Abstract
Intrinsically disordered proteins (IDPs) are now recognized to be prevalent in biology, and many potential functional benefits have been discussed. However, the frequent requirement of peptide folding in specific interactions of IDPs could impose a kinetic bottleneck, which could be overcome only by efficient folding upon encounter. Intriguingly, existing kinetic data suggest that specific binding of IDPs is generally no slower than that of globular proteins. Here, we exploited the cell cycle regulator p27Kip1 (p27) as a model system to understand how IDPs might achieve efficient folding upon encounter for facile recognition. Combining experiments and coarse-grained modeling, we demonstrate that long-range electrostatic interactions between enriched charges on p27 and near its binding site on cyclin A not only enhance the encounter rate (i.e., electrostatic steering), but also promote folding-competent topologies in the encounter complexes, allowing rapid subsequent formation of short-range native interactions en route to the specific complex. In contrast, nonspecific hydrophobic interactions, while hardly affecting the encounter rate, can significantly reduce the efficiency of folding upon encounter and lead to slower binding kinetics. Further analysis of charge distributions in a set of known IDP complexes reveals that, although IDP binding sites tend to be more hydrophobic compared to the rest of the target surface, their vicinities are frequently enriched with charges to complement those on IDPs. This observation suggests that electrostatically accelerated encounter and induced folding might represent a prevalent mechanism for promoting facile IDP recognition.
Introduction
Intrinsically disordered proteins (IDPs) are functional proteins that lack stable tertiary structures under physiological conditions 1;2. They often, but not always, fold into stable structures upon specific binding 3. IDPs are highly prevalent in proteomes, play crucial roles in cellular signaling and regulation, and are associated with numerous diseases 4–7. Substantial progress has been made in prediction, identification and general characterization of IDPs 8;9. However, significant challenges exist in detailed structural studies of disordered protein states, limiting the current understanding of specific conformational properties of unbound IDPs such as transient residual structures 10. Consequently, the physical basis of how intrinsic disorder mediates function remains poorly understood. Nevertheless, the prevalence of intrinsic disorder strongly supports the notion that protein conformational heterogeneity confers crucial functional advantages. This concept has been discussed extensively 11–14.
For cellular signaling and regulation, it is essential to achieve high association and disassociation rates 15. Nonspecific binding of unstructured and presumably extended conformations of IDPs could increase the capture radius to enhance the encounter rate, up to 1.6 fold 16. However, such “fly-casting” effect is offset by slower diffusion 17. Instead, conformational flexibility itself appears more important for binding kinetics. It could reduce the orientational restraints of forming specific complexes and increase the efficiency of peptide folding after initial encounter 17–20. Nonetheless, specific recognition that requires both binding and folding should be no faster than cases where folding is not required. Indeed, a recent dual-transition-rate theory 21 predicts that the (diffusion-limited) encounter rate constant represents the upper bound for that of a coupled binding and folding interaction. More importantly, the upper bound can be achieved only if the peptide readily folds upon encounter, which requires folding timescales beyond the µs “speed limit” estimated for folding of isolated proteins 22. Therefore, intrinsic disorder, while fulfilling certain functional constraints such as structural plasticity, could lead to a folding kinetic bottleneck that must be resolved for IDPs to viably function in signaling and regulation.
Intriguingly, existing kinetic data suggest that IDP binding is generally no slower, if not slightly faster, than that of globular proteins 17. The implication is that folding does not usually become rate limiting in IDP recognition, or, more specifically, IDPs can achieve efficient folding upon encounter. This concept is consistent with the observation that interacting domains of regulatory IDPs tend to be small and adopt simple folded topologies (i.e., with low contact order) in specific complexes. It is also consistent with an apparent prevalence of induced folding as the baseline mechanism of coupled binding and folding, despite the frequent presence of pre-folded structures in unbound IDPs 7;23. Nevertheless, it is not clear whether and how IDPs may exploit additional physical properties besides size and topology to achieve efficient folding as required for facile coupled binding and folding interactions.
The cyclin-dependent kinase (Cdk) regulators (CKRs) are among the first regulatory IDPs identified 24. They regulate the cell division cycle through direct interaction with Cdk/cyclin complexes 25, and play key roles in transcriptional regulation, cell differentiation, and apoptosis 26. The CKR kinase inhibitory domains (KIDs) fold upon specific binding to Cdk/cyclin complexes 24. A crystal structure of CKR p27Kip1 (p27) in complex with Cdk2/cyclin A is available 27 (Fig. 1B), which shows that subdomains 1 and 2 (D1, D2) of p27-KID interact with cyclin A and Cdk2, respectively. A linker helix (LH) connects D1 and D2. This subdomain exhibits partial helicity in the unbound state 28, and provides structural adaptability that is crucial for binding diverse Cdk/cyclin complexes 29. Structural, thermodynamic and kinetic studies together demonstrated that p27 recognized Cdk2/cyclin A via a sequential mechanism, initiated by rapid cyclin binding with an association rate constant of ka ~ 106 −1s−1 30. Subsequent binding to Cdk2 involves significant remodeling of the kinase structure, and is ~1000-fold slower 30. Nonetheless, much remains to be understood on the specific molecular steps involved in binding and folding of p27. Extensive biochemical and biophysical data exist to suggest intriguing complexities 25, and both nascent structures and conformational flexibility have been emphasized for promoting quick binding 31–33. Here, we exploit the CKRs as a model system and combine computation and experiment to investigate how the interplay of residual structures, conformational flexibility and electrostatic interactions of IDPs might support facile recognition and regulation.
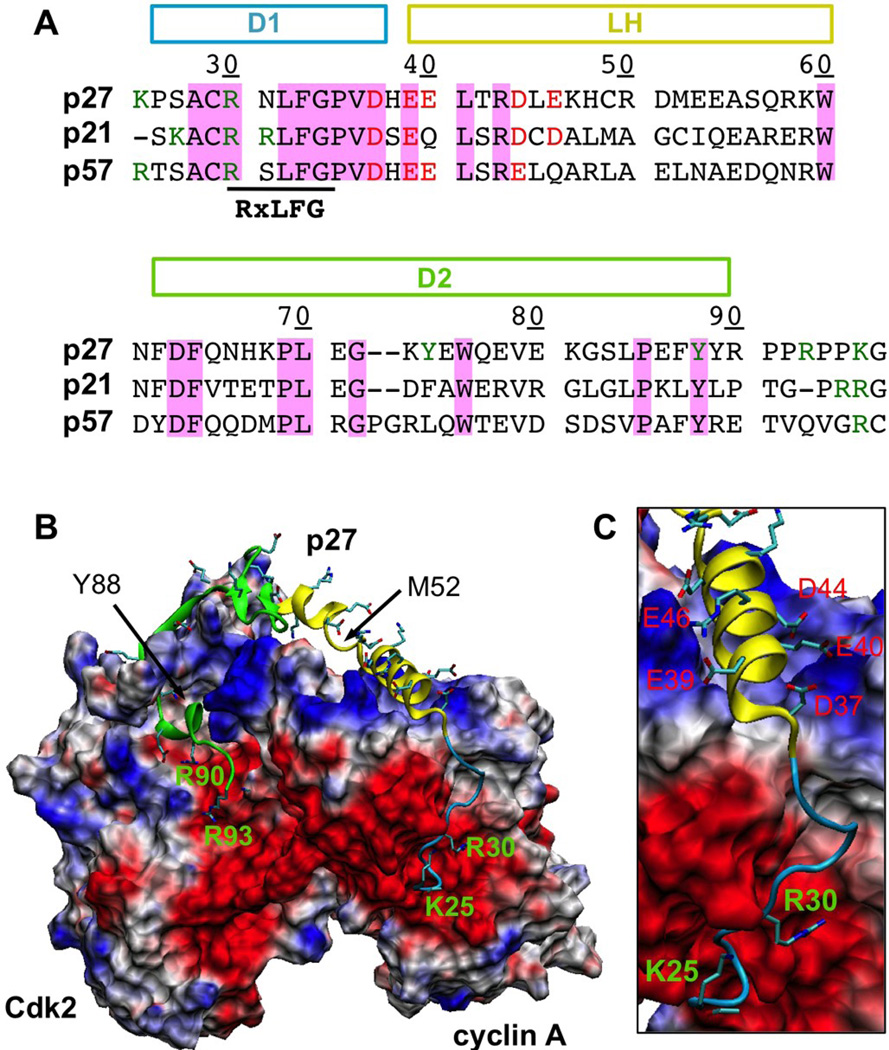
Fig. 1.
A. Sequence alignment of KIDs from three CKRs, The p27 residue numbering is shown, and the locations of three sub-domains (D1, LH and D2) are marked. Conserved residues are highlighted with a pink background. Key charges at the termini and the N-terminal segment are also colored. B. The structure of p27 in complex with Cdk2/cyclin A (PDB: 1jsu 27). The sub-domains D1 (residues 25–36), LH (residues 37–60) and D2 (residues 62–90) are colored in blue, yellow and green, respectively. All charged side chains of p27 are shown in sticks. Cdk2/cyclin A is shown in molecular surface and colored based on the surface electrostatic potential calculated without p27 using CHARMM PBEQ 66 (negative: red; positive: blue). C. A close-up view of the N-terminal half of p27 on cyclin A with key p27 charges labeled.
Results
Surface electrostatic potential: potential roles of electrostatic forces
Analysis of the surface electrostatic potential of the Cdk2/cyclin A complex (Fig. 1B) shows that several prominent features exist to complement a large number of conserved charges on p27 (Fig. 1A). For example, two large negative patches anchor two pairs of conserved charges on the p27-KID N- and C-termini; a positive patch on cyclin A complements five largely conserved negative charges within the LH N-terminus (Fig. 1C). In contrast, the Cdk2/cyclin A surface opposite to the p27 binding interface lacks any significant electrostatic features (data not shown). The highly evolved nature of the surface electrostatic characteristics strongly suggests key functional roles. Besides neutralizing enriched p27 charges to stabilize the folded state, long-range electrostatic interactions could directly mediate coupled binding and folding. For example, electrostatic force is known to be a dominant long-range force for accelerating protein binding 34, and can guide protein orientation in protein-DNA interactions 35;36. More importantly, electrostatic force can modulate protein folding 37. Well-located complementary charges can enhance the folding rate by promoting productive topologies early in the folding process 38 and/or by preventing the protein from accessing (folding) inefficient regions of the free energy landscape 39. Conversely, salt-induced premature formation of compact intermediates can slow down folding, over 10,000 fold for the ribosomal protein S6 40. The conformational ensembles of free IDPs are highly fluctuating and susceptible to weak electrostatic forces 41. It is conceivable that electrostatic forces exerted on enriched p27 charges modulate its coupled binding and folding and promote efficient folding upon encounter as required for facile recognition.
Salt dependence of p27/Cdk2/Cyclin A association kinetics: beyond electrostatic steering
The salt dependence of p27-Cdk2/cyclin A binding thermodynamics and kinetics was probed using isothermal titration calorimetry (ITC) and surface plasmon resonance (SPR) (Fig. S1 and Table 1). The result show that ka displayed the expected strong salt dependence, decreasing ~12 fold when the ionic strength was increased from 0.075 to 0.6 M. However, binding thermodynamics showed surprisingly small salt dependence. This is likely due to the complex multi-step nature of p27 coupled folding and binding 30. Indeed, inspection of the interaction of p27-D1 alone with Cdk2/cyclin A revealed the expected salt-dependence of binding thermodynamics (Table S1). The transient complex theory of protein binding kinetics predicts that the salt dependence of ka for a simple binding interaction (i.e., without significant protein conformational transitions) can be well described by a Debye-Huckel-like approximation, ln ka = ln ka0 – U0 / kT / (1 + a κ). Here, κ is the Debye-Huckel parameter, ka0 is the basal rate constant without electrostatic steering, U0 corresponds to the electrostatic interaction energy of the transient complex, and a is a fitting parameter 34. Interestingly, fit of ka from Table 1 to this empirical linear relation appears problematic, yielding unphysical parameters with a positive U0 (which suggests an electrostatically destabilized transient complex) (Fig. S2A). Restricting U0 to be negative leads to a much poorer fit (Fig. S2B). The implication is that the observed electrostatic acceleration of p27 binding to Cdk2/cyclin A arises from a more complex mechanism than simple electrostatic steering.
Table 1.
Salt-dependence of p27-Cdk2/cyclin A binding kinetics and thermodynamics.
| NaCl (mM) | ka (106M−1s−1) | KD (nM) | ΔG (kcal/mol) | ΔH (kcal/mol) | −TΔS (kcal/mol) |
|---|---|---|---|---|---|
| 75 | 10.6 ± 0.1 | 1.1 ± 0.6 | −12.3 ± 0.3 | −50.3 ± 3.6 | +38.0 ± 3.6 |
| 150 | 3.21 ± 0.01 | 1.3 ± 0.8 | −12.2 ± 0.4 | −49.5 ± 1.6 | +37.3 ± 1.8 |
| 300 | 1.26 ± 0.01 | 0.5 ± 0.6 | −12.9 ± 0.7 | − 51.0 ± 4.2 | +38.0 ± 4.3 |
| 600 | 0.894 ± 0.001 | 0.6 ± 0.3 | −12.7 ± 0.5 | −48.7 ± 3.3 | +36.0 ± 3.7 |
| 1000 | - | 0.6 ± 0.4 | −12.6 ± 0.3 | −45.4 ± 1.8 | +32.8 ± 2.1 |
The Gibbs free energy of binding ΔG was calculated from the dissociation constant, ΔG = RT ln KD, and the binding entropy from the binding free energy and enthalpy as −TΔS = ΔG–ΔH.
Coarse-grained modeling of p27 coupled binding and folding
We exploited topology-based coarse-grained modeling 42 to further understand the mechanism of folding and binding of p27 to Cdk2/cyclin A and how it is modulated by nonspecific electrostatic and hydrophobic interactions. It has been shown previously that the measured fast association kinetics arises from cyclin binding 30, which involves both subdomain D1 and the N-terminal segment of LH. LH exhibits a helix break at Met52, which naturally separates the cyclin A and Cdk2 interacting segments (Fig. 1B). Therefore, we included only D1 and the LH N-terminal segment of p27-KID (residues 25–51) and focused on its interaction with cyclin A in the current modeling study. Such a reduced model also avoids unnecessary complications that might arise from the need to properly model the Cdk2 binding step, which involves p27-induced Cdk2 remodeling but does not contribute to the rapid association phase. Starting from the p27/Cdk2/cyclin A structure, two sets of Gō-like models were constructed (Table 2). These models were carefully calibrated (see Methods), such that they yielded similar dissociation constants (KD) (Table 2) and residual structures in unbound p27 (Fig. S3). Such calibration is critical 43 for ensuring that the model does not inherently favor particular types of interactions, e.g., intra- vs. inter-molecular or native vs. nonspecific electrostatic. Note that the calculated KD value can be quite sensitive to small changes in the scaling of intermolecular interaction strengths (e.g., a few percent). Coupled with slow binding/folding (especially for uncharged models 1–7), it is computationally challenging to reproduce experimental KD values even with replica exchange sampling. Nonetheless, by simulating at corresponding melting temperature (Tm), remaining imperfections in the balance of various interactions are further suppressed, allowing reliable investigation of the effects of different native and non-native interactions on p27 binding and folding.
Table 2.
Calculated dissociation constants, melting temperatures and mean folding transition rates of all coarse-grained models of p27/cyclin A investigated in this study.
| Model | Charges | εHP | KD (nM) | Tm (K) | kTS (μs−1) |
|---|---|---|---|---|---|
| 1 | None | 0.0 | 154 ± 192 | 323 | 3.2 ± 0.4 |
| 2 | None | 0.05 | 6.24 | 327 | 3.6 ± 0.1 |
| 3 | None | 0.1 | 16 | 327 | 2.9 ± 0.4 |
| 4 | None | 0.2 | 242 | 325 | 2.5 ± 0.3 |
| 5 | None | 0.3 | 117 | 325 | 2.5 ± 0.04 |
| 6 | None | 0.4 | 20 | 325 | 2.6 ± 0.3 |
| 7 | None | 0.5 | 27 | 323 | 2.3 ± 0.4 |
| 8 | Explicit | 0.0 | 300 ± 148 | 325 | 30.0 ± 3.1 |
| 9 | Explicit | 0.05 | 68 | 330 | 28.1 ± 0.9 |
| 10 | Explicit | 0.1 | 86 | 327 | 23.7 ± 0.3 |
| 11 | Explicit | 0.2 | 174 | 327 | 23.4 ± 1.6 |
| 12 | Explicit | 0.3 | 83 | 327 | 23.6 ± 1.9 |
| 13 | Explicit | 0.4 | 148 | 325 | 21.3 ± 2.5 |
| 14 | Explicit | 0.5 | 153 | 325 | 20.9 ± 0.7 |
| 15 | Explicit; 0.15 M salt |
0.0 | 192 | 325 | 5.4 ± 0.6 |
| 16 | Explicit; (Mutant) |
0.0 | 98 | 325 | 22.4 ± 4.9 |
| 17 | Explicit; (Mutant) |
0.0 | 236 | 323 | 20.1 ± 1.1 |
KD was calculated from REX simulations (experimental KD = 25 ± 2.7 nM (29). kTS was calculated from production Langevin simulations at the corresponding Tm as kTS = NTS/ttot, where NTS is the number of reversible p27 binding and folding transitions observed during ttot. Models 16 and 17 were calibrated to yield ~30% and ~75% residual LH helicities in the free state, respectively. All uncertainties were estimated from differences between results calculated from the first and second halves of the data.
Mechanism of electrostatic acceleration
Kinetic parameters for binding derived from Langevin simulations using the calibrated Gō-like models recapitulate the salt dependence of ka from SPR. As shown in Table 2, uncharged models (Models 1–7), which mimic the high-salt condition with fully screened long-range electrostatic interactions, yield p27 binding/folding transition rates of kTS ~ 2.3–3.6 µs−1, compared with kTS ~ 20–30 µs−1 for models with explicit charges (Models 8–14), which mimic the low salt condition with unscreened electrostatic interactions. The predicted ~10 fold electrostatic acceleration, as reflected in the salt dependence, is in quantitative agreement with SPR. With 0.15 M salt (Model 15), the calculated transition rate is ~5 fold slower than that in the no salt case (Model 8). This also compares well with SPR results, even though there appears to be an over-prediction of salt-induced rate reduction. The over prediction is likely due the use of Cα-only models, and might be corrected with more detailed models where charges are placed near the side chain tips 37.
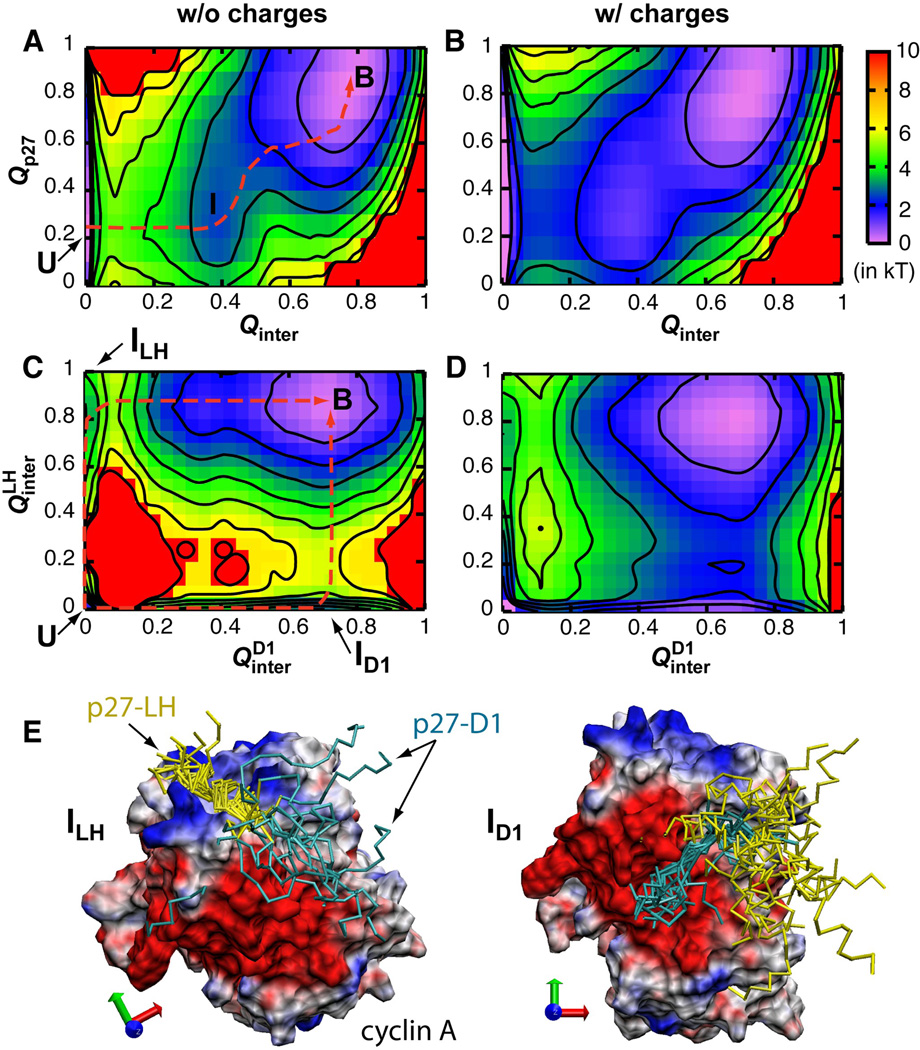
Extensive free energy and kinetic analysis was performed to obtain a mechanistic understanding of the observed electrostatic acceleration. As shown in Fig. 2, electrostatic forces substantially reduce the free energy barrier for coupled binding and folding of p27. The predicted ~2 kT barrier reduction is apparently consistent with ~10 fold rate acceleration derived from Langevin simulations, suggesting that Qinter is a good reaction coordinate. Analysis of free energy surfaces along combinations of various order parameters supports that p27 recognition follows an induced folding-like baseline mechanism, which is arguably necessary for achieving fast binding with ka ~ 106 M−1s−1. For example, Fig. 3A shows that Qp27 does not increase until Qinter ~ 0.4 (dashed line). That is, binding precedes folding. Further examination of the free energy surface along and supports the existence of two parallel pathways (Fig. 3C). Either subdomain D1 or LH can initiate binding through a key intermediate state, marked as ID1 and ILH, respectively. Coincidently, when projected along Qinter and Qp27, ID1 and ILH overlap to give rise to a single apparent intermediate basin centered at Qinter ~ 0.4, Qp27 ~ 0.3 (Fig. 3A). Importantly, long-range electrostatic interactions do not alter these basic mechanistic features of p27 coupled binding and folding, except to lower the free energy barriers separating various states (Fig. 3B and D).
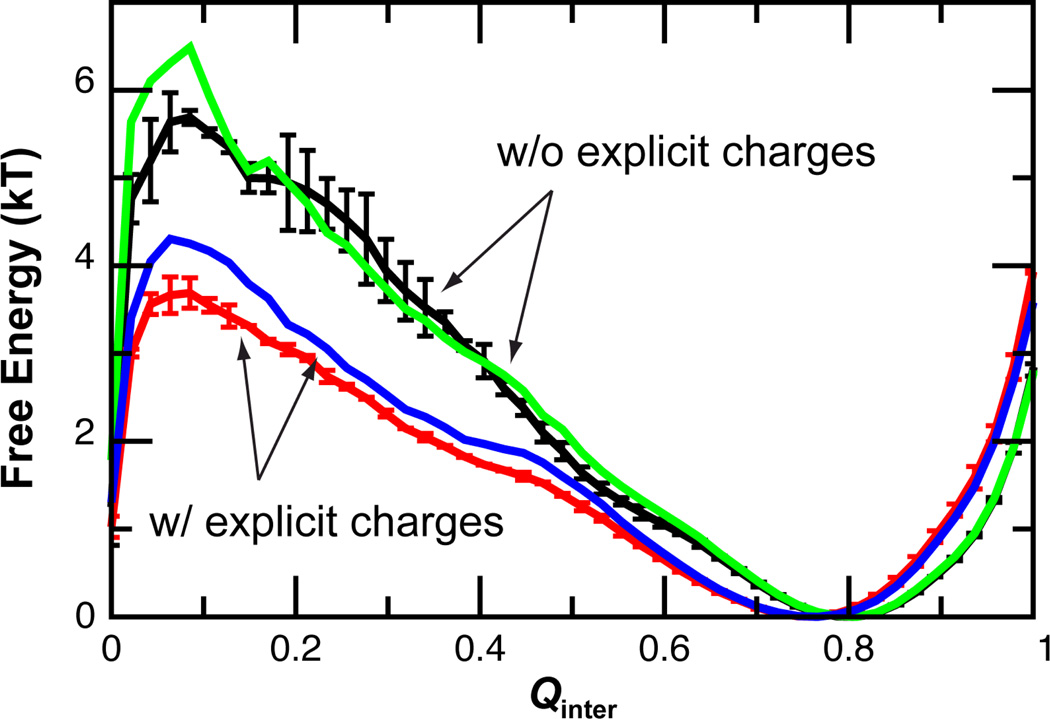
Fig. 2.
Free energy as a function of the fraction of native inter-molecular contacts formed (Qinter) at 300 K for four representative models: 1 (black), 7 (green), 8 (red), and 14 (blue) (see Table 2). These profiles were calculated from the REX simulations using WHAM. The error bars are not shown for the blue and green traces for clarity.
Fig. 3.
Free energy surfaces as a function of various fractions of native contacts computed using Gō-like models with (Model 8) and without (Model 1) explicit charges. and are the fractions of native inter-molecular contacts form by sub-domains D1 and LH, respectively. Q p27 is the fraction of intra-molecular contacts formed by p27. All surfaces were shifted such that the bound state is at zero. Contours are drawn every kT. Dashed lines in panels A and C indicate the minimal free energy paths. Panel E show two representative ensembles of ID1 and ILH with cyclin A, p27-D1 and p27-LH colored grey, cyan and yellow, respectively.
Based on the free energy analysis, the binding process was dissected to include an encounter step followed by multiple steps of p27 folding on the cyclin A surface. Conformations sampled during Langevin simulations were assigned to five states: unbound (U), collision complexes (CC), intermediates ID1 and ILH, and bound (B) (see Methods for details). The mean first passage times (MFPTs) and numbers of transitions between these states were then calculated to analyze the effects of electrostatic forces on different stages of p27 binding and folding. Representative results from Models 1 and 8, summarized in Table S2, show that long-range electrostatic force reduces the average encounter time from 1.03 ns to 0.26 ns, a ~3.8 fold acceleration. At the same time, the probability of advancing to the intermediate and bound states improves from 0.96% to 2.7%, providing an additional ~2.8 fold acceleration. Therefore, electrostatic forces enhance the binding kinetics not only by increasing the encounter rate, but also by enhancing the efficiency of folding upon encounter. Such a mechanistic understanding explains why the measured salt-dependence of ka is not well described by the Debye-Huckel-like approximation (Fig. S2).
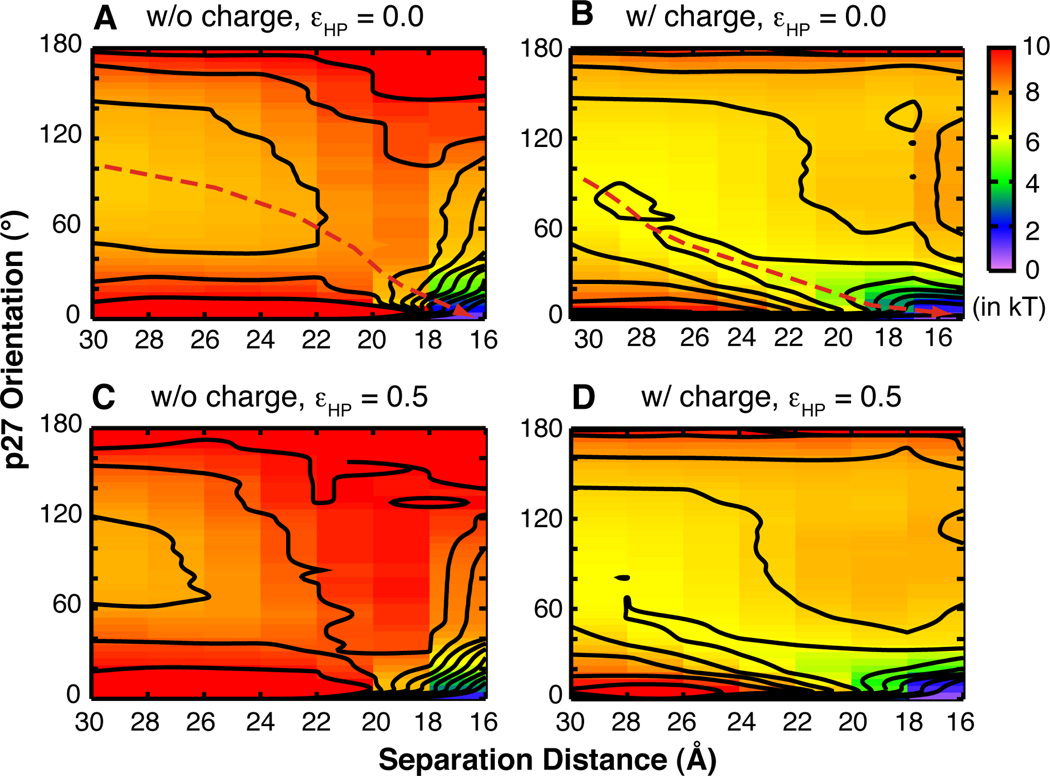
Detailed inspection of the conformational properties of the collision complexes provides further insights into the molecular basis for enhanced efficiency of p27 folding upon encounter. Comparison of Fig. 4 A and B shows that long-range electrostatic forces generate a strong free energy gradient that extends over 10 Å away from the native bound position (see dashed lines), such that p27 is more likely to adopt folded-like orientations as it approaches cyclin A. Without electrostatic guidance, p27 encounters cyclin A in largely random orientations (Fig. 4A), and thus cannot fold efficiently before the collision complex dissociates. Furthermore, the distributions of p27 on the cyclin A surface in the collision complexes (Fig. 5A and B) show that nonspecific electrostatic forces also significantly enhance the probability of capturing p27 near the native binding site. Together, these free energy and structural analyses suggest that long-range electrostatic interactions can promote productive topologies during early stages of coupled binding and folding and thus enhance the efficiency of p27 folding upon encounter.
Fig. 4.
Free energy surfaces of p27 coupled binding and folding as a function of p27-cyclin separation distance, defined as the distance between the centers of mass of p27 and all p27-contacting residues of cyclin A, and p27 orientation, defined using its principal axis with respect to the native bound conformation. Contours are dawn every kT, and dashed lines in panels A and B indicate the minimal free energy paths.
Fig. 5.
Distributions of p27 on the cyclin A surface in the collision complexes. Cyclin A is colored based on the probability of each residue in contact with p27 in the collision complex ensembles. The conformation of p27 in the native complex is shown in gray trace as a reference.
Effects of nonspecfic hydrophobic interactions
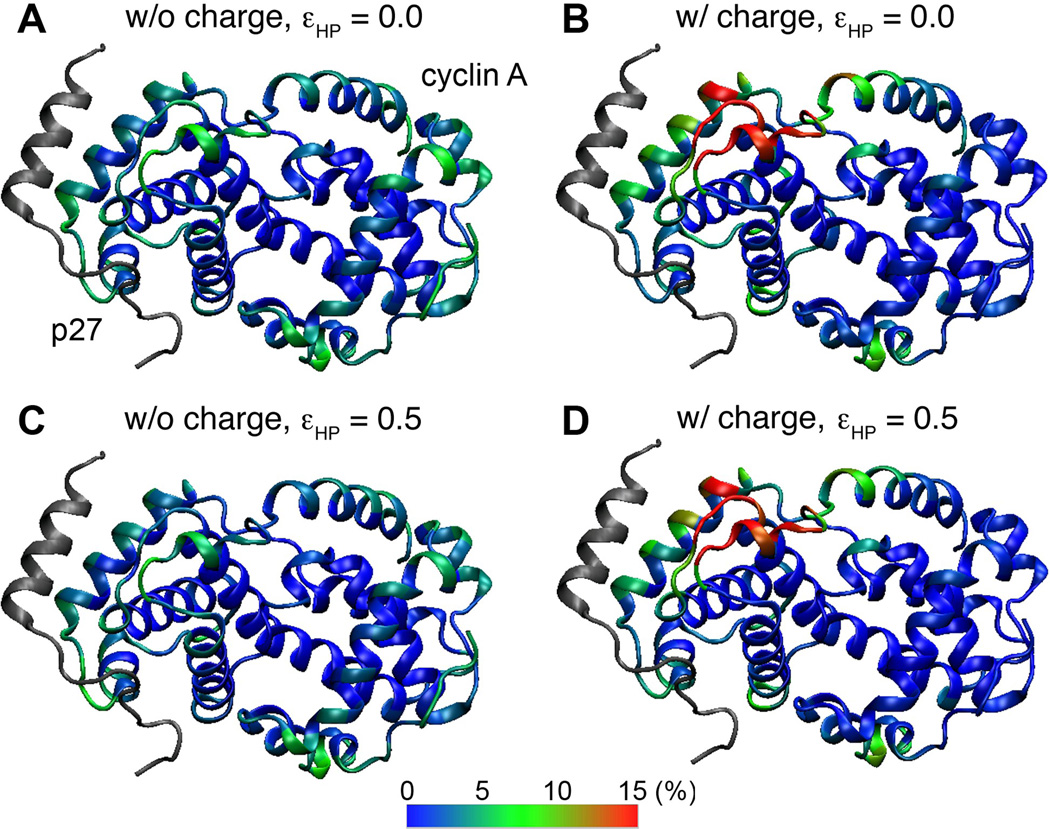
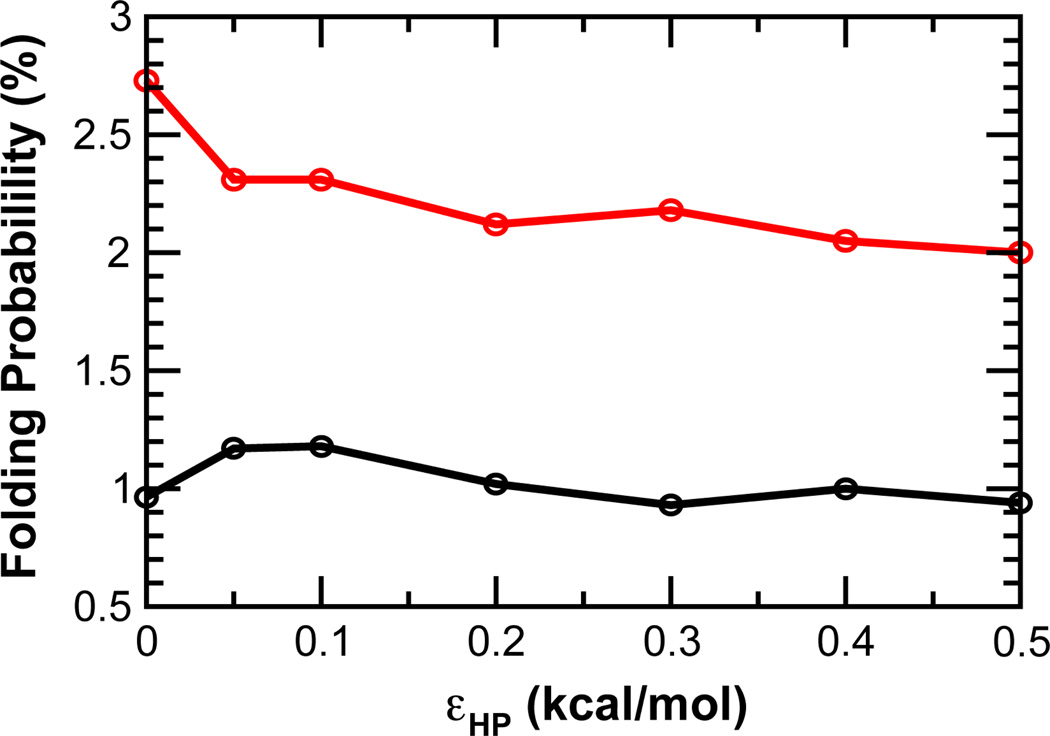
Recent modeling studies have suggested that weak nonspecific hydrophobic interactions can enhance IDP binding kinetics 44;45. For folding and binding of pKID to KIX, short-range nonspecific hydrophobic interactions minimally affected the encounter rate 44. However, the escape and folding rates of the “encounter state” are both reduced, but importantly, to different degrees, which causes the overall association rate to first increase and then dramatically decrease with stronger nonspecific hydrophobic interactions. A qualitatively similar trend was observed for p27 binding to cyclin A when the charges were not explicitly modeled (Models 1–7 in Table 2): kTS first slightly increases from 3.2 to 3.6 µs−1 with εHP = 0.05 kcal/mol, but then decreases significantly with larger values of εHP, to 2.3 µs−1 when εHP = 0.5 kcal/mol. However, in the pKID/KIX study 44, the binding rate did not decrease until nonspecific hydrophobic interactions became stronger than native ones. Besides technical differences in the Gō-like models employed, the dramatic difference in predicted optimal strengths of nonspecific hydrophobic interactions might be attributed to the protein size. Specifically, cyclin A is over three times larger than KIX. Only ~17% of cyclin A surface residues are involved in native contacts with p27, whereas ~30% of KIX surface residues contribute to specific pKID binding. The higher “non-reactive” to “reactive” surface ratio of cyclin A should render p27/cyclin A more sensitive to kinetic trapping from non-discriminative stabilization of encounter complexes. Indeed, nonspecific hydrophobic interactions do not help recruit p27 to the native binding interface at encounter (e.g., see the similar distributions in Fig. 5 A vs. C and B vs. D), and the efficiency of p27 folding upon binding decreases substantially with εHP > 0.1 kcal/mol after a small initial increase (Fig. 6, black trace).
Fig. 6.
Efficiency of p27 folding upon binding with increasing strength of nonspecific hydrophobic interactions. The red trace is for models with explicit charges and the black trace is for models without explicit charges. The folding efficiency was calculated as the probability of advancing to the intermediate and bound states (ID1, ILH, and B) from the collision complex (CC).
Intriguingly, when applied together with long-range electrostatic forces, nonspecific hydrophobic interactions appear to be always decelerating, even for very weak ones such as εHP = 0.05 kcal/mol (Table 2). This slowing effect can be mainly attributed to decreasing efficiency of folding upon encounter with larger εHP (Fig. 6, red trace). This result highlights the central role of electrostatic forces in modulating coupled binding and folding. Extensive free energy analysis supports that the mechanistic details of p27 binding and folding are robust against nonspecific hydrophobic interactions. The main effects include elevated free energy barriers (Fig. 2) and slightly stabilized intermediate states (e.g., comparing Fig. 3 and S4). In addition, nonspecific hydrophobic interactions can also generate a weak free energy gradient that extends a few Å from the native binding interface (Fig. 4C), which explains small increases in p27 folding efficiency observed for small values of εHP (Fig. 6).
Nascent structures, conformational flexibility vs. long-range electrostatic forces
A triple alanine mutant of p27 (E40A/D44A/K47A) was previously designed to investigate the role of nascent helices in binding kinetics 32. The mutations increased the residual helicity of subdomain LH from ~30% to ~75%. However, the rate of forming inhibited p27/Cdk2/cyclin A complex was reduced ~2–3 fold. This was interpreted as strong evidence for the binding kinetic advantage of intrinsic disorder 32. Interestingly, an un-intended effect of this mutant is removal of three key charges within the LH N-terminus, and potential consequences have not been considered. For this, two additional Gō-like models were designed to determine the differential contributions of nascent helices, conformational flexibility and electrostatic forces (Table 2). In model 16, the residual helicity of LH remained at ~30% and only charges on E40/D44/K47 were removed, allowing the consequence of charge removal to be selectively determined. In Model 17, the residual helicity of LH was re-calibrated to be ~75%, allowing the effects of both helix stabilization and charge removal to be studied. The results show that the charge removal alone reduces kTS from 30.0 µs−1 to 22.4 µs−1. Such a significant rate reduction is notable, as the net charge differs only by −1. Stabilization of the LH helix reduces kTS further to 20.1 µs−1. We note that the predicted overall rate reduction is smaller than 2–3 fold determined by the kinase activity assay 32. It is plausible that a flexible LH is important for the second, slower step of Cdk2 binding. Nonetheless, these calculations clearly support that both long-range electrostatic forces and conformational flexibility are important for p27 binding kinetics. We note that concerted involvement of flexibility and electrostatic interactions has also been shown to facilitate protein-DNA binding 46.
Enrichment of charges near IDP binding sites
IDPs are known to be significantly enriched with charged and polar residues 47. It is probable that electrostatically accelerated binding and folding observed for p27 might be prevalent in IDPs. To investigate this further, we analyzed the surface charge distributions for 51 complexes that involve a single IDP interacting with one or more structured protein partners (see Fig. S5 for a list of the PDB IDs). The results confirm the importance of hydrophobic contacts for intermolecular interactions involving IDPs 48, manifested as a depletion of charged residues at the binding interface. On average, 32.4% of substrate surface residues are charged, whereas only 27.6% at the IDP binding interface are charged. At the same time, charges are substantially enriched in the vicinity of the IDP binding site, where 34.2% of surface residues are charged. A continuum of electrostatic characteristics is also evident even within this modest set of IDP complexes. Some IDP-binding interfaces are heavily charged and surrounded by surfaces that are depleted of charged residues (e.g., complexes listed to the right end of Fig. S5). Nonetheless, a majority of these complexes (~60%) appears to possess electrostatic signatures similar to those observed for p27/Cdk2/cyclin A (Fig. 1). The implication is that electrostatic forces likely play a general role in IDP binding and folding and the surface electrostatic features of IDP-binding proteins have evolved to promote facile recognition.
Interestingly, a correlation between charge signatures and binding mechanisms appears to exist. Formation of complexes with charge enrichment near the binding site tends to follow induced folding-like baseline mechanisms. Previously characterized examples include 1dt7 49, 1jsu 33, 1dpj 50;51, 1kdx 52;53, 2rnl 54 and 1cee 55 (highlighted in red in Fig. S5). Note that complex 1cee (WASP/Cdc42) is the only (weak) exception, with a slight depletion of charges near the WASP binding site. This correlation is consistent with the notion that a key role of long-range electrostatic forces is to promote efficient folding of IDPs upon encounter (that is, induced folding) to achieve fast binding.
Discussion
One of the most important properties of IDPs is arguably that they can readily fluctuate among alternative conformational sub-states, spontaneously or in response to external stimuli such as binding of specific targets 13. Such conformational flexibility is particularly important for IDPs to overcome the potential kinetic bottleneck due to the requirement of peptide folding for specific recognition 19;20. Combining kinetic analysis using SPR, topology-based modeling and statistical analysis, we demonstrate that electrostatic interactions between enriched charges on IDPs and near their binding sites also play a key role in allowing IDPs to overcome the folding bottleneck. Specifically, long-range electrostatic forces do not only accelerate the encounter rate, but could also promote efficient folding upon encounter by modulating the early stages of coupled binding and folding. Importantly, previous studies have suggested that nonspecific (electrostatic) interactions can compete with native ones to destabilize the folded state and/or reduce folding kinetics56. These effects could translate into a competition between binding and folding rates as previously predicted in the case of protein-DNA assembly57. The observed electrostatic acceleration of folding in the p27/Cyclin A interaction requires sufficient level of self-consistency between charge distribution and folded topology. It is not clear how common IDP complexes possess such self-consistency for nonspecific electrostatic interactions to accelerate both binding and folding, even though our initial charge analysis of known IDP complexes clearly supports a prevalent role of electrostatic forces. Nonetheless, considering that interaction domains of IDPs are often small and adopt simple folded topologies (as required for fast folding), it should not be surprising that strong self-consistency between charge distributions and native folds has been frequently achieved in IDP complexes.
Interestingly, post-translational modifications frequently add or remove charges, and can thus profoundly impact IDP structure and interaction. For example, phosphorylation of p27 Tyr88 and possibly Tyr74 58;59 can lead to local unbinding and partial activation of p27/Cdk2/cyclin A (see Fig. 1). This allows subsequent intra-complex phosphorylation of p27 Thr187 by Cdk2, to promote p27 proteasomal degradation and allow the cell cycle to advance. An intriguing correlation also can be identified between patterns of charge conservations and multi-specificity of CKRs in Cdk/cyclin binding (Fig. S6). This correlation suggests that a generally conserved, electrostatics-guided binding and folding mechanism exists to mediate interactions of CKR with diverse Cdk/cyclin targets in cell cycle regulation. The mechanistic knowledge on CKR binding-induced folding from the current study will thus help understand their function and regulation in both normal and malignant cells.
Materials and Methods
Topology-based protein modeling: nonspecific electrostatic and hydrophobic interactions
A Cα-based sequenced-flavored Gō-like model 60 was first derived from the p27/Cdk2/cyclin A structure (PDB: 1jsu) 27. Details of this model have been described previously 27. The model was first reduced to include only cyclin A and the p27 N-terminal segment (residues 25–51; see Fig. 1). The model was then calibrated to balance intrinsic folding and intermolecular interactions using a previously described protocol 43. Briefly, the strengths of intra-molecular native contact interaction were first scaled to match the experimental helicity of unbound p27 (~30% in LH) 32. The strengths of inter-molecular contacts were then adjusted to reproduce the experimental binding affinity, Kd ~ 25 nM 30. The final model contains 47 inter-molecular (20 from D1 and 27 from LH) and 17 intra-molecular native contacts. Nonspecific electrostatic interactions are modeled using the Debye-Huckel potential to account for ionic screening 37. The dielectric constant is 80. Proper unit charges are assigned to charged residues (Lys, Arg, Glu and Asp) on both p27 and cyclin A. The strengths of native salt-bridge interactions were adjusted to 60% of the original values to avoiding double counting. Nonspecific interactions between all hydrophobic residues (Ala, Cys, Val, Leu, Ile, Met, Phe, Pro, Trp, Tyr) were modeled using 12-6 Lennard-Jones potentials with various strengths (εHP=0.05, 0.1, 0.2, 0.3, 0.4 and 0.5 kcal/mol). Models with different combinations of nonspecific interactions were independently calibrated using the same protocol to reproduce the experimental residual helicity and binding affinity (Table 2).
Simulation and Analysis
The complex was simulated in a 110 Å cubic box with periodic boundary conditions using CHARMM 61;62. Langevin dynamics was performed with a dynamic time step of 15 fs and a friction coefficient of 0.1 ps−1. To calibrate intra-molecular interactions, free p27 was simulated at 300 K for 750 ns. Replica exchange (REX) was used to calculate Kd and Tm, performed using the MMTSB Toolset 63 with eight replicas spanning 270 to 400 K. The length of calibration REX simulations is 3.15 µs. For each calibrated model (Table 2), a 22.5 to 30 µs production simulation was performed at Tm. All free energy (except Fig. 2) and kinetic data shown were calculated from the production simulations. A given native contact is considered formed if the inter-Cα distance is no more than 1.0 Å greater than the distance in the native complex. Helicity was calculated as the fraction of 1–5 (backbone) native contacts formed. Temperature weighted histogram analysis method (WHAM) 64 was used to compute the heat capacity curves and unbiased probability distributions from REX data. The dissociation constants were calculated from the bound and unbound probabilities 43, where the unbound state was identified as the one without any native intermolecular contacts formed. To investigate the effects of nonspecific interactions on different steps of coupled binding and folding, five conformational states were defined based on the number of various native and nonspecific contacts formed: U (Ninter < 1 and ), ID1 ( and ), ILH ( and ), B (Ninter > 30), and collision complexes (Ninter < 3, and not assigned to any of the above states). is the number of nonspecific inter-molecular contacts, defined when the inter-C〈 distance is below 10 Å. For charged models, slightly different criteria were used due to small shifts of some free energy basins (e.g., see Fig. 3): ID1 ( and ), ILH ( and ), B (Ninter > 25 and and ). Using these criteria, <5% of the snapshots were not assigned to any state and often corresponded to transition paths. All molecular visualization was prepared using VMD 65.
Sample preparation and procedures for SPR and ITC experiments
Human cyclin A (residues 173–432), full-length Cdk2, p27-KID and p27-D1 were expressed in E. coli BL21 (DE3) and purified according to established procedures 30. Surface Plasmon resonance (SPR) experiments were performed using a BIACORE 3000 instrument (GE Healthcare, Piscataway, NJ). His-tagged p27-KID was immobilized by direct, chemical capture on a carboxymethyl dextran-coated gold surface (CM4 Chip; GE Healthcare) and the association of Cdk2/cyclin A was monitored in real time. ITC experiments were performed using an Auto-ITC 200 microcalorimeter (GE Healthcare, Piscataway, NJ). His-tagged p27-KID or p27-D1 was titrated into solutions of Cdk2/cyclin A that was previously prepared using gel filtration chromatography. All SPR and ITC experiments were performed in triplicates, and the average values and standard deviations are reported. Details of the SPR and ITC procedures are provided under Supplementary Materials.
Supplementary Material
Highlights.
Efficient folding upon encounter is required for fast IDP association.
The p27/Cdk2/cyclin A complex contains highly-evolved electrostatics features.
Long-range electrostatic forces accelerate both encounter and induced folding.
Nonspecific hydrophobic forces tend to reduce the binding and folding rates.
IDP binding interfaces are frequently surrounded with enriched charges.
Acknowledgements
This work was supported by National Science Foundation through a CAREER Award (MCB 0952514 to J.C.) and by National Institutes of Health through P30-CA21765 (St. Jude Children’s Research Hospital) and 5R01-CA082491 (to R.W.K.). This work is contribution number 12-289-J from the Kansas Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright PE, Dyson HJ. Intrinsically unstructured proteins: Re-assessing the protein structure-function paradigm. Journal of Molecular Biology. 1999;293:321–331. doi: 10.1006/jmbi.1999.3110. [DOI] [PubMed] [Google Scholar]
- 2.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nature Reviews Molecular Cell Biology. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 3.Mittag T, Orlicky S, Choy WY, Tang XJ, Lin H, Sicheri F, Kay LE, Tyers M, Forman-Kay JD. Dynamic equilibrium engagement of a polyvalent ligand with a single-site receptor. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17772–17777. doi: 10.1073/pnas.0809222105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ, Campen AM, Ratliff CR, Hipps KW, Ausio J, Nissen MS, Reeves R, Kang CH, Kissinger CR, Bailey RW, Griswold MD, Chiu M, Garner EC, Obradovic Z. Intrinsically disordered protein. Journal of Molecular Graphics & Modelling. 2001;19:26–59. doi: 10.1016/s1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 5.Tompa P. Intrinsically unstructured proteins. Trends in Biochemical Sciences. 2002;27:527–533. doi: 10.1016/s0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 6.Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: Introducing the D-2 concept. Annual Review of Biophysics. 2008;37:215–246. doi: 10.1146/annurev.biophys.37.032807.125924. [DOI] [PubMed] [Google Scholar]
- 7.Click TH, Ganguly D, Chen JH. Intrinsically Disordered Proteins in a Physics-Based World. International Journal of Molecular Sciences. 2010;11:5293–5309. doi: 10.3390/ijms11125292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bracken C, Iakoucheva LM, Rorner PR, Dunker AK. Combining prediction, computation and experiment for the characterization of protein disorder. Current Opinion in Structural Biology. 2004;14:570–576. doi: 10.1016/j.sbi.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Eliezer D. Biophysical characterization of intrinsically disordered proteins. Current Opinion in Structural Biology. 2009;19:23–30. doi: 10.1016/j.sbi.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher CK, Stultz CM. Constructing ensembles for intrinsically disordered proteins. Current Opinion in Structural Biology. 2011;21:426–31. doi: 10.1016/j.sbi.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai CJ, Ma B, Sham YY, Kumar S, Nussinov R. Structured disorder and conformational selection. Proteins. 2001;44:418–427. doi: 10.1002/prot.1107. [DOI] [PubMed] [Google Scholar]
- 12.Tompa P, Szasz C, Buday L. Structural disorder throws new light on moonlighting. Trends in Biochemical Sciences. 2005;30:484–489. doi: 10.1016/j.tibs.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Boehr DD, Nussinov R, Wright PE. The role of dynamic conformational ensembles in biomolecular recognition. Nat Chem Biol. 2009;5:789–796. doi: 10.1038/nchembio.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilser VJ, Thompson EB. Intrinsic disorder as a mechanism to optimize allosteric coupling in proteins. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8311–8315. doi: 10.1073/pnas.0700329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou HX. Intrinsic disorder: signaling via highly specific but short-lived association. Trends in Biochemical Sciences. 2012;37:43–8. doi: 10.1016/j.tibs.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoemaker BA, Portman JJ, Wolynes PG. Speeding molecular recognition by using the folding funnel: The fly-casting mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8868–8873. doi: 10.1073/pnas.160259697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, Liu Z. Kinetic advantage of intrinsically disordered proteins in coupled folding-binding process: a critical assessment of the "fly-casting" mechanism. Journal of Molecular Biology. 2009;393:1143–1159. doi: 10.1016/j.jmb.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Qin S, Pang X, Zhou HX. Automated prediction of protein association rate constants. Structure. 2011;19:1744–1751. doi: 10.1016/j.str.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganguly D, Zhang W, Chen J. Synergistic folding of two intrinsically disordered proteins: searching for conformational selection. Mol Biosyst. 2012;8:198–209. doi: 10.1039/c1mb05156c. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Ganguly D, Chen J. Residual structures, conformational fluctuations, and electrostatic interactions in the synergistic folding of two intrinsically disordered proteins. Plos Computational Biology. 2012;8:e1002353. doi: 10.1371/journal.pcbi.1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou HX. From Induced Fit to Conformational Selection: A Continuum of Binding Mechanism Controlled by the Timescale of Conformational Transitions. Biophysical Journal. 2010;98:L15–L17. doi: 10.1016/j.bpj.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubelka J, Hofrichter J, Eaton WA. The protein folding 'speed limit'. Current Opinion in Structural Biology. 2004;14:76–88. doi: 10.1016/j.sbi.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Wright PE, Dyson HJ. Linking folding and binding. Current Opinion in Structural Biology. 2009;19:31–38. doi: 10.1016/j.sbi.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kriwacki RW, Hengst L, Tennant L, Reed SI, Wright PE. Structural studies of p21(Waf1/Cip1/Sdi1) in the free and Cdk2-bound state: Conformational disorder mediates binding diversity. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11504–11509. doi: 10.1073/pnas.93.21.11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galea CA, Wang Y, Sivakolundu SG, Kriwacki RW. Regulation of cell division by intrinsically unstructured proteins: Intrinsic flexibility, modularity, and signaling conduits. Biochemistry. 2008;47:7598–7609. doi: 10.1021/bi8006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP. Crystal structure of the p27(Kip1) cyclin-dependent-kinase inhibitor bound to the cyclin A Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 28.Sivakolundu SG, Bashford D, Kriwacki RW. Disordered p27(Kip1) exhibits intrinsic structure resembling the Cdk2/cyclin A-bound conformation. Journal of Molecular Biology. 2005;353:1118–1128. doi: 10.1016/j.jmb.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 29.Wang YF, Fisher JC, Mathew R, Ou L, Otieno S, Sublet J, Xiao LM, Chen JH, Roussel MF, Kriwacki RW. Intrinsic disorder mediates the diverse regulatory functions of the Cdk inhibitor p21. Nature Chemical Biology. 2011;7:214–221. doi: 10.1038/nchembio.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacy ER, Filippov I, Lewis WS, Otieno S, Xiao LM, Weiss S, Hengst L, Kriwacki RW. p27 binds cyclin-CDK complexes through a sequential mechanism involving binding-induced protein folding. Nature Structural & Molecular Biology. 2004;11:358–364. doi: 10.1038/nsmb746. [DOI] [PubMed] [Google Scholar]
- 31.Otieno S, Grace CR, Kriwacki RW. The Role of the LH Subdomain in the Function of the Cip/Kip Cyclin-Dependent Kinase Regulators. Biophysical Journal. 2011;100:2486–2494. doi: 10.1016/j.bpj.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bienkiewicz EA, Adkins JN, Lumb KJ. Functional consequences of preorganized helical structure in the intrinsically disordered cell-cycle inhibitor p27(Kip1) Biochemistry. 2002;41:752–759. doi: 10.1021/bi015763t. [DOI] [PubMed] [Google Scholar]
- 33.Verkhivker GM, Bouzida D, Gehlhaar DK, Rejto PA, Freer ST, Rose PW. Simulating disorder-order transitions in molecular recognition of unstructured proteins: Where folding meets binding. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5148–5153. doi: 10.1073/pnas.0531373100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schreiber G, Haran G, Zhou HX. Fundamental Aspects of Protein-Protein Association Kinetics. Chemical Reviews. 2009;109:839–860. doi: 10.1021/cr800373w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Shi Y, Chen XS, Warshel A. Simulating the electrostatic guidance of the vectorial translocations in hexameric helicases and translocases. Proceedings of the National Academy of Sciences. 2009;106:7449–7454. doi: 10.1073/pnas.0900532106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcovitz A, Levy Y. Frustration in protein-DNA binding influences conformational switching and target search kinetics. Proceedings of the National Academy of Sciences. 2011;108:17957–17962. doi: 10.1073/pnas.1109594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azia A, Levy Y. Nonnative electrostatic interactions can modulate protein folding: molecular dynamics with a grain of salt. Journal of Molecular Biology. 2009;393:527–542. doi: 10.1016/j.jmb.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Stoycheva AD, Onuchic JN, Brooks CL. Effect of gatekeepers on the early folding kinetics of a model beta-barrel protein. Journal of Chemical Physics. 2003;119:5722–5729. [Google Scholar]
- 39.Stoycheva AD, Brooks CL, Onuchic JN. Gatekeepers in the ribosomal protein S6: Thermodynamics, kinetics, and folding pathways revealed by a minimalist protein model. Journal of Molecular Biology. 2004;340:571–585. doi: 10.1016/j.jmb.2004.04.073. [DOI] [PubMed] [Google Scholar]
- 40.Otzen DE, Oliveberg M. Salt-induced detour through compact regions of the protein folding landscape. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11746–11751. doi: 10.1073/pnas.96.21.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao AH, Crick SL, Vitalis A, Chicoine CL, Pappu RV. Net charge per residue modulates conformational ensembles of intrinsically disordered proteins. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8183–8188. doi: 10.1073/pnas.0911107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy Y, Wolynes PG, Onuchic JN. Protein topology determines binding mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:511–516. doi: 10.1073/pnas.2534828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganguly D, Chen J. Topology-based modeling of intrinsically disordered proteins: Balancing intrinsic folding and intermolecular interactions. Proteins: Structure, Function, and Bioinformatics. 2011;79:1251–1266. doi: 10.1002/prot.22960. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y, Liu Z. Nonnative interactions in coupled folding and binding processes of intrinsically disordered proteins. PLoS ONE. 2010;5:e15375. doi: 10.1371/journal.pone.0015375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Sancho D, Best RB. Modulation of an IDP binding mechanism and rates by helix propensity and non-native interactions: association of HIF1alpha with CBP. Mol Biosyst. 2012;8:256–267. doi: 10.1039/c1mb05252g. [DOI] [PubMed] [Google Scholar]
- 46.Levy Y, Onuchic JN, Wolynes PG. Fly-casting in protein-DNA binding: frustration between protein folding and electrostatics facilitates target recognition. Journal of the American Chemical Society. 2007;129:738–739. doi: 10.1021/ja065531n. [DOI] [PubMed] [Google Scholar]
- 47.Uversky VN, Gillespie JR, Fink AL. Why are "natively unfolded" proteins unstructured under physiologic conditions? Proteins. 2000;41:415–427. doi: 10.1002/1097-0134(20001115)41:3<415::aid-prot130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Meszaros B, Tompa P, Simon I, Dosztanyi Z. Molecular principles of the interactions of disordered proteins. Journal of Molecular Biology. 2007;372:549–561. doi: 10.1016/j.jmb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Chen JH. Intrinsically disordered p53 extreme C-terminus binds to S100B(betabeta) through "fly-casting". Journal of the American Chemical Society. 2009;131:2088–2089. doi: 10.1021/ja809547p. [DOI] [PubMed] [Google Scholar]
- 50.Narayanan R, Ganesh OK, Edison AS, Hagen SJ. Kinetics of folding and binding of an intrinsically disordered protein: The inhibitor of yeast aspartic proteinase YPrA. Journal of the American Chemical Society. 2008;130:11477–11485. doi: 10.1021/ja803221c. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Wang Y, Chu X, Hagen SJ, Han W, Wang E. Multi-Scaled Explorations of Binding-Induced Folding of Intrinsically Disordered Protein Inhibitor IA3 to its Target Enzyme. Plos Computational Biology. 2011;7:e1001118. doi: 10.1371/journal.pcbi.1001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turjanski AG, Gutkind JS, Best RB, Hummer G. Binding-induced folding of a natively unstructured transcription factor. PLoS Comput Biol. 2008;4:e1000060. doi: 10.1371/journal.pcbi.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugase K, Dyson HJ, Wright PE. Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature. 2007;447:1021–1025. doi: 10.1038/nature05858. [DOI] [PubMed] [Google Scholar]
- 54.Bachmann A, Wildemann D, Praetorius F, Fischer G, Kiefhaber T. Mapping backbone and side-chain interactions in the transition state of a coupled protein folding and binding reaction. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3952–3957. doi: 10.1073/pnas.1012668108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Q, Lu HP, Wang J. Exploring the mechanism of flexible biomolecular recognition with single molecule dynamics. Physical Review Letters. 2007;98:128105. doi: 10.1103/PhysRevLett.98.128105. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, Chan HS. Competition between native topology and nonnative interactions in simple and complex folding kinetics of natural and designed proteins. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2920–2925. doi: 10.1073/pnas.0911844107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tόth-Petrόczy Ag, Simon I, Fuxreiter M, Levy Y. Disordered Tails of Homeodomains Facilitate DNA Recognition by Providing a Trade-Off between Folding and Specific Binding. Journal of the American Chemical Society. 2009;131:15084–15085. doi: 10.1021/ja9052784. [DOI] [PubMed] [Google Scholar]
- 58.Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S, Sun P, Tan CK, Hengst L, Slingerland J. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128:281–294. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grimmler M, Wang YF, Mund T, Cilensek Z, Keidel EM, Waddell MB, Jakel H, Kullmann M, Kriwacki RW, Hengst L. Cdk-inhibitory activity and stability of p27(Kip1) are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–280. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 60.Karanicolas J, Brooks CL. The origins of asymmetry in the folding transition states of protein L and protein G. Protein Science. 2002;11:2351–2361. doi: 10.1110/ps.0205402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. Charmm - a Program for Macromolecular Energy, Minimization, and Dynamics Calculations. Journal of Computational Chemistry. 1983;4:187–217. [Google Scholar]
- 62.Brooks BR, Brooks CL, Mackerell AD, Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch S, Caflisch A, Caves L, Cui Q, Dinner AR, Feig M, Fischer S, Gao J, Hodoscek M, Im W, Kuczera K, Lazaridis T, Ma J, Ovchinnikov V, Paci E, Pastor RW, Post CB, Pu JZ, Schaefer M, Tidor B, Venable RM, Woodcock HL, Wu X, Yang W, York DM, Karplus M. CHARMM: The Biomolecular Simulation Program. Journal of Computational Chemistry. 2009;30:1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feig M, Karanicolas J, Brooks CL. MMTSB Tool Set: enhanced sampling and multiscale modeling methods for applications in structural biology. Journal of Molecular Graphics & Modelling. 2004;22:377–395. doi: 10.1016/j.jmgm.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Gallicchio E, Andrec M, Felts AK, Levy RM. Temperature weighted histogram analysis method, replica exchange, and transition paths. Journal of Physical Chemistry B. 2005;109:6722–6731. doi: 10.1021/jp045294f. [DOI] [PubMed] [Google Scholar]
- 65.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. Journal of Molecular Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 66.Im W, Beglov D, Roux B. Continuum solvation model: Electrostatic forces from numerical solutions to the Poisson-Boltzmann equation. Comput. Phys. Comm. 1998;111:59–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.