Abstract
Human synovial fluid (SF) provides nutrition and lubrication to the articular cartilage. Particularly in arthritic diseases, SF is extensively accumulating in the synovial junction. During the last decade lipids have attracted considerable attention as their role in the development and resolution of diseases became increasingly recognized. Here, we describe a capillary LC-MS/MS screening platform that was used for the untargeted screening of lipids present in human SF of rheumatoid arthritis (RA) patients. Using this platform we give a detailed overview of the lipids and lipid – derived mediators present in the SF of RA patients. Almost 70 different lipid components from distinct lipid classes were identified and quantification was achieved for the lysophosphatidylcholine and phosphatidylcholine species. In addition, we describe a targeted LC-MS/MS lipid mediator metabolomics strategy for the detection, identification and quantification of maresin 1, lipoxin A4 and resolvin D5 in SF from RA patients. Additionally, we present the identification of 5S,12S-diHETE as a major marker of lipoxygenase pathway interactions in the investigated SF samples. These results are the first to provide a comprehensive approach to the identification and profiling of lipids and lipid mediators present in SF and to describe the presence of key anti-inflammatory and pro-resolving lipid mediators identified in SF from RA patients.
Keywords: Lipid Mediators, LC-MS/MS, Lipidomics, Rheumatoid Arthritis, Special Pro-resolving Mediators, Synovial Fluid
1. Introduction
SF is a rarely studied body fluid that is present in only minute amounts in healthy joints. Its physiological function is to provide nutrition and lubrication to the articular cartilage. SF is composed primarily of hyaluronic acid, secreted by fibroblasts in the synovial membrane, interstitial fluid filtered from plasma and a low number of cells. In pathologic circumstances , such as inflammatory conditions of the joints (e.g. arthritic diseases – rheumatoid arthritis (RA)), infections or trauma, SF can accumulate in the joint reflecting synovial pathology [1]. In RA, SF is enriched in inflammatory cytokines and immune cells which could further enhance synovial inflammation and subsequent cartilage and bone pathology [2]. Consistent with a pathological role for SF in arthritis, removal of SF during arthroscopy is an efficient intervention for immediate symptom relief [3]. During the last decade it has become clear that on-set and termination of inflammation are tightly controlled processes [4]. Particularly lipid species such as prostaglandins (PG) and lipid mediators play crucial roles in the tight regulation of inflammation. Prostaglandins, including PGE2, and leukotrienes, such as LTB4, are considered to play important roles in the onset and development of arthritic diseases [5],[6],[7]. Along these lines the major treatment strategies for RA include COX targeting substances such as NSAID, inhibiting the key enzyme(s) in the production of prostaglandins from AA. On the other hand, anti-inflammatory and pro-resolving lipid mediators, such as lipoxins and other specialized pro-resolving mediators are crucial for the active resolution of inflammatory processes[8]. The underlying mechanisms and their implications for the rheumatic diseases were summarized by Yacoubian and Serhan [9]. In this context, Lima-Garcia et al. recently provided evidence for the anti-hyperalgesic actions of both 17(R)HDoHE and AT-RvD1 in an arthritis rat model [10]. Of interest, a clinical trial showed that ω-3 polyunsaturated fatty acids (PUFA) had positive effects on reducing disease activity in RA patients [11]. Taken together, many clues from the literature point to an important role of lipids and particularly lipid mediators in arthritic diseases such as RA. Hence, we are interested in the lipid composition of human SF in RA patients in order to gain a better insight into the ongoing inflammatory processes occurring in the arthritic joint.
To date, the vast majority of published studies investigating SF were dedicated to proteome analysis [12, 13]. Only a few recent studies investigated non-peptide metabolites or mediators present in SF. Goto et al. determined the PGE2 levels in SF [14] after sodium hyaluronate injections, while Huffman et al. focused on glucose and lactate levels [15]. Referring to lipid species present in SF, earlier studies based on MALDI-MS mainly presented the detection of a limited number of different LPC and PC species in SF from RA patients, including altered LPC/PC ratios in RA patients [16, 17]. Other studies report differences between normal SF and SF from RA patients, in particular higher amounts of cholesterol, cholesterol esters and changes in the phospholipid composition[18]. A more recent study presented a more detailed investigation of LXA4 levels in SF[19]. In addition to this de Grauw et al. recently published an investigation of the lipid mediators present in the SF of horses with acute synovitis [20]. Thus all these studies investigated either total cholesterol- free FA- or TG- levels [21], and were mainly limited to LPC and PC species, focusing on a single substance, or have not investigated human materials. As emphasized above lipid species and lipid mediators are increasingly recognized as important key factors in the development and regulation of inflammatory processes [4]. Hence detailed profiling of the lipid species found in the SF of RA patients is of considerable interest and will enable further investigations and understanding of the underlying mechanisms.
Several analytical approaches are used for the profiling of lipids in human body fluids, including MALDI [16], LC-MS/MS, direct infusion experiments in combination with either FTICR-MS [22], or Orbitrap MS [23] and in some cases also GC-MS [24, 25]. The most versatile and frequently used technique certainly is LC-MS/MS in combination with ESI [26]. Besides commonly employed 2 mm i.d. columns, also nano-LC-MS/MS and capillary LC-MS/MS platforms have been described [27, 28].
In this manuscript, we report on the use of a capillary LC-ESI(±)-MS/MS platform, employing a fast scanning IT-MS and a core-shell based capillary RP18 phase for an in-depth characterization of the lipids present in SF of RA patients. The decoded lipid profiling data indicated the presence of PUFA, hFA and lipoxygenase products in human SF. Hence, we followed up on this finding with a targeted investigation of the biochemically expected downstream products by a dedicated LC-MS/MS platform for lipid mediator screening.
2. Experimental
2.1 Chemicals and Materials
Isopropanol LC-MS grade, methyl-tert.-butylether LC grade, MeOH gradient grade, glacial acetic acid p.a., formic acid p.a., ammonium formate p.a. grade, acetonitrile gradient grade, cholesterol linoleate and LC-MS grade water were from Sigma Aldrich (Schnelldorf, Germany). LPC(19:0/0:0), PG(14:0/14:0), PC(19:0/19:0), PC(14:0/14:0 d54), PS(16:0/18:1 d31) were from Avanti Polar Lipids (Alabaster, AL, USA). 5S-HETE, AAd8, LTB4, LTB4 d4, PGE2 d4 and LXA4 were from Cayman chemicals (Ann Arbor, MI, USA). C18 solid phase extraction cartridges (Sep-Pak C18 500 mg, 6 mL) were from Waters (Boston, MA, USA). MaR1 and RvD5 were prepared in house. PBS was from Life technologies (Paisley, UK).
2.2 Collection of Synovial Fluid
Synovial fluid was obtained as discarded waste material from knee arthroscopy of RA patients visiting the outpatient clinic at the department of Rheumatology in the LUMC. This procedure is part of the standard clinical care and the use of waste material for research was approved by the local ethical committee. Upon informed consent, SF samples were stored at −70 °C until use.
2.3 Lipid extraction
The methyl-tert.-butylether extraction as described by Matyash et al. [29] was applied with some modifications. The obtained SF samples were centrifuged at 13.200 × g for 3 min to remove tissue contaminations. To 50 μL SF were added 160 μL MeOH, 20 μL internal standard solution consisting of PC(14:0/14:0 d54), PS(16:0/18:1 d31) and AAd8 (33.3 μg/mL each) and 600 μL methyl-tert.-butylether in a 2 mL Eppendorf plastic centrifuge tube. The suspension was shaken for 30 min on a benchtop shaker at room temperature. For phase separation 200 μL water was added and the sample tube was centrifuged for 3 min at 13.200 × g. The upper layer was transferred into a 1.5 mL Eppendorf plastic tube and the extraction was repeated for an additional 5 min by adding 100 μL MeOH, 100 μL water and 300 μL methyl-tert.-butylether. The combined organic extracts were concentrated under a gentle stream of nitrogen and fully dried in an Eppendorf concentrator 5310 at 45 °C (approximately 30 min). To the dried residue 250 μL reconstitution solution (65% acetonitrile, 30% isopropanol, 5% water) was added and the tube was vortexed for 10 sec and ultrasonicated for 20 sec. The reconstituted sample was diluted 1:1 with water and transferred to the autosampler vial. A water sample treated in the same way was used as blank.
2.4 LC-MS/MS for untargeted lipidomics analysis
The HPLC system was a Dionex Ultimate 3000, consisting of a binary pump, connected to an autosampler equipped with a 1.0 μL injection loop and a column oven which was maintained at 50 °C. The column was an Ascentis express C-18, 5 cm × 300 μm, 2.7 μm from Sigma Aldrich (Schnelldorf, Germany). The flow rate was 9.0 μL/min. The gradient program started at 65 % eluent A (water: acetonitrile 80:20, containing 5 mM ammonium formate and 0.05 % formic acid) and 35 % eluent B (isopropanol: acetonitrile:water 90:9:1, containing 5 mM ammonium formate and 0.05 % formic acid) kept constant for 2 min, then linearly increasing to reach 95 % B at 30 min, held for 5 min.
The IT-MS was a Bruker amaZon speed, which was operated in the ultrascan mode (Bruker Daltonics, Bremen, Germany). The dry temperature was set to 180 °C. Nitrogen99.9990 % was used as dry gas (8 psi) and nebulizer gas (4 L/min). The capillary voltage was set to ± 3.5 kV. The MS was operated in the ESI± mode and auto MSn spectra collection was applied. The auto MSn settings were as follows: Collision energy - enhanced fragmentation mode ramping from 80-120 %, fragmentation time 30 msec, isolation width 1 amu, precursor exclusion after 3 collected spectra for 1 minute.
2.5 Validation experiments for the untargeted profiling platform and estimation of the present LPC and PC amounts in human SF
Experimental recovery for the methyl-tert.-butylether extraction method was determined according to Matuszewski et al. [30] by spiking a SF pool sample before and after the above described extraction procedure with 20 μL of the internal standard solution. The generated peak areas for the following ion-traces were compared, giving the nominal recoveries: m/z 311.2 (AAd8) and 731.0 (PC(14:0/14:0 d54)). In order to determine interday-repeatability, the peak areas of the following substances were monitored over three different days at a fortification level of 500 ng: PG(14:0/14:0), LPC(19:0/0:0) and PC(19:0/19:0), the samples were injected in triplicate. Linearity of the platform was determined by spiking SF pool samples with the same substances as used for the repeatability. The following amounts were spiked into a SF pool sample: 1, 0.5, 0.25,0.0625 and 0 μg. The so prepared samples were worked up on three consecutive days and analyzed in triplicate. To present an estimation of the platform’s sensitivity, the signal to noise ratios at the lowest measured concentration of the linearity determination as calculated by the Bruker data analysis software are given below. The calibration lines for LPC(19:0/0:0) and PC(19:0/19:0) were used to estimate the concentrations of the found LPC and PC lipids in human SF (cf table 3)
Table 3. Lipid species identified in human synovial fluid from a pooled sample of all investigated patients (n=5). d Referring to the initial SF sample, estimated from matrix matched calibration lines. n.d. not determined. LM lipid mediator. If the FAs present in the lipid molecule could not be specified unambiguously the lipid class is given with a general description of the found side chains, i.e. PC(18:1/18:2) vs. PC(36:3). All lipids have been identified to the best of our knowledge, in some cases the detected chromatographic peaks also contained lipids consisting of other FA combinations. In addition it has to be emphasized that this list of synovial lipids does not claim to be complete.
| Compo und # |
RT [mi n] |
a[M+H]+, b[M+NH4]+,c[ M-H]− |
Lip id cla ss |
Substance | Found mass - HRMS |
Calcul ated mass |
Conc - [μg/ mL]d |
ESI mo de |
|---|---|---|---|---|---|---|---|---|
| 1 | LP | 468.30 | 468.3 | + | ||||
| 2.2 | 468.4a | C | LPC(14:0) | 85 | 085 | 0.4 | ||
| 2 | LP | 494.32 | 494.3 | + | ||||
| 2.8 | 494.4a | C | LPC(16:1) | 42 | 241 | 0.9 | ||
| 3 | LP | 520.34 | 520.3 | + | ||||
| 3.1 | 520.3a | C | LPC(18:2) | 01 | 398 | 6.8 | ||
| 4 | LP | 544.33 | 544.3 | + | ||||
| 3.4 | 544.3a | C | LPC(20:4) | 75 | 398 | 2 | ||
| 5 | LP | + | ||||||
| 3.5 | 568.3a | C | LPC(22:6) | 0.3 | ||||
| 6 | LP | 496.33 | 496.3 | + | ||||
| 4.5 | 496.3a | C | LPC(16:0) | 99 | 398 | 43 | ||
| 7 | LP | 522.35 | 522.3 | + | ||||
| 5.2 | 522.3a | C | LPC(18:1) | 58 | 554 | 11 | ||
| 8 | LP | 510.35 | 510.3 | + | ||||
| 5.7 | 510.3a | C | LPC(17:0) | 57 | 554 | 1 | ||
| 9 | LP | 524.37 | 524.3 | + | ||||
| 7.3 | 524.3a | C | LPC(18:0) | 13 | 711 | 29 | ||
| 10 | 15. | 701.55 | 701.5 | n.d. | + | |||
| 3 | 701.6a | SM | SM(34:2) | 96 | 592 | |||
| 11 | 16. | 369.4 [M- | Ste | n.d. | + | |||
| 3 | H2O]+ | rol | Cholesterol | |||||
| 12 | 16. | 703.57 | 703.5 | n.d. | + | |||
| 5 | 703.6a | SM | SM(34:1) | 51 | 749 | |||
| 13 | 17. | 780.55 | 780.5 | 12 | + | |||
| 0 | 780.6a | PC | PC(16:0/20:5) | 21 | 538 | |||
| 14 | 17. | 729.59 | 729.5 | n.d. | + | |||
| 2 | 729.6a | SM | SM(36:2) PC(16:0/16:1) |
09 | 905 | |||
| 15 | 17. | and | 732.55 | 732.5 | + | |||
| 5 | 732.6a | PC | PC(14:0/18:1) | 41 | 538 | 15 | ||
| 16 | 17. | 782.56 | 782.5 | + | ||||
| 9 | 782.6a | PC | PC(16:0/20:4) | 85 | 695 | 55 | ||
| PC(18:2/20:4) | ||||||||
| 17 | 18. | and/or | 806.56 | 806.5 | + | |||
| 0 | 806.6a | PC | PC(16:0/22:6) | 74 | 695 | 22 | ||
| 18 | 18. | 758.57 | 758.5 | + | ||||
| 2 | 758.6a | PC | PC(16:0/18:2) | 00 | 695 | 160 | ||
| 19 | 18. | 784.58 | 784.5 | + | ||||
| 5 | 784.3a | PC | PC(16:0/20:3) PC(20:5/18:0) |
55 | 851 | 69 | ||
| 20 | 18. | and | 808.58 | 808.5 | + | |||
| 6 | 808.6a | PC | PC(16:0/22:5) | 34 | 851 | 23 | ||
| 21 | 18. | 734.56 | 734.5 | + | ||||
| 9 | 734.6a | PC | PC(16:0/16:0) | 97 | 695 | 22 | ||
| 22 | 19. | 760.58 | 760.5 | + | ||||
| 0 | 760.6a | PC | PC(16:0/18:1) PC(18:1/18:1) |
57 | 851 | 127 | ||
| 23 | 19. | and | 786.60 | 786.6 | + | |||
| 5 | 786.6a | PC | PC(18:0/18:2) | 10 | 008 | 112 | ||
| 24 | 19. | 810.60 | 810.6 | + | ||||
| 6 | 810.6a | PC | PC(18:0/20:4) | 09 | 008 | 42 | ||
| 25 | 19. | 834.59 | 834.6 | + | ||||
| 7 | 834.6a | PC | PC(22:6/18:0) | 85 | 008 | 9 | ||
| 26 | 20. | 812.61 | 812.6 | + | ||||
| 3 | 812.6a | PC | PC(18:0/20:3) | 67 | 164 | 23 | ||
| 27 | 20. | 788.61 | 788.6 | + | ||||
| 6 | 788.6a | PC | PC(18:0/18:1) | 69 | 164 | 35 | ||
| 29 | 22. | n.d. | + | |||||
| 2 | 801.8a | SM | SM(40:2) | |||||
| 28 | 23. | 813.68 | 813.6 | n.d. | + | |||
| 0 | 813.7a | SM | SM(42:2) | 50 | 844 | |||
| 30 | 23. | 815.70 | 815.7 | n.d. | + | |||
| 1 | 815.7a | SM | SM(42:1) | 02 | 001 | |||
| 31 | 27. | 792.70 | 792.7 | n.d. | + | |||
| 6 | 792.7b | TG | TG(46:2) | 81 | 075 | |||
| 32 | 27. | 818.72 | 818.7 | n.d. | + | |||
| 8 | 818.8b | TG | TG(48:3) | 33 | 232 | |||
| 33 | 27. | 688.60 | 688.6 | n.d. | + | |||
| 9 | 688.6b | CE | CE(20:5) | 28 | 027 | |||
| 34 | 28. | 844.73 | 844.7 | n.d. | + | |||
| 0 | 844.8b | TG | TG(50:4) | 98 | 388 | |||
| 35 | 28. | 820.73 | 820.7 | n.d. | + | |||
| 2 | 820.7b | TG | TG(48:2) | 88 | 388 | |||
| 36 | 28. | 794.72 | 794.7 | n.d. | + | |||
| 2 | 794.8b | TG | TG(46:1) | 26 | 232 | |||
| 37 | 28. | 664.60 | 664.6 | n.d. | + | |||
| 3 | 664.6b | CE | CE(18:3) | 31 | 027 | |||
| 38 | 28. | 846.8b | TG | TG(50:3) | 846.75 | 846.7 | n.d. | + |
| 4 | 48 | 545 | ||||||
| 39 | 28. | 690.61 | 690.6 | n.d. | + | |||
| 5 | 690.6b | CE | CE(20:4) | 85 | 183 | |||
| 40 | 28. | n.d. | + | |||||
| 7 | 640.6b | CE | CE(16:1) | |||||
| 41 | 28. | 666.61 | 666.6 | n.d. | + | |||
| 7 | 666.6b | CE | CE(18:2) | 87 | 183 | |||
| 42 | 29. | 874.78 | 874.7 | n.d. | + | |||
| 0 | 874.8b | TG | TG(52:3) | 68 | 858 | |||
| 43 | 29. | 848.77 | 848.7 | n.d. | + | |||
| 1 | 848.8b | TG | TG(50:2) | 09 | 701 | |||
| 44 | 29. | 850.78 | 850.7 | n.d. | + | |||
| 2 | 850.8b | TG | TG(50:1) | 56 | 858 | |||
| 45 | 29. | 876.80 | 876.8 | n.d. | + | |||
| 4 | 876.8b | TG | TG(52:2) | 23 | 014 | |||
| 46 | 29. | 668.63 | 668.6 | n.d. | + | |||
| 7 | 668.6b | CE | CE(18:1) | 44 | 340 | |||
| 47 | 29. | 642.61 | 642.6 | n.d. | + | |||
| 7 | 642.6b | CE | CE(16:0) | 86 | 183 | |||
| 48 | 29. | 878.81 | 878.8 | n.d. | + | |||
| 8 | 878.9b | TG | TG(52:1) | 76 | 171 | |||
| 49 | 30. | 670.65 | 670.6 | n.d. | + | |||
| 0 | 670.6b | CE | CE(18:4) | 01 | 496 | |||
| 50 | 29. | 904.83 | 904.8 | n.d. | f+ 1 | |||
| 9 | 904.8b | TG | TG(54:2) | 33 | 327 | |||
| 51 | 30. | 906.84 | 906.8 | n.d. | + | |||
| 3 | 906.8b | TG | TG(54:1) | 86 | 484 | |||
| 52 | L | - | - | n.d. | − | |||
| 1.6 | 335.3c | M | 5S,12S-diHETE | |||||
| 53 | LP | - | - | n.d. | − | |||
| 2.8 | 619.4c | I | LPI(20:4) | |||||
| 54 | hF | - | - | n.d. | − | |||
| 4.2 | 319.2c | A | hFA(20:5) | |||||
| 55 | LP | - | - | n.d. | − | |||
| 5.0 | 452.4c | E | LPE(16:0) | |||||
| 56 | LP | - | - | n.d. | − | |||
| 6.0 | 599.4c | I | LPI(18:0) | |||||
| 57 | LP | - | - | n.d. | − | |||
| 7.6 | 480.5c | E | LPE(18:0) | |||||
| 58 | 8.5 | 327.3c | FA | FA(22:6) | - | - | n.d. | − |
| 59 | 8.9 | 303.3c | FA | FA(20:4) | - | - | n.d. | − |
| 60 | 9.1 | 279.2c | FA | FA(18:2) | - | - | n.d. | − |
| 61 | 9.6 | 329.3c | FA | FA(22:5) | - | - | n.d. | − |
| 62 | 9.9 | 305.3c | FA | FA(20:3) | - | - | n.d. | − |
| 63 | 11. | n.d. | − | |||||
| 2 | 281.3c | FA | FA(18:1) | |||||
| 64 | 15. | 870.7 | n.d. | − | ||||
| 6 | [M+HCOO−]− | PC | PC(18:0/h20:4) | |||||
| 65 | 18. | n.d. | − | |||||
| 2 | 885.7c | PI | PI(18:0/20:4) |
2.6 LC-MS/MS system for targeted lipidomics
Targeted lipid mediator profiling was carried out as described in [31, 32] with some modifications. Briefly: Internal standards (PGE2 d4 and LTB4 d4) 500 pg each were added before extractions. Products were extracted from deidentified SF (125 μL) after protein precipitation with 500 μL MeOH using solid phase extraction (SPE). To 125 μL SF in a 10 mL glass vial were added 500 μL MeOH and the samples were stored at −20 °C for 30 min. The samples were centrifuged for 10 min at 4000 rounds per minute at 4 °C. The organic layer was poured into a fresh 10 mL glass vial and the extraction was repeated without freezing with another 500 μL MeOH. The organic extracts were combined and diluted to a final concentration of 10 % MeOH with 9 mL of MilliQ water. The samples were acidified by the addition of 25 μL 1 M HCl and immediately loaded onto the preconditioned Sep-Pak C18 500 mg SPE cartridges (cartridges initially washed with 6 mL MeOH, equilibrated with 6 mL MilliQ water). The SPE columns were subsequently washed with 10 mL of MilliQ water and 6 mL of n-hexane before elution of the analytes was accomplished with 8 mL of methylformate. The methylformate extract was roughly dried in a vacuum concentrator before final drying was carried out with a gentle stream of nitrogen and the frequent addition of MeOH for washing the analytes to the bottom of the vessel and enhancing the drying process. The dried samples were dissolved in 150 μL 60 % MeOH and centrifuged under the conditions stated above. 125 μL of the so prepared samples was transferred into a micro vial glass insert and subsequently analyzed on a QTrap 5500 mass spectrometer (AB Sciex, Boston, MA, USA) coupled to an Agilent Eclipse Plus C-18 column (4.6 mm × 50 mm × 1.8 μm) a quaternary 1100 pump (Agilent, Waldbronn, Germany) and a manual injection valve (Rheodyne, Oak Harbor, WA, USA). The following binary gradient of water (A) and MeOH (B) with 0.01% acetic acid was used: 0 min 55% B, held for 2 min, then ramped to 65% at 3 min, 88% at 15 min and 100% B at 16.5 min, held for 3.5 min. The flow rate was 500 μL/min. The injection volume was 20 μL. The MS was operated under the following conditions: The gas flow was set to medium, the drying temperature was 500 °C, the needle voltage −4000 V, the curtain gas was 10 psi, ion source gas 40 psi and the ion source gas 2 40 psi as well (nitrogen 99.9990%). Products were identified according to published criteria including retention times and at least 6 diagnostic ions present in the full MS/MS spectrum to match those of synthetic standards [31]. For quantitation the following MS/MS transitions with the specified collision energies were monitored: MaR1 (359→250, −22V), LXA4 (351→235, −22 V), 5S,12S-diHETE (335→195, −22 V), RvD5 (359→199, −22V), PGE2 d4 (355→193, −30 V) and LTB4 d4 (339→197, −24 V). The quantification of all analytes was performed against calibration lines constructed with synthetic standard material on the day of analysis using the following amounts: 0, 2, 10, 20, 50, 200, 400, 800 pg. 5S,12S-diHETE was quantified against LTB4, which shows identical MS fragmentation. All obtained values were corrected for their corresponding internal standard (MaR1, RvD5 and 5S,12S-diHETE were corrected with LTB4 d4, LXA4 was corrected with PGE2 d4). The recovery for the internal standards PGE2 d4 and LTB4 d4 was determined against an aqueous standard. In addition intra-day repeatability was determined for the two internal standards.
2.7 Preparation and identification of 5S,12S-diHETE
5S,12S-diHETE was prepared as in [33] from platelet incubations with some modifications: 5S-HETE was incubated with the platelets derived from 1 mL of fresh blood (heparin) collected from a healthy donor according to the ethical conventions of the LUMC. Platelet rich plasma was prepared by spinning 2 mL of blood at 100 × g for 15 minutes at room temperature. The collected plasma was then centrifuged at 2200 × g for 5 minutes at room temperature to obtain platelets. The collected platelets were resuspended in 200 μL of PBS. 100 μL platelet suspension was diluted to 500 μL with PBS containing magnesium and calcium. To this solution 500 ng of 5S-HETE (ethanolic solution, final ethanol content < 1%) was added and incubated for 4 hours at 37 °C. 100 μL of this solution was quenched with 100 μL of methanol and centrifuged at 13.000 × g for 3 minutes. 20 μL of the organic extract was injected into the LC-MS-IT (Dionex, Bruker) analysis platform equipped with the column and operated under the conditions described under 2.6 with some modifications: a split of 1:5 of the column effluent was applied before the AmaZon IT-MS, the dry temperature was 250 °C, the nebulizer gas was 5 L/min and the dry gas was set to 11 psi. As control solutions 500 ng 5S-HETE were incubated under the described conditions without platelets and platelets were incubated without 5S-HETE. To verify the identity of 5S,12S-diHETE in the human SF samples, the generated product solution from platelet incubations was spiked with LTB4 d4 and the obtained chromatogram compared to the one obtained with a SF sample. The IT-MS scanned from m/z 315-350 in ESI-mode with auto MSn scanning. In addition we also spiked sample RA-2 with synthetic LTB4 and LTB4d4 (25ng/mL).
2.8 Direct infusion experiments
To verify the lipid species detected with the untargeted LC-MS/MS screening system, a SF pool sample from all patients was worked up according to the methyl-tert.-butylether extraction protocol as described above, dissolved in reconstitution solvent and diluted 1:20 with isopropanol: acetonitrile:water (5 mM ammonium acetate, 0.05% AA) 90:9:1 followed by infusion at 5 μL/min into a 15T Bruker Solarix FTICR-MS (Bruker, Bremen, Germany).
2.9 Lipid identification
For lipid identification in the untargeted profiling mode, in first instance the auto MSn compound finding algorithm with a conservative intensity cut off set to 1.000.000 in positive ESI mode and 100.000 in negative ESI mode of the Bruker data analysis software was applied. This resulted in around 80 detected features in the positive ESI mode and around 50 in the negative ESI mode for a pooled SF sample from five RA patients. The generated compound list was subsequently classified into the different lipid classes by characteristic MS2 ions and neutral losses (NL). The diagnostic features used to primarily classify the different detected lipids are indicated in table 1. After the compounds had been classified each molecular feature was matched against the LIPIDMAPS and or MassBank libraries (www.lipidmaps.org; www.massbank.jp) identifying known lipid species on basis of their MS/MS spectra. Unmatched lipid species were (if possible) “manually” identified on basis of the well-known lipid fragmentation mechanisms [34]. The detected adducts of the different lipid classes were as described previously [26].
Table 1. Diagnostic features used to classify the detected lipids, a see text for a more detailed description.
In case of the targeted lipid analysis, compounds were identified on basis of characteristic MS/MS fragments (see supplementary material S1-S4), RRT comparison and co-injections [32, 41]. We calculated the relative retention times for all substances with respect to their internal standard and accepted a deviation of 0.5 %. If a deviation between 0.5 and 1.0 % was detected we conducted a co-injection of standard material. The results of the obtained data are summarized below.
3. Results and discussion
The employed IT-MS for the general lipid profiling allowed generating positive and negative ionization data, as well as the respective MS/MS data within a single LC run. Sample work up was carried out according to the methyl-tert.-butylether based protocol recently introduced by Matyash et al. [29]. The developed analysis platform was validated according to recovery, repeatability and linearity. The platform was applied to generate a detailed overview of the lipid species present in human SF of RA patients by analyzing a pooled sample of five patients. The so generated general profiling data and especially the detection of PUFA, hFAs and isomers of the pro-inflammatory lipid mediator - LTB4 (see below) suggested that a more detailed investigation of the downstream metabolic products of PUFA in individual SF samples of RA patients was warranted. These in-depth profiling analyses were carried out with a dedicated QTrap analysis platform as described below.
3.1 Validation
To validate the untargeted profiling platform, we determined linearity, reproducibility and recovery. Recovery for the two investigated substances AAd8 and PC(14:0/14:0 d54) was higher than 85%, while inter-day reproducibility was robust, fulfilling the criteria for a profiling platform. An overview of the obtained analytical figures of merit is given in Table 2. A blank sample (distilled water) processed according to the protocol did not show significant signals besides the internal standards.
Table 2. validation results of the developed LC-MS/MS platform, RSD relative standard deviation.
| Recovery (n=3) | Linearity R2 | Signal to Noise at 0.125 μg/mL |
Inter-day repeatability at 1 μg/mL (n=9) RSD |
|---|---|---|---|
| AAd8 92 ± 4% |
LPC(19:0/0:0) (ESI+) 0.996 |
LPC(19:0/0:0) (ESI+) 348 |
LPC(19:0 /0:0) (ESI+) 25% |
| PC(14:0/14:0 d54) 87± 11% |
PC(19:0/19:0) (ESI+) 0.996 |
PC(19:0/19:0) (ESI+) 152 |
PC(19:0/19:0) (ESI+) 13% |
| PG(14:0/14:0) (ESI−) 0.994 |
PG(14:0/14:0) (ESI−) 73 |
PG(14:0/14:0) (ESI−) 15% |
3.2 Lipid content of Synovial Fluid
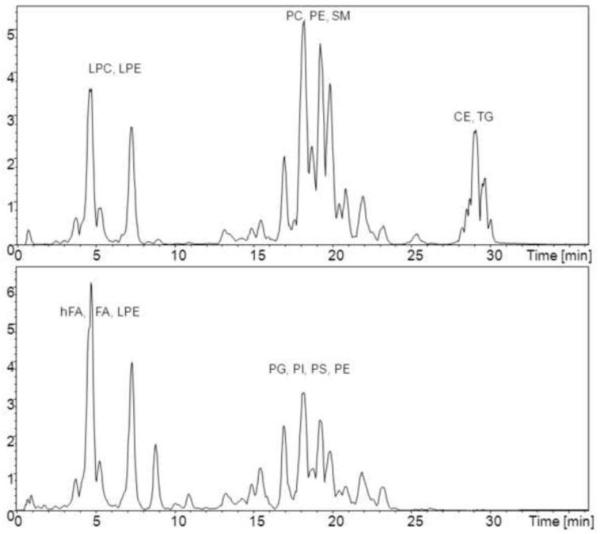
Initially we conducted a general lipid profiling of human SF from five RA patients (Figure 1). This screening approach led to the identification of the lipids presented in table 3. Identification of the different lipid species was carried out by clustering into different lipid classes according to their characteristic fragment ions, or neutral losses as shown in Table 1 and figure 2. Further identification was carried out by comparison of the obtained mass spectra with the LIPIDMAPS database, the MassBank database, or by “denovo” identification (www.lipidmaps.org; www.massbank.jp). The assignment of the sn-1 and sn-2 FAs given in this manuscript is tentative. Even though it has been described that sn-2 FAs give higher relative intensities in the negative ESI mode, when compared to the sn-1 chain [38], known limitations to this approach hamper the absolute assignment of the sn-1 and sn-2 position difficult [34]. Nevertheless this concept was used to give a tentative assignment of the sn-1 and sn-2 position and most of the PUFA contained in PC species were found in the sn-2 position. In the case of lyso-lipids FA side chain assignments were mainly based on ESI-fragment ions. The assignment whether a sn-1 or sn-2 acylation was present has not been carried out. Figure 1 shows the base peak intensity chromatogram of human synovial fluid in the positive and negative ESI mode. Besides the main species in the ESI+ mode being PC and LPC components (including most of the earlier described PC and LPC species [16, 17]) a series of CE and TG species were detected. Direct infusion experiments confirmed the identity of these molecular species, furthermore verifying the identities of the presented (acyl-) PC lipids, by deconvoluting them from possible ether or plasmalogen species, which usually have a mass difference of around 0.05 Da, easily distinguishable by FTICR-MS. As isotopic signals, particularly of the second isotope might lead to false positive identifications, we only accepted signals which were at least two times as high as expected for an isotopic signal.
Figure 1.
Base Peak Intensity chromatograms (BPI) of the ESI+ mode (above) and the ESI-mode (below), the abbreviations mark the elution windows of the different lipid classes, assignments are given in the ESI+, ESI- or both modes according to table 1
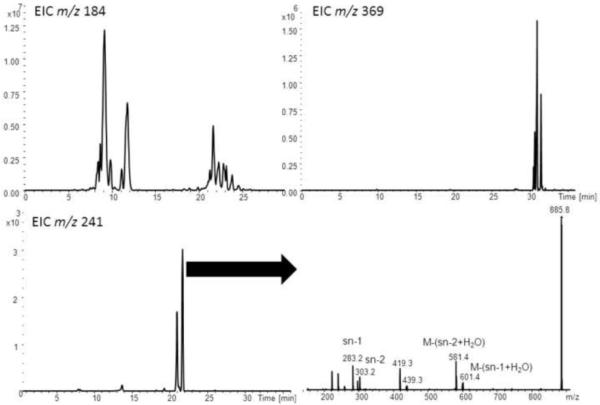
Figure 2.
comparison of different MS2 extracted ion chromatograms (EIC), lower right corner mass spectrometric identification of PI(18:0/20:4) [46]
Especially in the ESI-mode LPE, PI and LPI components could be detected. The neutral loss scanning (− m/z 87) for PS components revealed no other species than the internal standard PS(16:0/18:1 d31). Furthermore, no PG species could be detected by product ion scanning. On the other hand some prominent species identified in the negative ion mode were PUFA. Especially DHA (FA 22:6), eicosapentaenoic acid (FA 20:5) and their further metabolic products together with 5S,12S-diHETE.
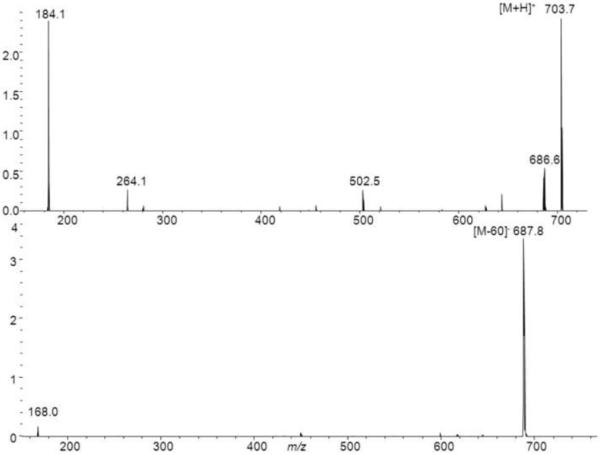
Other than LPE lipids PE could not be unambiguously identified. This might be explained by the fact that these co-elute with PC lipids, which possibly suppress their detection by causing ion-suppression. For the detection of SM species we monitored odd numbered [M+H]+ and [M+HCOO]− ions in the positive and negative ESI mode, respectively. SM lipids give rise to a m/z 184 ion in the positive ESI mode, a strong [M-60]− and a characteristic m/z 168 fragment ion in the negative ESI mode [42]. The formation of the m/z 168 fragment ion is not observed for PC lipids which instead give rise to fragment ions corresponding to their FA side chains in the negative ESI mode. Figure 3 shows the MS/MS spectrum of SM(34:1), as can be seen we obtain a fragment ion at m/z 264 which most likely is related to a d18:1-SM lipid. Although the fragment ion at m/z 264 is presumably characteristic for a d18:1-SM the analysis of the sphingosine backbone is usually carried out after hydrolysis as for example described by Blaas et al.[35]. For a more detailed profiling of the SM content, the methods of Bielawski [43] or Liebisch et al. [44] might be applied.. With this approach several SM lipids could be identified in human SF.. Since the ESI-MS response of phospholipids is largely class dependent [36, 45] we used the matrix matched calibration lines for LPC(19:0/0:0) and PC(19:0/19:0) to estimate the concentrations of the LPC and PC species found in human SF. Although some of the measured compounds were present in concentrations higher than the actual calibration range, this data still gives an estimate of the present concentrations. To provide a more accurate quantitation of the present lipid species a triple quadrupole based analysis platform should be used [36].
Figure 3.
MS/MS spectra of SM(34:1) in the positive and negative ESI mode
3.3 Targeted lipidomics analysis - lipid mediator screening
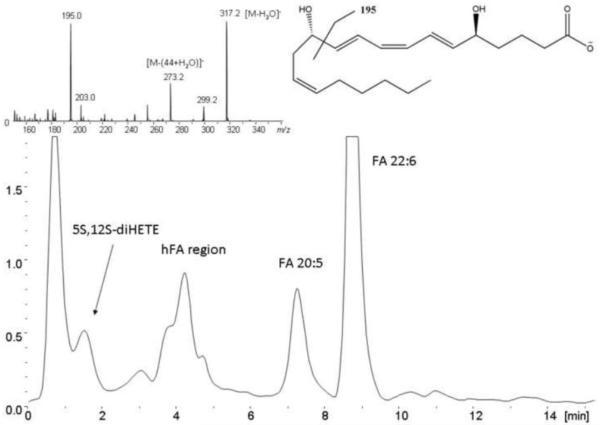
SFs from RA patients proved to be a complex body fluid with respect to lipid analysis. Figure 4 shows the extracted ion chromatogram of a human SF sample obtained with the general profiling platform, depicting the ion traces m/z 301, 327, 343, 319, 317 and 335 in the negative ESI mode, targeting for FA 20:5, FA 22:6, the hFAs h22:6, h20:4, h20:5 and what we initially considered to be the lipid mediator - LTB4. However, additional experiments revealed that the signal we detected actually belonged to the well-known isomer of LTB4 which we identified to be 5S,12S-diHETE (see below and supplementary material S6-S9). Moreover significant amounts of PUFA an hFA can be detected in the SF of the pooled RA patient sample. In addition the isomer of the pro-inflammatory lipid mediator - LTB4 was identified by the untargeted screening approach. PUFA are of particular interest from a biochemical perspective, as they are the starting point for the biosynthesis of numerous biologically active lipid mediators and their biomarkers, involved in both initiation and termination of the inflammatory processes [47], [48]. Therefore, we used a targeted lipidomics approach [31] to investigate the presence and concentration of the following lipid mediators in human SF of five RA patients: 5S,12S-diHETE, LXA4, MaR1 and RvD5. These lipid mediators were identified based on matching RRTs (deviation < 0.5%) and characteristic MS/MS fragmentations with those of synthetic standards prepared by total organic synthesis. Furthermore identification of 5S,12S-diHETE was conducted by co-injection of biosynthetic standard whilst MaR1 was identified following MS3 measurements and a co-injection of standard material. The spectral information for all target analytes as well as standard substances can be found in the supplementary material S1-S4, and figure 5 for MaR1. The identification of MaR1 in a human SF sample is shown in figure 5. In the case of 5S,12S-diHETE we carried out the identification by producing standard material through the incubation of 5S-HETE with human platelets and compared the obtained chromatograms after spiking with authentic LTB4 and LTB4 d4. We also spiked our sample materials with LTB4 and analyzed it on the AmaZon speed IT-MS to investigate whether LTB4 can be found in our samples and if it might contribute to the signal which was quantified as 5S,12S-diHETE. As can be seen in figure S9 (supplementary material), the experiments showed that LTB4 can partially be resolved from 5S,12S-diHETE and that the unspiked sample did not contain significant amounts of LTB4 at the amounts chromatographed.
Figure 4.
Extracted ion chromatogram (ESI-) of the ion traces m/z 327.2, 343.3, 301.2, 317.2, 319.2 and 335.3 referring to FA 22:6, FA 20:5, their respective hydroxylated species and 5S,12S-diHETE in the RA pool sample. The upper left corner shows the obtained MS/MS spectrum for the precursor m/z 335 at 1.6 min. The upper right corner shows 5S,12S-diHETE and its preferred fragmentation yielding m/z 195.
Figure 5.
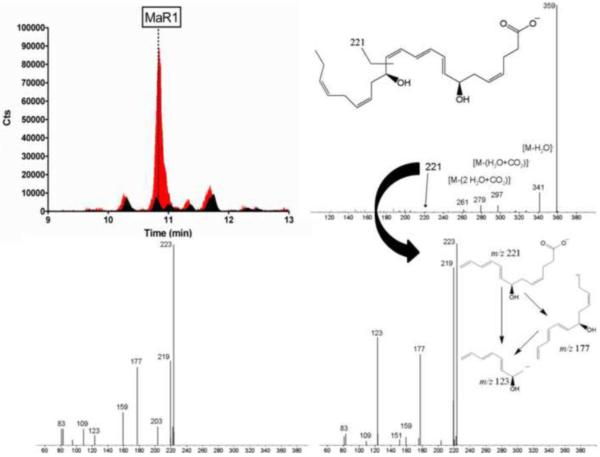
Identification of MaR1 in human synovial fluid, upper left corner selected reaction monitoring chromatograms of the transition m/z 359→221, black – unspiked sample, red - co-injection of a synthetic MaR1 standard (100 pg on column spike level), upper right corner – MS/MS spectrum of MaR1, lower right corner - MS3 spectrum of the characteristic MaR1 fragment m/z 221 corresponding to the sample, lower left corner MS3 spectrum of synthetic MaR1 (m/z 221) – standard sample (10 pg on column).
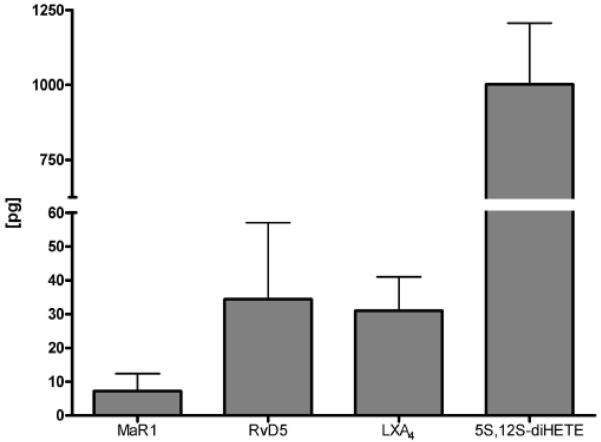
As depicted in figure 6, a range of amounts of lipid mediators were identified in human SF obtained from five different RA patients. The minimal recoveries determined for the internal standards PGE2 d4 and LTB4 d4 were 82 and 72 %, respectively. Intra-day repeatability for the internal standards was determined to be 13 and 14 % relative standard deviation for LTB4 d4 and PGE2 d4. The linearity for all measured analytes was better than R2 = 0.994. All samples displayed a rather similar pattern with respect to the presence of lipid mediators, indicating activation of pro-resolving mediator pathways during disease.
Figure 6.
Lipid mediator amounts identified and quantified in five different human SF samples. Absolute amounts in pg on column are given. Error bars show one standard deviation (n=5). For the individual values see supplementary material S5.
Interestingly, all of the RA patients investigated presented significant amounts of the aforementioned lipid mediators. In addition, the detected high levels of lipid mediators were in agreement with significant amounts of FA 22:6 and FA 20:5 as determined in the general lipid profiling platform. These findings are in line with an earlier report describing the presence of 5-lipoxygenase and 15-lipoxygenase, key enzymes involved in the biosynthesis of lipid mediators identified herein, in the synovium of arthritis patients[49]. In this respect, it is also noteworthy that the formation of the identified lipid mediators is not simply an oxidation driven process, but for the most part enzymatically controlled. It is important to note that most of the lipid mediators identified herein were accompanied by numerous substances giving the same mass spectrometric transitions. These substances are most likely isomers of the described lipid mediators. Naturally this fact makes the quantification and identification of the presented lipid mediators challenging. We therefore have carried out the identification of the presented substances using rigorous criteria (>6 characteristic MS/MS fragments, RRT < 0.5% deviation, co-injection of standard material if RRT deviated more than 0.5%).
4. Conclusions
The main points described in this manuscript are threefold: A) a capillary LC-MS/MS platform that we applied for the detailed characterization of various lipid species present in SF of RA patients is presented. This method employs a core-shell particle column and a fast scanning IT-MS for the convenient generation of ESI+ and ESI-data, including MS/MS spectra in a single analysis. Importantly our screening platform did not only allow investigating higher molecular weight lipid species, but also the biochemically important hFAs and their oxygenated products such as 5S,12S-diHETE. B) the developed screening platform led to identification of approximately 70 distinct lipids in human SF of RA patients, belonging to a range of lipid and lipid mediator classes. Although largely descriptive, our study provides the basis for future investigations aimed at understanding the biological effect of different lipids on joint-associated structures in rheumatic diseases. C) most importantly, we provide mass spectrometric evidence for the presence and determine the concentration of several important lipid mediators in human SF of RA patients. In addition we found high amounts of 5S,12S-diHETE, an isomer of LTB4 which is primarily a product of trans-cellular biosynthesis for example in human platelets and neutrophils [33, 50], pinpointing to the involvement of both cell types. It should be emphasized that although LTB4 could not be identified with the present approach, it might be present at concentrations below the detection limits of our methods or more likely further metabolized to ω-20 oxidation products i.e. 20-OH-LTB4 and 20-COOH-LTB4. Moreover it is noteworthy that the quantified area beneath the peak co-eluting with 5S,12S-diHETE should mainly consist of this compound alone. The presence of other LTB4 isomers, for example 5S,12R-diHETE [51] remains possible but was not the subject of the presented study. Our main goal was to investigate whether or not we are able to identify LTB4 itself and in this context we separated 5S,12S-diHETE from LTB4. Remarkably, this study shows that pro-resolving mediators are present in a chronic inflammatory disease such as RA (cf figure 6). Although further investigations are required to validate the prevalence of these mediators in a larger RA population, our results indicate that pro-resolving pathways could play a role in disease pathogenesis in RA. The presence of pro-resolving lipid mediators which exert anti-inflammatory actions stopping further leukocyte infiltration and enhancing the pro-resolving actions of macrophages might suggest a role for these mediators in preventing further joint inflammation and limiting tissue damage.
Extended analyses of other lipid mediators and their precursors in SF are needed to gain further insight into the pro-inflammatory and pro-resolving pathways activated in this disease and therefore affording a deeper understanding of the possible biological relevance of these pathways in RA and how they may be gender and individual dependent. Importantly, these SPM profiles are very likely to reflect treatment and disease progression. Likewise, it will be important to establish the modulatory effect the lipid (mediator) fraction has on tissues and cells involved in joint pathology in RA in order to evaluate the potential that these mediators might hold as novel anti-rheumatic treatments. For example, the use of lipoxin stable analogs or leukotriene inhibitors has been proposed for the treatment of scleroderma lung disease, which is characterized by an overproduction of leukotrienes [9, 52]. In addition such substances have also been proposed as possible treatment in fibrotic diseases [53].
Supplementary Material
Highlights.
LC-MS/MS profiling of lipids in synovial fluid of rheumatoid arthritis patients
LC-MS/MS platform incorporating a ion trap MS and a core-shell particle column
Targeted investigation of pro-resolving lipid mediators in patient samples
Identification and quantification of MaR1, 5S,12S-diHETE, RvD5 and LXA4 in synovial fluid
First description of pro-resolving lipid mediators in rheumatoid arthritis patients
5. Acknowledgements
We thank Hulda Jónasdóttir, Simone Nicolardi (FTICR MS analysis) and Willem Jonker for technical assistance. The work was supported in part by U.S. National Institutes of Health grants 1P01GM095467 (C.N.S.). Top Institute Pharma and the Dutch Arthritis Association (A.I.F.), a grant from Centre for Medical Systems Biology (CMSB) within the framework of the Netherlands Genomics Initiative (NGI), FP7 program Masterswitch, and the IMI EU funded project BeTheCure, contract no 115142-2 (R.E.M.T.) . Disclosure: CNS is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. CNS is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. CNS’ interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Abbreviations
- 5S-HETE
5S-6E,8Z,11Z,14Z-hydroxyeicosatetraenoic acid
- 5S,12S-diHETE
5S,12S-6E,8Z,10E,14Z-dihydroxyeicosatetraenoic acid
- 17(R)HDoHE
17(R)-hydroxy docosahexaenoic acid
- AAd8
arachidonic acid d8
- AT-RvD1
7S,8R,17R-trihydroxy-4Z,9E,11E,13Z,15E19Z-docosahexaenoic acid
- CE
cholesterol ester
- DHA
docosahexaenoic acid
- ESI(±)
electrospray ionization (positive/negative)
- FA
fatty acid
- FTICR-MS
fourier transform ion cyclotron mass spectrometry
- GC-MS
gas chromatography mass spectrometry
- hFA
hydroxylated fatty acid
- HRMS
high resolution mass spectrometry
- IT-MS
ion trap mass spectrometer
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LOX
lipoxygenase
- LPC
lysophosphatidylcholine
- LPC(19:0)
1-nonadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine
- LPE
lysophosphatidylethanolamine
- LPI
lysophosphatidylinositol
- LTB4
5S,12R-dihydroxy-6Z-8E,10E,14--Z-eicosatetraenoic acid
- LTB4 d4
5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic-6,7,14,15-d4 acid
- LXA4
5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid
- MALDI
matrix assisted laser desorption ionization
- MaR
maresin, macrophage mediator in resolving inflammation
- MaR1
7R,14S-dihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid MeOH, methanol
- PBS
phosphate buffered saline
- PC
phosphatidylcholine
- PC(14:0/14:0 d54)
1,2-dimyristoyl(d54)-sn-glycero-3-phosphocholine
- PC(19:0/19:0)
2-dinonadecanoyl-sn-glycero-3-phosphocholine
- PE
phosphatidylethanolamine
- PG
phosphatidylglycerol
- PG(14:0/14:0)
1,2-dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol)(sodium salt)
- PGE2 d4
prostaglandin E2 d4
- PI
phosphatidylinositol
- PIP
phosphatidylinositolphosphate
- PS
phosphatidylserine
- PS(16:0/18:1 d31)
1-palmitoyl(d31)-2-oleoyl-sn-glycero-3-[phospho-L-serine] (sodium salt)
- PUFA
poly-unsaturated-fatty acid
- RA
rheumatoid arthritis
- RP18
reversed phase octadecyl silica
- RT
retention time
- RRT
Relative retention time
- RvD5
7S,17S-dihydroxy-docosa-5Z,8E,10Z,13Z,15E,19Z-hexaenoic acid
- SF
synovial fluid
- SM
sphingomyelin
- TG
triglyceride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL. A systems biology approach to synovial joint lubrication in health, injury, and disease, Wiley Interdiscip. Rev. Syst. Biol. Med. 2012;4:15–37. doi: 10.1002/wsbm.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- [3].Bird HA, Ring EF. Therapeutic value of arthroscopy. Ann. Rheum. Dis. 1978;37:78–79. doi: 10.1136/ard.37.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J .Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- [7].Serhan CN. Eicosanoids. In: Koopman MWJ, L W, editors. Arthitis and Allied Conditions: A Textbook of Rheumatology. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 515–535. [Google Scholar]
- [8].Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yacoubian S, Serhan CN. New endogenous anti-inflammatory and proresolving lipid mediators: implications for rheumatic diseases. Nat. Clin. Pract. Rheum. 2007;3:570–579. doi: 10.1038/ncprheum0616. [DOI] [PubMed] [Google Scholar]
- [10].Lima-Garcia JF, Dutra RC, da Silva K, Motta EM, Campos MM, Calixto JB. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Brit. J. Pharmacol. 2011;164:278–293. doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leeb BF, Sautner J, Andel I, Rintelen B. Intravenous application of omega-3 fatty acids in patients with active rheumatoid arthritis. The ORA-1 trial. An open pilot study, Lipids. 2006;41:29–34. doi: 10.1007/11745-006-5066-x. [DOI] [PubMed] [Google Scholar]
- [12].Hu S, Loo JA, Wong DT. Human body fluid proteome analysis. Proteomics. 2006;6:6326–6353. doi: 10.1002/pmic.200600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liao H, Wu J, Kuhn E, Chin W, Chang B, Jones MD, O’Neil S, Clauser KR, Karl J, Hasler F, Roubenoff R, Zolg W, Guild BC. Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:3792–3803. doi: 10.1002/art.20720. [DOI] [PubMed] [Google Scholar]
- [14].Goto M, Hanyu T, Yoshio T, Matsuno H, Shimizu M, Murata N, Shiozawa S, Matsubara T, Yamana S, Matsuda T. Intra-articular injection of hyaluronate (SI-6601D) improves joint pain and synovial fluid prostaglandin E2 levels in rheumatoid arthritis: a multicenter clinical trial. Clin. Exp. Rheumatol. 2001;19:377–383. [PubMed] [Google Scholar]
- [15].Huffman KM, Bowers JR, Dailiana Z, Huebner JL, Urbaniak JR, Kraus VB. Synovial fluid metabolites in osteonecrosis. Rheumatology (Oxford) 2007;46:523–528. doi: 10.1093/rheumatology/kel302. [DOI] [PubMed] [Google Scholar]
- [16].Fuchs B, Schiller J, Wagner U, Hantzschel H, Arnold K. The phosphatidylcholine/lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: investigations by 31P NMR and MALDI-TOF MS. Clin. Biochem. 2005;38:925–933. doi: 10.1016/j.clinbiochem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [17].Fuchs B, Bondzio A, Wagner U, Schiller J. Phospholipid compositions of sera and synovial fluids from dog, human and horse: a comparison by 31P-NMR and MALDI-TOF MS. J. Anim. Physiol. Anim. Nutr. (Berl.) 2009;93:410–422. doi: 10.1111/j.1439-0396.2008.00822.x. [DOI] [PubMed] [Google Scholar]
- [18].Prete PE, Gurakar-Osborne A, Kashyap ML. Synovial fluid lipids and apolipoproteins: a contemporary perspective. Biorheology. 1995;32:1–16. doi: 10.3233/bir-1995-32101. [DOI] [PubMed] [Google Scholar]
- [19].Hashimoto A, Hayashi I, Murakami Y, Sato Y, Kitasato H, Matsushita R, Iizuka N, Urabe K, Itoman M, Hirohata S, Endo H. Antiinflammatory mediator lipoxin A4 and its receptor in synovitis of patients with rheumatoid arthritis. J. Rheumatol. 2007;34:2144–2153. [PubMed] [Google Scholar]
- [20].de Grauw JC, van de Lest CH, van Weeren PR. A targeted lipidomics approach to the study of eicosanoid release in synovial joints. Arthritis Res. Ther. 2011;13:R123. doi: 10.1186/ar3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wise CM, White RE, Agudelo CA. Synovial fluid lipid abnormalities in various disease states: review and classification. Semin. Arthritis Rheum. 1987;16:222–230. doi: 10.1016/0049-0172(87)90024-2. [DOI] [PubMed] [Google Scholar]
- [22].Jain M, Petzold CJ, Schelle MW, Leavell MD, Mougous JD, Bertozzi CR, Leary JA, Cox JS. Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proc. Natl. Acad. Sci. USA. 2007;104:5133–5138. doi: 10.1073/pnas.0610634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Graessler J, Schwudke D, Schwarz PE, Herzog R, Shevchenko A, Bornstein SR. Top-down lipidomics reveals ether lipid deficiency in blood plasma of hypertensive patients. PLoS One. 2009;4:e6261. doi: 10.1371/journal.pone.0006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Giera M, Plossl F, Bracher F. Fast and easy in vitro screening assay for cholesterol biosynthesis inhibitors in the post-squalene pathway. Steroids. 2007;72:633–642. doi: 10.1016/j.steroids.2007.04.005. [DOI] [PubMed] [Google Scholar]
- [25].Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bird SS, Marur VR, Sniatynski MJ, Greenberg HK, Kristal BS. Lipidomics profiling by high-resolution LC-MS and high-energy collisional dissociation fragmentation: focus on characterization of mitochondrial cardiolipins and monolysocardiolipins. Anal. Chem. 2011;83:940–949. doi: 10.1021/ac102598u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee JY, Min HK, Moon MH. Simultaneous profiling of lysophospholipids and phospholipids from human plasma by nanoflow liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011;400:2953–2961. doi: 10.1007/s00216-011-4958-7. [DOI] [PubMed] [Google Scholar]
- [28].Sommer U, Herscovitz H, Welty FK, Costello CE. LC-MS-based method for the qualitative and quantitative analysis of complex lipid mixtures. J. Lipid Res. 2006;47:804–814. doi: 10.1194/jlr.M500506-JLR200. [DOI] [PubMed] [Google Scholar]
- [29].Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- [31].Yang R, Chiang N, Oh SF, Serhan CN. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr. Protoc. Immunol. 2011;14:Unit–14. doi: 10.1002/0471142735.im1426s95. Chapter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Borgeat P, Fruteau de Laclos B, Picard S, Drapeau J, Vallerand P, Corey EJ. Studies on the mechanism of formation of the 5S,12S-dihydroxy-6,8,10,14(E,Z,E,Z)-icosatetraenoic acid in leukocytes. Prostaglandins. 1982;23:713–724. doi: 10.1016/s0090-6980(82)80009-9. [DOI] [PubMed] [Google Scholar]
- [34].Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom. Rev. 2003;22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- [35].Blaas N, Schuurmann C, Bartke N, Stahl B, Humpf HU. Structural profiling and quantification of sphingomyelin in human breast milk by HPLC-MS/MS. J. Agric. Food Chem. 2011;59:6018–6024. doi: 10.1021/jf200943n. [DOI] [PubMed] [Google Scholar]
- [36].Retra K, Bleijerveld OB, van Gestel RA, Tielens AG, van Hellemond JJ, Brouwers JF. A simple and universal method for the separation and identification of phospholipid molecular species. Rapid Commun. Mass Spectrom. 2008;22:1853–1862. doi: 10.1002/rcm.3562. [DOI] [PubMed] [Google Scholar]
- [37].Welti R, Wang X, Williams TD. Electrospray ionization tandem mass spectrometry scan modes for plant chloroplast lipids. Anal. Biochem. 2003;314:149–152. doi: 10.1016/s0003-2697(02)00623-1. [DOI] [PubMed] [Google Scholar]
- [38].Lisa M, Cifkova E, Holcapek M. Lipidomic profiling of biological tissues using off-line two-dimensional high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A. 2011;1218:5146–5156. doi: 10.1016/j.chroma.2011.05.081. [DOI] [PubMed] [Google Scholar]
- [39].Cui Z, Thomas MJ. Phospholipid profiling by tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877:2709–2715. doi: 10.1016/j.jchromb.2009.06.034. [DOI] [PubMed] [Google Scholar]
- [40].Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom. Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- [41].Masoodi M, Mir AA, Petasis NA, Serhan CN, Nicolaou A. Simultaneous lipidomic analysis of three families of bioactive lipid mediators leukotrienes, resolvins, protectins and related hydroxy-fatty acids by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008;22:75–83. doi: 10.1002/rcm.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brügger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc. Natl. Acad. Sci. USA. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- [44].Liebisch G, Lieser B, Rathenberg J, Drobnik W, Schmitz G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim. Biophys. Acta. 2004;1686:108–117. doi: 10.1016/j.bbalip.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [45].Gao F, Tian X, Wen D, Liao J, Wang T, Liu H. Analysis of phospholipid species in rat peritoneal surface layer by liquid chromatography/electrospray ionization ion-trap mass spectrometry. Biochim. Biophys. Acta. 2006;1761:667–676. doi: 10.1016/j.bbalip.2006.03.022. [DOI] [PubMed] [Google Scholar]
- [46].Hsu FF, Turk J. Characterization of phosphatidylinositol, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate by electrospray ionization tandem mass spectrometry: a mechanistic study. J. Am. Soc. Mass Spectrom. 2000;11:986–999. doi: 10.1016/S1044-0305(00)00172-0. [DOI] [PubMed] [Google Scholar]
- [47].Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim. Biophys. Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gheorghe KR, Korotkova M, Catrina AI, Backman L, af Klint E, Claesson HE, Radmark O, Jakobsson PJ. Expression of 5-lipoxygenase and 15-lipoxygenase in rheumatoid arthritis synovium and effects of intraarticular glucocorticoids. Arthritis Res. Ther. 2009;11:R83. doi: 10.1186/ar2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Marcus AJ, Broekman MJ, Safier LB, Ullman HL, Islam N, Serhan CN, Rutherford LE, Korchak HM, Weissmann G. Formation of leukotrienes and other hydroxy acids during platelet-neutrophil interactions in vitro. Biochem. Biophys. Res. Commun. 1982;109:130–137. doi: 10.1016/0006-291x(82)91575-3. [DOI] [PubMed] [Google Scholar]
- [51].Dixon RAF, Diehl RE, Opas E, Rands E, Vickers PJ, Evans JF, Gillard JW, Miller DK. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- [52].Kowal-Bielecka O, Kowal K, Distler O, Gay S. Mechanisms of Disease: leukotrienes and lipoxins in scleroderma lung disease[mdash]insights and potential therapeutic implications. Nat. Clin. Pract. Rheum. 2007;3:43–51. doi: 10.1038/ncprheum0375. [DOI] [PubMed] [Google Scholar]
- [53].Kronke G, Reich N, Scholtysek C, Akhmetshina A, Uderhardt S, Zerr P, Palumbo K, Lang V, Dees C, Distler O, Schett G, Distler JH. The 12/15-lipoxygenase pathway counteracts fibroblast activation and experimental fibrosis. Ann. Rheum. Dis. 2012;71:1081–1087. doi: 10.1136/annrheumdis-2011-200745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.