Abstract
The gap junction protein, connexin43 (Cx43) controls both bone formation and osteoclastogenesis via osteoblasts and/or osteocytes. Cx43 has also been proposed to mediate an anti-apoptotic effect of bisphosphonates, potent inhibitors of bone resorption. We studied whether bisphosphonates are effective in protecting mice with a conditional Cx43 gene deletion in osteoblasts and osteocytes (cKO) from the consequences of ovariectomy on bone mass and strength. Ovariectomy resulted in rapid loss of trabecular bone followed by a slight recovery in wild type (WT) mice, and a similar degree of trabecular bone loss, albeit slightly delayed, occurred in cKO mice. Treatment with either risedronate (20µg/kg) or alendronate (40µg/kg) prevented ovariectomy-induced bone loss in both genotypes. In basal conditions, bones of cKO mice have larger marrow area, higher endocortical osteoclast number, and lower cortical thickness and strength relative to WT. Ovariectomy increased endocortical osteoclast number in WT but not in cKO mice. Both bisphosphonates prevented these increases in WT mice, and normalized endocortical osteoclast number, cortical thickness and bone strength in cKO mice. Thus, lack of osteoblast/osteocyte Cx43 does not alter bisphosphonate action on bone mass and strength in estrogen deficiency. These results support the notion that one of the main functions of Cx43 in cortical bone is to restrain osteoblast and/or osteocytes from inducing osteoclastogenesis at the endocortical surface.
Keywords: Gap junctions, Conditional Knockout, bisphosphonate
Introduction
Gap junctions are intercellular channels that provide aqueous continuity between the cytoplasm of adjacent cells. These channels are comprised of hexameric arrays of connexin proteins, known as connexons, which align in the membranes of adjacent cells to form a transcellular channel, or gap junction [1]. Connexons can also exist as individual “hemichannels” without forming a gap junction, thus acting as a membrane channel with large molecular permeability [2]. The hemichannel activity of Cx43, in particular the src-ERK-dependent opening of Cx43 hemichannels, has been linked to an anti-apoptotic action of bisphosphonates in osteocytes [3–5]. Bisphosphonates are potent inhibitors of bone resorption and they represent the mainstay therapy for osteoporosis and fracture prevention. Their primary action is on osteoclasts, where they directly interfere with cell function and decrease survival [6]. The concept that bisphosphonates can also act on osteocytes is attractive and could contribute to explain their anti-fracture efficacy independent of their effect on bone resorption [7]. However, the significance of the anti-apoptotic effect, which is seen at low doses, to the overall pharmacologic action of bisphosphonates remains to be determined [8]. Furthermore, the anti-apoptotic effect of bisphosphonates in osteocytes is difficult to reconcile with the pro-apoptotic action in osteoclasts and, at high doses, in osteoblastic cells [9].
This topic has been recently studied in vivo in a model of corticosteroid-induced bone loss in mice with a conditional deletion of the Cx43 gene ( Gja1 ) in osteoblasts and osteocytes [5]. Although that study confirmed that Cx43 is involved in the anti-apoptotic effect of alendronate, treatment with this bisphosphonate prevented the corticosteroid-induced bone loss similarly well in wild type (WT) and mutant animals, suggesting that Cx43 is not required for preservation of bone mineral density (BMD). However corticosteroid bone disease is a complex condition characterized by inhibition of bone formation and a relatively smaller increase of bone resorption, with overall decreased bone turnover [10], and whether Cx43 is involved in modulating bone strength was not studied [5].
In the present work, we have tested the effect of two bisphosphonates on ovariectomy (OVX)-induced bone loss in mice with conditional Gja1 ablation driven by the 2.3kb Col1α1 promoter, which we have previously shown to efficiently induce gene recombination in osteoblast and osteocytes [11]. These conditional Cx43-deficient mice (cKO) have increased endocortical bone resorption and periosteal bone formation resulting in bone marrow area expansion, increased cortical area and decreased thickness [12, 13]. This phenotype is consistently observed in other models of Gja1 ablation in the osteogenic lineage [14–16]. In contrast to corticosteroid treatment, estrogen deficiency increases bone turnover, thus making it easier, in principle, to determine if the therapeutic effect of bisphosphonates requires Cx43. A second goal of this study was to determine whether and to what extent inhibition of bone resorption by bisphosphonates can affect the phenotypic changes present in conditionally Gja1 ablated mice, and thus what is the biologic relevance of paracrine Cx43 modulation of bone resorption.
We find that OVX Cx43 deficient mice experienced a similar increase in BMD as did WT mice upon treatment with either alendronate or risedronate started immediately after surgery. Both agents prevented trabecular bone loss following OVX in WT and cKO, and actually rescued some of the abnormalities of cKO bone, normalizing cortical thickness and bone strength. These results further support the notion that increased osteoclast activation is responsible for the widened marrow area and thinner cortex of Cx43-deficient bones, and thus modulation of bone resorption is a major function of Cx43. Our results do not support a major role of Cx43 in modulating effects of bisphosphonates on bone forming cells and bone formation in vivo.
Material and Methods
Transgenic Mice
For conditional Gja1 ablation, a mouse strain harboring a mutant “floxed” Gja1 allele (Gja1flox) [17] was mated to Gja1+/− mice expressing Crerecombinase under the control of the 2.3-kb Col1α1 promoter (ColCre) [18], yielding ColCre::Gja1flox/− (cKO), Gja1flox/+, which was used as wild type (WT), and other phenotypes that were not used in these studies [11]. All mouse lines used in this study were in a mixed C57BL/6-C129/J background and littermates were used as controls. Mice were fed regular chow ad libitum and housed in a room maintained at constant temperature (25°C) on a 12 hours of light and 12 hours of dark schedule. All procedures were approved by the Animal Studies Committee of Washington University in St Louis. Genotyping was performed by PCR on genomic DNA extracted from mouse tails using the HotSHOT method [19]. We used previously described methods to detect the ColCre transgene and Gja1+, Gja1−, and Gja1flox alleles [11].
Animal Procedures
Mice were randomly assigned to treatment groups within each genotype. Ovariectomy or sham operations were performed on 4-month-old females as detailed previously [20]. Briefly, the ovaries were exposed through an abdominal approach and either resected after clipping the blood vessels or left in place (sham operation). The muscle and skin of the abdomen were sutured. Mice were given a subcutaneous injection of buprenex immediately after surgery and ibuprofen was supplied for a week post-surgery in the drinking water. Starting immediately after OVX, vehicle (phosphate buffered saline), 20µg/kg risedronate or 40µg/kg alendronate (both provided by Procter&Gamble Pharmaceuticals, Cincinnati, OH) was injected intra-peritoneally every 4 days for 4 weeks. These doses were chosen based on the consideration that 20 µg/kg dose of risedronate produced significant suppression of bone remodeling in mice overexpressing Runx2 [21], and that risedronate is approximately twice more potent than alendronate in anti-resorptive activity in different species [22, 23]. This 1:2 dose ratio is also consistent with approved clinical doses for treatment of osteoporosis.
Bone Mass and Microstructure
Whole body bone mineral density (BMD) was monitored by dual-energy X-ray absorptiometry (DXA) using a PIXImus scanner (GE/Lunar, Madison, WI), under anesthesia as previously described [24]. Analysis of femoral bone structure was performed using a µCT system (µCT 40; Scanco Medical AG), as previously described [12].
Bone Biomechanics
Mechanical testing in a 3-point bending to failure was conducted on femora after µCT. Briefly, hydrated femora were stabilized over supports 7mm apart and a loading force was applied in the anteroposterior direction midway between the supports (Instron, Norwood, MA, USA). Test curves were analyzed to determine ultimate force to failure and stiffness as described previously [25].
Bone Histology and Histomorphometry
To label mineralizing fronts, mice were injected with calcein (15 mg/kg i.p., Sigma-Aldrich) 7 and 2 days before euthanasia, which was performed under light anaesthesia by exsanguination through dorsal aortic puncture. Blood was collected and the serum stored at −80°C for later assays. Bone samples were prepared and decalcified as previously described [11]. Decalcified tibias were embedded in paraffin and 2-µm thick longitudinal sections of the whole bone were cut and stained for tartrate resistant acid phosphatase (TRACP). Undecalcified femora were embedded in methyl methacrylate and 4-µm thick longitudinal sections of the whole bone were cut and left unstained for assessment of calcien fluorescence. Quantitative histomorphometry was performed using a commercial software (OSTEO II, Bioquant, Nashville, TN, USA), and standard parameters of bone remodeling were determined as detailed elsewhere [13].
Biochemical Markers of Bone Turnover
Serum C-terminus cross-linked telopeptides of type I collagen (CTX) and osteocalcin were measured as indices of bone resorption and formation, respectively. For these assays, serum was obtained from mice that had been deprived of food and water for 6 hours. Analyses were performed by ELISA, using CTX [RatLaps™ EIA] (Immunodiagnostics Systems Inc., Fountain Hills, AZ) and osteocalcin [Mouse Osteocalcin EIA Kit](Biomedical Technologies Inc., Stoughton, MA) kits according to the manufacturer’s instructions.
Statistics analysis
Group means were compared by t-test for unpaired samples. Data on repeated measures were analyzed by ANOVA, followed by a post-hoc multiple Holm-Sidak method t-test. Data were analysed using SigmaPlot Vs11.0 (Systat Software GmbH, Germany). All data are expressed as the mean±s.d.
Results
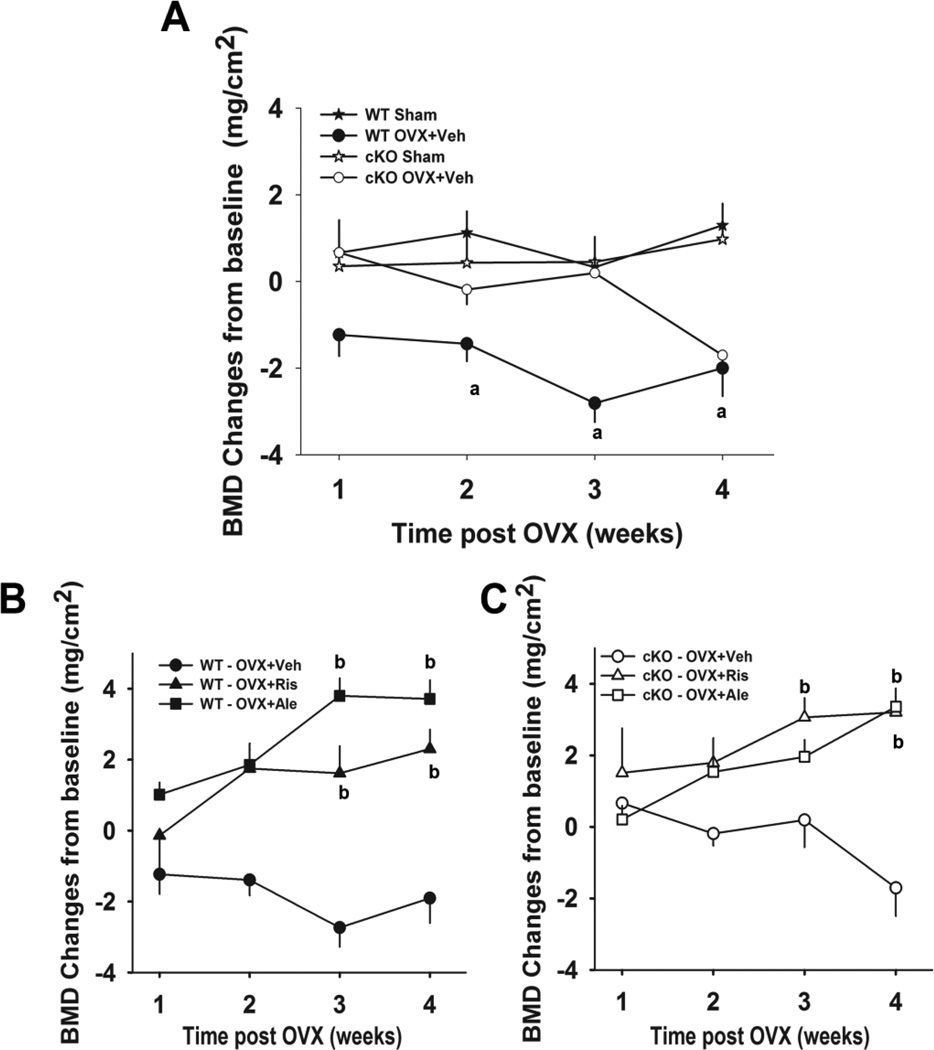
As expected in WT mice, surgically induced estrogen loss caused rapid (within 2 weeks) and significant loss of whole body BMD. Bone loss was seen as early as 2 weeks post OVX and was sustained through 4 weeks post OVX (Figure 1A). The cKO mice, however, showed a delay in bone loss, which became significant only 4 weeks post OVX (Fig. 1A). There was no significant bone loss in either the sham-operated WT or sham-operated cKO mice. Treatment of the OVX WT group with either risedronate or alendronate resulted in significant gains in BMD above baseline, which were significant two weeks post OVX compared to Sham operated WT mice (WT Sham; Fig 1B). The gain in BMD was more pronounced on alendronate treatment at the end of the study, although this was not statistically significant (Fig. 1B). Likewise, treatment of cKO OVX mice with either risedronate or alendronate resulted in significant increases of BMD. However, probably because of the loss of BMD in the cKO, the bisphosphonate-induced bone gain only reached statistical significance after 4 weeks post OVX (Fig. 1C).
Figure 1. Effect of OVX and bisphosphonate treatment on whole body bone mineral density (BMD) in wild type (WT) and Gja1 conditional knockout (cKO) mice.
(A) Four month-old female WT and cKO mice were either ovariectomised (OVX+veh) or sham operated (sham). (B) WT and (C) cKO OVX were treated with vehicle (veh), 20µg/kg risedronate (OVX+Ris), or 40µg/kg alendronate (OVX+Ale), and BMD monitored by whole body DXA (n=8–10). Data are presented as absolute BMD change (mean±sd) from baseline. There was a significant effect of OVX on BMD and of treatement (risedronate and alendronate) on BMD changes in WT and cKO groups, but no effect of genotype (two-way ANOVA). Significant differences at each time point, assessed by post-hoc analysis (Holm-Sidak multiple t-test) are also shown. a, p< 0.05 vs sham; b, p<0.05 vs OVX+veh.
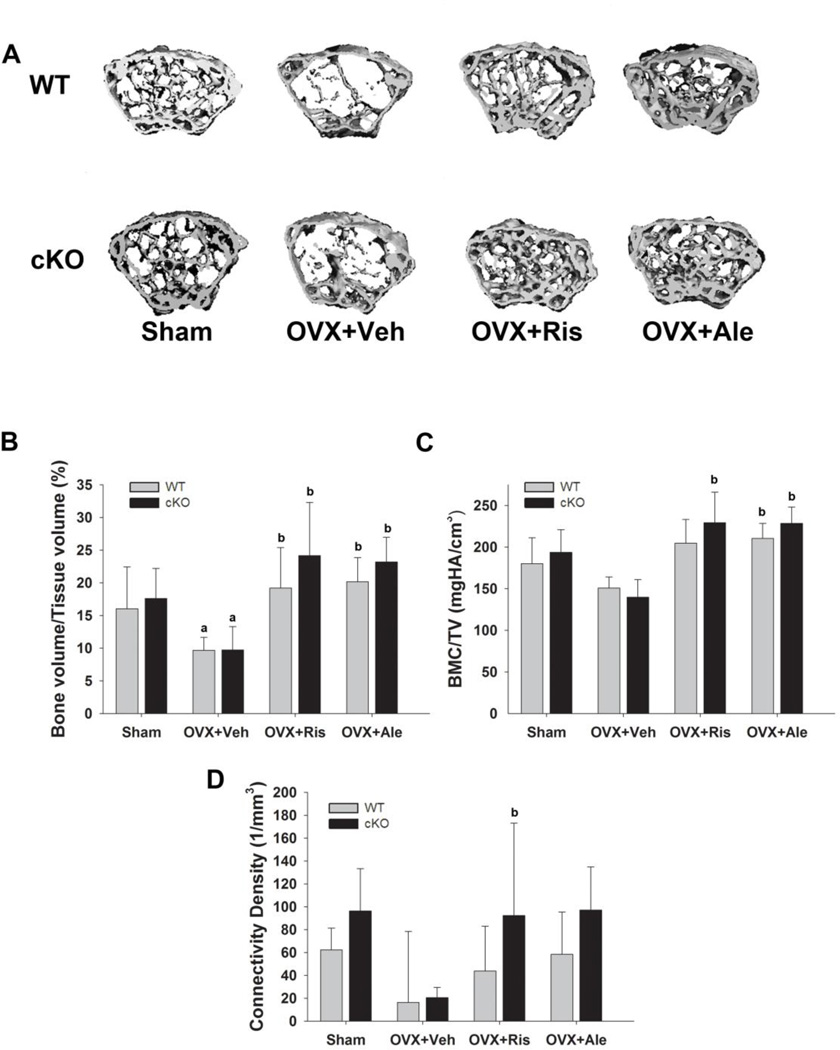
The magnitude of trabecular bone loss in WT and cKO sham mice four weeks after OVX is exemplified in the 3D reconstruction of the femoral proximal epiphyses by µCT (Fig. 2A, left panels). This trabecular bone loss was not observed in alendronate or risedronate treated animals of either genotype (Fig. 2A, right panels). OVX induced an approximately 35% decrease in trabecular bone volume (BV/TV) in both WT and cKO mice when compared to sham operated mice (Fig. 2B). The decrease in BV/TV in the OVX groups was associated with significant reductions in trabecular thickness (Supp. Fig 1A) and number (Supp. Fig 1C), without significant changes in trabecular spacing (Supp. Fig. 1B). Tissue mineral density and connectivity density, assessed by µCT, were lower in both OVX groups but the differences did not reach statistical significance (Fig. 2C, D). Treatment with either bisphosphonate prevented all these changes in both genotypes, and increased trabecular bone volume and tissue mineral density above the respective sham group values, without significant genotypes differences in responsiveness to either bisphosphonate (Fig. 2B–D).
Figure 2. Effect of OVX and bisphosphonate treatment on trabecular bone in wild type (WT) and Gja1 conditional knockout (cKO) mice.
(A) Representative µCT reconstructions of femoral trabecular bone from 4-month-old WT and cKO mice that were either ovariectomised (OVX) or sham operated (sham), and treated with either vehicle (OVX+Veh), 20µg/kg risedronate (OVX+Ris), or 40µg/kg alendronate (OVX+Ale) for 4 weeks. (B) Trabecular bone volume (BV/TV); (C) Bone mineral content per tissue volume (BMC/TV); and (D) connectivity density determined at the end of the study by µCT in the different treatment groups (n=5). a, p< 0.05 vs WT; b, p< 0.05 vs Sham, 2-way ANOVA, and post-hoc multiple t-test (Holm-Sidak).
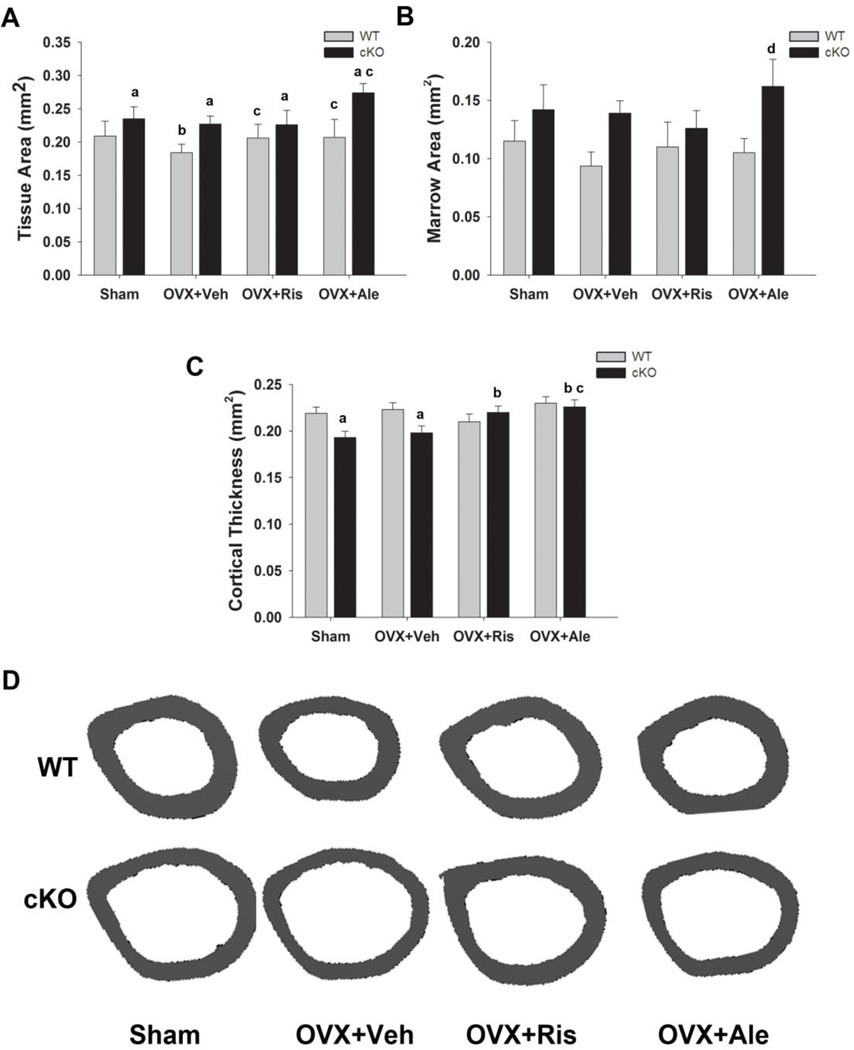
Analysis of cortical bone at the diaphysis revealed larger tissue area and lower cortical thickness in cKO, as previously reported [12, 13], but the increase in marrow area was not statistically significant (Fig. 3A–C). In WT mice, OVX decreased tissue and marrow area, though again the latter failed to reach significance (p=0.069). However, no changes in cortical thickness were observed. In cKO mice, ovariectomy had no effect on tissue and marrow area or cortical thickness (Fig. 3A–C). In WT mice, treatment with either risedronate or alendronate prevented the decrease in tissue area, but had no effect on cortical thickness. Tissue and marrow area were not significantly different in risedronate treated mice compared to Sham and OVX cKO groups, whereas tissue and marrow area were larger in the alendronate cKO group (Fig. 3A, B). However, treatment with either bisphosphonate significantly increased cortical thickness of cKO mice to levels greater than either the sham or OVX cKO groups, and comparable to WT groups (Fig 3C). Examples of tibial cortical structure in the different treatment groups are shown in Fig. 3D.
Figure 3. Effect of OVX and bisphosphonate treatment on cortical bone in wild type (WT) and Gja1 conditional knockout (cKO) mice.
(A) Total tissue area; (B) marrow area; and (C) cortical thickness of femurs, determined by µCT, of 4-month-old WT and cKO mice (n=5) that were either ovariectomised (OVX) or sham operated (sham), and treated with either vehicle (OVX=Veh), 20µg/kg risedronate (OVX+Ris), or 40µg/kg alendronate (OVX+Ale) for 4 weeks. (D) Representative µCT cross-sections of femoral diaphysis of the different genotypes and treatment groups. a, p< 0.05 vs WT; b, p< 0.05 vs Sham; c, p<0.05 vs OVX+Veh; d, p< 0.05 vs OVX+Ris, 2-way ANOVA, and post-hoc multiple t-test (Holm-Sidak).
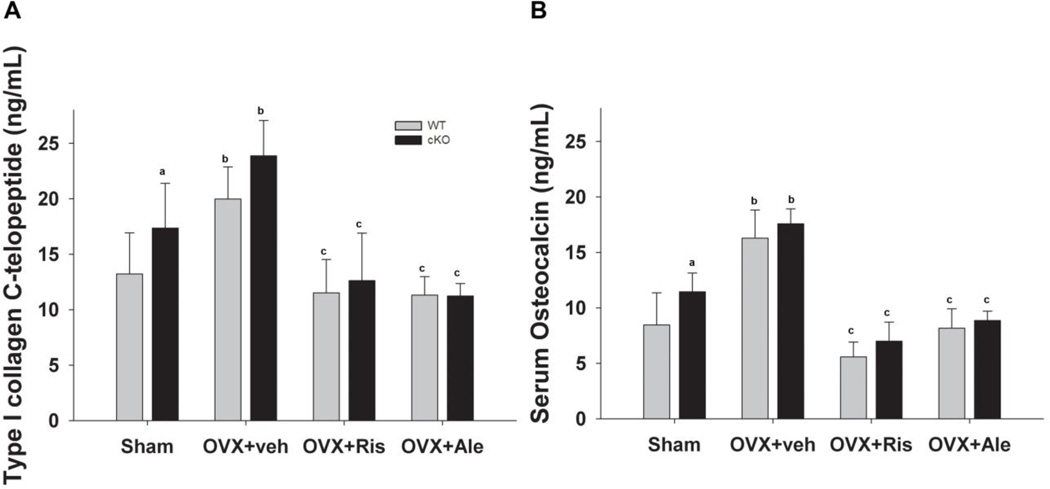
Serum CTX increased significantly in both WT and cKO mice after OVX, and treatment with either bisphosphonate prevented such changes in both genotypes (Fig. 4A). We also observed similar changes in serum osteocalcin after OVX, and bisphosphonate treatment reduced serum osteocalcin in both OVX groups to levels below those present in sham operated mice (Fig. 4B). Of note, baseline levels of both serum CTX and osteocalcin were significantly higher in the cKO compared to WT levels (Fig 4A, 4B).
Figure 4. Effect of OVX and bisphosphonate treatment on biochemical markers of bone turnover in wild type (WT) and Gja1 conditional knockout (cKO) mice.
(A) Serum C-terminal telopeptides of type I collagen (CTX); and (B) serum osteocalcin in the different genotypes and treated groups (n=5–9), (See Fig. 2 for abbreviations). a, p< 0.05 vs WT; b, p< 0.05 vs Sham; c, p<0.05 vs OVX+Veh, 2-way ANOVA, and post-hoc multiple t-test (Holm-Sidak).
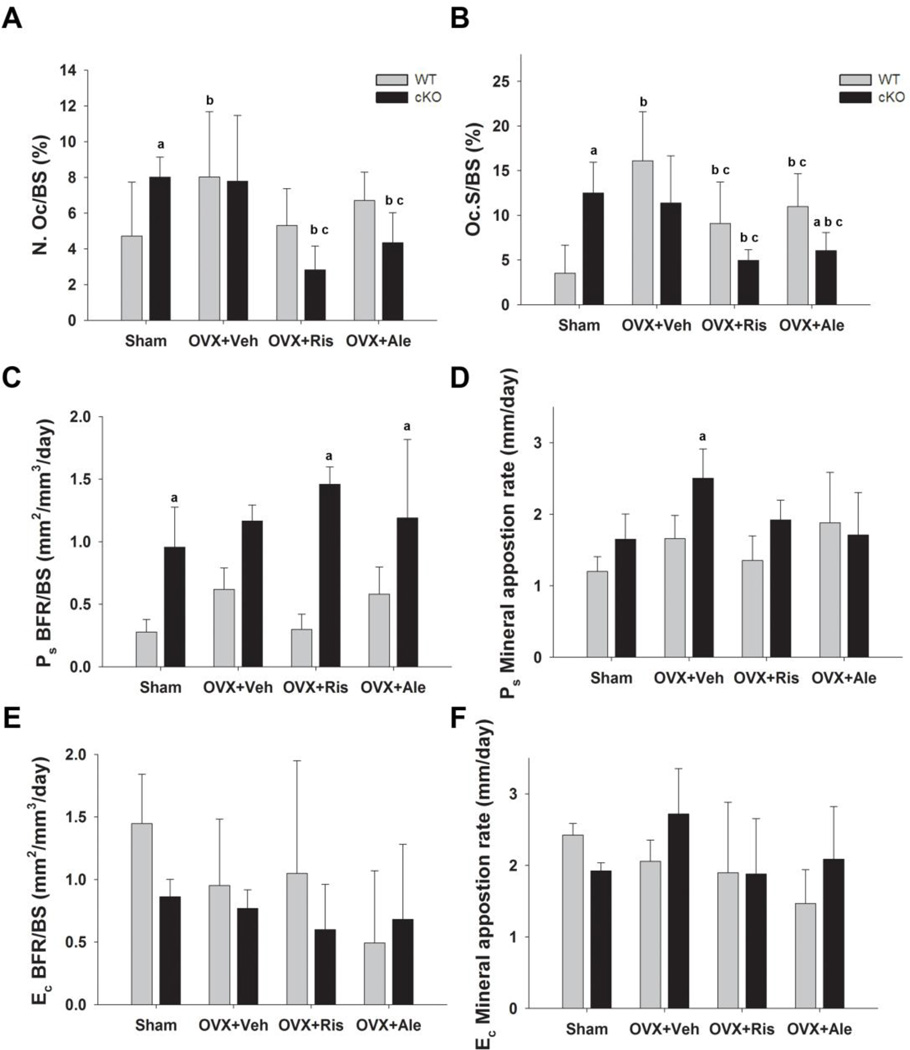
Histological analysis of cortical bone confirmed significantly higher TRACP+ osteoclast number and osteoclast covered surface on the endocortical bone in cKO compared to WT mice [13]. These parameters significantly increased after OVX in the WT but not in cKO mice (Fig. 5A, B), consistent with lack of changes in cortical µCT indices. More to the point, bisphosphonate treatment prevented the OVX-dependent increase in OC number and surface in WT mice. Both bisphosphonates also produced significant decreases in OC number and surface in the cKO, relative to both sham and OVX cKO control groups (Fig. 5A, B). As anticipated from previous studies [13, 14], periosteal bone formation rate was significantly greater in the sham cKO relative to WT. Neither OVX nor bisphosphonate treatment significantly altered periosteal bone formation, which remained elevated in cKO compared to WT in all groups (Fig 5C). Mineral apposition rate was not significantly different in the cKO, also confirming previous findings [13, 14], but it did increase after OVX in the cKO group.Both bisphosphonates seemed to prevent this increase though the differences vs OXV+VEH cKO were not statistically significant (Fig. 5D). There were no significant differences in endocortical bone formation between the two genotypes, and no significant effects with ovariectomy or bisphosphonate treatment, although variability was high (Fig. 5E, F).
Figure 5. Effect of OVX and bisphosphonate treatment on cortical bone turnover in wild type (WT) and Gja1 conditional knockout (cKO) mice.
(A) Osteoclast number and (B) osteoclast surface as percentage of endocortical surface area, assessed by TRAPC+ staining of femur section in the different genotypes and treated groups (See Fig. 2 for abbreviations). (C) Periosteal bone formation rate (BFR); (D) periosteal mineral appostion rate (MAR); (E) endosteal BFR; and (F) endosteal MAR in the different genotypes and treated groups at the end of the study (n=4). a, p< 0.05 vs WT, b, p< 0.05 vs Sham; c, p<0.05 vs OVX+Veh, 2-way ANOVA, and post-hoc multiple t-test (Holm-Sidak).
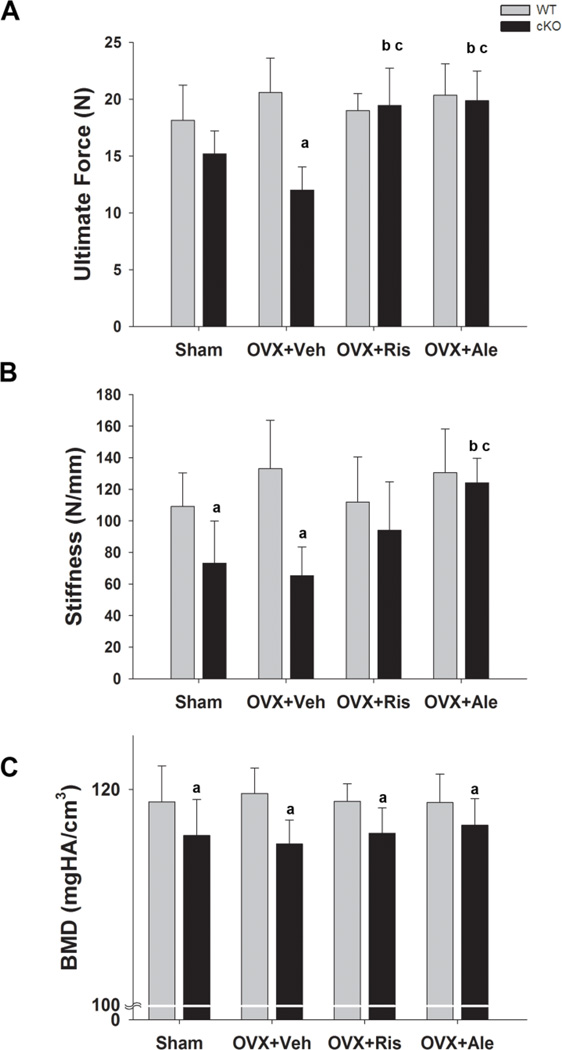
Cortical bone biomechanical properties were tested via three-point bending. As previously shown [12, 13], ultimate force and stiffness were reduced in the sham cKO mice relative to WT; OVX further reduced ultimate force in cKO but not in WT mice (Fig. 6A, B) Importantly, these changes in cKO mice were completely prevented by administration of either risedronate or alendronate. In fact ultimate force in bisphosphonate treated cKO mice was higher than in sham cKO mice and equivalent to that in sham WT mice (Fig. 6A). Similarly, alendronate treatment in cKO mice normalized bone stiffness to WT values, while risedronate had a lesser effect (Fig. 6B). Also confirming previous reports [13], cortical bone mineral density assessed by µCT was significantly lower in cKO compared to WT, and this difference remained after OVX or bisphosphonate treatment (Fig. 6C).
Figure 6. Effect of OVX and bisphosphonate treatment on bone mechanical properties in wild type (WT) and Gja1 conditional knockout (cKO) mice.
(A) Ultimate force and (B) bone stiffness of femurs, measured in a three-point bending protocol in the different genotypes and treated groups (n=4–7) (See Fig. 2 for abbreviations). (C) Cortical bone mineral density (BMD), measured by µCT in the different genotypes at the end of the study (n=5). a, p< 0.05 vs WT; b, p< 0.05 vs Sham; c, p<0.05 vs OVX+Veh; 2-way ANOVA, and post-hoc multiple t-test(Holm-Sidak).
Discussion
We find that lack of osteoblast/osteocyte Cx43 delays and attenuates OVX-induced trabecular bone loss and that treatment with bisphosphonate not only prevents OVX-dependent bone loss but also rescues the cortical thinning and reduced bone strength caused by Cx43 deficiency. Hence, increased bone resorption is a key mechanism of the cortical phenotype that develops postnatally in mice deficient of Cx43 in osteoblasts and osteocytes.
As we and others have previously reported, the most consistent and important phenotypic feature consequent to Gja1 ablation in the osteoblastic lineage is an enlargement of cortical tissue area and thinning of cortical bone [12, 14–16]. This is due to increased endocortical bone resorption coupled to increased periosteal bone formation [14, 15]. On the other hand, in the present study the consequences of estrogen deficiency were seen primarily on trabecular rather than cortical bone, and bone loss following OVX was similar in WT and cKO mice. Since the cortical component contributes more to whole body bone mass than trabecular bone [26], the delayed, attenuated bone loss detected by DXA in cKO mice most likely reflects the fact that no changes in cortical bone mass occurred after OVX in cKO, thus in part masking the trabecular bone loss. Accordingly, the increased biochemical bone turnover markers after OVX in cKO mice in the face of no changes in endocortical osteoclast number likely reflects the prevalent effect of estrogen deficiency on trabecular vs. cortical bone. Consistent with this finding, we have observed attenuated activation of cortical (but not trabecular) bone resorption after mechanical skeletal unloading in cKO mice [13]. As hypothesized in that study, it is likely that the increased endocortical osteoclast number present in cKO mice prevents further activation by other osteoclastogenic stimuli, such as estrogen loss or skeletal unloading. Lack of OVX effect on cortical bone mass or strength is consistent with similar reports [27, 28]. However, other studies have shown that OVX reduces bone mass and strength in mice [29, 30]. Such discrepancies may be explained in great part by different sensitivity to OVX by different mouse strains, as well as length of time after OVX the measurements are taken [31].
We had previously reported Gja1 ablation in trabecular osteoblasts and osteocytes and decreased trabecular bone mass by histomorphometry in the same genetic model [11]. However, such differences did not emerge when trabecular bone was evaluated by µCT [13], a result confirmed in the present study. The apparent discrepancy may be related to the different techniques used, a problem particularly evident for trabecular thickness [32, 33]. Furthermore, we have observed less efficient recombination of Gja1 in the trabecular compartment relative to cortical bone using another promoter to ablate Gja1 [14]. We also found that trabecular bone loss following muscle paralysis is largely independent of Cx43 [13]. Thus, Cx43 appears to be more important for cortical than for trabecular bone, and it is thus not too surprising to find that prevention of OVXinduced trabecular bone loss by bisphosphonates is not affected by the absence of Cx43.
In a model of corticosteroid-induced bone loss, Cx43 ablation in osteoblasts and osteocytes prevented bisphosphonate inhibition of apoptosis but it did not alter their preventative effect on bone loss [5]. In our studies, bisphosphonate treatment of Cx43 deficient mice actually improved bone mass and strength without affecting either endocortical or periosteal bone formation. Thus, if osteoblast/osteocyte Cx43 plays a role in modulating apoptosis [3, 5], this does not seem to play a major role in the overall pharmacologic action of bisphosphonates on bone mass and strength, at least in this model. Osteocyte survival is clearly a key factor in bone homeostasis, as induction of osteocyte apoptosis by targeted expression of diphtheria toxin receptor decreases trabecular bone volume and cortical thickness and impairs bone strength [34]. However, the inhibitory action of Cx43 on osteocyte (and osteoblast) apoptosis contrasts with the finding of insufficient apoptosis as the mechanism for syndactyly in oculodentodigital dysplasia [35], a disease linked to loss-of-function Gja1 mutations [36]. These discrepancies reflect the seemingly contrasting role for Cx43 and gap junctions in apoptosis in various tissues, with Cx43 both increasing and decreasing apoptosis [37].
A key finding from this study is the reversal of some key features of the Gja1 cKO cortical phenotype after a 4-week treatment with either alendronate or risedronate in OVX groups. There was restoration of cortical thickness to WT levels, inhibition of endocortical osteoclasts, and improvement of bone strength to WT levels. Hence, the increased cortical thickness is sufficient to normalize mechanical properties of cKO bones, even though the abnormally low mineralization is not improved by bisphosphonates. This is consistent with the notion that bisphosphonates are potent osteoclast inhibitors but do not alter production of a hypomineralized bone matrix by Cx43 deficient bone forming cells. It is likely that the bisphosphonate-induced increase in cortical thickness and area compensates for the decreased bone mineral content of cKO mice, thus resulting in an effective rescue of the decreased bone strength in Gja1 deficient mice. Therefore, bisphosphonate inhibition of endocortical bone resorption reverses one of the major phenotypic changes in Cx43 deficient mice. This result is in keeping with recent work with mice in which Gja1 was deleted in osteoblasts and osteocytes using the human osteocalcin promoter, showing that alendronate restored cortical thinning and enlarged marrow cavity area of mutant mice to WT levels [15]. In this context, we were unable to detect any significant difference between alendronate and risedronate, which were equally efficient at the doses used (a 2-fold higher molar dose of alendronate vs. risedronate) at inhibiting endocortical osteoclastic bone resorption in Cx43 deficient mice.
In summary, this study does not support a major role of Cx43 in modulating effects of bisphosphonates on bone forming cells, but demonstrates that one of the main functions of Cx43 is to restrain osteoblast and/or osteocytes from inducing osteoclastogenesis at the endocortical surface. This represents a key mechanism by which Cx43 can shape the structure and strength of long bones in the adult skeleton.
Supplementary Material
Highlights.
Trabecular bone loss after ovariectomy similar to wildtype in osteoblasts/osteocytes specific Cx43 gene deletion mice (cKO), although slightly delayed
Endocortical osteoclast numbers does not increase following ovariectomy in cKO
Bisphosphonates normalize endocortical osteoclast number, cortical thickness and bone strength in cKO mice
Lack of osteoblast/osteocyte Cx43 does not alter bisphosphonate action on bone mass and strength in estrogen deficiency
Bisphosphonate correction of most cortical bone defects in cKO mice demonstrates one major action of osteoblasts/osteocyte Cx43 is osteoclast regulation
Acknowledgements
This work was supported by NIH grant R01-AR041255, by a grant from Procter&Gamble Pharmaceutical, and by the Research Center for Auditory and Vestibular Studies Histology Core, Washington University (supported by P30-DC004665).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Roberto Civitelli receives research support from Pfizer, Inc. and Amgen, and holds stock of Amgen, Eli-Lilly and Merck & Co. Roger J. Phipps and Frank H. Ebetino were employed by Procter&Gamble at the time most this study was performed.
Part of this work has been presented at the 31st annual meeting of the American Society for Bone and Mineral Research, Denver, Colorado, September 2009.
References
- 1.Lowenstein WR. Junctional intercellular communication: the cell-to-cell membrane channel. Physiological Reviews. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- 2.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nature Reviews in Molecular and Cell Biology. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 3.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. Journal of Biological Chemistry. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 4.Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. Journal of Biological Chemistry. 2005;280:7317–7325. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- 5.Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. Journal of Bone and Mineral Research. 2008;23:1712–1721. doi: 10.1359/JBMR.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers MJ, Frith JC, Luckman SP, Coxon FP, Benford HL, Monkkonen J, Auriola S, Chilton KM, Russell RG. Molecular mechanisms of action of bisphosphonates. Bone. 1999;24:73S–79S. doi: 10.1016/s8756-3282(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 7.Bellido T, Plotkin LI. Novel actions of bisphosphonates in bone: preservation of osteoblast and osteocyte viability. Bone. 2011;49:50–55. doi: 10.1016/j.bone.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporosis International. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 9.Idris AI, Rojas J, Greig IR, Van't Hof RJ, Ralston SH. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int. 2008;82:191–201. doi: 10.1007/s00223-008-9104-y. [DOI] [PubMed] [Google Scholar]
- 10.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 11.Chung DJ, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. Journal of Cell Science. 2006;119:4187–4198. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 12.Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1) J Bone Miner Res. 2008;23:879–886. doi: 10.1359/JBMR.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimston SK, Goldberg DB, Watkins M, Brodt MD, Silva MJ, Civitelli R. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. Journal of Bone and Mineral Research. 2011;26:2151–2160. doi: 10.1002/jbmr.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E, Civitelli R. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Molecular Biology of the Cell. 2011;22:1240–1251. doi: 10.1091/mbc.E10-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun LR, Rhee Y, Bellido T, Plotkin LI. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. Journal of Bone and Mineral Research. 2011 doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Paul EM, Sathyendra V, Davison A, Sharkey N, Bronson S, Srinivasan S, Gross TS, Donahue HJ. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One. 6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theis M, Mas C, Doring B, Kruger O, Herrera P, Meda P, Willecke K. General and conditional replacement of connexin43-coding DNA by a lacZ reporter gene for cellautonomous analysis of expression. Cell Communication and Adhesion. 2001;8:383–386. doi: 10.3109/15419060109080758. [DOI] [PubMed] [Google Scholar]
- 18.Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Developmental Dynamics. 2002;224:245–251. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- 19.Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- 20.Fang LC, Cheng SL, Mbalaviele G, Donsante C, Watkins M, Radice GL, Civitelli R. Accentuated ovariectomy induced bone loss and altered osteogenesis in hyeterozygous N-cadherin null mice. Journal of Bone and Mineral Research. 2006;21:1897–1906. doi: 10.1359/jbmr.060906. [DOI] [PubMed] [Google Scholar]
- 21.Geoffroy V, Paschalis EP, Libouban H, Blouin S, Ostertag A, Chappard D, Cros M, Phipps R, de Vernejoul MC. Effects of risedronate in Runx2 overexpressing mice, an animal model for evaluation of treatment effects on bone quality and fractures. Calcified Tissue International. 2011;88:464–475. doi: 10.1007/s00223-011-9480-6. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs RK, Phipps RJ, Burr DB. Recovery of trabecular and cortical bone turnover after discontinuation of risedronate and alendronate therapy in ovariectomized rats. Journal of Bone and Mineral Research. 2008;23:1689–1697. doi: 10.1359/JBMR.080501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Castro CH, Shin CS, Stains JP, Cheng SL, Sheikh S, Mbalaviele G, Szejnfeld VL, Civitelli R. Targeted expression of a dominant-negative N-cadherin in vivo delays peak bone mass and increases adipogenesis. Journal of Cell Science. 2004;117:2853–2864. doi: 10.1242/jcs.01133. [DOI] [PubMed] [Google Scholar]
- 25.Willinghamm MD, Brodt MD, Lee KL, Stephens AL, Ye J, Silva MJ. Age-related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int. 86:470–483. doi: 10.1007/s00223-010-9359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan EF, Bouxsein M. Biomechanics of Bone and Age-related Fractures. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of Bone Biology. 3rd ed. San Diego, CA: Academic Press; 2008. pp. 29–51. [Google Scholar]
- 27.Aitchison KJ, Gonzalez FJ, Quattrochi LC, Sapone A, Zhao JH, Zaher H, Elizondo G, Bryant C, Munro J, Collier DA, Makoffa AI, Kerwin RW. Identification of novel polymorphisms in the 5' flanking region of CYP1A2, characterization of interethnic variability, and investigation of their functional significance. Pharmacogenetics. 2000;10:695–704. doi: 10.1097/00008571-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Fonseca H, Moreira-Goncalves D, Vaz M, Fernandes MH, Ferreira R, Amado F, Mota MP, Duarte JA. Changes in proximal femur bone properties following ovariectomy and their association with resistance to fracture. J Bone Miner Metab. 2011 doi: 10.1007/s00774-011-0308-2. [DOI] [PubMed] [Google Scholar]
- 29.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca D, Ward WE. Daidzein together with high calcium preserve bone mass and biomechanical strength at multiple sites in ovariectomized mice. Bone. 2004;35:489–497. doi: 10.1016/j.bone.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 31.Li CY, Schaffler MB, Wolde-Semait HT, Hernandez CJ, Jepsen KJ. Genetic background influences cortical bone response to ovariectomy. J Bone Miner Res. 2005;20:2150–2158. doi: 10.1359/JBMR.050819. [DOI] [PubMed] [Google Scholar]
- 32.Chappard D, Retailleau-Gaborit N, Legrand E, Basle MF, Audran M. Comparison insight bone measurements by histomorphometry and microCT. J Bone Miner Res. 2005;20:1177–1184. doi: 10.1359/JBMR.050205. [DOI] [PubMed] [Google Scholar]
- 33.Muller R, Van Campenhout H, Van Damme B, Van Der Perre G, Dequeker J, Hildebrand T, Ruegsegger P. Morphometric analysis of human bone biopsies: a quantitative structural comparison of histological sections and micro-computed tomography. Bone. 1998;23:59–66. doi: 10.1016/s8756-3282(98)00068-4. [DOI] [PubMed] [Google Scholar]
- 34.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Dobrowolski R, Hertig G, Lechner H, Worsdorfer P, Wulf V, Dicke N, Eckert D, Bauer R, Schorle H, Willecke K. Loss of connexin43-mediated gap junctional coupling in the mesenchyme of limb buds leads to altered expression of morphogens in mice. Hum Mol Genet. 2009;18:2899–2911. doi: 10.1093/hmg/ddp227. [DOI] [PubMed] [Google Scholar]
- 36.Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha JN, Jabs EW. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Human Mutation. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- 37.Decrock E, Vinken M, De Vuyst E, Krysko DV, D'Herde K, Vanhaecke T, Vandenabeele P, Rogiers V, Leybaert L. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;16:524–536. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.