Electrical activity underlies the control of the frequency, strength, and duration of contraction of the heart. During the cardiac cycle, a regular rhythmic pattern must be established in time-dependent changes in ionic conductances to ensure events that underlie normal cardiac function. Electrical impulses, originating at the sino atrial node are conducted throughout the atria until they converge at the atrio-ventricular node, pass through the bundle of His and the Purkinje fiber conducting system, and eventually excite the working myocardial cells of both ventricles. Current flow through a large number of ion channels, exchange mechanisms, and pumps underlies and coordinates these electrical signals and alteration of the critical balance of these multiple current pathways can lead to disruptive, often fatal, rhythm disturbances: the cardiac arrhythmias. Although the cardiac arrhythmias form a complicated and diverse group reflecting the complexities of the ionic mechanisms underlying the electrical activity of the human heart, a surprisingly large number of rhythm disturbances are caused either directly or indirectly by mechanisms that prolong the duration of the action potential of the working myocardium (1, 2). Whereas prolongation of the cardiac ventricular action potential under controlled conditions can be, in principle, an effective mechanism to prevent certain types of re-entrant arrhythmias (3), excessive prolongation can be fatal. In this issue of PNAS, Mitcheson et al. (4) provide the first detailed report of a structural basis for the most common form of arrhythmia that is caused by drug-induced prolongation of the cardiac action potential that is reflected in prolongation of the QT interval of the electrocardiogram. This important study thus paves the way for a rational approach to the development of compounds that can be safely administered with minimum risk of QT prolongation and the induction of life-threatening arrhythmias.
Action potentials of the heart are long lasting, and particularly in the ventricle, they are characterized by a period of slowly changing and maintained depolarization, the action potential plateau. The plateau is crucial in determining the strength and duration of contraction of the myocardium, in setting the proper relationship between systolic contraction and diastolic filling times, and in providing a cardio-protective window in which re-excitation cannot take place via voltage-dependent sodium or calcium channels (5). The duration of the plateau phase is controlled in part by the activation of two types of delayed potassium channel currents IKr and IKs (6).
The congenital long QT syndrome (LQTS) is a disease that prolongs ventricular repolarization and predisposes individuals to episodes of syncope, polymorphous ventricular tachycardia (torsades de pointes), and sudden death (7, 8). Multiple genes that encode ion channel subunits are linked to LQTS (9–11), including KCNQ1 (KvLQT1) and KCNE1 (minK) KCNH2 and KCNE2, the genes encoding the α and β subunit of the delayed rectifier potassium channels that conduct IKr and IKs (12–16). It is clear that mutation-induced reduction in these key currents underlies the disease phenotype (17); however, the prevalence of these inherited disorders is rare (18). A more common and related disorder is excessive delay in repolarization caused inadvertently by drug-induced inhibition of HERG channels by commonly used medications. This disorder is referred to as drug-induced LQTS (19). As shown schematically in Fig. 1, the essence of this disorder is that drug-induced action potential prolongation may vary within a patient population. A drug concentration, which may slightly prolong the action potential plateau and actually be antiarrhythmic in some patients, may produce excessive prolongation in others, leading to arrhythmogenic activity (3). This drug activity is not limited to cardiac antiarrhythmic drugs but also has been reported for antianginal agents such as bepridil and prenylamine as well as nonsedating antihistamines such as terfenadine and astemizole (20, 21). Previous investigations have identified sporadic ion channel mutations in patients with drug-induced torsades de pointes (22, 23), indicating that patients with “acquired” LQTS can have a genetic predisposition to arrhythmia because of mutation in subunits of their IKr potassium channels.
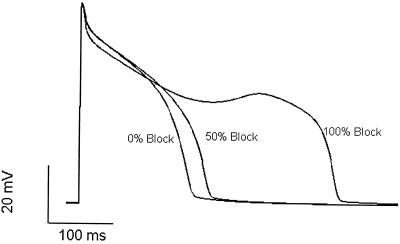
Figure 1.
Simulation of drug-induced LQTS. Cardiac ventricular action potentials generated by using the Luo—Rudy cellular model (28) representing control, drug-free conditions (0% block) and conditions in which the same drug concentration blocks 50% and 100% of available IKr channels. Modest action potential prolongation caused by 50% channel block can be antiarrhythmic, but extreme prolongation caused by block of all available channels induces secondary depolarizing activity, which can be proarrhythmic.
One approach in the management of acquired LQTS is thus to develop effective and efficient genetic screens that will detect single-nucleotide polymorphisms and/or other mutations of genes that code for key ion channels that might confer distinct drug sensitivity on assembled channels as reported (24, 25). Another approach is to identify the molecular mechanisms responsible for drug-channel interactions that underlie these effects with the goal of altering drug structures to minimize the risk of unwanted channel inhibition. This is the approach that has been taken in the study by Mitcheson et al. (4).
Their paper reports a creative combination of alanine scanning mutagenesis of key residues of the S6 segment and key residues of the pore helix predicted to line the channel cavity and inner pore regions of the HERG channel with a homology model using the KcsA crystal structure (26) as a template. The result is a model of drug-channel interaction that predicts drug specificity dictated by constraints on channel structure imposed by amino acid residues that are unique to the HERG channel. The model suggests that limitation of the space of the channel cavity between the selectivity filter and the activation gate, a region previously suggested to be the site of action of these drugs (27), is the key factor that underlies the discrimination between HERG and other channels by these drugs and direct electrostatic interactions between drug molecules and the aromatic rings of specific residues on segment S6. This novel combination of functional analysis of drug interactions with homology modeling of the HERG channel pore region thus paves the way to begin investigating, in a systematic manner, drug structures that are designed to minimize binding to (and block of) HERG channels, but that retain desired pharmacological efficacy with other targets. A structural approach to minimization of risk of acquired LQTS represents a significant change in the design of drugs that interact either intentionally or inadvertently with ion channels in the heart.
Footnotes
See companion article on page 12329.
References
- 1.el Sherif N, Turitto G. Pacing Clin Electrophysiol. 1999;22:91–110. doi: 10.1111/j.1540-8159.1999.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 2.Viskin S. Lancet. 1999;354:1625–1633. doi: 10.1016/S0140-6736(99)02107-8. [DOI] [PubMed] [Google Scholar]
- 3.Colatsky T J, Follmer C H, Starmer C F. Circulation. 1990;82:2235–2242. doi: 10.1161/01.cir.82.6.2235. [DOI] [PubMed] [Google Scholar]
- 4.Mitcheson J S, Chen J, Lin M, Culberson C, Sanguinetti M C. Proc Natl Acad Sci USA. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kass R S. In: Physiology and Pathophysiology of the Heart. Sperelakis N, editor. Norwell, MA: Kluwer Academic; 1995. pp. 77–90. [Google Scholar]
- 6.Sanguinetti M C, Jurkiewicz N K. J Gen Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss A J, Schwartz P J, Crampton R S, Tzivoni D, Locati E H, MacCluer J, Hall W J, Weitkamp L, Vincent M, Garso A, et al. Circulation. 1991;84:1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz P J, Periti M, Malliani A. Am Heart J. 1975;89:378–390. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- 9.Catterall W A. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 10.Duff H J. Can J Cardiol. 2000;16:304–306. [PubMed] [Google Scholar]
- 11.Yan G X, Antzelevitch C. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Zou A, Splawski I, Keating M T, Sanguinetti M C. J Biol Chem. 1999;274:10113–10118. doi: 10.1074/jbc.274.15.10113. [DOI] [PubMed] [Google Scholar]
- 13.Splawski I, Tristani-Firouzi M, Lehmann M H, Sanguinetti M C, Keating M T. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 14.Sanguinetti M C, Curran M E, Zou A, Shen J, Spector P S, Atkinson D L, Keating M T. Nature (London) 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Curran M E, Splawski I, Burn T C, Millholland J M, Vanraay T J, Shen J, Timothy K W, Vincent G M, Dejager T, et al. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 16.Sanguinetti M C. J Cardiovasc Electrophysiol. 2000;11:710–712. doi: 10.1111/j.1540-8167.2000.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 17.Viswanathan P C, Shaw R M, Rudy Y. Circulation. 1999;99:2466–2474. doi: 10.1161/01.cir.99.18.2466. [DOI] [PubMed] [Google Scholar]
- 18.Zareba W, Moss A J, Schwartz P J, Vincent G M, Robinson J L, Priori S G, Benhorin J, Locati E H, Towbin J A, Keating M T, et al. N Engl J Med. 1998;339:960–965. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 19.Camm A J, Janse M J, Roden D M, Rosen M R, Cinca J, Cobbe S M. Eur Heart J. 2000;21:1232–1237. doi: 10.1053/euhj.2000.2222. [DOI] [PubMed] [Google Scholar]
- 20.Coumel P. Am J Cardiol. 1992;69:75D–78D. doi: 10.1016/0002-9149(92)90963-y. [DOI] [PubMed] [Google Scholar]
- 21.Woosley R L, Chen Y, Freiman J P, Gillis R A. J Am Med Assoc. 1993;269:1532–1536. [PubMed] [Google Scholar]
- 22.Napolitano C, Schwartz P J, Brown A M, Ronchetti E, Bianchi L, Pinnavaia A, Acquaro G, Priori S G. J Cardiovasc Electrophysiol. 2000;11:691–696. doi: 10.1111/j.1540-8167.2000.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 23.Donger C, Denjoy I, Berthet M, Neyroud N, Cruaud C, Bennaceur M, Chivoret G, Schwartz K, Coumel P, Guicheney P. Circulation. 1997;96:2778–2781. doi: 10.1161/01.cir.96.9.2778. [DOI] [PubMed] [Google Scholar]
- 24.Abbott G W, Sesti F, Splawski I, Buck M E, Lehmann M H, Timothy K W, Keating M T, Goldstein S A. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 25.Sesti F, Abbott G W, Wei J, Murray K T, Saksena S, Schwartz P J, Priori S G, Roden D M, George A L, Jr, Goldstein S A. Proc Natl Acad Sci USA. 2000;97:10613–10618. doi: 10.1073/pnas.180223197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle D A, Cabral J M, Pfuetzner R A, Kuo A, Gulbis J M, Cohen S L, Chait B T, MacKinnon R. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 27.Mitcheson J S, Chen J, Sanguinetti M C. J Gen Physiol. 2000;115:229–240. doi: 10.1085/jgp.115.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo C H, Rudy Y. Circ Res. 1994;74:1097–1113. doi: 10.1161/01.res.74.6.1097. [DOI] [PubMed] [Google Scholar]