Abstract
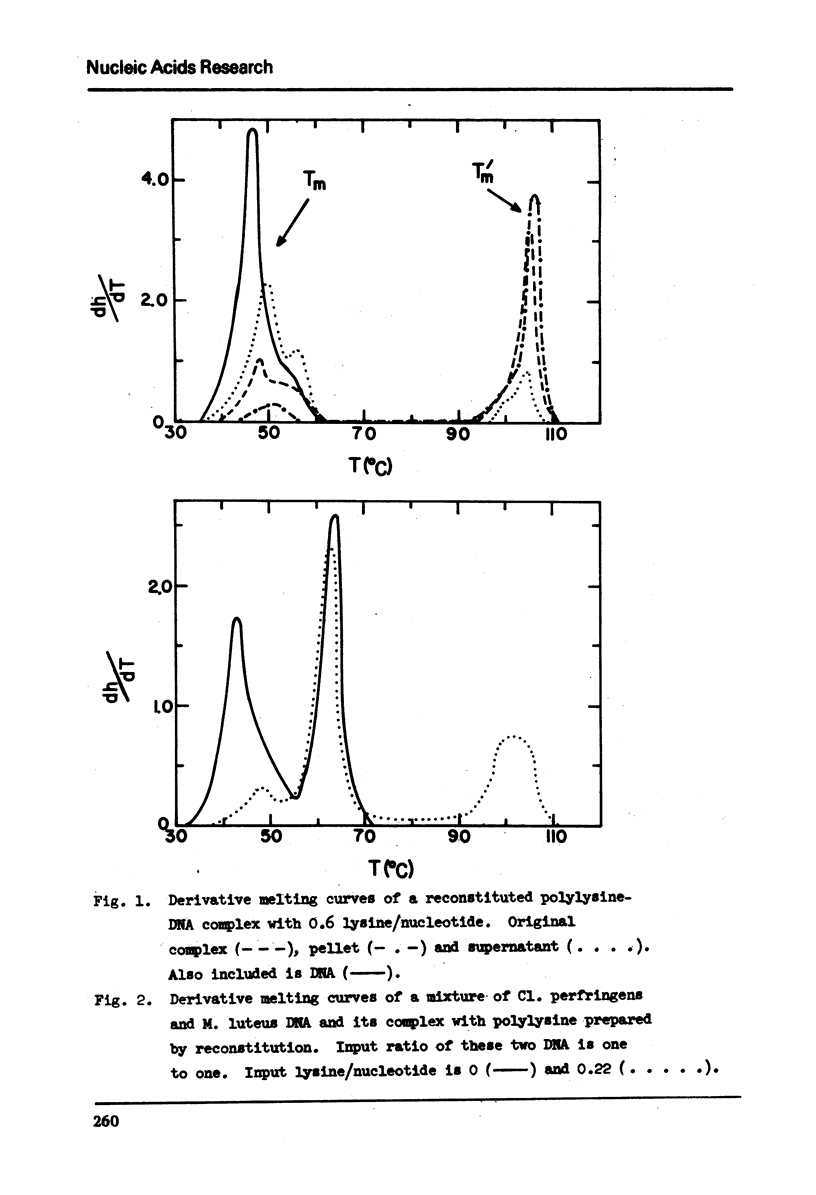
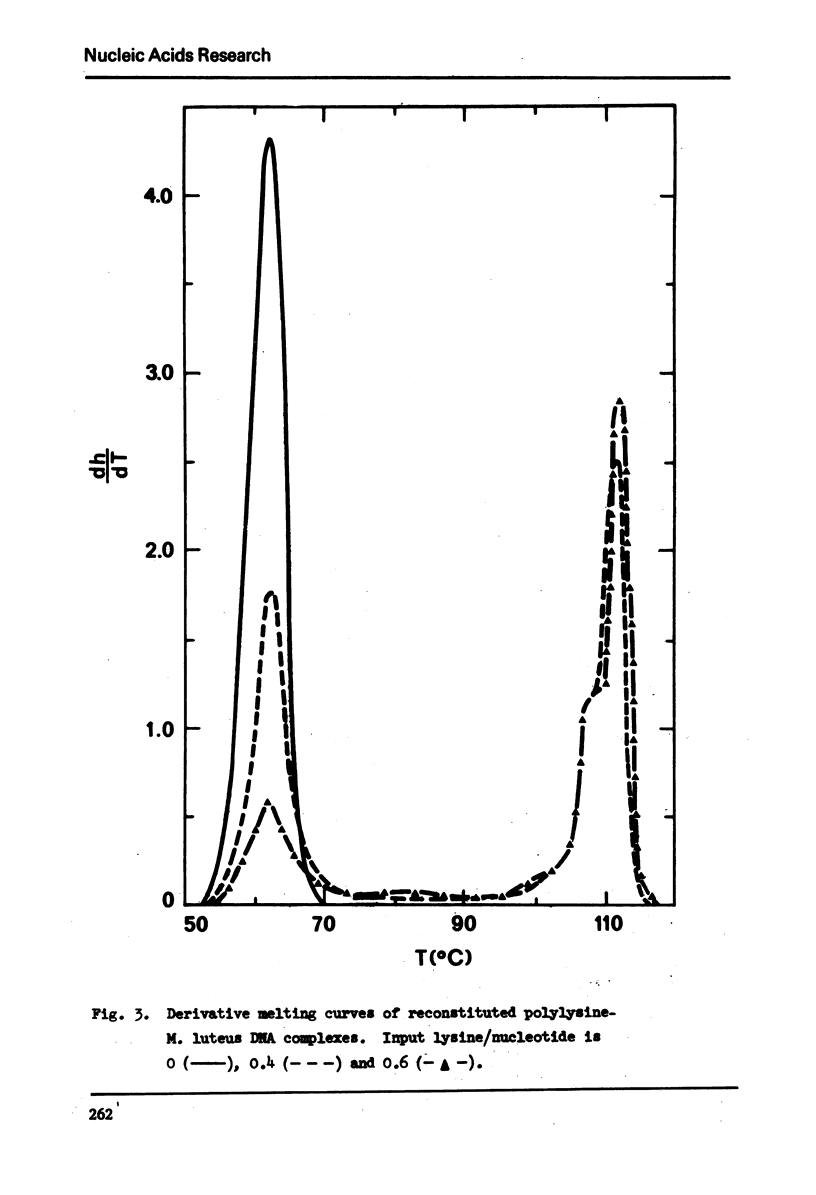
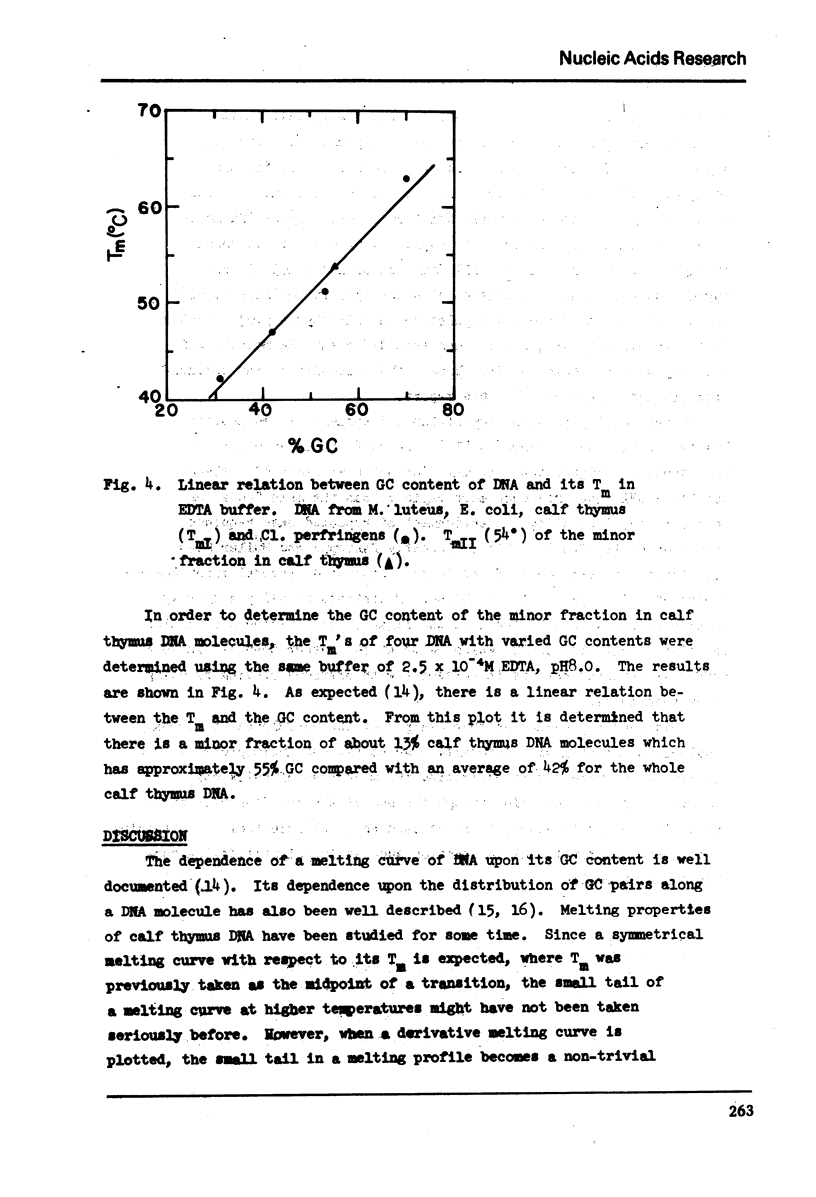
In 2.5 × 10−4M EDTA buffer, the derivative melting curve of calf thymus DNA shows a major band at 47° with a shoulder at about 54°. The fraction of melting area of this shoulder is about 13%. For reconstituted polylysine-calf thymus DNA complexes, in addition to the melting of free DNA regions at about 50° (Tm) there is another melting at about 106° (Tm) of polylysine-bound regions. The melting band of the complex at Tm is not symmetrical. As more polylysine is bound to DNA the melting amplitude is diminished greatly on the major band at 47° but only slightly on the shoulder at 54°. The insensitivity of this shoulder appears to result from the existence of a 13% fraction of calf thymus DNA containing 55% GC. It is not favorably bound by polylysine. It remains in the supernatant after centrifugation and melts at about 54-56°. This conclusion is further supported by two facts: the reconstitution method provides a condition for selective binding of polylysine to AT-rich DNA, and it yields a fully symmetric melting band at Tm for complexes of polylysine with homogeneous bacterial DNA such as the one from M. luteus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- CROTHERS D. M., KALLENBACH N. R., ZIMM B. H. THE MELTING TRANSITION OF LOW-MOLECULAR-WEIGHT DNA: THEORY AND EXPERIMENT. J Mol Biol. 1965 Apr;11:802–820. doi: 10.1016/s0022-2836(65)80037-7. [DOI] [PubMed] [Google Scholar]
- Crothers D. M. Calculation of melting curves for DNA. Biopolymers. 1968 Oct;6(10):1391–1404. doi: 10.1002/bip.1968.360061003. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G., Hirschman S. Z. A neighbor-interaction analysis of the hypochromism and spectra of DNA. J Mol Biol. 1965 Sep;13(2):407–427. doi: 10.1016/s0022-2836(65)80106-1. [DOI] [PubMed] [Google Scholar]
- Leng M., Felsenfeld G. The preferential interactions of polylysine and polyarginine with specific base sequences in DNA. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1325–1332. doi: 10.1073/pnas.56.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. J., Chang C., Weiskopf M. Helix-coil transition in nucleoprotein-chromatin structure. Biochemistry. 1973 Apr 24;12(9):1763–1772. doi: 10.1021/bi00733a016. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Olins D. E. Interaction of lysine-rich histones and DNA. J Mol Biol. 1969 Aug 14;43(3):439–460. doi: 10.1016/0022-2836(69)90351-9. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L., Von Hippel P. H. Model nucleoprotein complexes: studies on the interaction of cationic homopolypeptides with DNA. J Mol Biol. 1967 Mar 14;24(2):157–176. doi: 10.1016/0022-2836(67)90324-5. [DOI] [PubMed] [Google Scholar]
- Polli E., Ginelli E., Bianchi P., Corneo G. Renaturation of calf thymus satellite DNA. J Mol Biol. 1966 May;17(1):305–308. doi: 10.1016/s0022-2836(66)80114-6. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Thermal denaturation and template properties of DNA complexes with purified histone fractions. J Mol Biol. 1970 Mar;48(3):469–487. doi: 10.1016/0022-2836(70)90059-8. [DOI] [PubMed] [Google Scholar]


