Abstract
Objective
Cardiac subsarcolemmal (SSM) and interfibrillar (IFM) mitochondrial subpopulations possess distinct biochemical properties and differ with respect to their protein and lipid compositions, capacities for respiration and protein synthesis, and sensitivity to metabolic challenge, yet their responsiveness to mitochondrially active cardioprotective therapeutics has not been characterized. This study assessed the differential responsiveness of the two mitochondrial subpopulations to diazoxide, a cardioprotective agent targeting mitochondria.
Methods
Mitochondrial subpopulations were freshly isolated from rat ventricles and their morphologies assessed by electron microscopy and enzymatic activities determined using standard biochemical protocols with a plate reader. Oxidative phosphorylation was assessed from State 3 respiration using succinate as a substrate. Calcium dynamics and the status of Ca2+-dependent mitochondrial permeability transition (MPT) pore and mitochondrial membrane potential were assessed using standard Ca2+ and TPP+ ion-selective electrodes.
Results
Compared to IFM, isolated SSM exhibited a higher sensitivity to Ca2+ overload-mediated inhibition of adenosine triphosphate (ATP) synthesis with decreased ATP production (from 375±25 to 83±15 nmol ATP/min/mg protein in SSM, and from 875±39 to 583±45 nmol ATP/min/mg protein in IFM). In addition, SSM exhibited reduced Ca2+-accumulating capacity as compared to IFM (230±13 vs. 450±46 nmol Ca2+/mg protein in SSM and IFM, respectively), suggestive of increased Ca2+ sensitivity of MPT pore opening. Despite enhanced susceptibility to stress, SSM were more responsive to the protective effect of diazoxide (100 μM) against Ca2+ overload-mediated inhibition of ATP synthesis (67% vs. 2% in SSM and IFM, respectively).
Conclusion
These results provide evidence for the distinct sensitivity of cardiac SSM and IFM toward Ca2+-dependent metabolic stress and the protective effect of diazoxide on mitochondrial energetics.
Introduction
Two distinct mitochondrial subpopulations – subsarcolemmal (SSM), situated underneath the sarcolemmal membrane, and interfibrillar (IFM), distributed between myofibrils – have been previously identified in myocardium [1]–[5]. These mitochondrial subpopulations differ in respect to their protein and lipid compositions, capacities for respiration and protein synthesis, and in their sensitivity to metabolic challenge [4]–[13]. Differences in the responsiveness of mitochondrial subpopulations to metabolic stress with enhanced susceptibility of SSM have been demonstrated in the heart [14], [15]. SSM appears to be more vulnerable to ischemic injury and mitochondrial Ca2+ overload when compared to IFM [5], [8]–[11], [16]–[20]. Despite distinct biochemical properties and sensitivity to stress, the differences between SSM and IFM in responsiveness to mitochondrially active therapeutics have not been completely characterized. Here, we demonstrate that diazoxide, a cardioprotective mitochondria-targeting agent [21]–[24], effectively protects mitochondria against Ca2+ loading and restores Ca2+-inhibited oxidative phosphorylation to a greater extent in SSM than in IFM. These results thus provide evidence of distinct sensitivity of cardiac mitochondrial subpopulations toward the protective effect of diazoxide, indicating that SSM could be the preferred target for drug treatment.
Materials and Methods
Ethic statement
The study was approved by the Mayo Clinic Institutional Animal Care and use Committee (Protocol # A28201), and all procedures were in accordance with recommendations published in Guide for the Care and Use of Laboratory Animals, National Academic Press, Washington, D.C., 1996.
Mitochondrial isolation
Mitochondria were isolated from the hearts of pentobarbital (100 mg/kg intraperitoneal injection)-anesthetized male adult rats (Sprague-Dawley; Harlan Laboratories, Indianapolis, IN). Following thoracotomy, the heart was rapidly removed from the chest and ventricles were trimmed of atria and connective tissue. The ventricles were placed in ice-cold media containing (in mmol/L): sucrose 50, mannitol 200, KH2PO4 5, EGTA 1, 0.2% BSA, MOPS 5 (pH = 7.3) as described by Holmuhamedov et al. [22]. SSM and IFM were isolated from Polytron®-homogenized (Brinkmann Instruments, Westbury, NY) ventricles using differential centrifugation as previously described [9], [21], [22]. Briefly, isolation of SSM was achieved by mechanical rupture of ventricular tissue with Polytron followed by differential centrifugation, whereas IFM isolation was performed in tissue depleted of SSM by an additional enzymatic digestion with Nagarse and mechanical disruption of residual ventricular tissue to release IFM. A subset of experiments was repeated to rule out nonspecific effect of enzymatic treatment with Nagarse on basic mitochondrial functions in isolated SSM. No significant differences were observed in mitochondrial respiration and Ca2+ handling of isolated SSM in the presence or absence of Nagarse treatment. Protein concentration was determined using DC™ Protein Determination Kit (Bio-Rad Laboratories, Hercules CA).
Electron microscopy
SSM and IFM were fixed using Trump's buffer (1% glutaraldehyde, 4% formaldehyde, 0.1-M phosphate buffer, pH 7.2), rinsed and post-fixed in phosphate-buffered 1% osmium tetroxide [25]–[27]. Samples were stained en bloc with 2% uranyl acetate for 30 min at 60°C, rinsed, dehydrated, and embedded in Spurr's resin. Thin sections were cut on an Ultracut E ultramicrotome (Reichert-Jung, Vienna, Austria), placed on copper grids and stained with lead citrate. Mitochondria were micrographed with a 1200 EX II electron microscope (Jeol, Tokyo, Japan).
Citrate synthase activity
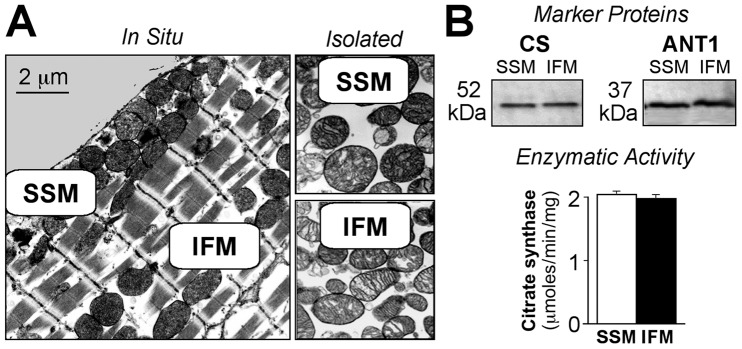
The activity of citrate synthase (CS) in SSM and IFM was determined as described by Short et al. [28] with minor modifications. Aliquots of mitochondria were transferred into the incubation buffer, which contained (in mmol/L): 5,5′-dithiobis-(2-nitrobenzoic acid) = 0.1; acetyl-Co-A = 0.12; oxaloacetate = 0.5; TRIZMA = 100; Triton X-100 = 0.1%; pH = 8.1. The activity of CS was monitored as absorbance change of 412 nm and expressed as µmoles of thionitrobenzoic acid (TNB)/min/mg protein.
Western blot
Aliquots of mitochondria solubilized in Laemmle sample buffer were separated on polyacyrlamide gels (Criterion™, Bio-Rad Laboratories) and then transferred to polyvinylidene fluoride membranes as described by Short et al. [28]. Briefly, membranes were blocked in 5% milk in Tris-buffered saline with 0.1% Tween-20 for 1 hour and then incubated overnight with primary antibody. Dilutions for the primary antibodies were: citrate synthase 1∶1000 (a kind gift from J.O. Holloszy, MD, Washington University, St. Louis, MO), adenine nucleotide transporter 1 (ANT1) 1∶500 (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were subsequently exposed to secondary horseradish peroxidase-labeled antibodies at 1∶10,000 (Amersham Biosciences, Piscataway, NJ) and then chemiluminescent substrate (ECL Plus™, Amersham) was used for detection. Images captured on Kodak Omat film (Kodak Scientific, Rochester, NY) were then used for densitometry of bands using Kodak Image Station 1000.
Respiration, membrane potential and calcium transport
Respiration, membrane potential and Ca2+ transport of isolated mitochondria were determined using a multichannel system (ABMT-USA, Durham, NC) equipped with oxygen-, tetraphenylphosphonium (TPP+)- and Ca2+-selective minielectrodes, as previously described [21]–[23]. Briefly, mitochondria (1 mg/ml) were added into the incubation buffer containing (in mM): KCl 110, K2HPO4 5, succinate 5, pyruvate 5, and MOPS 10 (pH = 7.35) and respiration was measured using calibrated Clark-type O2 minielectrode. Mitochondrial membrane potential was measured simultaneously with respiration using TPP+-sensitive minielectrode, manufactured and calibrated as described by Kamo et al. [29]. Concentration of TPP+ was 200 nM, and mitochondrial membrane potential was calculated as previously described [21], [22], [29]. Mitochondrial Ca2+ uptake was measured from changes in the free Ca2+ concentration within the suspension using calibrated Ca2+-selective minielectrodes (Microelectrodes Inc., Bedford, NH) as described [22], [25]. Mitochondrial Ca2+-accumulating capacity was determined as the total amount of Ca2+ accumulated into the matrix from a train of 50-μM Ca2+ pulses added at 1-min intervals until the load reached a threshold pulse after which mitochondria underwent irreversible and rapid Ca2+ release [30], [31].
Adenosine triphosphate synthesis
Adenosine triphosphate (ATP) production in mitochondria was determined using K2CO3/MOPS-neutralized HClO4-soluble mitochondrial extracts by high-pressure liquid chromatography (Hewlett-Packard, Waldbronn, Germany) as described by Holmuhamedov et al. [23]. Briefly, 200 μl of mitochondrial suspension were treated with 20 μl of 3.3-M HClO4, and precipitated proteins were removed by centrifugation (60 s, 14,000 rpm, 4°C). After neutralization of the supernatant with 80 μl of a mixture containing 2.5-M K2CO3 in 1-M HEPES, the precipitate was separated by centrifugation (60 s, 14,000 rpm, 4°C), and the concentration of ATP within the extract was determined in coupled enzymatic reactions [32]. The time course of adenosine diphosphate (ADP)-to-ATP conversion within mitochondrial suspension was monitored from changes in NADPH fluorescence (Ascent FL, Scientific Resources, Saint Paul, MN) in a coupled hexokinase/glucose-6-phosphate dehydrogenase assay [21]–[23], [32].
Drugs
Diazoxide (Research Biochemical International, Natick, MA) was dissolved as a concentrated stock solution in dimethylsulfoxide (DMSO), and the maximal concentration of DMSO in the incubation medium was kept under 0.5%. All other chemicals were from Sigma Chemicals (St. Louis, MO).
Statistical analysis
Data are expressed as mean ± standard error of mean, and “n” represents the number of mitochondrial isolations. Comparison between groups was made using analysis of variance (ANOVA) with post-hoc test. ANOVA was performed for multiple comparisons between groups using two-way comparison of means by Tukey-Kramer HSD test, and p<0.05 was considered to be statistically significant.
Results
Biochemical similarity and differences in isolated cardiac SSM and IFM
Electron micrographs of the heart muscle demonstrate intracellular localization of mitochondrial subpopulations and morphological appearance of SSM and IFM in situ and after isolation (Fig. 1A). Intracellular SSM have a round shape and a less electron-dense “light” matrix, while IFM are elongated and rod-shaped with the matrix containing a greater electron-dense material (Fig. 1A). The content of CS and ANT1, specific mitochondrial matrix and membrane proteins were all determined by Western blot (Fig. 1B, top panels). Both subpopulations of isolated mitochondria demonstrated similar levels of expression of CS and ANT1 (Fig. 1B, top panels). In addition, the activity of CS in mitochondrial subpopulations was similar (2.04±0.03 vs. 1.98±0.04 µmoles TNB/min/mg protein in SSM and IFM, respectively; Fig. 1B, lower panel, n = 6, p = NS).
Figure 1. Morphological and biochemical characteristics of two mitochondrial populations in heart muscle.
A, Electron micrographs of subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria within the heart cell (In Situ) and after isolation (Isolated). Magnification: x10, 000. B, Expression of mitochondria-specific marker proteins in SSM and IFM. Top panel displays Western blots of citrate synthase (CS) and adenine nucleotide transporter 1 (ANT1). Bottom panel displays bar graphs of the activity of CS (SSM, open bars; IFM, filled bars).
Ca2+ handling and oxidative phosphorylation capacity of SSM and IFM
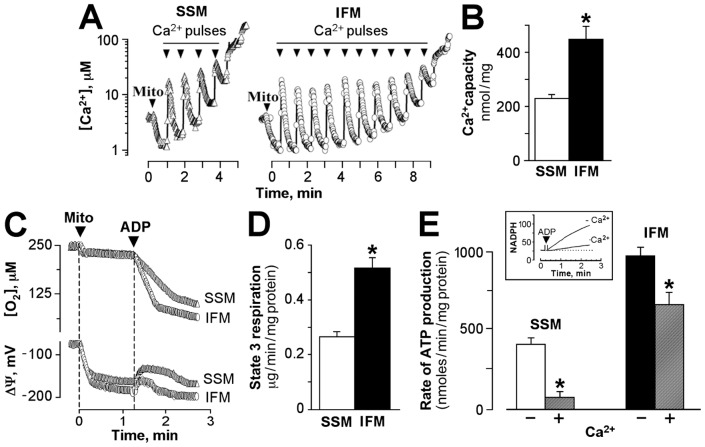
The sensitivity of mitochondria toward Ca2+-induced mitochondrial permeability transition (MPT) pore opening was determined from the number of Ca2+ pulses required to reach the threshold for rapid and spontaneous Ca2+ release [31]. There was no difference in the baseline content of endogenous Ca2+ in isolated SSM and IFM measured immediately after isolation (2.1±0.9 vs. 2.2±1.1 nmol Ca2+/mg protein, respectively, n = 6, data not shown). However, the maximal Ca2+-accumulating capacity of mitochondrial subpopulations (determined from experiments with multiple Ca2+ pulses, described in Materials and Methods) was significantly decreased in SSM and was 230±13 nmol Ca2+/mg protein as compared with 450±46 nmol Ca2+/mg protein in IFM (Fig. 2A and 2B, n = 6, p<0.05).
Figure 2. Ca2+ handling and oxidative phosphorylation capacity of subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria.
A, Typical tracing of Ca2+ loading in SSM (left panel) and IFM (right panel). Mitochondria were loaded with consecutive Ca2+ pulses (arrows), and each pulse delivers 50 nmol of Ca2+. B, Average Ca2+-accumulating capacity of SSM (open bar) and IFM (filled bar), n = 6, p<0.05. C, Typical tracing of ADP-induced changes in oxygen consumption (top) and changes in membrane potential from baseline (bottom) of SSM and IFM. D, Average ADP-stimulated (State 3) respiration of SSM and IFM (n = 6, p<0.05). E, The rate of ATP production in SSM (left bars) and IFM (right bars) before (B/W bars) and after (gray bars) loading with 150 nmol Ca2+/mg protein, n = 3, p<0.05. Inset: Alternative monitoring of ATP production in mitochondria using coupled enzymes. Asterisks signify statistical differences with p<0.05.
The capacity for oxidative phosphorylation assessed from ADP-mediated increase in mitochondrial respiration and membrane depolarization was also different in SSM and IFM (Fig. 2C and 2D). On average, ADP-stimulated respiration (State 3) in SSM was 49% lower than in IFM (267±15 vs. 518±37 ng-atoms O2/min/mg protein, respectively; Fig. 2D, n = 6, p<0.05). Decreased State 3 respiration in SSM correlated with a longer period of ADP-to-ATP conversion, as monitored from reversible and transient ADP-induced membrane depolarization from the baseline (76±3 sec in SSM versus 36±3 sec in IFM; Fig. 2C, lower panel). Although the rates of respiration upon completion of ADP-to-ATP conversion (State 4) were different in these populations (67±5 and 123±11 ng-atoms O2/min/mg protein in SSM and IFM, respectively), the resulting respiratory control ratio was not significantly different (3.2±0.7 vs. 4.2±0.5, n = 6), demonstrating similar coupling of oxidative phosphorylation in SSM and IFM.
Loading of mitochondria with 150 nmol Ca2+/mg protein inhibited oxidative phosphorylation in both SSM and IFM, but the extent of inhibition was significantly higher in SSM. The rate of ATP synthesis in the presence of Ca2+ was decreased from 375±25 to 83±15 nmol ATP/min/mg protein in SSM, and from 875±39 to 583±45 nmol ATP/min/mg protein in IFM (Fig. 2E, n = 6, p<0.05). Thus, compared to IFM, SSM demonstrated lesser Ca2+ handling capacity, enhanced susceptibility to MPT pore opening, decreased rate of oxidative phosphorylation and enhanced sensitivity to inhibition of mitochondrial energetics by excessive Ca2+ loading.
Diazoxide decreases Ca2+ loading preferentially in SSM
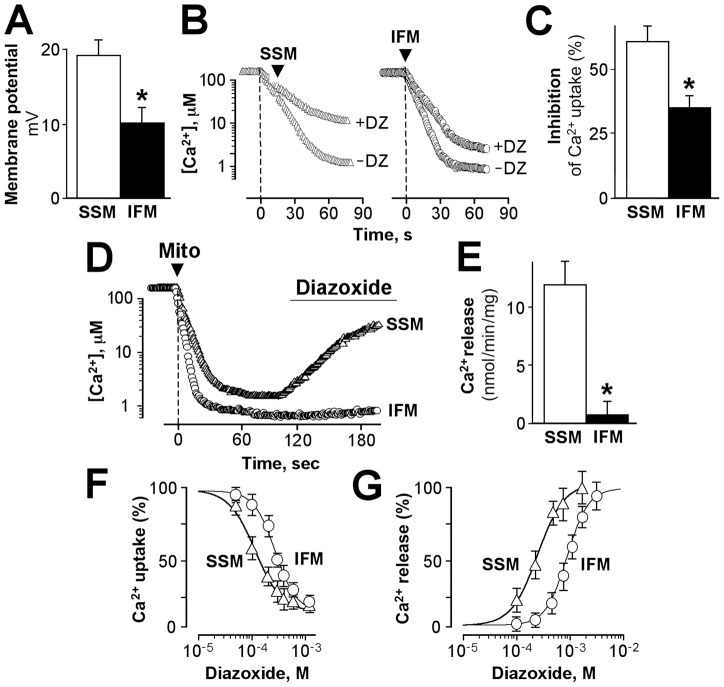
Diazoxide, an opener of sarcolemmal ATP-sensitive K+ channels with cardioprotective properties also known to target and depolarize isolated mitochondria [21], [22], [33]–[36], differentially affected mitochondrial membrane potential and Ca2+ handling in these two mitochondrial subpopulations. The SSM-oxidizing succinate, demonstrated a higher sensitivity to diazoxide (100 μM)-induced depolarization of the inner membrane (19±2 mV) compared with IFM (10±2 mV; Fig. 3A, n = 6, p<0.05). Similarly, when added to mitochondria prior to Ca2+ loading, diazoxide (100 µM) suppressed the rate of Ca2+ uptake preferentially in SSM (from 347±9 to 137±7 nmol Ca2+/min/mg protein) than in IFM (from 503±13 to 326±10 nmol Ca2+/min/mg protein), demonstrating a 61% vs. 35% inhibition of Ca2+ uptake in SSM and IFM, respectively (Fig. 3B and 3C, n = 6, p<0.05). In the absence of diazoxide, neither SSM nor IFM demonstrated release of accumulated Ca2+ during 20 min of observation (data not shown). However, diazoxide had a differential effect on Ca2+ release from Ca2+-loaded mitochondria. In mitochondria preloaded with 100 nmol Ca2+/mg protein, the same concentration of diazoxide (100 µM) induced faster release of accumulated Ca2+ from SSM than from IFM (Fig. 3D). On average, the rate of diazoxide-induced Ca2+ release was fivefold higher (10±2 vs. 2±1 nmol Ca2+/min/mg protein) in SSM and IFM, respectively (Fig. 3E, n = 6, p<0.05). Overall, the effect of diazoxide on mitochondrial Ca2+ uptake and release was dose-dependent, and the concentration of diazoxide causing 50% inhibition of Ca2+ uptake (IC50) was estimated at 104±15 and 289±32 µM in SSM and IFM, respectively (Fig. 3F, n = 6, p<0.05), whereas the concentration of diazoxide causing 50% Ca2+ release was 114±21 and 377±44 µM in SSM and IFM, respectively (Fig. 3G, n = 6, p<0.05). Thus, SSM and IFM exhibit differential responsiveness toward diazoxide-mediated membrane depolarization, Ca2+ uptake, and release of accumulated Ca2+.
Figure 3. Effect of diazoxide on the membrane potential and Ca2+ handling in subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria.
A, Depolarizing effect of diazoxide (100 µM) in SSM (open bar) and IFM (filled bar), n = 6, p<0.05. B, Mitochondrial Ca2+ uptake in SSM and IFM in the absence (-DZ) and presence (+DZ) of diazoxide (100 µM). C, Inhibition of the rate of Ca2+ uptake in SSM (open bar) and IFM (filled bar), n = 6, p<0.05. D, Diazoxide (100 µM) mediated Ca2+ release from preloaded SSM and IFM. E, Average rate of diazoxide-mediated Ca2+ release from mitochondria, n = 3, p<0.05. F and G, Dose-dependent effect of diazoxide on Ca2+ uptake (F) and Ca2+ release (G) in SSM (triangles) and IFM (circles), n = 6.
Diazoxide restores Ca2+-inhibited ATP production
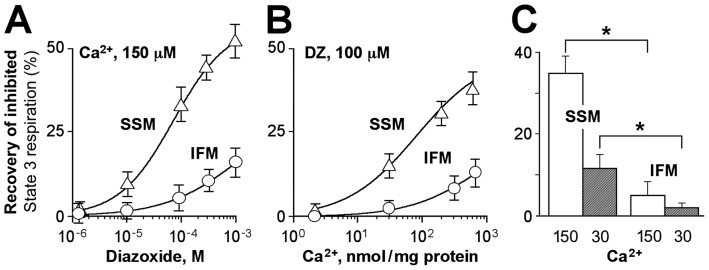
Excessive mitochondrial Ca2+ loading inhibited oxidative phosphorylation (Fig. 2) and diazoxide in a dose-dependent manner restored State 3 respiration in both SSM and IFM (Fig. 4A). The rescuing effect of diazoxide on Ca2+-inhibited State 3 respiration was more prominent in SSM compared to IFM (Fig. 4) and was dependent upon the level of mitochondrial Ca2+ overload (Fig. 4B and 4C). In mitochondria loaded with 150 nmol Ca2+/mg protein, diazoxide restored Ca2+-inhibited ADP-stimulated respiration by 35±4% in SSM compared with 5±3% in IFM (Fig. 4C, p<0.05, n = 6, open bars), whereas at 30 nmol Ca2+/mg protein, the protective effect of diazoxide was only by 12±3% and 4±0.9% in SSM and IFM, respectively (Fig. 4C, n = 6, hatched bars). ATP synthesis in mitochondria, monitored using coupled enzymatic reactions [22], [23], confirmed that diazoxide was more efficient in recovering the rate of Ca2+-inhibited ATP production in SSM than IFM. In mitochondria loaded with 150 nmol Ca2+/mg protein, diazoxide restored the rate of ATP production by 48±5% in SSM compared with 5±3% in IFM. Thus, diazoxide restores Ca2+-inhibited mitochondrial ATP synthesis in both SSM and IFM populations, but the magnitude of this protective effect is markedly higher in SSM compared to IFM.
Figure 4. Diazoxide-mediated recovery of Ca2+-inhibited ATP synthesis.
A, Dose-dependent effect of diazoxide on restoration of Ca2+-inhibited State 3 respiration in subsarcolemmal (SSM) (triangles) and interfibrillar (IFM) (circles) mitochondria. B, Effect of diazoxide on Ca2+-inhibited State 3 respiration in SSM and IFM preloaded with (0–150 nmol Ca2+/mg protein). C, Bar graphs of diazoxide (100 µM)-mediated recovery of Ca2+-inhibited State 3 respiration in SSM and IFM loaded with 150 (open bars) and 30 nmol Ca2+/mg protein (hatched bars), n = 3. Asterisks signify statistical differences with p<0.05.
Discussion
This study demonstrates that SSM and IFM, two subpopulations of cardiac mitochondria, exhibit differential susceptibility to Ca2+-dependent inhibition of oxidative phosphorylation, opening of MPT pore and sensitivity to the protective effect of diazoxide, a mitochondrially active cardioprotective agent. These results provide additional insights into the functional and pharmacological differences between the mitochondrial subpopulations previously shown to differ in their biochemical characteristics, protein and lipid composition, and susceptibility toward metabolic challenge [6], [8]–[12], [17], [37]. Here we demonstrate that SSM were more vulnerable to the damaging effects of Ca2+ overload and inhibition of oxidative phosphorylation when compared to IFM, in line with previous observations [7], [10], [11], [16]–[18]. While Ca2+-mediated inhibition of oxidative phosphorylation was more prominent in SSM compared to IFM [8], [10], diazoxide was more effective in restoring Ca2+-inhibited oxidative phosphorylation in SSM than IFM (Figs. 3 and 4). This finding could be of great clinical relevance, as SSM has been shown to be more susceptible to injury than the IFM [6], [10], [11], [17], [38]. Mitochondrial energy production is determined by the activity of key enzymes of tricarboxylic cycle, which are regulated by Ca2+ ions in the physiological range of Ca2+ concentrations [39], [40]. However, excessive Ca2+ loading under pathological conditions has a detrimental effect on mitochondrial ATP synthesis [39]–[44]. Diazoxide has been demonstrated to protect mitochondrial energetic function and preserve cellular ATP level under metabolic stress [22], [45]–[48]. Here, we demonstrate that diazoxide-mediated decrease in mitochondrial Ca2+ loading is accompanied by partial restoration of Ca2+-inhibited ATP production in both mitochondrial subsets, but the responsiveness of SSM to diazoxide was much greater. The levels of expression of the mitochondrial-specific matrix enzyme (citrate synthase) and membrane protein (adenine nucleotide transporter) were not different in the two subpopulations, suggesting that distinct properties were not introduced due to differences in the isolation protocol or the number of mitochondria but are intrinsic features of mitochondrial subpopulations. The salvaging effect of diazoxide on ATP production in Ca2+-loaded mitochondria was greater in mitochondria with a higher level of Ca2+ load, indicating that the effect of diazoxide is condition-selective, and it is rather the release of inhibited oxidative phosphorylation than the activation of mitochondrial ATP synthesis. In accordance with this notion is the fact that in the absence of Ca2+ loading, diazoxide had little effect on State 3 respiration in both populations, and may even slow the rate of ATP production as reported previously [22], [23], [33], [49], [50].
The precise mechanism of diazoxide action on mitochondria in vivo remains unknown, [26], [33], [34], [46], [47], [51] and likely involves multiple effects, including mitochondrial uncoupling by a protonophoric effect [24], potassium transport, and substrate metabolism reported in isolated rat hearts [24], [33], [34], [36], [50], [52] and mitochondria [23], [26]. Additional factors may influence the overall effect of diazoxide on cardiac energetics and protection, including the intracellular locale of biochemically and functionally different mitochondrial subpopulations exposed to a different degree of Ca2+ load and metabolic stress. This is of significance in view of the different responsiveness of subsarcolemmal and interfibrillar mitochondria in intact cardiomyocytes [8]–[11], [16], [17], [53] and the heterogeneity in mitochondrial Ca2+ loading demonstrated in various cellular microdomains [54]–[58]. Our observation that SSM are more sensitive toward diazoxide-mediated protection from Ca2+ injury is consistent with reports on the higher vulnerability and reduced tolerance of this mitochondrial population toward Ca2+-mediated functional and structural damages. Therefore, our observation indicates that by preferentially targeting SSM (the more vulnerable subpopulation of cardiac mitochondria) diazoxide will be protective against ischemia/reperfusion-mediated injury.
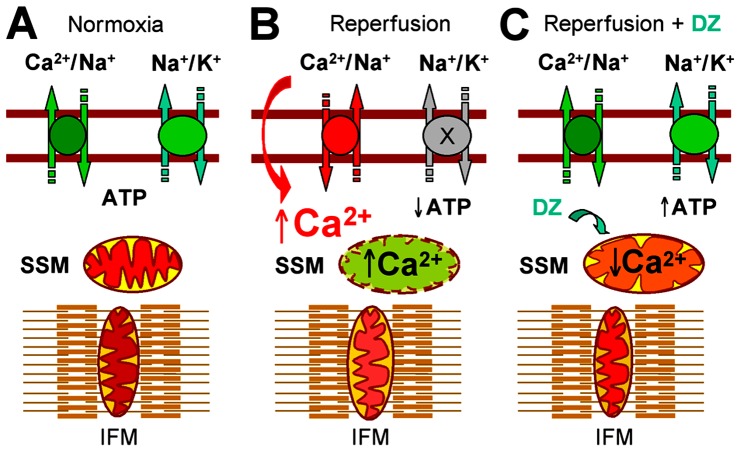
Under normal conditions, both SSM and IFM are efficient in meeting demands of the cellular ATP-dependent processes and maintaining ionic homeostasis of cells (Fig. 5A). During ischemic insult and decreased delivery of oxygen, mitochondrial ATP production drops and ionic pumps fail to maintain required gradients of Na+ and K+ ions across the sarcolemmal membrane, resulting in increased cytosolic Ca2+ (Fig. 5B). At reperfusion, oxygen availability quickly restores mitochondrial membrane potential and leads to excessive uptake of Ca2+ from the cytosol. By promoting MPT pore opening or inhibition of oxidative phosphorylation, this oxygen availability causes greater injury to the more vulnerable SSM than IFM, resulting in additional structural and functional derangements that limit the capacity of SSM, which are located in the close vicinity of plasma membrane ionic pumps [4], [5], [9]–[11], [13] in order to synthesize ATP, a critical function for maintaining homeostasis at the time of reperfusion (Fig. 5B). From our findings, we speculate that during and/or following metabolic stress, cardioprotective diazoxide moderately depolarizes mitochondria and prevents SSM against excessive Ca2+ overload by decreasing the rate of Ca2+ uptake or releasing accumulated Ca2+ or both, resulting in preservation of ATP production in the more vulnerable and strategically distributed SSM (Fig. 5), thus rescuing the energy source for ATP-dependent cellular processes, such as the maintenance of transsarcolemmal ionic homeostasis.
Figure 5. Schematic illustration of preferential targeting of subsarcolemmal mitochondria (SSM) by diazoxide.
A, Before ischemic insult (Normoxia), SSM and interfibrillar mitochondria (IFM) produce sufficient ATP to feed ionic pumps and maintain ionic homeostasis of cell. During ischemic period and decreased supply of substrates and oxygen, the mitochondrial ATP formation decreases and disables ionic pumps, resulting in increased cytosolic Ca2+. B, At reperfusion (Reperfusion), restored supply of substrates and oxygen reenergizes the SSM, resulting in excessive Ca2+ uptake (from high Ca2+ cytosol) and inhibition of ATP production. C, In the presence of diazoxide during reperfusion (Reperfusion + DZ) Ca2+ uptake into SSM will be decreased, thus preserving mitochondrial ability to produce ATP, which is required for the activity of ionic pumps and restoration of normal cellular homeostasis.
In summary, SSM are a preferential target for the cardioprotective drug diazoxide in the setting of ischemia and heart reperfusion. Our data suggest that the mechanism of protective action of diazoxide could be through decreased Ca2+ uptake, reduction of mitochondrial Ca2+ loading and through release of excessively loaded Ca2+ and restoration of Ca2+-inhibited ATP production in postischemic heart muscle.
Acknowledgments
Authors express their gratitude for technical assistance provided by Dr. C. Ozcan. The authors also acknowledge Joe Grundle and Katie Klein of Aurora Cardiovascular Services for editorial preparation of the manuscript and Brian Miller and Brian Schurrer for their help with figures.
Funding Statement
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL089542-04 and HL101240-03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Palmer JW, Tandler B, Hoppel CL (1977) Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252: 8731–8739. [PubMed] [Google Scholar]
- 2. Duan J, Karmazyn M (1989) Relationship between oxidative phosphorylation and adenine nucleotide translocase activity of two populations of cardiac mitochondria and mechanical recovery of ischemic hearts following reperfusion. Can J Physiol Pharmacol 67: 704–709. [DOI] [PubMed] [Google Scholar]
- 3. Hoppel CL, Moghaddas S, Lesnefsky EJ (2002) Interfibrillar cardiac mitochondrial comples III defects in the aging rat heart. Biogerontology 3: 41–44. [DOI] [PubMed] [Google Scholar]
- 4. Hoppel CL, Tandler B, Fujioka H, Riva A (2009) Dynamic organization of mitochondria in human heart and in myocardial disease. Int J Biochem Cell Biol 41: 1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riva A, Tandler B, Loffredo F, Vazquez E, Hoppel C (2005) Structural differences in two biochemically defined populations of cardiac mitochondria. Am J Physiol Heart Circ Physiol 289: H868–H872. [DOI] [PubMed] [Google Scholar]
- 6. Duan JM, Karmazyn M (1989) Acute effects of hypoxia and phosphate on two populations of heart mitochondria. Mol Cell Biochem 90: 47–56. [DOI] [PubMed] [Google Scholar]
- 7. Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL (2001) Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol 280: H2770–H2778. [DOI] [PubMed] [Google Scholar]
- 8. McMillin-Wood J, Wolkowicz PE, Chu A, Tate CA, Goldstein MA, et al. (1980) Calcium uptake by two preparations of mitochondria from heart. Biochim Biophys Acta 591: 251–265. [DOI] [PubMed] [Google Scholar]
- 9. Palmer JW, Tandler B, Hoppel CL (1985) Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch Biochem Biophys 236: 691–702. [DOI] [PubMed] [Google Scholar]
- 10. Palmer JW, Tandler B, Hoppel CL (1986) Heterogeneous response of subsarcolemmal heart mitochondria to calcium. Am J Physiol 250: H741–H748. [DOI] [PubMed] [Google Scholar]
- 11. Piper HM, Sezer O, Schleyer M, Schwartz P, Hütter JF, et al. (1985) Development of ischemia-induced damage in defined mitochondrial subpopulations. J Mol Cell Cardiol 17: 885–896. [DOI] [PubMed] [Google Scholar]
- 12. Servais S, Couturier K, Koubi H, Rouanet JL, Desplanches D, et al. (2003) Effect of voluntary exercise on H2O2 release by subsarcolemmal and intermyofibrillar mitochondria. Free Radic Biol Med 35: 24–32. [DOI] [PubMed] [Google Scholar]
- 13. Williamson CL, Dabkowski ER, Baseler WA, Croston TL, Alway SE, et al. (2010) Enhanced apoptotic propensity in diabetic cardiac mitochondria: influence of subcellular spatial location. Am J Physiol Heart Circ Physiol 298: H633–H642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kavazis AN, McClung JM, Hood DA, Powers SK (2008) Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol 294: H928–H935. [DOI] [PubMed] [Google Scholar]
- 15. Kavazis AN, Alvarez S, Talbert E, Lee Y, Powers SK (2009) Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am J Physiol Heart Circ Physiol 297: H144–H152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL (2001) Mitochondrial dysfunction in cardiac disease: ischemia – reperfusion, aging, and heart failure. J Mol Cell Cardiol 33: 1065–1089. [DOI] [PubMed] [Google Scholar]
- 17. Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, et al. (1997) Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol 273: H1544–H1554. [DOI] [PubMed] [Google Scholar]
- 18. Judge S, Leeuwenburgh C (2007) Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol 292: C1983–C1992. [DOI] [PubMed] [Google Scholar]
- 19. Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C (2005) Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J 19: 419–421. [DOI] [PubMed] [Google Scholar]
- 20. Bizeau ME, Willis WT, Hazel JR (1998) Differential responses to endurance training in subsarcolemmal and intermyofibrillar mitochondria. J Appl Physiol 85: 1279–1284. [DOI] [PubMed] [Google Scholar]
- 21. Holmuhamedov EL, Jovanović S, Dzeja PP, Jovanović A, Terzic A (1998) Mitochondrial ATP-sensitive K+ channels modulate cardiac mitochondrial function. Am J Physiol 275: H1567–H1576. [DOI] [PubMed] [Google Scholar]
- 22. Holmuhamedov EL, Wang L, Terzic A (1999) ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J Physiol 519 Pt 2: 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holmuhamedov EL, Ozcan C, Jahangir A, Terzic A (2001) Restoration of Ca2+-inhibited oxidative phosphorylation in cardiac mitochondria by mitochondrial Ca2+ unloading. Mol Cell Biochem 220: 135–140. [DOI] [PubMed] [Google Scholar]
- 24. Holmuhamedov EL, Jahangir A, Oberlin A, Komarov A, Colombini M, et al. (2004) Potassium channel openers are uncoupling protonophores: implication in cardioprotection. FEBS Lett 568: 167–170. [DOI] [PubMed] [Google Scholar]
- 25. Jahangir A, Ozcan C, Holmuhamedov EL, Terzic A (2001) Increased calcium vulnerability of senescent cardiac mitochondria: protective role for a mitochondrial potassium channel opener. Mech Ageing Dev 122: 1073–1086. [DOI] [PubMed] [Google Scholar]
- 26. Ozcan C, Holmuhamedov EL, Jahangir A, Terzic A (2001) Diazoxide protects mitochondria from anoxic injury: implications for myopreservation. J Thorac Cardiovasc Surg 121: 298–306. [DOI] [PubMed] [Google Scholar]
- 27. Preston CC, Oberlin AS, Holmuhamedov EL, Gupta A, Sagar S, et al. (2008) Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech Ageing Dev 129: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, et al. (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 102: 5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamo N, Muratsugu M, Hongoh R, Kobatake Y (1979) Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol 49: 105–121. [DOI] [PubMed] [Google Scholar]
- 30. Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, et al. (2006) The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J 273: 2077–2099. [DOI] [PubMed] [Google Scholar]
- 31. Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, et al. (2004) Preconditioning delays Ca2+-induced mitochondrial permeability transition. Cardiovasc Res 61: 115–122. [DOI] [PubMed] [Google Scholar]
- 32. He ZH, Chillingworth RK, Brune M, Corrie JE, Trentham DR, et al. (1997) ATPase kinetics on activation of rabbit and frog permeabilized isometric muscle fibres: a real time phosphate assay. J Physiol 501 (Pt 1): 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grover GJ, Garlid KD (2000) ATP-Sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol 32: 677–695. [DOI] [PubMed] [Google Scholar]
- 34. Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, et al. (1997) Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res 81: 1072–1082. [DOI] [PubMed] [Google Scholar]
- 35. Costa AD, Garlid KD (2009) MitoKATP activity in healthy and ischemic hearts. J Bioenerg Biomembr 41: 123–126. [DOI] [PubMed] [Google Scholar]
- 36. Gross GJ, Fryer RM (1999) Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res 84: 973–979. [DOI] [PubMed] [Google Scholar]
- 37. Suh JH, Heath SH, Hagen TM (2003) Two subpopulations of mitochondria in the aging rat heart display heterogenous levels of oxidative stress. Free Radic Biol Med 35: 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruiz-Meana M, Garcia-Dorado D, Miró-Casas E, Abellán A, Soler-Soler J (2006) Mitochondrial Ca2+ uptake during simulated ischemia does not affect permeability transition pore opening upon simulated reperfusion. Cardiovasc Res 71: 715–724. [DOI] [PubMed] [Google Scholar]
- 39. McCormack JG, Denton RM (1989) The role of Ca2+ ions in the regulation of intramitochondrial metabolism and energy production in rat heart. Mol Cell Biochem 89: 121–125. [PubMed] [Google Scholar]
- 40. McCormack JG, Denton RM (1993) The role of intramitochondrial Ca2+ in the regulation of oxidative phosphorylation in mammalian tissues. Biochem Soc Trans 21 (Pt 3): 793–799. [DOI] [PubMed] [Google Scholar]
- 41. De Gómez-Puyou MT, Gavilanes M, Gómez-Puyou A, Ernster L (1980) Control of activity states of heart mitochondrial ATPase. Role of the proton-motive force and Ca2+. Biochim Biophys Acta 592: 396–405. [DOI] [PubMed] [Google Scholar]
- 42. Ferrari R, Pedersini P, Bongrazio M, Gaia G, Bernocchi P, et al. (1993) Mitochondrial energy production and cation control in myocardial ischaemia and reperfusion. Basic Res Cardiol 88: 495–512. [DOI] [PubMed] [Google Scholar]
- 43. Miyata H, Lakatta EG, Stern MD, Silverman HS (1992) Relation of mitochondrial and cytosolic free calcium to cardiac myocyte recovery after exposure to anoxia. Circ Res 71: 605–613. [DOI] [PubMed] [Google Scholar]
- 44. Griffiths EJ, Rutter GA (2009) Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta 1787: 1324–1333. [DOI] [PubMed] [Google Scholar]
- 45. Sato T, Marbán E (2000) The role of mitochondrial K(ATP) channels in cardioprotection. Basic Res Cardiol 95: 285–289. [DOI] [PubMed] [Google Scholar]
- 46. Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD (2001) Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am J Physiol Heart Circ Physiol 280: H649–H657. [DOI] [PubMed] [Google Scholar]
- 47. Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, et al. (2002) Mechanisms by which opening the mitochondrial ATP-sensitive K(+) channel protects the ischemic heart. Am J Physiol Heart Circ Physiol 283: H284–H295. [DOI] [PubMed] [Google Scholar]
- 48. Akao M, O'Rourke B, Kusuoka H, Teshima Y, Jones SP, et al. (2003) Differential actions of cardioprotective agents on the mitochondrial death pathway. Circ Res 92: 195–202. [DOI] [PubMed] [Google Scholar]
- 49. Tanonaka K, Taguchi T, Koshimizu M, Ando T, Morinaka T, et al. (1999) Role of an ATP-sensitive potassium channel opener, YM934, in mitochondrial energy production in ischemic/reperfused heart. J Pharmacol Exp Ther 291: 710–716. [PubMed] [Google Scholar]
- 50. Fryer RM, Eells JT, Hsu AK, Henry MM, Gross GJ (2000) Ischemic preconditioning in rats: role of mitochondrial K(ATP) channel in preservation of mitochondrial function. Am J Physiol Heart Circ Physiol 278: H305–H312. [DOI] [PubMed] [Google Scholar]
- 51. Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P (2009) Cardioprotective signaling to mitochondria. J Mol Cell Cardiol 46: 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Minners J, Lacerda L, Yellon DM, Opie LH, McLeod CJ, et al. (2007) Diazoxide-induced respiratory inhibition – a putative mitochondrial K(ATP) channel independent mechanism of pharmacological preconditioning. Mol Cell Biochem 294: 11–18. [DOI] [PubMed] [Google Scholar]
- 53. Gallitelli MF, Schultz M, Isenberg G, Rudolf F (1999) Twitch-potentiation increases calcium in peripheral more than in central mitochondria of guinea-pig ventricular myocytes. J Physiol 518 (Pt 2): 433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rizzuto R, Brini M, Murgia M, Pozzan T (1993) Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262: 744–747. [DOI] [PubMed] [Google Scholar]
- 55. Rizzuto R, Bastianutto C, Brini M, Murgia M, Pozzan T (1994) Mitochondrial Ca2+ homeostasis in intact cells. J Cell Biol 126: 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rutter GA, Burnett P, Rizzuto R, Brini M, Murgia M, et al. (1996) Subcellular imaging of intramitochondrial Ca2+ with recombinant targeted aequorin: significance for the regulation of pyruvate dehydrogenase activity. Proc Natl Acad Sci U S A 93: 5489–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Montero M, Alonso MT, Albillos A, Cuchillo-Ibáñez I, Olivares R, et al. (2001) Control of secretion by mitochondria depends on the size of the local [Ca2+] after chromaffin cell stimulation. Eur J Neurosci 13: 2247–2254. [DOI] [PubMed] [Google Scholar]
- 58. Montero M, Alonso MT, Carnicero E, Cuchillo-Ibáñez I, Albillos A, et al. (2000) Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol 2: 57–61. [DOI] [PubMed] [Google Scholar]