16:133–137. doi: 10.1007/s10787-007-0017-6. [DOI] [PubMed] [Google Scholar]
- 37.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]