Abstract
Angiogenesis plays a crucial role during tumorigenesis and much progress has been recently made in elucidating the role of VEGF and other growth factors in the regulation of angiogenesis. Recently, microRNAs (miRNAs) have been shown to modulate a variety of physiogical and pathological processes. We identified a set of differentially expressed miRNAs in microvascular endothelial cells co-cultured with tumour cells. Unexpectedly, most miRNAs were derived from tumour cells, packaged into microvesicles (MVs), and then directly delivered to endothelial cells. Among these miRNAs, we focused on miR-9 due to the strong morphological changes induced in cultured endothelial cells. We found that exogenous miR-9 effectively reduced SOCS5 levels, leading to activated JAK-STAT pathway. This signalling cascade promoted endothelial cell migration and tumour angiogenesis. Remarkably, administration of anti-miR-9 or JAK inhibitors suppressed MV-induced cell migration in vitro and decreased tumour burden in vivo. Collectively, these observations suggest that tumour-secreted miRNAs participate in intercellular communication and function as a novel pro-angiogenic mechanism.
Keywords: angiogenesis, endothelium, JAK-STAT, microRNA, tumour
Introduction
Angiogenesis is a key requirement for tumour growth (Folkman, 1995; Chung et al, 2010). Much evidence indicates that VEGF is a major regulator of angiogenesis (Chung and Ferrara, 2011). Various inhibitors of the VEGF signalling pathway have been developed and approved for treatment of cancer or intraocular neovascular disorders (Ferrara et al, 2007; Ebos and Kerbel, 2011). Recently, microRNAs (miRNAs) have been shown to provide a new layer of regulation of gene expression in many physiological and pathological processes, including angiogenesis (Wang and Olson, 2009; Anand and Cheresh, 2011; Small and Olson, 2011; Weis and Cheresh, 2011).
miRNAs are a family of noncoding RNAs ∼22 nt in length, which can suppress gene expression by pairing to the 3′ untranslated regions (UTRs) of target mRNAs (Bartel, 2004, 2009). Accumulating evidence indicates that endothelial miRNAs are involved in developmental and tumour angiogenesis, thus providing opportunities for further control of the tumour vasculature. Ablation of the miRNA precursor-processing enzyme Dicer in endothelium reduces tumour angiogenesis and progression (Suarez et al, 2008), suggesting that specific miRNAs in endothelial cells regulate angiogenic processes. In this regard, miR-296 and miR-132, both induced by VEGF, have been proposed to facilitate angiogenic switching (Wurdinger et al, 2008; Anand et al, 2010). It would be of considerable interest to identify VEGF-independent angiomirs, which might serve as anti-angiogenic targets in combination with current anti-VEGF therapies.
We performed a quantitative PCR-based miRNA screen and identified a set of differentially expressed miRNAs in microvascular endothelial cells co-cultured with tumour cells. Unexpectedly, most miRNAs were derived from tumour cells, packaged into microvesicles (MVs), and then directly delivered to endothelial cells. Tumour secreted miRNAs were functional in recipient endothelial cells as assessed by the ability to promote migration and neovascularization. Thus, our data support a novel cellular communication involving directional transfer of miRNAs during tumour angiogenesis.
Results
Tumour cells induce upregulation of miRNAs in endothelial cells
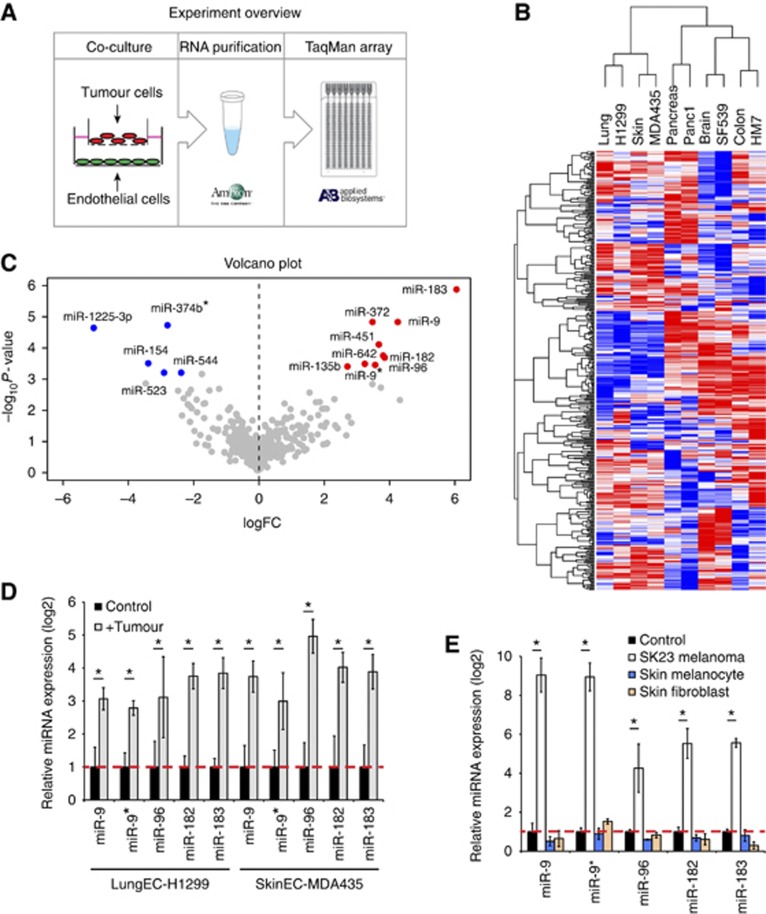
To mimic some of the activation steps in angiogenesis, we established tumour-endothelial co-cultures, in which tumour and endothelial cells are separated by a transwell membrane insert (Figure 1A). We paired five human tumour cell lines (H1299, non-small cell lung cancer (NSCLC); MDA435, melanoma; Panc1, pancreatic cancer; SF-539, glioblastoma; HM7, colorectal cancer) with microvascular endothelial cells isolated from normal tissues matching each tumour type examined (Figure 1B). After 24 h, we profiled endothelial miRNAs in the presence or absence of tumour cells using Taqman micro fluidic cards (Figure 1A). Hierarchical clustering indicated that different endothelial cells have surprisingly distinct miRNA expression (Figure 1B), while tumour cell stimulation only affected a subset of miRNAs (Supplementary Table 1). Volcano plot of rank products analysis showed a group of most significantly differentially expressed miRNAs among the five endothelial cell types (Figure 1C), the majority of which are highly conserved across species and were confirmed by quantitative RT–PCR (Figure 1D; Supplementary Figure 1A). Importantly, these miRNAs appeared to be selectively activated by tumour cells, since SK23 melanoma cells, but not normal skin melanocytes or fibroblasts, promoted miRNA expression (Figure 1E).
Figure 1.
Tumour cells induce upregulation of miRNAs in endothelial cells. (A) Experimental overview. (B) Hierarchical clustering of miRNAs in control or tumour-stimulated endothelial cells. (C) Volcano plot of miRNA changes across five pairs of tumour-endothelial cells. (D) Verification of tumour-induced miRNAs by quantitative RT–PCR. Lung endothelial cells co-cultured with H1299 and skin endothelial cells co-cultured with MDA435 for 24 h are shown. The experiment was repeated three times. *P<0.05, Student’s t-test. (E) Quantitative PCR of miRNAs in skin microvesicular endothelial cells stimulated with SK23 melanoma cells, normal skin melanocytes or fibroblasts. The experiment was repeated twice. *P<0.05, Student’s t-test.
To rule out the possibility that our observations may be restricted to a few tumour cell lines, we screened a large panel of NSCLC lines and found that the vast majority were able to induce miRNA changes in endothelial cells, although to different degrees (Supplementary Figure 1B). Furthermore, human umbilical vein endothelial cells (HUVECs), when co-cultured with human or mouse tumour cells, also exhibited miRNA changes comparable to those observed in microvascular endothelial cells (Supplementary Figure 1C). Therefore, our observations are consistent with a general endothelial miRNA upregulation induced by tumour cells, which may play a role in tumour angiogenesis.
Tumour-derived miRNAs are delivered into endothelial cells via MVs
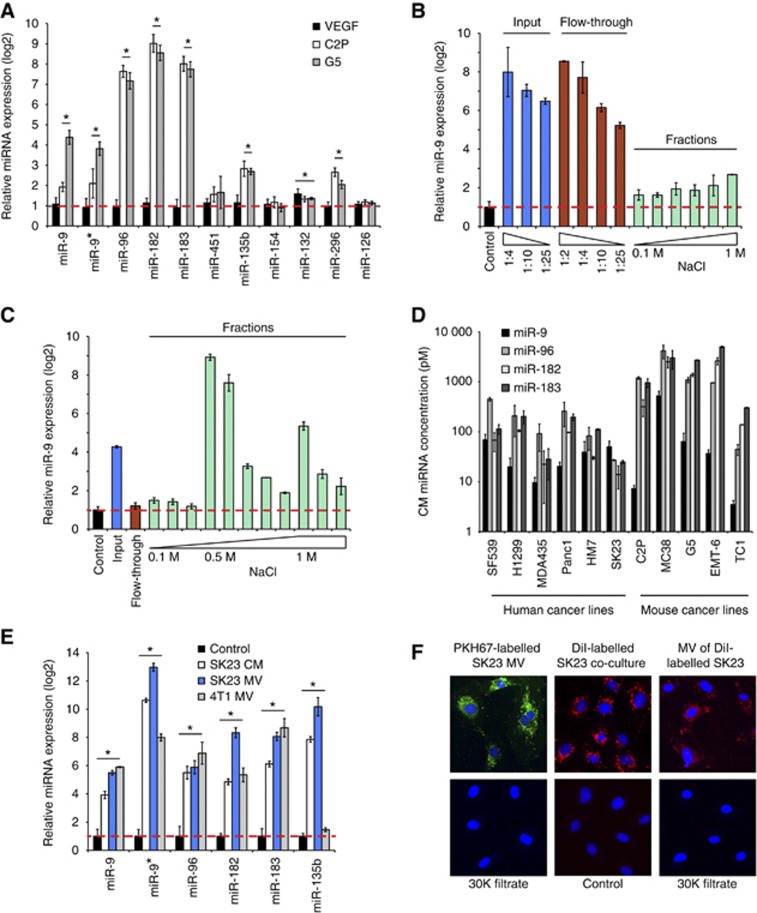
To follow-up our observations with tumour-endothelial co-cultures, we tested conditioned media from several tumour cell lines and found that they also resulted in enhanced expression of miRNAs in endothelial cells (Supplementary Figure 2A), raising the possibility that soluble factors are responsible for this effect. Recombinant VEGF had no effect on the miRNAs identified in this study. Additionally, wild-type (C2P) and VEGF-null (G5) mouse fibrosarcoma cells (Dong et al, 2004) had similar abilities to induce these miRNAs in HUVECs (Figure 2A).
Figure 2.
Tumour-derived miRNAs are delivered to endothelial cells via microvesicles. (A) Quantitative PCR of miRNAs in HUVECs stimulated with VEGF (50 ng/ml), or co-cultured with parental (C2P) or VEGF null (G5) fibrosarcoma cells. *P<0.05, Student’s t-test. (B) Quantitative PCR of miR-9 in HUVECs co-cultured with different fractions of SK23 conditioned medium after anion exchange chromatography. The experiment was repeated twice. (C) Quantitative PCR of miR-9 in HUVECs co-cultured with different fractions of SK23 conditioned medium after heparin-sepharose chromatography. The experiment was repeated twice. (D) Conditioned medium was collected from each cancer cell line and miRNAs concentration was measured by quantitative RT–PCR against spike-in synthetic oligonucleotides. (E) Quantitative PCR of miRNAs in HUVECs treated with SK23 conditioned medium, SK23 microvesicles or 4T1 microvesicles. Three biological replicates were used for each condition. *P<0.05, Student’s t-test. (F) SK23 microvesicles were labelled with PKH67 (green), concentrated with Amicon filters (30KDa) and incubated with HUVECs for 24 hours (left panel). SK23 cells were labelled with DiI (red) and co-cultured with HUVECs (middle panel). Purified microvesicles from DiI-labelled SK23 were concentrated with Amicon filters (30KDa) and added to HUVECs (right panel). The experiment was repeated twice.
We initially considered the possibility that induction of miRNAs in endothelial cells might be mediated by growth factor(s) other than VEGF. We tested several angiogenic factors (bFGF, TNFα, HGF, PDGF-A, PDGF-B, PDGF-C and angiopoietin-1) and found that none of these was able to alter the expression of these miRNAs (Supplementary Figure 2B).
We also undertook an initial biochemical characterization of such activity. We chose SK23 tumour cells as a source because they elicited a strong induction of endothelial miRNAs (Figure 1E) and could also be easily maintained in serum-free medium. The inducing activity was completely retained by Amicon filters with up to 50 kDa MW cutoff (Supplementary Figure 2C), which indicates that this factor(s) has a relatively large molecular mass. We then subjected the tumour cell conditioned media to anion exchange chromatography on a Hi-Trap Q column, but the miRNA inducing activity did not bind to the column and was almost completely recovered in the flow-through (Figure 2B). In contrast, a heparin-sepharose (HS) column bound the activity, which was eluted by 0.5 M NaCl (Figure 2C). These results prompted us to hypothesize that endothelial miRNAs could be stimulated by tumour-derived soluble factors with heparin-binding properties.
However, in conflict with this hypothesis, we did not detect any significant changes in endothelial pri-miRNA or pre-miRNA in response to conditioned medium or HS fractions. Therefore, to determine the source of upregulated miRNAs, we knocked down Drosha, which is required for miRNA biogenesis. Surprisingly, Drosha knockdown in tumour cells impaired miRNA induction (Supplementary Figure 2D). Accordingly, when we individually inhibited miR-9 or miR-183 in SK23 melanoma cells, tumour cells were less capable of inducing endothelial miR-9 or miR-183, respectively (Supplementary Figure 2D). The level of inhibition induced by siDrosha or antagomirs on endothelial miRNAs was ∼50%. This incomplete inhibition may be due to the residual miRNAs in knockdown conditions. These results suggest that the observed increases in endothelial miRNAs largely originate within tumour cells in the co-culture system. Indeed, we were able to detect miRNAs in tumour conditioned media by RT–PCR (Figure 2D). Considering that our data suggest these miRNAs are in relatively large molecular weight complexes, we hypothesized that tumour-secreted MVs might be the carriers (Balaj et al, 2011; Lee et al, 2011; Chen et al, 2012), transporting miRNAs into endothelial cells. In agreement with this hypothesis, isolated MVs from either SK23 or 4T1 cells induced miRNAs in endothelial cells (Figure 2E). To further verify that the miRNAs were indeed assembled within the MVs, we treated purified MVs with RNase and found that miRNAs were substantially protected from RNase digestion. Pre-incubation with 0.5% SDS, but not with proteinase K, significantly reduced miRNA levels after RNase treatment (Supplementary Figure 2E). To determine whether MVs are truly taken up by recipient endothelial cells, we labelled isolated MVs with PKH67 green fluorescence and visualized them while internalized into endosome-like structures by HUVECs (Figure 2F, left panel). Similar results were observed when we incubated SK23 cells with DiI and co-cultured the labelled cells with HUVECs (Figure 2F, middle panel). Furthermore, we verified that purified MVs from DiI-labelled tumour cells are also transferred into HUVECs (Figure 2F, right panel). Following miR-9 knockdown in SK23 cells, we observed a concurrent decrease in miR-9 levels in purified MVs or HUVECs co-cultured with MVs (Supplementary Figure 2F). Taken together, these data demonstrate that miRNAs are secreted by tumour cells and are delivered into endothelial cells via a specific population of MVs that bind heparin.
Tumour secreted miR-9 promotes endothelial cell migration
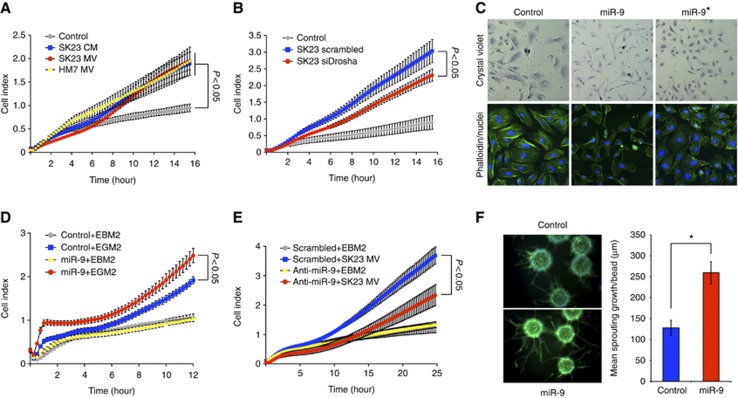
To determine whether MVs carrying miRNA contribute to angiogenesis, we assessed endothelial cell migration in real time using the xCELLigence system. Consistent with previous reports (Skog et al, 2008; Grange et al, 2011), both SK-23 tumour conditioned media and isolated MVs led to enhanced migration of HUVECs (Figure 3A). Following knockdown of Drosha in tumour cells, tumour-derived MVs showed reduced ability to promote endothelial cell migration, indicating that miRNAs in MVs play an important role in this process (Figure 3B).
Figure 3.
Tumour secreted miR-9 promotes endothelial cell migration. (A) HUVEC migration in response to SK23 conditioned medium, SK23 microvesicles or HM7 microvesicles was analysed by xCELLigence system. Each group had three biological replicates. The experiment was repeated twice. For statistical analysis, ANOVA was performed followed by Tukey’s post-test to compare two groups. (B) SK23 cells were transfected with scrambled or Drosha siRNAs and purified microvesicles were tested to induce HUVEC migration. Each group had five biological replicates. (C) HUVECs were transfected with miR-9 or miR-9* and stained by crystal violet or FITC-phalloidin. (D) Cell migration of HUVECs overexpressing miR-9. Each group had three biological replicates. The experiment was repeated three times. ANOVA was performed followed by Tukey’s post-test to compare two groups (P<0.05). (E) HUVECs were transfected with miR-9 antagomirs and cell migration stimulated by SK23 microvesicles was assessed. Each group had three biological replicates. The experiment was repeated twice. ANOVA was performed followed by Tukey’s post-test to compare two groups (P<0.05). (F) Phalloidin staining of HUVEC sprouts and quantification of mean sprouting length per bead. *P<0.05, Student’s t-test.
To identify functionally important miRNAs, we overexpressed each miRNA found to be upregulated in endothelial cells and determined its ability to affect endothelial cell motility. We found that the vast majority of miRNAs had no obvious effect (data not shown). However, miR-9 induced striking morphological changes in HUVECs, including elongated cellular shape, fewer stress fibres and increased lamellipodia at the cell edge (Figure 3C). These observations suggested a potential role for miR-9 in modulating endothelial cell motility. To explore these events in greater details, we performed gain- and loss-of-function studies in cell migration assays. We found that overexpression of miR-9 in HUVECs results in enhanced cell motility (Figure 3D), while miR-9 knockdown significantly inhibits MV-induced cell migration (Figure 3E). Additionally, inhibition of miR-9 in SK23 cells partially suppressed migration of co-cultured endothelial cells in transwell assays (Supplementary Figure 3A). We also evaluated the role of miR-9 in an angiogenic sprouting assay. Expression of miR-9 markedly increased sprouting angiogenesis of HUVECs, suggesting an increase in the number of tip cells and/or branching activities of endothelial cells (Figure 3F). We tested the hypothesis that miR-9 may indirectly affect endothelial cells by regulating tumour cell proliferation. However, we found no changes in tumour cell growth in the presence of anti-miR-9 or siDrosha (Supplementary Figure 3B). Also, the composition of the MVs did not appear to be markedly affected by miR-9 inhibition, as assessed by antibody arrays to evaluate a panel of angiogenesis-related proteins (Supplementary Figure 3C). Interestingly, angiogenic factors such as IL8 and VEGF were readily detectable in purified SK23 MVs, which may account for the incomplete inhibition of endothelial cell migration by anti-miR-9. These findings indicate that tumour cells activate endothelial cell migration at least in part through miR-9.
miR-9 induces tumour angiogenesis in vivo
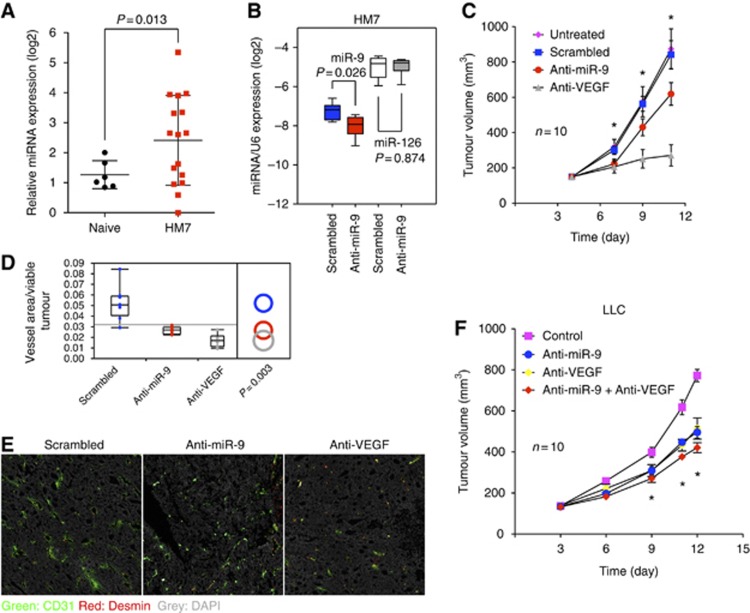
Focusing on miR-9, we conducted a series of in-vivo experiments to determine whether this miRNA affects tumour angiogenesis. Initially, we extracted miRNAs from plasma of naïve or tumour-bearing mice. Subcutaneous implantation of HM7 human colorectal tumours resulted in elevated miR-9 levels in the plasma, suggesting that tumour cells also secrete miRNAs in vivo (Figure 4A). Next, we intratumorally injected miR-9 antagomirs, which specifically decreased miR-9 but not miR-126 in HM7 tumours (Figure 4B). We observed a statistically significant delay in tumour growth (Figure 4C), in spite of the fact that the knockdown of miR-9 was only ∼50%. Tumour angiogenesis, as assessed by CD31 immunostaining, was significantly decreased in the presence of miR-9 antagomirs (Figure 4D and E). To specifically quantify functional tumour vasculature, we performed FITC-lectin perfusion before collecting tumour tissues. Both the numbers of perfused vessels and FITC-labelled vessel areas were significantly reduced in anti-VEGF or anti-miR-9 treated tumours, suggesting that the function of tumour vessels was compromised (Supplementary Figure 4A). We found that inhibition of miR-9 significantly reduces tumour cell proliferation without perturbing apoptosis, as assessed by Ki67 and cleaved caspase3 staining, respectively (Supplementary Figure 4B). A likely explanation for the decreased tumour cell proliferation is inhibition of tumour angiogenesis. Indeed targeting miR-9 did not affect HM7 growth in cell culture (Supplementary Figure 3B). The ability of miR-9 antagomirs to inhibit tumour growth was confirmed in the LLC, a murine lung carcinoma model that, in agreement with previous studies (Shojaei et al, 2007), was only moderately responsive to anti-VEGF treatment (Figure 4F). Interestingly, the combination of miR-9 antagomirs with anti-VEGF showed a clear trend towards reduced tumour progression compared to single agent treatment, although it did not achieve statistical significance (P=0.06). We conclude that miR-9 plays a role in promoting tumour angiogenesis in vivo.
Figure 4.
miR-9 induces tumour angiogenesis in vivo. (A) Quantification of miR-9 in plasma of naïve (n=6) and HM7 tumour bearing mice (n=16). (B) Quantitative PCR of miR-9 and miR-126 in HM7 tumours treated with miR-9 or control antagomirs, six mice per group. (C) Mice (n=10 per group) bearing HM7 tumours were treated with miR-9 antagomirs or anti-VEGF twice a week and tumour volumes were measured by digital caliper. *P<0.05, ANOVA followed by Tukey’s post-test. The experiment was repeated twice. (D) Vessel density in tumour sections was assessed by CD31-positive area over total viable tumour tissues. (E) Representative images of CD31 staining in HM7 tumours. (F) Mice bearing LLC tumours were treated with miR-9 antagomirs and/or anti-VEGF twice a week and tumour volumes were measured by digital caliper. *P<0.05, ANOVA followed by Tukey’s post-test.
miR-9 regulates the JAK-STAT pathway in endothelial cells
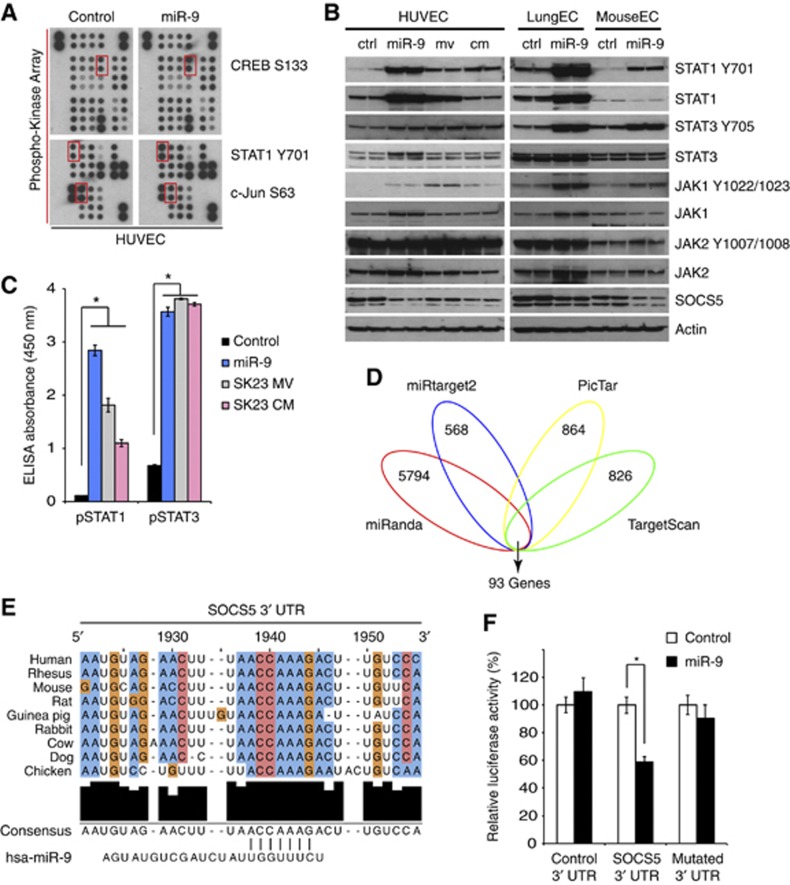
To investigate the molecular mechanisms by which miR-9 enhances endothelial cell migration, we undertook an unbiased approach by overexpressing miR-9 in HUVECs and examining the major signalling pathways involved in endothelial cell function. Among 46 components tested, cAMP response element binding (CREB) and signal transducer and activator of transcription 1 (STAT1) exhibited the most marked increases in phosphorylation, while c-Jun showed noticeable decreases (Figure 5A). Using a different antibody, we confirmed that phospho-STAT1 is augmented following miR-9 transfection in human or mouse endothelial cells. Phospho-STAT3 was also dramatically elevated (Figure 5B). In agreement with these findings, MVs or tumour conditioned media increased STAT1 and STAT3 phosphorylation in HUVECs (Figure 5B), a finding that was further verified by ELISA (Figure 5C). Other STAT members were either unchanged or undetectable. Upstream of STAT1 and STAT3, JAK2 (Janus Kinase 2) was phosphorylated in basal conditions in endothelial cells. We also found that JAK1 phosphorylation is upregulated upon miR-9 overexpression (Figure 5B). Suppressor of cytokine signalling 5 (SOCS5), a negative regulator of JAK-STAT pathway, was substantially decreased in response to miR-9, MVs or conditioned media (Figure 5B).
Figure 5.
miR-9 regulates the JAK-STAT pathway in endothelial cells. (A) Antibody array analysis of HUVECs transfected with miR-9 or control sequence. Red squares indicate changes in protein phosphorylation. (B) Endothelial cells were transfected with miR-9 or stimulated with SK23 microvesicles or conditioned medium. They were then analysed by western blot with the indicated antibodies. The experiment was repeated twice. (C) Phospho-STAT1 and phospho-STAT3 levels were quantified by ELISA. Each condition had seven biological replicates. *P<0.05, Student’s t-test. (D) Venn diagram of predicted miR-9 targets by four programs. (E) Alignment of a predicted miR-9 binding site in SOCS5 3′ UTR. (F) Dual luciferase assay on wild-type or mutated SOCS5 3′ UTR in COS-7 cells transfected with control or miR-9. *P<0.05, Student’s t-test. Figure source data can be found with the Supplementary data.
In addition, we predicted the gene targets of miR-9 in silico (Xiao et al, 2009) using four independent algorithms: miRanda (Betel et al, 2008), miRtarget2 (Wang and El Naqa, 2008), PicTar (Krek et al, 2005) and TargetScan (Lewis et al, 2005). Each program yielded a large number of genes. However, the 93 top candidates were common to all four methods (Figure 5D). Interestingly, SOCS5 was identified as one of the top candidates, having two putative miR-9 binding sites in its 3′ UTR. Similar to miR-9, the two predicted binding sites, separated by ∼80 bases, are highly conserved (one shown in Figure 5E). Ectopic expression of miR-9 suppressed the activity of a luciferase reporter in frame with wild-type SOCS5 3′ UTR by ∼40%, but not SOCS5 3′ UTR with mutated miR-9 binding sites, suggesting that SOCS5 gene is a direct target of miR-9 (Figure 5F). Taken together, we conclude that miR-9 targets SOCS5 in endothelial cells and activates the JAK-STAT signalling pathway.
Pharmacological inhibition of JAK-STAT impairs cell migration and angiogenesis
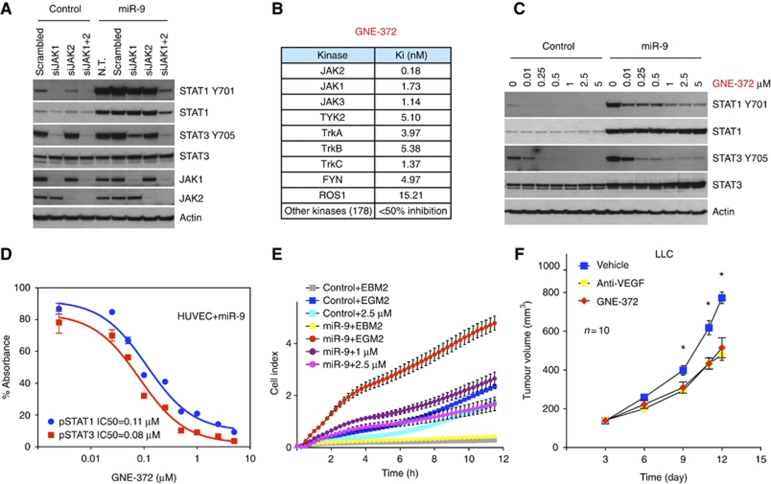
In addition to miR-9, multiple miRNAs identified in our screening were able to activate STATs, at least to various extents (Supplementary Figure 5A). Therefore, JAK-STAT appears to be a major signalling pathway regulated by miRNAs in endothelial cells. These findings prompted us to determine whether interfering pharmacologically with JAK-STAT signalling could impair miR-9 induced cell migration and tumour angiogenesis. First, we showed that JAK proteins play an essential role in mediating miR-9 induced STATs activation by knocking down JAK1 or JAK2. When we combined both siRNAs, STAT1 and STAT3 phosphorylations triggered by miR-9 were completely abolished (Figure 6A), indicating an indispensible role of JAK kinases in mediating miR-9 induced STATs activation.
Figure 6.
Pharmacological inhibition of JAK-STAT signalling impairs endothelial cell migration and angiogenesis. (A) JAK1 and/or JAK2 were knocked down in control or miR-9 overexpressing HUVECs. Proteins were probed as indicated. (B) Kinase profiling of the JAK2 inhibitor GNE-372. (C) Inhibition of phospho-STAT1 and phospho-STAT3 by GNE-372 in HUVECs. The experiment was repeated twice. (D) IC50 of GNE-372 was calculated on the basis of phospho-STAT1 and phospho-STAT3 ELISA. (E) Migration of HUVECs in the presence of GNE-372. Each group had four biological replicates. The experiment was repeated twice. For statistical analysis, ANOVA was performed followed by Tukey’s post-test to compare two groups. (F) Mice bearing LLC tumours were treated with vehicle control, GNE-372 (100 mg/kg, twice daily) or anti-VEGF (10 mg/kg, twice weekly). Each group comprised 10 mice. The experiment was repeated twice. *P<0.05, ANOVA followed by Tukey’s post-test. Figure source data can be found with the Supplementary data.
GNE-372 is a potent JAK2 inhibitor (manuscript in preparation) that specifically targets JAK family kinases including JAK2 and JAK1 (Figure 6B). As expected, this compound effectively inhibited STAT1 and STAT3 phosphorylation in miR-9 transfected endothelial cells (Figure 6C). We used ELISA to quantify its efficacy in cell-based assays. GNE-372 inhibited pSTAT1 with an IC50 of 0.11 μM and pSTAT3 with an IC50 of 0.08 μM (Figure 6D). Addition of the inhibitor to HUVECs overexpressing miR-9 significantly reduced cell migration (Figure 6E). Also, GNE-372 inhibited endothelial cell proliferation and survival (Supplementary Figure 5B and C). To assess the impact of JAK inhibition on tumour growth in vivo, we treated mice bearing LLC tumours with GNE-372. Such treatment significantly inhibited tumour growth relative to vehicle-treated controls, and was comparable to the anti-VEGF cohorts (Figure 6F).
Discussion
We systematically profiled endothelial miRNAs significantly changed by tumour cells in our co-culture system and discovered a subset of miRNAs directly transferred from tumour to endothelial cells. Furthermore, we describe a novel role of miR-9 in regulating tumour angiogenesis by modulating the JAK-STAT pathway in endothelial cells. These findings support the hypothesis that tumour-secreted miRNAs constitute a molecular mechanism implicated in the regulation of tumour neovascularization.
Previous studies have shown that miRNAs are stably detectable in human bloodstream and that alterations in circulating miRNAs are associated with certain cancers (Mitchell et al, 2008; Zhu et al, 2009; Komatsu et al, 2011; Mostert et al, 2011). Therefore, miRNAs may represent novel non-invasive biomarkers for cancer diagnosis (Mitchell et al, 2008; Hu et al, 2010; Boeri et al, 2011; Liu et al, 2011). Several investigations suggest that tumour-derived miRNAs enter the circulation predominantly through tumour-released MVs/exosomes (Kosaka et al, 2010; Muralidharan-Chari et al, 2010; Grange et al, 2011). Our data further indicate that tumour-derived miRNAs may be packaged into specific populations of MVs and function as important mediators of tumour-stroma communication.
The finding that endothelial-targeting miRNAs are within a heparin-binding complex is particularly intriguing. It is well established that heparan sulphate proteoglycans are widely distributed in the extracellular matrix (ECM) and that interactions with such moieties are of fundamental importance for a variety of processes, including angiogenesis (Munoz and Linhardt, 2004). Heparin binding is known to facilitate targeting of angiogenic molecules to the ECM and cell surface of endothelial cells (Munoz and Linhardt, 2004). Also, other studies have shown that, once bound to heparin, growth factors may become much more stable and resistant to degradation induced by various agents (Gospodarowicz and Cheng, 1986). All of these properties might be crucially important for MV stability and miRNA functions described in our study. Further biochemical characterization of this complex is necessary to elucidate the biological significance of our observation.
It is noteworthy that MV-mediated delivery, as observed for miR-9, is unlike the mechanism of endothelial up-regulation of miR-296 and miR-132, which have been characterized as positive regulators of angiogenesis. Both miRNAs have been reported to be upregulated by VEGF in endothelial cells (Wurdinger et al, 2008; Anand et al, 2010). In our co-culture screens, miR-296 and miR-132 were induced by some but not all tumour cell lines, with much lower fold changes compared to miR-9. Furthermore, other miRNAs have been shown to regulate endothelial cell recruitment indirectly, through modulation of expression of angiogenic factors within tumour cells (Dews et al, 2006; Png et al, 2012). Therefore, during angiogenesis delivery of genetic information mediated by miRNAs may occur through multiple mechanisms. It remains to be determined whether different tumours preferentially choose one or other mechanism and how these miRNAs are coordinated in regulating angiogenesis.
Our data implicate miR-9 in endothelial biology and tumour angiogenesis. miR-9 is increased in breast cancer and many other tumour types (Ma et al, 2010; Shigehara et al, 2011). It has been reported that miR-9 promotes tumour cell motility and metastasis by repressing E-cadherin expression. In the present study, we found that miR-9 also enhances endothelial cell migration and angiogenesis. Although miR-9 was proposed to increase vegf transcription in tumour cells (Ma et al, 2010), our results suggested that this is not a universal mechanism since miR-9 knockdown did not induce vegf mRNA changes in any of the tumour cell lines we tested. On the other hand, miR-9 was consistently upregulated in tumour-associated endothelial cells, which resulted in reduced SOCS5, a predicted target of miR-9. Consequently, the JAK-STAT signalling cascade was aberrantly activated. Therefore, it is tempting to speculate that miR-9, similar to miR-17-92 cluster (Olive et al, 2010), acts as a potent oncomir by simultaneously activating multiple tumorigenic pathways.
The JAK-STAT pathway is recognized as one of the major oncogenic signalling pathways activated in a variety of human malignancies (Yu and Jove, 2004). STAT proteins not only play a crucial role in tumour cell proliferation, survival and invasion, but also significantly contribute to the formation of a unique tumour microenvironment (Lee et al, 2009; Yu et al, 2009). A link between STATs activation in endothelial cells and tumour angiogenesis has been described in several studies (Bartoli et al, 2003; Leong et al, 2009; Dong et al, 2010). In addition, JAK1 was identified as an endothelial miR-17-92 target and was functionally validated as a proangiogenic molecule (Doebele et al, 2010), providing a rationale for targeting this pathway. Intriguingly, our data indicate that miR-9 prominently triggers JAK-STAT activities, and that inhibition of both JAK1 and JAK2 is sufficient to abrogate the effects. These findings highlight the importance of understanding the major target of miRNAs and suggest that, although it is challenging to therapeutically inhibit miR-9 in patients, JAK inhibitors might be an alternative approach. Indeed, we show that a JAK2 inhibitor efficiently blocked miR-9-induced pSTAT1 and pSTAT3. As a result, endothelial cell migration and tumour burden in mice were significantly reduced. In agreement with our findings, a recent study demonstrated that another JAK2 inhibitor also reduced tumour growth, as least in part through inhibition of angiogenesis (Xin et al, 2011). Of note, several JAK inhibitors are currently in late-stage trials for treating different diseases and generally have shown acceptable toxicological profiles (Garber, 2011).
Tumour angiogenesis is a complex process, requiring the involvement of multiple mediators. The present study extends our understanding of how this process is regulated by tumour-secreted miRNAs, which represent a new class of modulators in recipient endothelial cells. Further characterization of intercellular miRNA communication and downstream signalling events would help us identify novel mechanisms of angiogenesis and develop new therapeutic strategies.
Materials and methods
Cell culture
Primary microvascular endothelial cells from human brain, colon, and pancreas and HUVECs were purchased from Lonza (Walkersville, MD). Human dermal and lung microvascular endothelial cells were from Cell Systems (Kirkland, WA). Endothelial cells were cultured in EGM-2 or EGM-2MV medium (Lonza). Tumour cell lines were obtained from the Genentech cell line repository (gCell) and were cultured in DMEM (high glucose) supplemented with 10% fetal bovine serum (Invitrogen). The HM7 cell line is a variant of LS 174T human colorectal carcinoma cell line, as previously described (Bresalier et al, 1991; Warren et al, 1995). Tumour-endothelial co-culture experiments were performed in six-well plates with 8-micron inserts (BD Biosciences). miRNA mimics (Applied Biosystems) and siRNA sequences (Dharmacon) were transfected with Lipofectamine RNAiMAX reagent (Invitrogen).
miRNA profiling and quantitative PCR
Total RNA was prepared with mirVana miRNA isolation kit (Ambion) according to manufacturer’s protocol. miRNA in 500 ng total RNA was subjected to reverse transcription using the Megaplex RT primer pools and the Taqman microRNA reverse transcription kit (Applied Biosystems). The resultant cDNA was pre-amplified using Megaplex PreAmp primers and TaqMan PreAmp master mix (Applied Biosystems), and then loaded into the Taqman array microRNA cards. The real-time PCR was run on the Applied Biosystems 7900HT machine. Relative expression levels of each miRNA were normalized to U6 snRNA. Expression of individual miRNAs was quantified by TaqMan miRNA assays (Applied Biosystems) following manufacturer’s instructions. U6 snRNA was used as the endogenous control for all experiments. At least three biological replicates were included for each condition.
Cell migration assay
Real-time monitoring of endothelial cell migration was performed using the xCELLigence system with the CIM-Plate 16 (Roche). The upper chamber was coated with 20 μg/ml Fibronectin (BD Biosciences) and seeded with 50 000 HUVECs. When cells migrated through the membrane into the bottom chamber in response to attractants, they contacted and adhered to the electronic sensors, resulting in an increase in impedance. The cell-index values reflecting impedance changes were automatically and continuously recorded every 15 min.
HUVEC sprouting assay
Twenty hours after transfection, HUVECs were mixed with Cytodex microcarrier beads (Sigma-Aldrich) in a ratio of 106 cells per 2500 beads and incubated for 4 h at 37°C. Coated beads were then cultured in a six-well dish overnight. The following day, ∼200 coated beads were dissolved in a solution of 2 mg/ml fibrinogen (Sigma-Aldrich) in EGM-2, and added to 0.625 U/ml of thrombin (Sigma-Aldrich) in one well of a 24-well plate. Skin fibroblasts (D551) were then plated on top of the clot and incubated with D551 conditioned medium and EGM-2 (3:1). HUVEC sprouts were visualized by immunostaining with Alexa Fluor 488 phalloidin (1:100) and Hoecsht 33258 (1:1000) (Invitrogen) overnight at 4°C. Image Xpress Micro was used for capturing images and HUVEC sprouting was analysed in MetaXpress software.
MV isolation
Tumour cells were cultured in serum-free DMEM. Conditioned medium was collected after 48 h and concentrated 10-fold. MVs were isolated using two independent methods. For differential centrifugation, conditioned medium was filtered through a 0.22-μm membrane and centrifuged for 30 min at 16 500 g. Supernatants were filtered again and MVs were pelleted by ultracentrifugation for 2 h at 110 000 g. In the other approach, ExoQuick precipitation reagent (System Biosciences) was mixed with concentrated conditioned medium and MVs were precipitated by centrifugation.
Chromatography
Approximately 80% confluent SK23 cells were incubated in serum-free medium for 3 days. Conditioned media were collected and concentrated 10-fold as starting material for chromatography. The heparin-sepharose column (Hi-Trap, HS, 5 ml) was pre-equilibrated with 20 mM Tris, pH 7.4. The Hi-Trap Q sepharose column, 1 ml (GE Healthcare) was pre-equilibrated with 20 mM Tris, pH 8.0. Samples were injected into the column and bound material was eluted with a linear gradient of NaCl. Aliquots of fractions were tested on HUVECs for miR-9 expression.
Western blot
Cells were lysed in RIPA buffer (Tris pH 7.4 50 mM, NaCl 150 mM, NP-40 1%, SDS 0.1%, EDTA 2 μM) containing proteinase inhibitors (Roche) and phosphatase inhibitors (Sigma). The cell lysates (20 ug protein) were subjected to SDS–PAGE and western blot. Antibodies against the following proteins were used: pTyr-701 STAT1, STAT1, pTyr-705 STAT3, STAT3, pTyr-1022/1023 JAK1, JAK1, pTyr-1007/1008 JAK2, JAK2, PARP, Caspase-3, Actin (Cell Signaling Technology); SOCS5 (Santa Cruz Biotechnology). Human phospho-kinase array and human angiogenesis antibody array were performed following manufacturer’s instructions (R&D Systems). For phosphor-kinase array, ∼300 μg HUVEC lysates were analysed 48 h after transfection of control oligo or miR-9. For human angiogenesis array, MVs were purified from 5 × 106 SK23 cells transfected with scrambled sequence or anti-miR-9, and subjected to the antibody array.
Dual luciferase reporter assay
For miR-9 target validation, 3′ UTR segment of human SOCS5 was cloned into a mammalian expression vector with dual luciferase reporter system (GeneCopoeia). COS-7 cells were transfected in six-well plates using Lipofectamine 2000 (Invitrogen). Transfections were performed using 1 μg dual luciferase reporter plasmids and a final concentration of 10 nM synthetic miRNA mimics (Applied Biosystems). Forty-eight hours after transfection, dual luciferase assays were performed using Luc-Pair miR luciferase assay kit (GeneCopoeia) according to manufacturer's instructions. Firefly luciferase activity was first normalized to Renilla luciferase expression control. For each reporter construct, the normalized value for miR-9 transfection was then normalized to the value obtained from the same reporter construct co-transfected with control miRNA. Mean values, standard deviations and Student’s t-test were calculated from seven independent transfections for each condition. The QuikChange II XL site-directed mutagenesis kit (Agilent) was used to mutate miR-9 binding sites at SOCS5 3′ UTR.
Tumour models
Tumour cells (1 × 106) were subcutaneously implanted in the dorsal flank of BALB/c Nude mice. Anti-VEGF mAb B-20.4.1 (Liang et al, 2006) or anti-Ragweed control was IP injected at the dose of 10 mg/kg twice weekly. Antagomirs (Exiqon) were mixed with in vivo-jetPEI transfection reagent (Polyplus) and injected intratumorally at 2 μg per mouse twice a week. JAK2 inhibitors GNE-372 (100 mg/kg) or vehicle control (0.5% methylcellulose/0.2% Tween-80) were administered twice daily by oral gavage. Tumour volumes (10 animals per group) were measured with digital caliper and calculated as length × width2 × 0.52. To quantify perfused tumour vessels, fluorescein-labelled lectin (Vector Lab) was injected intravenously (2 mg/kg) 10 min before collecting tumours. All animal protocols were approved by the Genentech animal care and use committee.
Vascular staining and quantification
Tumours from six mice per treatment group were collected and embedded in OCT blocks. Tissues were cryo-sectioned to 16 μm thickness on Leica CM3050S, and stained with CD31 antibody (BD Biosciences). Images were acquired with Zeiss AxioImager Z1 fluorescence microscope controlled by TissueFAXS software. Image files were loaded into the TissueStudio analysis package (v1.5, Definiens). Necrotic tissues and staining artifacts such as skin tissues and folds were automatically identified and excluded based on nuclei staining. Blood vessel density was calculated as the ratio of CD31-positive pixels to the total viable tumour area. Statistical analysis was performed in JMP software.
Statistical analysis
Rank products analysis was performed on the miRNA profiling results to identify most significantly changed miRNAs in response to tumour stimulation, and a permutation approach was used to compute the empirical P-values (Breitling et al, 2004). The results were graphed in volcano plot, and miRNAs with P<0.001 were highlighted. In all experiments, comparisons between two groups were based on two-sided Student’s t-test and one-way analysis of variance (ANOVA) was used to test for differences among more groups. P-values of <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank the Genentech animal facility, the antibody purification group and the gCell lab. We are grateful to W Ye and D Dornan for their critical reading of our manuscript. We acknowledge X Qu, A Chung, H Tran, R Lesley, B Haley, L Komuves, J Lill, M Nagel and L Lee for their help.
Author contributions: GZ and NF designed the study and wrote the manuscript. GZ, XW, JY, YG, ZM and CB performed the experiments. ZJ and IK provided statistical and imaging analyses, respectively. JO and DS developed the JAK2 inhibitor. All authors contributed to finalise the manuscript.
Footnotes
All authors are employees of Genentech Inc.
References
- Anand S, Cheresh DA (2011) MicroRNA-mediated regulation of the angiogenic switch. Curr Opin Hematol 18: 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN, Lapinski PE, King PD, Weis SM, Cheresh DA (2010) MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med 16: 909–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J (2011) Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat commun 2: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297 [DOI] [PubMed] [Google Scholar]
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli M, Platt D, Lemtalsi T, Gu X, Brooks SE, Marrero MB, Caldwell RB (2003) VEGF differentially activates STAT3 in microvascular endothelial cells. FASEB J 17: 1562–1564 [DOI] [PubMed] [Google Scholar]
- Betel D, Wilson M, Gabow A, Marks DS, Sander C (2008) The microRNA.org resource: targets and expression. Nucleic Acids Res 36: D149–D153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, Calabro E, Croce CM, Pastorino U, Sozzi G (2011) MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci USA 108: 3713–3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling R, Armengaud P, Amtmann A, Herzyk P (2004) Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett 573: 83–92 [DOI] [PubMed] [Google Scholar]
- Bresalier RS, Niv Y, Byrd JC, Duh QY, Toribara NW, Rockwell RW, Dahiya R, Kim YS (1991) Mucin production by human colonic carcinoma cells correlates with their metastatic potential in animal models of colon cancer metastasis. J Clin Invest 87: 1037–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liang H, Zhang J, Zen K, Zhang CY (2012) Secreted microRNAs: a new form of intercellular communication Trends Cell Biol 22: 125–132 [DOI] [PubMed] [Google Scholar]
- Chung AS, Ferrara N (2011) Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 27: 563–584 [DOI] [PubMed] [Google Scholar]
- Chung AS, Lee J, Ferrara N (2010) Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer 10: 505–514 [DOI] [PubMed] [Google Scholar]
- Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A (2006) Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet 38: 1060–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebele C, Bonauer A, Fischer A, Scholz A, Reiss Y, Urbich C, Hofmann WK, Zeiher AM, Dimmeler S (2010) Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood 115: 4944–4950 [DOI] [PubMed] [Google Scholar]
- Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC, Bai W, Yu L, Kowalski J, Liang X, Fuh G, Gerber HP, Ferrara N (2004) VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J 23: 2800–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Lu B, Zhang X, Zhang J, Lai L, Li D, Wu Y, Song Y, Luo J, Pang X, Yi Z, Liu M (2010) Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis 31: 2097–2104 [DOI] [PubMed] [Google Scholar]
- Ebos JM, Kerbel RS (2011) Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 8: 210–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Mass RD, Campa C, Kim R (2007) Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med 58: 491–504 [DOI] [PubMed] [Google Scholar]
- Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1: 27–31 [DOI] [PubMed] [Google Scholar]
- Garber K (2011) Pfizer's JAK inhibitor sails through phase 3 in rheumatoid arthritis. Nat Biotechnol 29: 467–468 [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D, Cheng J (1986) Heparin protects basic and acidic FGF from inactivation. J Cell Physiol 128: 475–484 [DOI] [PubMed] [Google Scholar]
- Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G (2011) Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res 71: 5346–5356 [DOI] [PubMed] [Google Scholar]
- Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y, Chen Y, Xu L, Zen K, Zhang C, Shen H (2010) Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol 28: 1721–1726 [DOI] [PubMed] [Google Scholar]
- Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Morimura R, Nagata H, Kosuga T, Iitaka D, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E (2011) Circulating microRNAs in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer 105: 104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Ochiya T (2010) Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 101: 2087–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N (2005) Combinatorial microRNA target predictions. Nat Genet 37: 495–500 [DOI] [PubMed] [Google Scholar]
- Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H (2009) Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell 15: 283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, D'Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J (2011) Microvesicles as mediators of intercellular communication in cancer–the emerging science of cellular 'debris'. Semin Immunopathol 33: 455–467 [DOI] [PubMed] [Google Scholar]
- Leong H, Mathur PS, Greene GL (2009) Green tea catechins inhibit angiogenesis through suppression of STAT3 activation. Breast Cancer Res Treat 117: 505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20 [DOI] [PubMed] [Google Scholar]
- Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G (2006) Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem 281: 951–961 [DOI] [PubMed] [Google Scholar]
- Liu R, Chen X, Du Y, Yao W, Shen L, Wang C, Hu Z, Zhuang R, Ning G, Zhang C, Yuan Y, Li Z, Zen K, Ba Y, Zhang CY (2011) Serum MicroRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin Chem 58: 610–618 [DOI] [PubMed] [Google Scholar]
- Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA (2010) miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 12: 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105: 10513–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert B, Sieuwerts AM, Martens JW, Sleijfer S (2011) Diagnostic applications of cell-free and circulating tumor cell-associated miRNAs in cancer patients. Expert Rev Mol Diagn 11: 259–275 [DOI] [PubMed] [Google Scholar]
- Munoz EM, Linhardt RJ (2004) Heparin-binding domains in vascular biology. Arterioscler Thromb Vasc Biol 24: 1549–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy JW, Sedgwick A, D'Souza-Schorey C (2010) Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 123: 1603–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive V, Jiang I, He L (2010) mir-17-92, a cluster of miRNAs in the midst of the cancer network. Int J Biochem Cell Biol 42: 1348–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Png KJ, Halberg N, Yoshida M, Tavazoie SF (2012) A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 481: 190–194 [DOI] [PubMed] [Google Scholar]
- Shigehara K, Yokomuro S, Ishibashi O, Mizuguchi Y, Arima Y, Kawahigashi Y, Kanda T, Akagi I, Tajiri T, Yoshida H, Takizawa T, Uchida E (2011) Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PloS ONE 6: e23584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Shanz S, Fuh G, Gerber H-P, Ferrara N (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+myeloid cells. Nature Biotechnol 25: 911–920 [DOI] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10: 1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EM, Olson EN (2011) Pervasive roles of microRNAs in cardiovascular biology. Nature 469: 336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez Y, Fernandez-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC (2008) Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci USA 105: 14082–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Olson EN (2009) AngiomiRs–key regulators of angiogenesis. Curr Opin Genet Dev 19: 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, El Naqa IM (2008) Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics 24: 325–332 [DOI] [PubMed] [Google Scholar]
- Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N (1995) Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 95: 1789–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17: 1359–1370 [DOI] [PubMed] [Google Scholar]
- Wurdinger T, Tannous BA, Saydam O, Skog J, Grau S, Soutschek J, Weissleder R, Breakefield XO, Krichevsky AM (2008) miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell 14: 382–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Zuo Z, Cai G, Kang S, Gao X, Li T (2009) miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res 37: D105–D110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Herrmann A, Reckamp K, Zhang W, Pal S, Hedvat M, Zhang C, Liang W, Scuto A, Weng S, Morosini D, Cao ZA, Zinda M, Figlin R, Huszar D, Jove R, Yu H (2011) Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Cancer Res 71: 6601–6610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Jove R (2004) The STATs of cancer–new molecular targets come of age. Nat Rev Cancer 4: 97–105 [DOI] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9: 798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Qin W, Atasoy U, Sauter ER (2009) Circulating microRNAs in breast cancer and healthy subjects. BMC Res Notes 2: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.