Abstract
α-Synuclein causes Parkinson’s disease if mutated or aberrantly produced in neurons. α-Synuclein–lipid interactions are important for the normal function of the protein, but can also contribute to pathogenesis. We previously reported that deletion of the first 10 N-terminal amino acids dramatically reduced lipid binding in vitro, as well as membrane binding and toxicity in yeast. Here we extend this study to human neuroblastoma SHSY-5Y cells, and find that in these cells the first 10 N-terminal residues do not affect α-synuclein membrane binding, self-association and cell viability, contrary to yeast. Differences in lipid composition, membrane fluidity and cytosolic factors between yeast and neuronal cells may account for the distinct binding behavior of the truncated variant in these two systems. Retinoic acid promotes differentiation and α-synuclein oligomer formation in neuroblastoma cells, while addition of a proteasomal inhibitor induces neurite outgrowth and toxicity to certain wild-type and truncated α-synuclein clones. Yeast recapitulate several features of α-synuclein (patho)biology, but its simplicity sets limitations; verification of yeast results in more relevant model systems is, therefore, essential.
Keywords: α-synuclein, membrane binding, N-terminus, Parkinson’s disease, toxicity
The α-synuclein protein (α-syn) causes familial Parkinson’s disease (PD), when mutated (A30P, A53T, E46K) (Polymeropoulos et al. 1997; Kruger et al. 1998; Zarranz et al. 2004) or over-produced as a result of gene multiplication (Singleton et al. 2003; Chartier-Harlin et al. 2004). Moreover, α-syn is the major fibrillar component of Lewy bodies, present in cases of sporadic PD (Spillantini et al. 1997). In vitro, α-syn is intrinsically unstructured (Weinreb et al. 1996), but adopts an α-helical structure upon lipid binding to its amphipathic repeats comprising ca. 100 N-terminal residues (Davidson et al. 1998), or forms β-sheet aggregates upon prolonged incubation (Conway et al. 2000). We previously reported that deletion of as few as 10 N-terminal residues (DVFMKGLSKA) dramatically altered the properties of α-syn in vitro and in yeast. N-terminal deletion decreases binding to anionic lipid vesicles, α-helical structure propensity (Vamvaca et al. 2009), and aggregation (Zibaee et al. 2007). Over-produced wild-type α-syn is toxic to Saccharomyces cerevisiae, localizes to the plasma membrane and forms cytosolic inclusions (Outeiro and Lindquist 2003), whereas the N-terminally truncated variant (del2-11) is nontoxic to yeast and homogenously dispersed throughout the cytosol (Vamvaca et al. 2009).
The binding of α-syn to phospholipids via its N-terminus plays a key role in protein function. α-Syn localizes to synaptic vesicles, where it interacts with certain protein molecules (synphilin-1, CSPalpha, SNARE complex) to modulate synaptic plasticity and neurotransmitter release (Ribeiro et al. 2002; Chandra et al. 2005; Burre et al. 2010). In pathologic situations, however, high local concentrations of α-syn at the membrane may promote abnormal protein–protein or protein–lipid interactions. For instance, A30P and A53T α-syn can oligomerize into fibrillar pores, which permeabilize lipid vesicles like bacterial toxins (Lashuel et al. 2002; Volles and Lansbury 2002). Excessive coating of the membrane with non-fibrillar α-syn monomer may also disrupt cellular homeostasis by hindering normal membrane-based processes (Volles and Lansbury 2007). At high concentrations, wild-type and A53T α-syn bind to mitochondrial membranes as well, inducing oxidative stress and apoptosis in SHSY-5Y cells (Parihar et al. 2008).
α-Syn toxicity can be exacerbated by proteasome inhibitors. For example, A53T and A30P mutants cause proteasomal dysfunction in M17 neuronal cells, thereby increasing sensitivity to proteasome inhibitors (Petrucelli et al. 2002). Insoluble filaments, soluble oligomers, and to a lesser extent, monomers of α-syn impair proteasomal function (Snyder et al. 2003; Lindersson et al. 2004; Emmanouilidou et al. 2010). Blockage of the proteasomal degradation pathway either by external inhibitors or by endogenous α-syn itself, could lead to accumulation of α-syn and further suppression of proteolytic activity.
We created stable SHSY-5Y clones, constitutively producing either wild-type or del2-11 α-syn, and monitored membrane binding, self-association, and toxicity in cycling and differentiated cells, both in the absence and presence of a proteasomal inhibitor. Contrary to previous data in yeast, the first N-terminal amino acids are neither essential for α-syn membrane binding nor do they significantly affect aggregαtion and toxicity of the protein in neuronal cells.
Materials and methods
Generation of stable cell lines
The cDNA sequences of wild-type and del2-11 α-syn were subcloned into the pIRES2-enhanced green fluorescent protein (EGFP) vector (Clontech, Mountain View, CA, USA) between its unique restriction sites SacI/SalI and NheI/BglII, respectively. Both constructs were verified by DNA sequencing; they contain the Kozak consensus sequence GCCACC preceding the ATG start codon to increase translation efficiency. Naïve SHSY-5Y cells (gift from Dr D. Yamashiro, Columbia University, New York, NY, USA) were transfected with pIRES2-EGFP (empty vector) or pIRES2-EGFP bearing the wild-type or del2-11 α-syn gene in OptiMEM (Gibco-Invitrogen, Carlsbad, CA, USA) using Lipofectamine 2000 reagent (Invitrogen). Stable transformants were selected in the presence of 300 μg/mL G418 sulfate (Calbiochem, San Diego, CA, USA). Individual clones were pre-screened by EGFP fluorescence, followed by western blot analysis.
Cell culture
Human neuroblastoma SHSY-5Y cells were cultured in RPMI 1640 medium (Gibco-Invitrogen), supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO, USA), 2 mmol/L L-glutamine (Sigma), 1000 U/mL penicillin and 1 mg/mL streptomycin solution (Biochrom AG, Berlin, Germany). Cells were grown in a humidified incubator at 37°C, in a 5% CO2 atmosphere. Stable clones were maintained in 300 μg/mL G418 sulfate. Cells were differentiated with 10 μmol/L all-trans retinoic acid (RA; Calbiochem). The proteasome inhibitor (PSI; benzyloxycarbonyl-L-isoleucyl-gammα-t-butyl-L-glutamyl-L-alanyl-L-leucinal; Calbiochem) was added at a concentration of 50 nmol/L 5 days after differentiation was induced. In each experiment, RA-containing medium (± PSI) was replaced every 2 days.
Microscopy
EGFP fluorescence was monitored with a LEICA DM IRE2 fluorescence microscope, using an excitation wavelength band-pass filter of 450–490 nm, a 20-fold magnification lens, and a 250 ms exposure.
Western blot analysis
Proliferating and differentiated (RA added for 6 days) cells were washed with phosphate-buffered saline (PBS; Gibco-Invitrogen), treated with 0.25% trypsin (wt/vol) – 1 mmol/L EDTA solution (Gibco) and harvested by centrifugation (2000 g, 5 min). The pellet was washed with PBS and subsequently resuspended in 150 mmol/ L Tris–HCl pH 7.6, 150 mmol/L NaCl, 2 mmol/L EDTA, 1% Triton X-100 (vol/vol), containing protease inhibitor mix (complete mini EDTA-free; Roche Molecular Biochemicals, Indianapolis, IN, USA). Following 20-min incubation on ice, the cell lysate was cleared by centrifugation (13 000 rpm, 10 min). Protein concentrtion was determined by Coomasie blue (Bio-Rad, Hercules, CA, USA). The soluble protein fraction was loaded on 16.5% (wt/vol) tris-tricine sodium dodecyl sulfate (SDS)–polyacrylamide gel (7 μg protein/lane), which is suitable for resolution of proteins smaller than 30 kDa (Schagger 2006). Running buffer contained 100 mmol/L Tris–HCl, 100 mmol/L tricine (Applichem, Darms-tadt, Germany), and 0.1% (wt/vol) SDS. Separated proteins were transferred from the gel onto a nitrocellulose membrane (Whatman Protran, Dassel, Germany). Transfer buffer was composed of 100 mmol/L Tris–HCl, 100 mmol/L tricine, 0.05% (wt/vol) SDS, and 20% (vol/vol) methanol. Membranes were blocked in PBS containing 5 % non-fat milk (wt/vol) and 0.1% (vol/vol) Tween 20 (Applichem), and then probed with antibodies: (i) rabbit polyclonal α-syn C-20 (1 : 1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), (ii) mouse monoclonal β-actin (1 : 5000 dilution; Sigma), (iii) mouse monoclonal GFP B-2 (1 : 1000 dilution; Santa Cruz), (iv) mouse monoclonal lamp2 H4B4 (1 : 1000 dilution; Development Studies Hybridoma Bank, University of Iowa), (v) ERK2 rabbit polyclonal (1 : 4000 dilution; Santa Cruz). Proteins were detected with horseradish peroxidase-conjugated secondary antibodies (Pierce, Rockford, IL, USA).
Membrane fractionation
Proliferating and differentiated (after a 6-day RA treatment) cells were lysed in 50 mmol/L Tris-HCl (pH 8), 1 mmol/L EDTA, containing protease inhibitor mix, and homogenized by passing through a 29-gauge needle 10 times. The lysate was centrifuged at 600 g for 5 min, and supernatant underwent ultracentrifugation at 100 000 g for 2 h to separate into membrane (pellet) and cytosolic (supernatant) fractions. The membrane fraction was washed thrice with lysis buffer at 4°C, to remove remaining cytosolic proteins. Soluble membrane proteins were extracted with 150 mmol/L Tris–HCl pH 7.6, 150 mmol/L NaCl, 2 mmol/L EDTA, 1% Triton X-100 (vol/vol), containing protease inhibitors, and isolated by centrifugation (14 000 g, 15 min). Protein concentration was determined by the Bradford assay. Samples (14 μg protein) were separated by 16.5% (wt/vol) tris-tricine SDS polyacrylamide gel electrophoresis and analyzed by western immunoblotting.
Cell survival assay
The viability of selected clones was compared with controls. 105 cells were plated onto 12-well plates and differentiated with RA. After 3, 6 and 9 days, cells were washed with PBS, treated with 40 μL trypsin-EDTA solution (Gibco), which was subsequently inactivated with 80 μL RPMI/10% fetal bovine serum, containing 1 mmol/L EDTA (Sigma). Cell viability was assayed by trypan blue [0.4% (wt/vol) solution; Sigma] exclusion; intact cells were counted using a hematocytometer (Hausser Scientific, Horsham, PA, USA). Cell counts were performed at least in triplicate, and the data are presented as the mean value ± standard deviation.
Results
Stable SHSY-5Y cell lines produce high levels of wild-type and del2-11 α-syn
Wild-type and del2-11 α-syn cDNA sequences were each cloned into the pIRES2 EGFP vector, between the immediate early promoter of human cytomegalovirus (PCMV IE) and the internal ribosome entry site (IRES) sequence, which precedes the EGFP coding region. PCMV IE allows constitutive gene expression, and IRES enables translation of α-syn and EGFP genes from a single bicistronic mRNA molecule. In this way, α-syn and EGFP are synthesized in parallel without being covalently attached. EGFP serves as a reporter for transfected cells without disrupting the conformation of α-syn.
SHSY-5Y cells share many characteristics of dopaminergic neurons, including dopamine synthesis and differentiation into a neuronal phenotype upon exposure to certain molecules. Transient transfections confirmed that both α-syn proteins and EGFP can be produced at high amounts under all conditions (±RA, ±PSI; data not shown). Stable clones were isolated after a 3-week treatment with G418 antibiotic (pIRES2 contains neomycin resistance gene), while all non-transfected control cells had died. For each cell line, three clones exhibiting homogeneous EGFP fluorescence and high α-syn levels were selected. Polyclonal cell lines were also collected to average clonal variability; α-syn levels in these lines were about 30-fold higher than endogenous α-syn levels in naïve cells (Figure S1).
RA induces α-syn oligomer formation in stably transfected cells
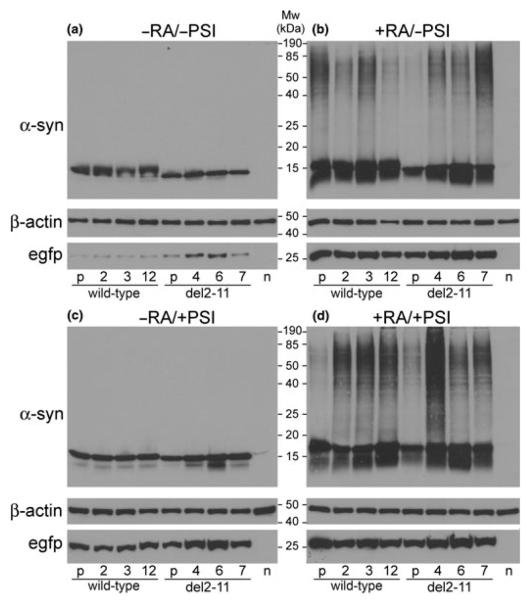
The generated cell lines were analyzed by western blotting under four different conditions (±RA, ±PSI; Fig. 1). Stable clones produced similar levels of wild-type and del2-11 α-syn, which markedly increased in the presence of RA, along with EGFP levels. Moreover, RA induced α-syn oligomer formation [soluble in 1% (vol/vol) Triton X-100; Figs. 1b and d], while PSI alone did not (Fig. 1c). Despite clonal differences, wild-type and del2-11 α-syn overall oligomerized at a similar extent.
Fig. 1.
Western blots of wild-type and del2-11 α-syn clones (a), plus RA (b), plus PSI (c), plus RA and PSI (d). Cells were treated with 10 μmol/L RA for 6 days and PSI (50 nmol/L) was added on day 5. Polyclonal cell lines (depicted as ‘p’) and naïve SHSY-5Y cells (depicted as ‘n’) were also analyzed. Each lane contains 7 μg of soluble protein. β-Actin served as a loading control. All four blots were processed in parallel, using identical conditions and exposure times.
N-terminal truncation does not affect α-syn membrane binding in SHSY-5Y cells
Membrane-bound proteins (with slow dissociation rate) were separated from their cytosolic counterparts, using an established fractionation protocol (Liu et al. 2009). The lysosomal-associated membrane protein lamp2 was almost exclusively detected in the membrane fraction, whereas β-actin was mostly present in the cytosol. Western blot analysis revealed (on average) similar levels of membrane bound monomeric wild-type and del2-11 α-syn, both in proliferating and in differentiated cells (Fig. 2). We estimated for each clone (±RA) the relative amount of α-syn monomer in the cytosolic and membrane fractions by densitometry, and found no significant differences in the relative ratio of membrane bound to total α-syn monomer between wild-type and del2-11 clones (data not shown). Oligomeric and truncated α-syn species appear to be relatively less populated in the membrane than in the cytosol, suggesting weaker membrane binding than the full-length monomeric protein. As previously observed (Fig. 1b), oligomers were detected in the differentiated state and no significant differences between wild-type and del2-11 α-syn were observed in terms of their oligomerization propensity. In this experiment, however, few oligomeric species were visible in the cytosolic fraction of proliferating cells (Fig. 2a), probably because cell lysis was performed under milder conditions (mechanical shearing in a detergent-free buffer).
Fig. 2.
Western-blot analysis of cytosolic (a, b) and membrane (c, d) fractions from proliferating and differentiated (6-day RA-treatment) SHSY-5Y cells, respectively. Naïve (marked as ‘n’) and polyclonal cells (marked as ‘p’) were included in the analysis. Each lane contains 14 μg of soluble protein. Loading controls for the cytosolic and membrane fractions were β-actin and lamp2 respectively. All four blots were processed in parallel, using identical conditions and exposure times.
Certain wild-type and del2-11 α-syn clones are toxic to SHSY-5Y cells, only in the presence of RA and PSI
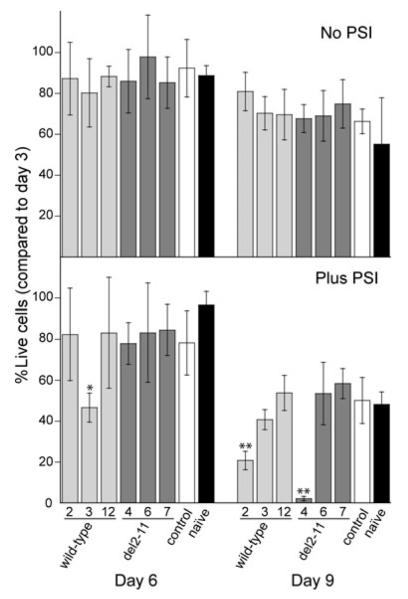
Stable SHSY-5Y clones constitutively expressing wild-type or del2-11 α-syn represent a good system for toxicity studies, given that the expression levels for the chromosomally integrated α-syn genes are ca. 30-fold greater than for the endogenous α-syn gene (Figure S1). An EGFP fluorescent empty-vector clone with low endogenous α-syn levels served as a negative control, along with naïve cells. Proliferating wild-type and del2-11 α-syn clones showed no growth disadvantage over naïve or empty vector control SHSY-5Y cells (data not shown), as previously reported (Vekrellis et al. 2009). Following a 6- to 9-day RA-treatment, α-syn clones did not die faster than controls (Fig. 3 top). PSI addition, however, promoted cell death of wild-type clone 3 at day 6 and caused massive cell death of wild-type clone 2 and del2-11 clone 4 at day 9 (Fig. 3, bottom). The three clones displaying increased vulnerability to PSI did not express more monomeric or oligomeric α-syn compared with other clones (Figs. 1 and 2). Despite individual clonal differences, there was no overall difference in toxicity between wild-type and del2-11 α-syn clones.
Fig. 3.
Cell viability measurements of wild-type and del2-11 clones treated with 10 μmol/L RA for 6 and 9 days; 50 nmol/L PSI was added on day 5 (bottom plot). Empty-vector control and naïve SHSY-5Y cells were used as a reference. The percentage of live cells on days 6 and 9 is expressed as a function of viable cells on day 3 (the % of live cells on day 3 is set to 100%). The data are presented as mean value ± standard deviation of at least three independent experiments (n = 3–9). p-Values (*p < 0.05, **p < 0.01) were derived from a one-way ANOVA analysis followed by Tukey’s test, comparing individual clones with empty vector control on days 6 and 9.
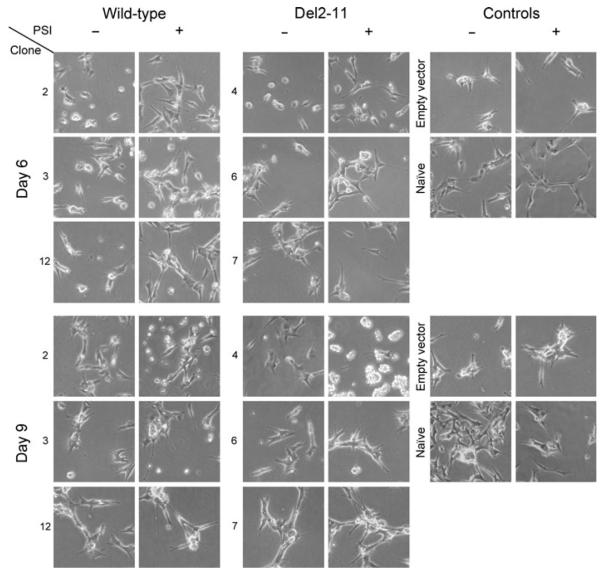
Cell morphology changes of wild-type and del2-11 α-syn clones upon RA/PSI treatment
Naïve SHSY-5Y cells formed elongated processes upon differentiation. Following a 6-day treatment with RA, wild-type clone 2 and del2-11 clone 4 remained primarily round-shaped and lacked neuritic processes, whereas the rest of the clones adopted a differentiated phenotype (Fig. 4 top), similar to that of controls. PSI addition (day 5) induced further neurite outgrowth, detectable after 24 h. On day 9, wild-type clone 2 and del2-11 clone 4 PSI-treated cells massively detached from the plate surface, indicating cell death (Fig. 4 bottom). The effect was more pronounced in del2-11 clone 4, in accordance with the toxicity data (Fig. 3). All clones and controls retained EGFP fluorescence until the end of the experiment (Figure S2).
Fig. 4.
Microscopy images of wild-type and del2-11 clones after a 6-(top) and 9-day (bottom) RA-treatment in the presence or absence of PSI (added on day 5). Empty-vector and naïve SHSY-5Y cells were used as controls. PSI induced neurite outgrowth in all cells.
Discussion
Deletion of the first 10 N-terminal amino acids from the α-syn primary sequence did not affect self-association, membrane binding or toxicity of the protein in neuroblastoma cells, contrary to previous findings in vitro and in yeast (Vamvaca et al. 2009). The behavior of the wild-type protein and the truncated variant was studied both in proliferating and differentiated SHSY-5Y cells. RA induces differentiation, inhibiting cell growth and promoting neuritic outgrowth (Pahlman et al. 1984). RA treatment resulted in higher levels of α-syn and EGFP, whereas β-actin control levels remained constant (Fig. 1). The PCMV IE promoter contains a RA-responsive element (Ghazal et al. 1992), whose activation probably accounts for the elevated α-syn levels in RA-treated cells. Changes in cellular metabolism (protein synthesis vs. degradation rates) upon differentiation may also contribute. Interestingly, only in the presence of RA did α-syn form soluble oligomers, while N-terminal truncation did not affect the propensity of the protein to self-associate (Fig. 1). The observed increase in α-syn concentration, which favors protein–protein interactions, as well as other RA-induced cellular responses are likely to promote α-syn oligomerization. For instance, tyrosine hydroxylase – a key enzyme in dopamine biosynthesis – is much more abundant in differentiated than in proliferating neuroblastoma cells, suggesting a strong increase in dopamine levels upon differentiation (Bourdeaut et al. 2009). Dopamine stabilizes soluble α-syn oligomers both in vitro (Conway et al. 2001) and in RA-treated SHSY-5Y cells (Mazzulli et al. 2006). N-terminal truncation did not affect α-syn oligomerization in differentiated SHSY-5Y cells, while it dramatically decreased inclusion formation in yeast (Vamvaca et al. 2009) and decelerated aggregation in vitro (Zibaee et al. 2007). Intracellular factors present in differentiated neuroblastoma cells but absent in yeast, such as dopamine, may promote del2-11 α-syn oligomerization.
The membrane provides a nucleation site for oligomerization, given the high effective concentration of lipid-bound protein. In fact, membrane-bound α-syn can seed aggregation of the cytosolic form (Lee et al. 2002). The folding of α-syn upon lipid binding is a two step process (Bartels et al. 2010; Bodner et al. 2010). First, residues 3–25 anchor the protein to the membrane, and subsequently, residues 26- to 100-fold into an α-helix. The first conformation has a high propensity to aggregate because of exposed hydrophobic residues (61– 95), whereas the second can undergo an α-helix to β-sheet conversion, yielding fibrillar aggregates (Abedini and Raleigh 2009). Oligomeric α-syn was less populated in the membrane than in the cytosolic fraction of differentiated SHSY-5Y cells, relative to monomeric α-syn (Fig. 2), possibly due to greater dissociation of oligomers from the membrane to the cytosol, and/or lesser association of oligomers from the cytosol to the membrane.
Surprisingly, del2-11 α-syn did not bind weaker than wild-type to neuroblastoma cell membranes, contrary to previous data in yeast and in vitro (Vamvaca et al. 2009). In a related study, deletion of amino acids 2–9 did not alter α-syn membrane binding in SHSY-5Y cells (Wang et al. 2010). The weaker affinity of del2-11 α-syn for yeast membranes, compared with neuronal cell membranes, is probably caused by differences in membrane lipid composition, fluidity, and curvature between the two cell types (Kjaer et al. 2009). α-Syn preferentially binds to anionic phospholipids embedded in a liquid-disordered rather than liquid-ordered domains (Stockl et al. 2008). Cholesterol plays a key role in the interaction of α-syn with lipid rafts, mediating synaptic localization of the protein (Fortin et al. 2004). In contrast to neuronal cells, lipid rafts in S. cerevisiae yeast plasma membrane are highly-ordered gel domains lacking ergosterol, the yeast counterpart of cholesterol (Aresta-Branco et al. 2011). Moreover, proteins in brain cytosol – likely absent in yeast – promote A30P α-syn membrane binding (Wislet-Gendebien et al. 2008).
Over-produced α-syn is highly toxic to yeast (Outeiro and Lindquist 2003). In differentiated SHSY-5Y cells, however, the toxic effects of α-syn are observed only in certain PSI-treated clones (Fig. 3), probably because of differences in the nature of gene integration sites. Although N-terminal truncation ameliorated toxicity in yeast, faster dying SHSY-5Y cells included both wild-type and del2-11 α-syn clones. Notably, these clones did not have significantly higher α-syn monomer or oligomer levels than the rest (Fig. 1). PSI inhibits proteasomal degradation, leading to abnormal protein accumulation. The impaired clearance of regulatory proteins likely affects cell-cycle progression, promoting neurite outgrowth in SHSY-5Y cells (Fig. 4). Similarly, PSI induced process extension in PC12 cells (Giasson et al. 1999), and several other proteasomal inhibitors also favored morphological differentiation (Saito and Kawashima 1989; Fenteany et al. 1994). PSI-associated α-syn toxicity may be induced by abnormal protein accumulation itself and/or by processes linked to differentiation. Interestingly, clones with increased vulnerability to α-syn are round-shaped cells lacking neurites after a 6-day RA-treatment, as opposed to controls, and form processes only in the presence of PSI (Fig. 4). These clones possibly ‘resist’ differentiation by RA in order to survive, whereas PSI ‘forces’ them to differentiate, thereby promoting cell death.
Yeast is a simple PD model system, which reproduces several aspects of α-syn pathobiology observed in higher eukaryotes, including cell death, proteasomal dysfunction, vesicle trafficking defects, accumulation of lipid droplets and reactive oxygen species (Outeiro and Lindquist 2003; Flower et al. 2005). A genomewide screen in yeast identified proteins involved in endoplasmic reticulum-to-Golgi vesicle trafficking as major α-syn toxicity modifiers. Strikingly, a mammalian homolog of one such protein (Rab1) rescued neuronal loss in a fly, worm and rat PD model (Cooper et al. 2006). Moreover, yeast data established a genetic link between α-syn and another PD-associated gene (PARK9), suggesting participation of α-syn in a highly conserved interaction network (Gitler et al. 2009).
Although yeast is a useful tool for gaining insight into α-syn toxic function, this study revealed a discrepancy between data in yeast and in neurons. Deletion of the first 10 N-terminal amino acids of α-syn dramatically reduced membrane binding, inclusion formation and toxicity in S. cerevisiae, while it caused no such effects in human neuroblastoma cells. Findings in yeast, therefore, do not necessarily reflect the situation in neurons, data extrapolation is not always valid, and initial results require verification in more relevant PD models. SHSY-5Y stable clones reported herein produce high levels of α-syn and EGFP, being amenable to screening applications and α-syn isolation for biochemical studies.
Supplementary Material
Acknowledgements
We thank Dr Michael J. Volles and Stefanis laboratory members for useful discussions. This work was supported by a Morris K. Udall Parkinson’s Disease Research Center for Excellence grant (NS038375; PTL) and a MEFOPA grant (FP7-Health 2009 241791; LS). The authors declare no conflict of interest.
Abbreviations used
- α-syn
α-synuclein
- del2-11
α-syn variant lacking the first 10 N-terminal amino acids
- EGFP
enhanced green fluorescent protein
- PBS
phosphate-buffered saline
- PD
Parkinson’s disease
- PSI
proteasome inhibitor
- RA
all-trans retinoic acid
- S. cerevisiae
yeast strain Saccharomyces cerevisiae
- SDS
sodium dodecyl sulfate
Footnotes
Supporting information Additional supporting information may be found in the online version of this article:
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Abedini A, Raleigh DP. A role for helical intermediates in amyloid formation by natively unfolded polypeptides? Phys. Biol. 2009;6:015005. doi: 10.1088/1478-3975/6/1/015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aresta-Branco F, Cordeiro AM, Marinho HS, Cyrne L, Antunes F, de Almeida RF. Gel domains in the plasma membrane of Saccharomyces cerevisiae: highly ordered, ergosterol-free, and sphingolipid-enriched lipid rafts. J. Biol. Chem. 2011;286:5043–5054. doi: 10.1074/jbc.M110.154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels T, Ahlstrom LS, Leftin A, Kamp F, Haass C, Brown MF, Beyer K. The N-terminus of the intrinsically disordered protein alpha-synuclein triggers membrane binding and helix folding. Biophys. J. 2010;99:2116–2124. doi: 10.1016/j.bpj.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner CR, Maltsev AS, Dobson CM, Bax A. Differential phospholipid binding of alpha-synuclein variants implicated in Parkinson’s disease revealed by solution NMR spectroscopy. Biochemistry. 2010;49:862–871. doi: 10.1021/bi901723p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeaut F, Janoueix-Lerosey I, Lucchesi C, et al. Cholinergic switch associated with morphological differentiation in neuroblastoma. J. Pathol. 2009;219:463–472. doi: 10.1002/path.2614. [DOI] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Südhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- Conway KA, Rochet J-C, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein oligomers are targeted to, and impair, the 26S proteasome. Neurobiol. Aging. 2010;31:953–968. doi: 10.1016/j.neurobiolaging.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Fenteany G, Standaert RF, Reichard GA, Corey EJ, Schreiber SL. A betα-lactone related to lactacystin induces neurite outgrowth in a neuroblastoma cell line and inhibits cell cycle progression in an osteosarcoma cell line. Proc. Natl Acad. Sci. USA. 1994;91:3358–3362. doi: 10.1073/pnas.91.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower TR, Chesnokova LS, Froelich CA, Dixon C, Witt SN. Heat shock prevents alpha-synuclein-induced apoptosis in a yeast model of Parkinson’s disease. J. Mol. Biol. 2005;351:1081–1100. doi: 10.1016/j.jmb.2005.06.060. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J. Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P, DeMattei C, Giulietti E, Kliewer SA, Umesono K, Evans RM. Retinoic acid receptors initiate induction of the cytomegalovirus enhancer in embryonal cells. Proc. Natl Acad. Sci. USA. 1992;89:7630–7634. doi: 10.1073/pnas.89.16.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Bruening W, Durham HD, Mushynski WE. Activation of stress-activated protein kinases correlates with neurite outgrowth induced by protease inhibition in PC12 cells. J. Neurochem. 1999;72:1081–1087. doi: 10.1046/j.1471-4159.1999.0721081.x. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat. Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer L, Giehm L, Heimburg T, Otzen D. The influence of vesicle size and composition on alpha-synuclein structure and stability. Biophys. J. 2009;96:2857–2870. doi: 10.1016/j.bpj.2008.12.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Choi C, Lee SJ. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J. Biol. Chem. 2002;277:671–678. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- Lindersson E, Beedholm R, Hojrup P, Moos T, Gai W, Hendil KB, Jensen PH. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J. Biol. Chem. 2004;279:12924–12934. doi: 10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Meray RK, Grammatopoulos TN, Fredenburg RA, Cookson MR, Liu Y, Logan T, Lansbury PT., Jr Membrane-associated farnesylated UCH-L1 promotes alpha-synuclein neurotoxicity and is a therapeutic target for Parkinson’s disease. Proc. Natl Acad. Sci. USA. 2009;106:4635–4640. doi: 10.1073/pnas.0806474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Mishizen AJ, Giasson BI, Lynch DR, Thomas SA, Nakashima A, Nagatsu T, Ota A, Ischiropoulos H. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J. Neurosci. 2006;26:10068–10078. doi: 10.1523/JNEUROSCI.0896-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlman S, Ruusala AI, Abrahamsson L, Mattsson ME, Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984;14:135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell. Mol. Life Sci. 2008;65:1272–1284. doi: 10.1007/s00018-008-7589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucelli L, O’Farrell C, Lockhart PJ, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Ribeiro CS, Carneiro K, Ross CA, Menezes JR, Engelender S. Synphilin-1 is developmentally localized to synaptic terminals, and its association with synaptic vesicles is modulated by alpha-synuclein. J. Biol. Chem. 2002;277:23927–23933. doi: 10.1074/jbc.M201115200. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kawashima S. The neurite-initiating effect of a tripeptide aldehyde protease inhibitor on PC12h cells. J. Biochem. 1989;106:1035–1040. doi: 10.1093/oxfordjournals.jbchem.a122960. [DOI] [PubMed] [Google Scholar]
- Schagger H. Tricine–SDS–PAGE. Nat. Protoc. 2006;1:16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B. Aggregated and monomeric alpha-synuclein bind to the S6’ proteasomal protein and inhibit proteasomal function. J. Biol. Chem. 2003;278:11753–11759. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stockl M, Fischer P, Wanker E, Herrmann A. Alpha-synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. J. Mol. Biol. 2008;375:1394–1404. doi: 10.1016/j.jmb.2007.11.051. [DOI] [PubMed] [Google Scholar]
- Vamvaca K, Volles MJ, Lansbury PT., Jr The first N-terminal amino acids of alpha-synuclein are essential for alpha-helical structure formation in vitro and membrane binding in yeast. J. Mol. Biol. 2009;389:413–424. doi: 10.1016/j.jmb.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekrellis K, Xilouri M, Emmanouilidou E, Stefanis L. Inducible over-expression of wild type alpha-synuclein in human neuronal cells leads to caspase-dependent non-apoptotic death. J. Neurochem. 2009;109:1348–1362. doi: 10.1111/j.1471-4159.2009.06054.x. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT., Jr Relationships between the sequence of alpha-synuclein and its membrane affinity, fibrillization propensity, and yeast toxicity. J. Mol. Biol. 2007;366:1510–1522. doi: 10.1016/j.jmb.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moualla D, Wright JA, Brown DR. Copper binding regulates intracellular alpha-synuclein localisation, aggregation and toxicity. J. Neurochem. 2010;113:704–714. doi: 10.1111/j.1471-4159.2010.06638.x. [DOI] [PubMed] [Google Scholar]
- Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- Wislet-Gendebien S, Visanji NP, Whitehead SN, Marsilio D, Hou W, Figeys D, Fraser PE, Bennett SA, Tandon A. Differential regulation of wild-type and mutant alpha-synuclein binding to synaptic membranes by cytosolic factors. BMC Neurosci. 2008;9:92. doi: 10.1186/1471-2202-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, et al. The new mutation, E46K, of alpha-synuclein causes parkinson and Lewy body dementia. Ann. Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zibaee S, Jakes R, Fraser G, Serpell LC, Crowther RA, Goedert M. Sequence determinants for amyloid fibrillogenesis of human alpha-synuclein. J. Mol. Biol. 2007;374:454–464. doi: 10.1016/j.jmb.2007.09.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.