Summary
Many types of cancer cells constitutively express major molecular chaperones at high levels. Recent findings demonstrate that specific depletion of individual chaperones, including various members of the Hsp70 family, small heat shock proteins, or VCP/p97, leads to activation of p53 pathway and subsequently triggers cellular senescence. Here we discuss a possibility that in cancer cells high levels of chaperones serve to keep the p53 signaling under control, thus allowing cancer cells to evade the default senescence and form tumors.
It is generally accepted that apoptosis plays the major role in control of cancer development. In fact, cells encounter multiple apoptotic stimuli during cancer progression, including activation of proapoptotic myc oncogene (1–3), anoikis, i.e. substrate detachment-induced apoptosis upon invasion and metastases (4), apoptotic stimuli of tumor milieu, like TNF, FAS, hypoxia, nutrient deprivation, etc. (5,6). Accordingly, it was suggested that the well-documented anti-apoptotic potential of the heat shock proteins may play a critical role in suppression of apoptosis in cancer cells (7,8). In line with this suggestion, recent research from many labs dissected multiple effects of the major heat shock proteins, Hsp72 and Hsp27, on various components of multiple apoptotic pathways (9–11), including Bax, JNK, FAS receptor, Smad, lysosome stability, and others. However, direct involvement of Hsps in suppression of cancer-related apoptosis has not been clearly confirmed. More recently, the attention of the cancer community has shifted towards a novel role of cell senescence in control of cancer development, and with this shift our view on the role of Hsps in cancer has also evolved towards appreciation of the major role of Hsps in regulation of the senescence program.
Cellular senescence was originally described as a limit to a number of divisions that a normal cell can undergo. For example, normal fibroblasts can divide about 60 times in culture before acquiring a specific “flat” morphology and becoming permanently growth arrested (12,13). Epithelial, endothelial or hematopoetic cells also have a limit on the number of divisions, which is usually below twenty (14–19). Originally it was thought that the replicative senescence is an ultimate result of the telomers shortening, but at present it became clear that senescence could be triggered by various types of DNA damage that results in accumulation of cell cycle inhibitors p16 and p21 (20–24). Senescence is a very complex program with multiple end points that include not only growth arrest, but also enlargement of cells, extensive vacuolization, repression and de-repression of certain sets of genes, secretion of various signaling molecules, inhibition of the heat shock response, and other manifestations.
The senescence program seems to represent one of the major breaks on cancer emergence. Indeed, limiting cell divisions seems to be a perfect way of preventing tumor growth (25). Another alternative to achieve the same goal is through activation of apoptosis. Mammalian cells appear to utilize both programs to counteract action of the major oncogenes. In fact, as counterintuitive, as it sounds overexpression of major oncogenes could either activate apoptosis, as seen with myc, or trigger senescence as seen with Ras, Her-2, PTEN, Raf, and others oncogenes of the Ras pathway (26–32).
The discovery that cancer cells could become senescent was quite unexpected since tumor cells by definition divide indefinitely. Nevertheless, being unrestrained by the replicative senescence, many cancer cells still could undergo senescence (so-called premature senescence) in response to activation of oncogenes (Fig. 1), e.g. Ras or Her-2, or to various DNA-damaging drugs, like doxorubicin (33,34). In fact, the major mechanism of action of many anti-cancer drugs seems to be activation of the premature senescence program (35,36). These findings demonstrate that the senescence program remains functional even after cancer transformation. Premature senescence in cancer cells depends upon activation of the cell cycle inhibitor p21, while p16 seems to be involved only in senescence of normal cells.
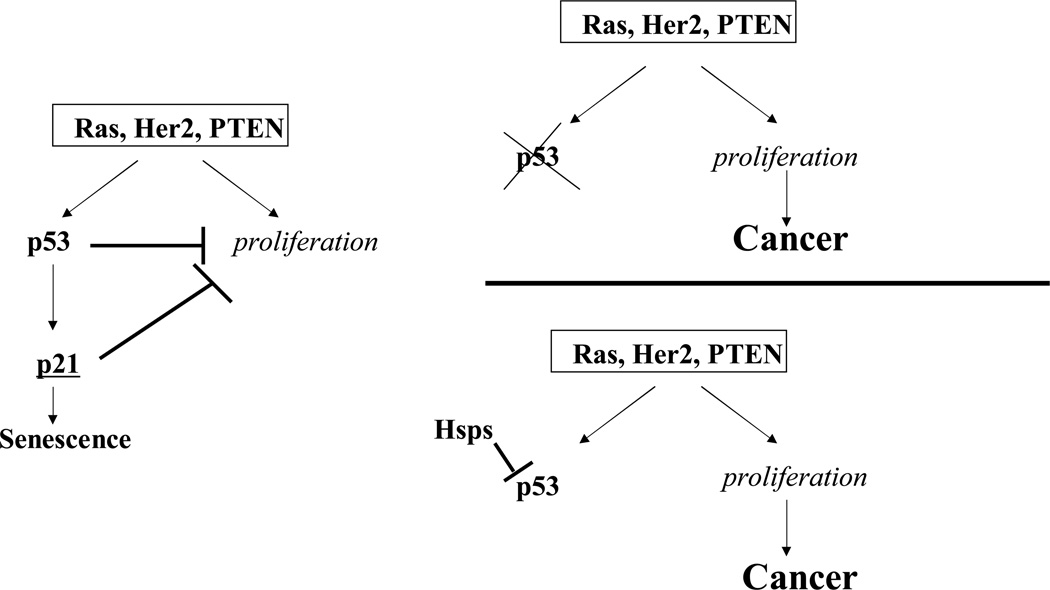
Fig. 1.
Hsps can suppress p53 and allow proliferation of cells with activated oncogenes. Oncogenes of Ras pathway activate two parallel pathways, including the proliferation response and the growth inhibitory p53 pathway. Normally p53 induces p21 and causes growth arrest and senescence, thus preventing cell proliferation. However, proliferating cells acquire mutations in the p53 pathway. Alternatively, high levels of Hsps inhibit p53 and allow cells to proliferate.
In general oncogenes of Ras pathway activate the senescence program via several mechanisms that involve activation of p53, the transcriptional activator of p21. For example, these oncogenes can activate the DNA damage response kinases ATM and ATR because of excessive activation of DNA replication forks, and these kinases phosphorylate and stabilize p53 (37). Another mechanism involves induction of p19ARF that inhibits the p53 ubiquitin ligase Hdm2, also leading to stabilization of p53 (38). In turn stabilization of p53 leads to its accumulation and activation. Since cancer cells have active oncogenes that can trigger cell senescence, in order to proliferate they have to acquire mechanisms that completely or partially suppress p21. Indeed, it is well known that mutations in p21 or mutations that result in disabling of p21 transcriptional regulator, p53, are extremely common in human cancers. However, in many cancer types the p53 pathway remains functional, and therefore alternative mechanisms of suppression of p21 must operate in these tumors.
How cancer cells with activated oncogenes and intact p53 pathway escape senescence? Cancer cells constitutively overexpress several major chaperones (39). It appears that these chaperones play an important role in suppressing the senescent program, thus allowing cancer cells to proliferate. A critical observation on the role of chaperones in suppression of cellular senescence was the finding that a specific downregulation of Hsp70-2, a member of Hsp70 family, leads to rapid senescence of various cancer cell lines (40,41). Hsp70-2 was originally described as a testis-specific chaperone, but more recently it became clear that it is widely expressed in a variety of cancer cell types (40,41). Depletion of this protein using siRNA approach caused permanent G1 arrest, cell enlargement and flattening typical for senescent cells. These alterations were associated with up-regulation of p53. Gene array analysis showed that expression of a large number of genes was significantly altered (up- or downregulated) upon Hsp70-2 depletion. The pattern of the gene expression alterations had a lot of similarities to that found in senescence caused by doxorubicin (40,42,43). One of the genes upregulated at both conditions was MIC-1, a cytokine of TGFβ family, which appeared to be critical for senescence. In fact, expression of siRNA against MIC-1 prevented development of flat morphology and other hallmarks of senescence after depletion of Hsp70-2. Overproduction of MIC-1 is controlled by p53. Therefore the primary effect of Hsp70-2 depletion seems to involve p53 activation, which in turn triggers the senescence program. It is likely that this process involves induction of p21, because it is a direct p53 transcriptional target. On the other hand, MIC-1 appears to serve as an additional critical p53-dependent co-stimulator of cell senescence. It is likely that MIC-1 is secreted from cells and further promotes the senescence program, thus providing both autocrine and, possibly, paracrine stimulation. Thus Hsp70-2 seems to play a role in keeping p53 pathway suppressed, and Hsp70-2 depletion leads to the abrogation of this control and reactivation of the default senescence program.
A distinct member of the Hsp70 family, the major inducible heat shock protein Hsp72 plays a similar function in cancer cells, though the ways of controlling the p53 pathway by Hsp70-2 and Hsp72 appear to be different (see below). Depletion of Hsp72 led to a strong activation of p53, induction of p21, cell cycle arrest at both G1 and G2 phases, appearance of acidic β-gal activity and other hallmarks of senescence (44). Previously, Hsp72 was shown to bind p53 (45,46) and retain it in cytoplasm, implying that depletion of Hsp72 would release p53 and allow its nuclear translocation and activation of the transcription targets. In addition, p53 was stabilized upon depletion of Hsp72, due to reduction of activity of the p53 ubiquitin ligase Hdm2. Defining the primary effect of Hsp72 depletion on p53 pathway is rather tricky since p53 and Hdm2 represent a negative feedback loop, where p53 induces Hdm2, while Hdm2 facilitate degradation of p53. However, it was shown that suppression of Hdm2 upon Hsp72 depletion is the primary event, since under these conditions inhibition of Hdm2 was seen even in p53 knockout cells (44).
Interestingly, though p53 and p21 clearly contributed to development of senescence in Hsp72-depleted cells, other pathways also appear to be involved since a significant senescence was seen after Hsp72 depletion in p53 knockout colon carcinoma HCT-116 cells. The p53-independent senescence pathway under these conditions was associated with the reduction of expression and increase in the inhibitory phosphorylation of the cdc2 kinase (44). In line with this finding, partial depletion of cdc2 also caused senescence in p53 knockout cells.
There are reports that yet another member of the Hsp70 family, i.e. mitochondrial chaperone Grp75 also could activate senescence pathway through the control of p53. Being the major mitochondrial chaperone, Grp75 shows a pan-cytosolic localization in normal cells. Upon immortalization, however, it changes its localization and moves into the perinuclear zone (47,48). Association of this protein with cell immortalization is reflected in its alternative name, mot-2 (mortalin). Interestingly, overexpression of mot-2 protein in C.elegans significantly extended the worm’s life span (49). Importantly, overexpression of mot-2 in normal human lung fibroblasts led to a temporal escape of fibroblasts from the replicative senescence, allowing extra 12–18 doublings (50). Effects of mot-2 on temporal suppression of senescence were associated with inhibition of p53. Mot-2 directly interacts with p53 and suppresses its activity by sequestering p53 in cytosol (51–53). Accordingly, disruption of mot-2 –binding to p53 by specific peptides leads to p53 translocation to nucleus and concurrent activation (54). Therefore, several major Hsp70 family members appear to be involved in the control of p53.
Members of the Hsp70 family are not unique in their ability to interfere with senescence, as depletion of Hsp27 in transformed cells, similarly led to activation of the senescence program. In fact, upon siRNA-mediated depletion of Hsp27 human colon tumor cells HCT-116 acquired all major hallmarks of senescence associated with activation of p53 and induction of p21 (55). Similar effects were seen upon depletion in HeLa cells of a distinct chaperone vcp/p97 that controls delivery of ubiquitinated substrates to proteasome. Vcp/p97 downregulation led to activation of p53 and subsequent p21 induction and cell cycle arrest accompanied by cell flattening, vacuolization, and enlargement (56).
As discussed previously, some chaperons could directly interact with p53 and suppress its activity. In addition, the fact that depletion of different chaperones results in activation of p53 and leads to senescence suggests that these effects could be mediated through the chaperones’ general involvement in protein folding and degradation. In fact, there is a possibility that depletion of individual chaperones may reduce a refolding/degradation capacity of cells, thus causing a build-up of abnormal polypeptides, proteotoxicity and subsequent activation of p53. In line with this suggestion, certain protein damaging stresses that cause a build-up of abnormal polypeptides, like heat shock or oxidative stress, lead to activation of p53 (57–59). However, surprisingly, targeted depletion of individual chaperones Hsp72 or Hsp27 in HCT-116 cells did not result in accumulation of abnormal protein species. Accordingly, no accumulation of oxidized or ubiquitinated proteins were detected (55). Ubiquitin-proteasome degradation and refolding of model substrates were normal, indicating that UPS and the chaperone machinery were not overwhelmed. Moreover, there was no activation of the heat shock response, suggesting the lack of the proteotoxic stress (55). Furthermore, depletion of either Hsp72 or Hsp27 had only minor effects on heat shock sensitivity of these cells, as judged by activation of apoptosis. Therefore, effects of depletion of individual chaperones on the p53 pathway appear to control this pathway by specific mechanisms, rather than through evoking general proteotoxic stress. Findings that distinct chaperones are involved in control of the p53 and possibly other senescence pathways may help to explain why chaperones are often overexpressed in a variety of cancers. In fact, high expression of Hsp72 and Hsp27 often correlates with the grade of tumors, drug resistance and overall poor prognosis. Similarly, Hsp70-2, mot-2 and vcp/p97 were reported to be highly expressed in various tumors, and with some tumors they could be used as prognostic factors (39). Therefore cancer cells in contrast to normal cells selectively overproduce these chaperones individually or together. The overproduction of chaperones in tumors could be achieved through various mechanisms, including overexpression of the major heat shock transcription factor Hsf1 (60,61), phosphorylation of Hsf1 by Akt kinase activated by certain oncogenes (62), expression of a splicing isoform of p63 (63) and probably other mechanisms.
What could be the specific physiological significance of increased chaperone expression in cancers? As mentioned above, activation of certain oncogenes (e.g. Ras, Her-2 or PTEN mutation) in normal cells stimulate p53 and forces cells to senesce, which serves as an important defense mechanism against cancer transformation. Depletion of various chaperones triggers senescence suggesting that chaperones serve as endogenous suppressors of a latent senescence program. In other words, in many cancers various chaperones keep p53 under control, thus allowing cancer transformation to occur (Fig. 1). It is possible that tumors accumulate chaperones as a means to avoid the oncogene-induced senescence. This mechanism of evading activation of the senescence program in tumors could be an alternative to acquiring mutations in p53 pathway, which takes place in a large fraction of tumors. All together these data imply that heat shock proteins could serve as new important targets for anticancer therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2(10):764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 2.Pelengaris S, Rudolph B, Littlewood T. Action of Myc in vivo - proliferation and apoptosis [Review] Opinion in Genetics & Development. 2000;10(1):100–105. doi: 10.1016/s0959-437x(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 3.Prendergast GC. Mechanisms of apoptosis by c-Myc. Oncogene. 1999;18(19):2967–2987. doi: 10.1038/sj.onc.1202727. [DOI] [PubMed] [Google Scholar]
- 4.Valentijn AJ, Zouq N, Gilmore AP. Anoikis. Biochem Soc Trans. 2004;32(Pt3):421–425. doi: 10.1042/BST0320421. [DOI] [PubMed] [Google Scholar]
- 5.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2(8):589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 6.Zhang GJ, Kimijima I, Tsuchiya A, Abe R. THE ROLE OF BCL-2 EXPRESSION IN BREAST CARCINOMAS (REVIEW) [Review] Oncology Reports. 1998;5(5):1211–1216. doi: 10.3892/or.5.5.1211. [DOI] [PubMed] [Google Scholar]
- 7.Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92(19):1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- 8.Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23(16):2907–2918. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- 9.Beere HM. Death versus survival: functional interaction between the apoptotic and stressinducible heat shock protein pathways. J Clin Invest. 2005;115(10):2633–2639. doi: 10.1172/JCI26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beere HM. "The stress of dying": the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117(Pt 13):2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- 11.Gabai VL, Sherman MY. Invited review: Interplay between molecular chaperones and signaling pathways in survival of heat shock. J Appl Physiol. 2002;92(4):1743–1748. doi: 10.1152/japplphysiol.01101.2001. [DOI] [PubMed] [Google Scholar]
- 12.Hahn WC. Telomere and telomerase dynamics in human cells. Curr Mol Med. 2005;5(2):227–231. doi: 10.2174/1566524053586572. [DOI] [PubMed] [Google Scholar]
- 13.Stewart SA, Weinberg RA. Telomeres: cancer to human aging. Annu Rev Cell Dev Biol. 2006;22:531–557. doi: 10.1146/annurev.cellbio.22.010305.104518. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Goligorsky MS. Premature senescence of endothelial cells: Methusaleh's dilemma. Am J Physiol Heart Circ Physiol. 2006;290(5):H1729–H1739. doi: 10.1152/ajpheart.01103.2005. [DOI] [PubMed] [Google Scholar]
- 15.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66(2):286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40(12):926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Sherr CJ, Bertwistle D, W DENB, Kuo ML, Sugimoto M, Tago K, Williams RT, Zindy F, Roussel MF. p53-Dependent and -independent functions of the Arf tumor suppressor. Cold Spring Harb Symp Quant Biol. 2005;70:129–137. doi: 10.1101/sqb.2005.70.004. [DOI] [PubMed] [Google Scholar]
- 18.Piacibello W, Gammaitoni L, Pignochino Y. Proliferative senescence in hematopoietic stem cells during ex-vivo expansion. Folia Histochem Cytobiol. 2005;43(4):197–202. [PubMed] [Google Scholar]
- 19.Chen J. Senescence of hematopoietic stem cells and bone marrow failure. Int J Hematol. 2005;82(3):190–195. doi: 10.1532/IJH97.05094. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs JJ, de Lange T. p16INK4a as a second effector of the telomere damage pathway. Cell Cycle. 2005;4(10):1364–1368. doi: 10.4161/cc.4.10.2104. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37(5):961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Sharpless NE. Ink4a/Arf links senescence and aging. Exp Gerontol. 2004;39(11–12):1751–1759. doi: 10.1016/j.exger.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Roninson IB. Oncogenic functions of tumour suppressor p21(Waf1/Cip1/Sdi1): association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett. 2002;179(1):1–14. doi: 10.1016/s0304-3835(01)00847-3. [DOI] [PubMed] [Google Scholar]
- 24.Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63(11):2705–2715. [PubMed] [Google Scholar]
- 25.Parkinson EK, Munro J, Steeghs K, Morrison V, Ireland H, Forsyth N, Fitzsimmons S, Bryce S. Replicative senescence as a barrier to human cancer. Biochem Soc Trans. 2000;28(2):226–233. doi: 10.1042/bst0280226. [DOI] [PubMed] [Google Scholar]
- 26.Sebastian T, Johnson PF. Stop and go: anti-proliferative and mitogenic functions of the transcription factor C/EBPbeta. Cell Cycle. 2006;5(9):953–957. doi: 10.4161/cc.5.9.2733. [DOI] [PubMed] [Google Scholar]
- 27.Benanti JA, Galloway DA. The normal response to RAS: senescence or transformation? Cell Cycle. 2004;3(6):715–717. [PubMed] [Google Scholar]
- 28.Mason DX, Jackson TJ, Lin AW. Molecular signature of oncogenic ras-induced senescence. Oncogene. 2004;23(57):9238–9246. doi: 10.1038/sj.onc.1208172. [DOI] [PubMed] [Google Scholar]
- 29.Peeper DS, Dannenberg JH, Douma S, te Riele H, Bernards R. Escape from premature senescence is not sufficient for oncogenic transformation by Ras. Nat Cell Biol. 2001;3(2):198–203. doi: 10.1038/35055110. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436(7051):725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen CL, Gardie B, Yaswen P, Stampfer MR. Raf-1-induced growth arrest in human mammary epithelial cells is p16-independent and is overcome in immortal cells during conversion. Oncogene. 2002;21(41):6328–6339. doi: 10.1038/sj.onc.1205780. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12(19):2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, Roninson IB. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 1999;18(34):4808–4818. doi: 10.1038/sj.onc.1203078. [DOI] [PubMed] [Google Scholar]
- 34.Chang BD, Broude EV, Dokmanovic M, Zhu H, Ruth A, Xuan Y, Kandel ES, Lausch E, Christov K, Roninson IB. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999;59(15):3761–3767. [PubMed] [Google Scholar]
- 35.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432(7015):307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 36.Narita M, Lowe SW. Executing cell senescence. Cell Cycle. 2004;3(3):244–246. [PubMed] [Google Scholar]
- 37.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d'Adda di Fagagna F. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444(7119):638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 38.Bihani T, Mason DX, Jackson TJ, Chen SC, Boettner B, Lin AW. Differential oncogenic Ras signaling and senescence in tumor cells. Cell Cycle. 2004;3(9):1201–1207. [PubMed] [Google Scholar]
- 39.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31(3):164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Rohde M, Daugaard M, Jensen MH, Helin K, Nylandsted J, Jaattela M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005;19(5):570–582. doi: 10.1101/gad.305405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daugaard M, Jaattela M, Rohde M. Hsp70-2 is required for tumor cell growth and survival. Cell Cycle. 2005;4(7):877–880. doi: 10.4161/cc.4.7.1838. [DOI] [PubMed] [Google Scholar]
- 42.Chang BD, Swift ME, Shen M, Fang J, Broude EV, Roninson IB. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc Natl Acad Sci U S A. 2002;99(1):389–394. doi: 10.1073/pnas.012602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang BD, Watanabe K, Broude EV, Fang J, Poole JC, Kalinichenko TV, Roninson IB. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc Natl Acad Sci U S A. 2000;97(8):4291–4296. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaglom JA, Gabai VL, Sherman MY. High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res. 2007;67:2373–2381. doi: 10.1158/0008-5472.CAN-06-3796. [DOI] [PubMed] [Google Scholar]
- 45.Nihei T, Sato N, Takahashi S, Ishikawa M, Sagae S, Kudo R, Kikuchi K, Inoue A. Demonstration of selective protein complexes of p53 with 73 kDa heat shock cognate protein, but not with 72 kDa heat shock protein in human tumor cells. Cancer Lett. 1993;73(2–3):181–189. doi: 10.1016/0304-3835(93)90262-8. [DOI] [PubMed] [Google Scholar]
- 46.Ehrhart JC, Duthu A, Ullrich S, Appella E, May P. Specific interaction between a subset of the p53 protein family and heat shock proteins hsp72/hsc73 in a human osteosarcoma cell line. Oncogene. 1988;3(5):595–603. [PubMed] [Google Scholar]
- 47.Wadhwa R, Akiyama S, Sugihara T, Reddel RR, Mitsui Y, Kaul SC. Genetic differences between the pancytosolic and perinuclear forms of murine mortalin. Exp Cell Res. 1996;226(2):381–386. doi: 10.1006/excr.1996.0239. [DOI] [PubMed] [Google Scholar]
- 48.Wadhwa R, Takano S, Mitsui Y, Kaul SC. NIH 3T3 cells malignantly transformed by mot-2 show inactivation and cytoplasmic sequestration of the p53 protein. Cell Res. 1999;9(4):261–269. doi: 10.1038/sj.cr.7290025. [DOI] [PubMed] [Google Scholar]
- 49.Yokoyama K, Fukumoto K, Murakami T, Harada S, Hosono R, Wadhwa R, Mitsui Y, Ohkuma S. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002;516(1–3):53–57. doi: 10.1016/s0014-5793(02)02470-5. [DOI] [PubMed] [Google Scholar]
- 50.Kaul SC, Yaguchi T, Taira K, Reddel RR, Wadhwa R. Overexpressed mortalin (mot-2)/mthsp70/GRP75 and hTERT cooperate to extend the in vitro lifespan of human fibroblasts. Exp Cell Res. 2003;286(1):96–101. doi: 10.1016/s0014-4827(03)00101-0. [DOI] [PubMed] [Google Scholar]
- 51.Wadhwa R, Takano S, Robert M, Yoshida A, Nomura H, Reddel RR, Mitsui Y, Kaul SC. Inactivation of tumor suppressor p53 by mot-2, a hsp70 family member. J Biol Chem. 1998;273(45):29586–29591. doi: 10.1074/jbc.273.45.29586. [DOI] [PubMed] [Google Scholar]
- 52.Wadhwa R, Yaguchi T, Hasan MK, Mitsui Y, Reddel RR, Kaul SC. Hsp70 family member, mot-2/mthsp70/GRP75, binds to the cytoplasmic sequestration domain of the p53 protein. Exp Cell Res. 2002;274(2):246–253. doi: 10.1006/excr.2002.5468. [DOI] [PubMed] [Google Scholar]
- 53.Kaul SC, Aida S, Yaguchi T, Kaur K, Wadhwa R. Activation of wild type p53 function by its mortalin-binding, cytoplasmically localizing carboxyl terminus peptides. J Biol Chem. 2005;280(47):39373–39379. doi: 10.1074/jbc.M500022200. [DOI] [PubMed] [Google Scholar]
- 54.Wadhwa R, Sugihara T, Yoshida A, Nomura H, Reddel RR, Simpson R, Maruta H, Kaul SC. Selective toxicity of MKT-077 to cancer cells is mediated by its binding to the hsp70 family protein mot-2 and reactivation of p53 function. Cancer Res. 2000;60(24):6818–6821. [PubMed] [Google Scholar]
- 55.O'Callahan C, Gabai VL, Sherman MY. Protection from the doxorubicin toxicity exposes the role of Hsp27 in suppression of cellular senescence. Cancer Res. 2007 submitted. [Google Scholar]
- 56.Wojcik C, Yano M, DeMartino GN. RNA interference of valosin-containing protein (VCP/p97) reveals multiple cellular roles linked to ubiquitin/proteasome-dependent proteolysis. J Cell Sci. 2004;117(Pt 2):281–292. doi: 10.1242/jcs.00841. [DOI] [PubMed] [Google Scholar]
- 57.Miyakoda M, Suzuki K, Kodama S, Watanabe M. Activation of ATM and phosphorylation of p53 by heat shock. Oncogene. 2002;21(7):1090–1096. doi: 10.1038/sj.onc.1205196. [DOI] [PubMed] [Google Scholar]
- 58.McNeill-Blue C, Wetmore BA, Sanchez JF, Freed WJ, Merrick BA. Apoptosis mediated by p53 in rat neural AF5 cells following treatment with hydrogen peroxide and staurosporine. Brain Res. 2006;1112(1):1–15. doi: 10.1016/j.brainres.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Das KC, Dashnamoorthy R. Hyperoxia activates the ATR-Chk1 pathway and phosphorylates p53 at multiple sites. Am J Physiol Lung Cell Mol Physiol. 2004;286(1):L87–L97. doi: 10.1152/ajplung.00203.2002. [DOI] [PubMed] [Google Scholar]
- 60.Tang D, Khaleque MA, Jones EL, Theriault JR, Li C, Wong WH, Stevenson MA, Calderwood SK. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;10(1):46–58. doi: 10.1379/CSC-44R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoang AT, Huang J, Rudra-Ganguly N, Zheng J, Powell WC, Rabindran SK, Wu C, Roy-Burman P. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol. 2000;156(3):857–864. doi: 10.1016/S0002-9440(10)64954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khaleque MA, Bharti A, Sawyer D, Gong J, Benjamin IJ, Stevenson MA, Calderwood SK. Induction of heat shock proteins by heregulin beta1 leads to protection from apoptosis and anchorage-independent growth. Oncogene. 2005;24(43):6564–6573. doi: 10.1038/sj.onc.1208798. [DOI] [PubMed] [Google Scholar]
- 63.Wu G, Osada M, Guo Z, Fomenkov A, Begum S, Zhao M, Upadhyay S, Xing M, Wu F, Moon C, Westra WH, Koch WM, Mantovani R, Califano JA, Ratovitski E, Sidransky D, Trink B. DeltaNp63alpha up-regulates the Hsp70 gene in human cancer. Cancer Res. 2005;65(3):758–766. [PubMed] [Google Scholar]