Abstract
The synthesis and properties of four new fluorescent reagents capable of forming moderately stable links to the 3′ oxidized end of RNA are reported. All are hydrazide derivatives: pyrene butyric acid hydrazide, proflavine monosemicarbazide, proflavine monosuccinic acid hydrazide, and anthracene-9-carboxaldehyde carbohydrazone. In addition, procedures are given for coupling the bifunctional reagent carbohydrazide to the 3′ end of RNA. These carbohydrazide adducts can easily be coupled in turn to a wide variety of fluorescent reagents having specificity for aliphatic amino groups, including isothiocyanates and sulfonyl halides. Thus a route exists for the preparation of an enormous variety of 3′ fluorescent labeled RNAs. The carbohyrazide adducts are also useful for other synthetic procedures such as preparation of covalent tRNA dimers.
Full text
PDF






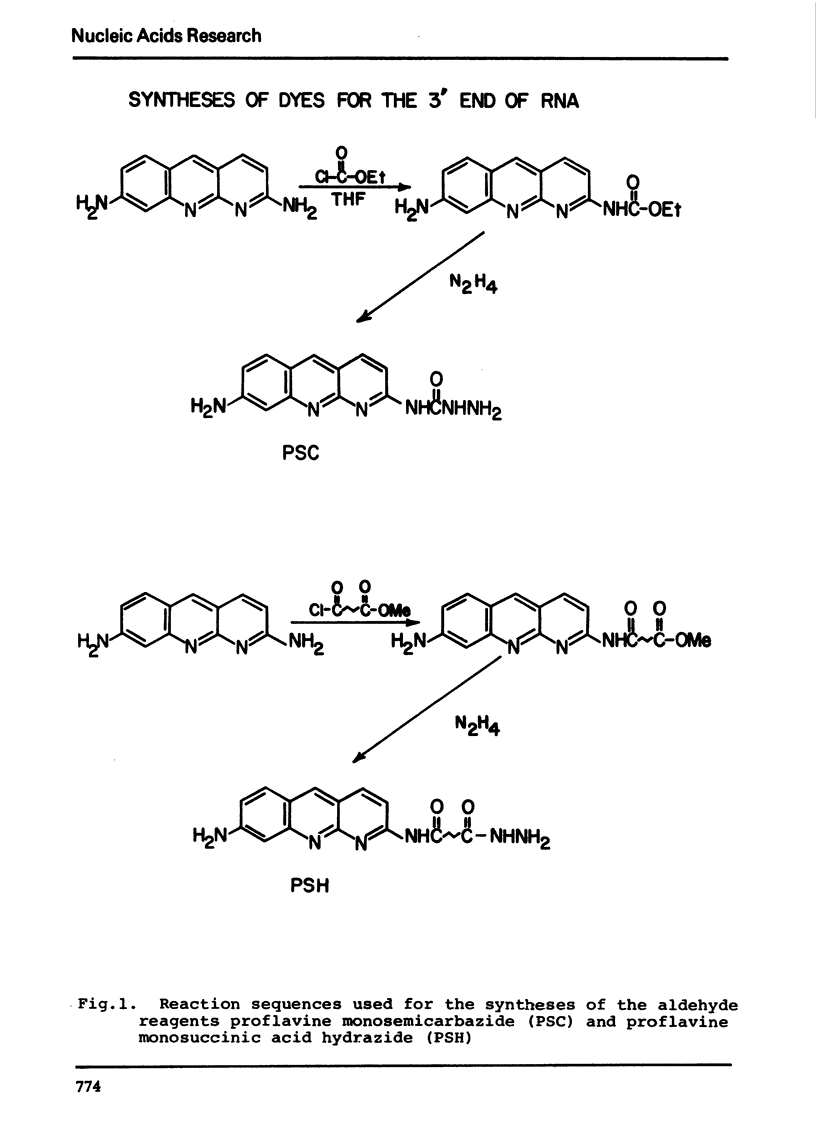
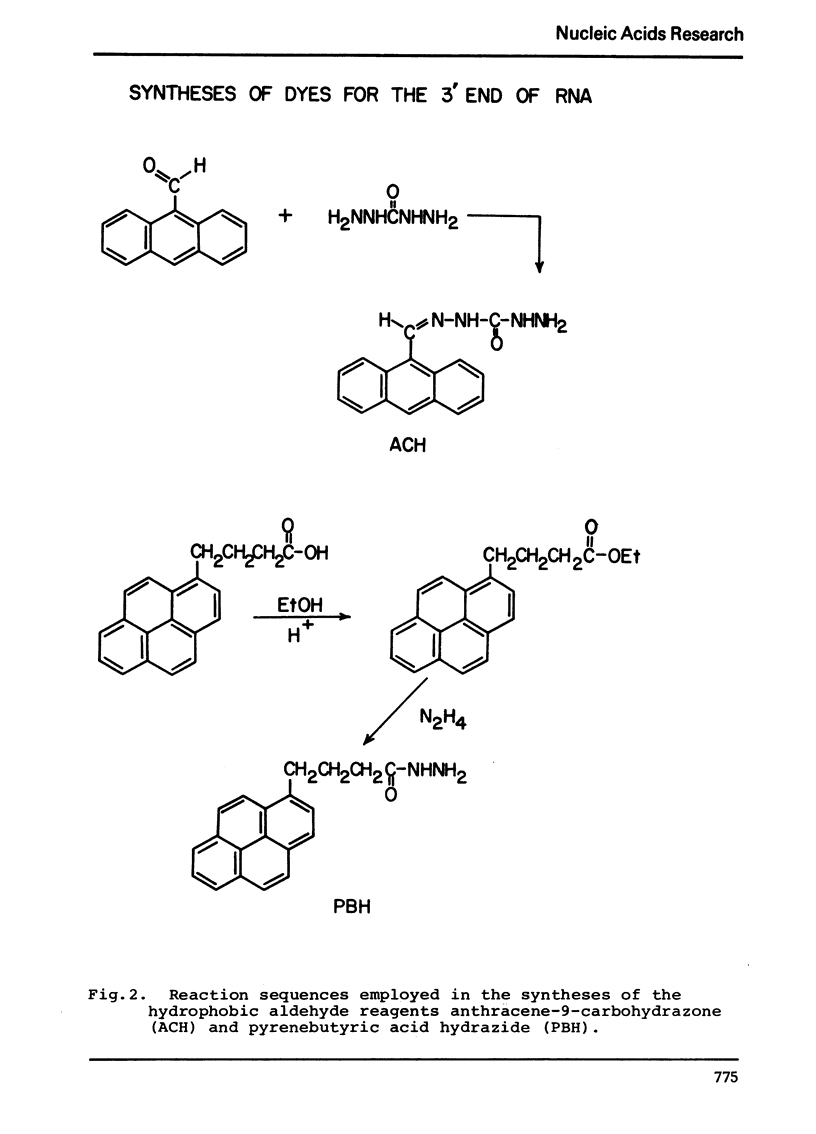
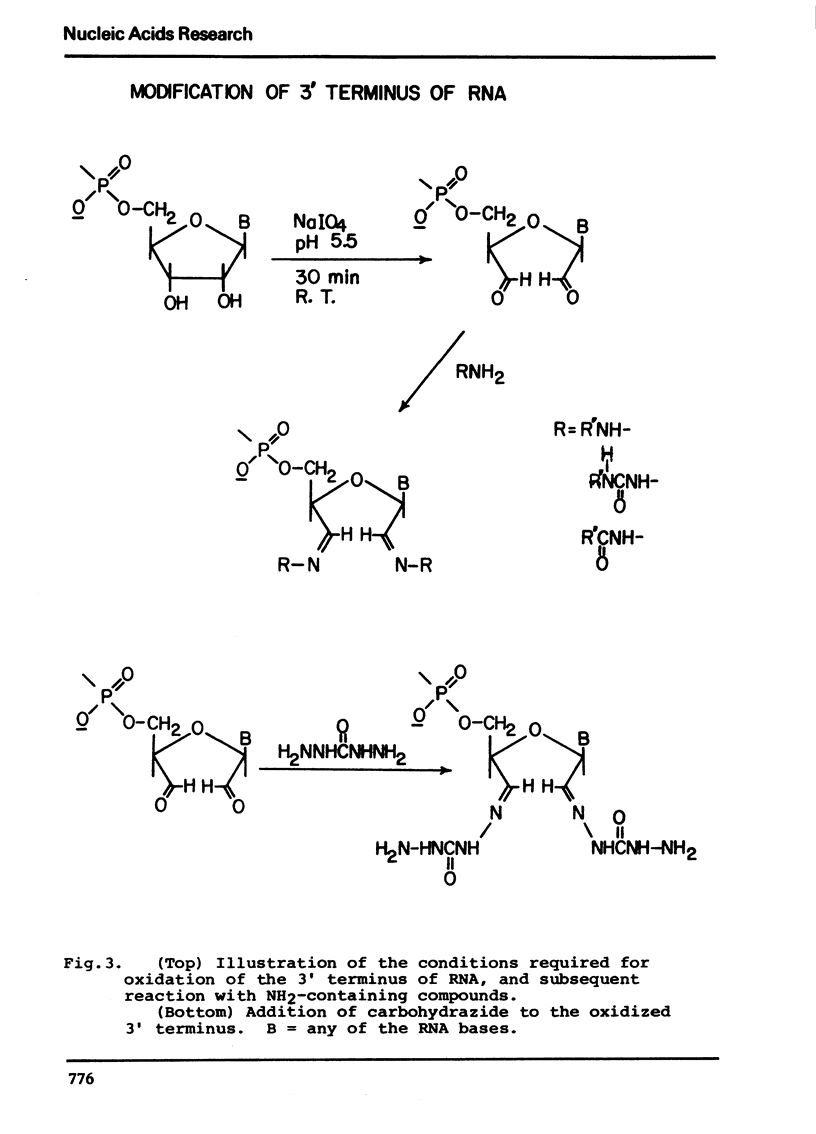

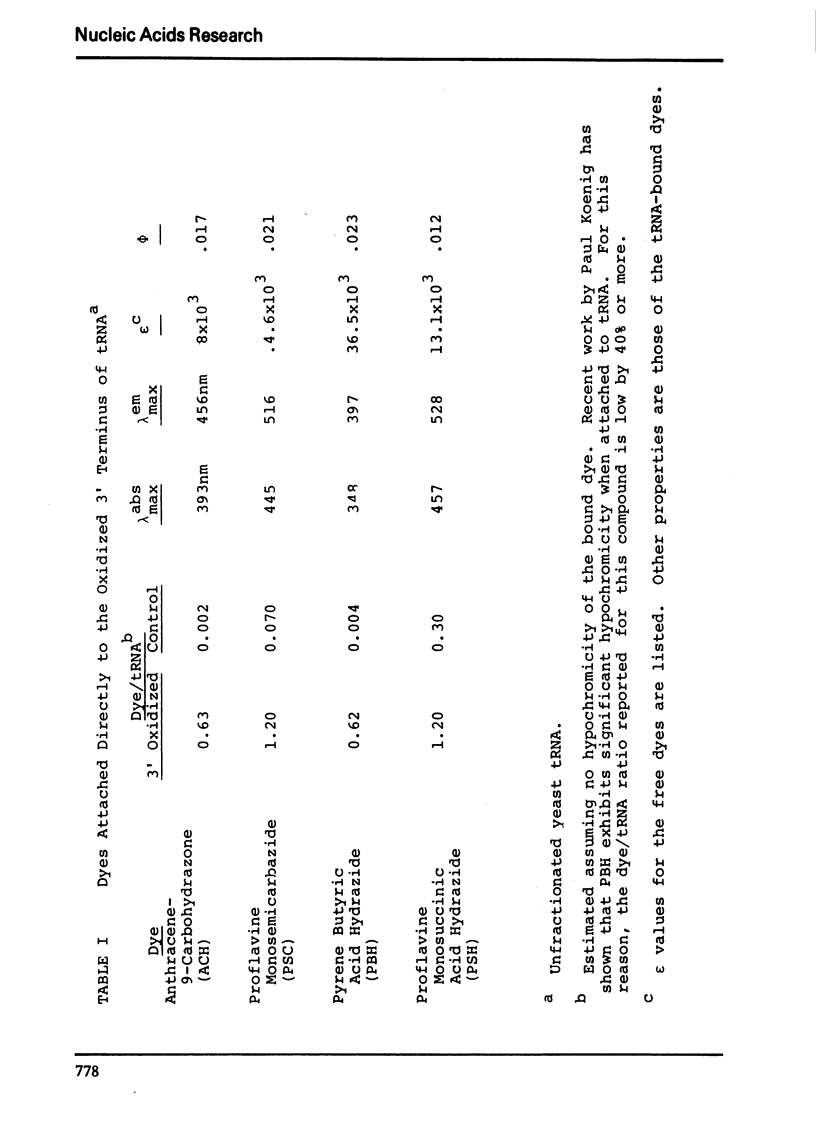
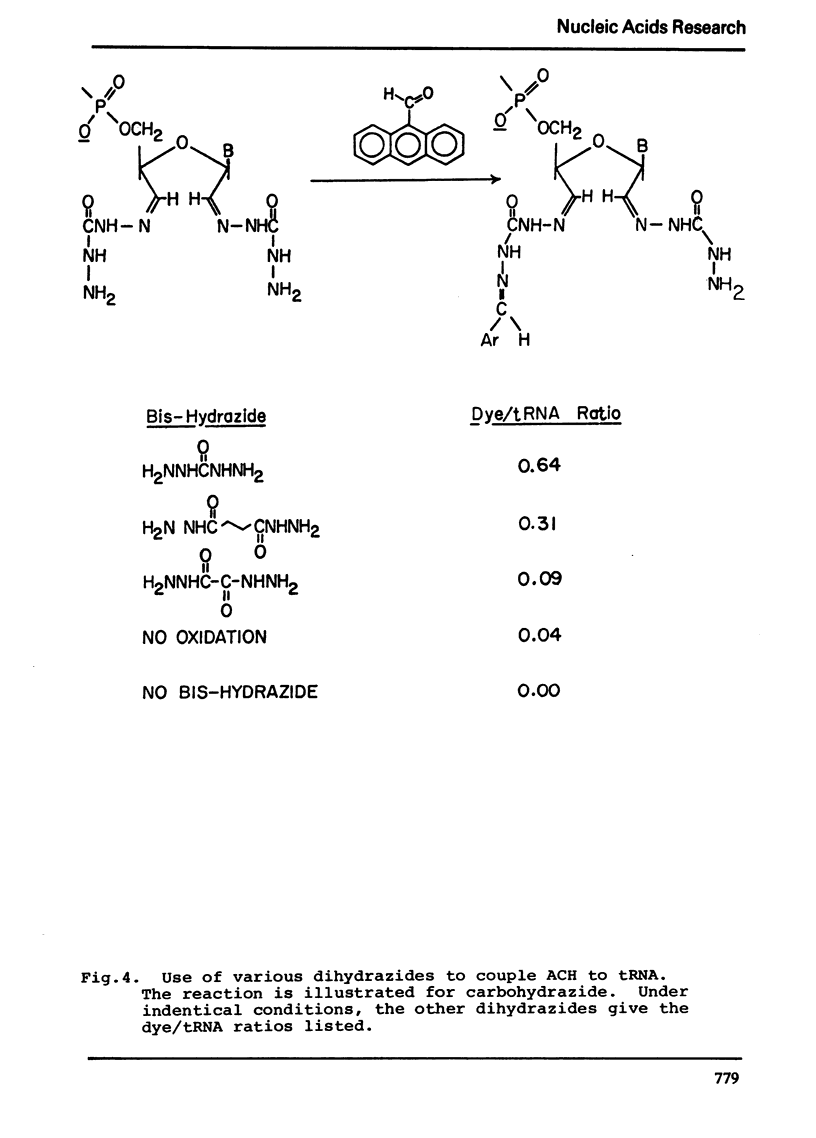

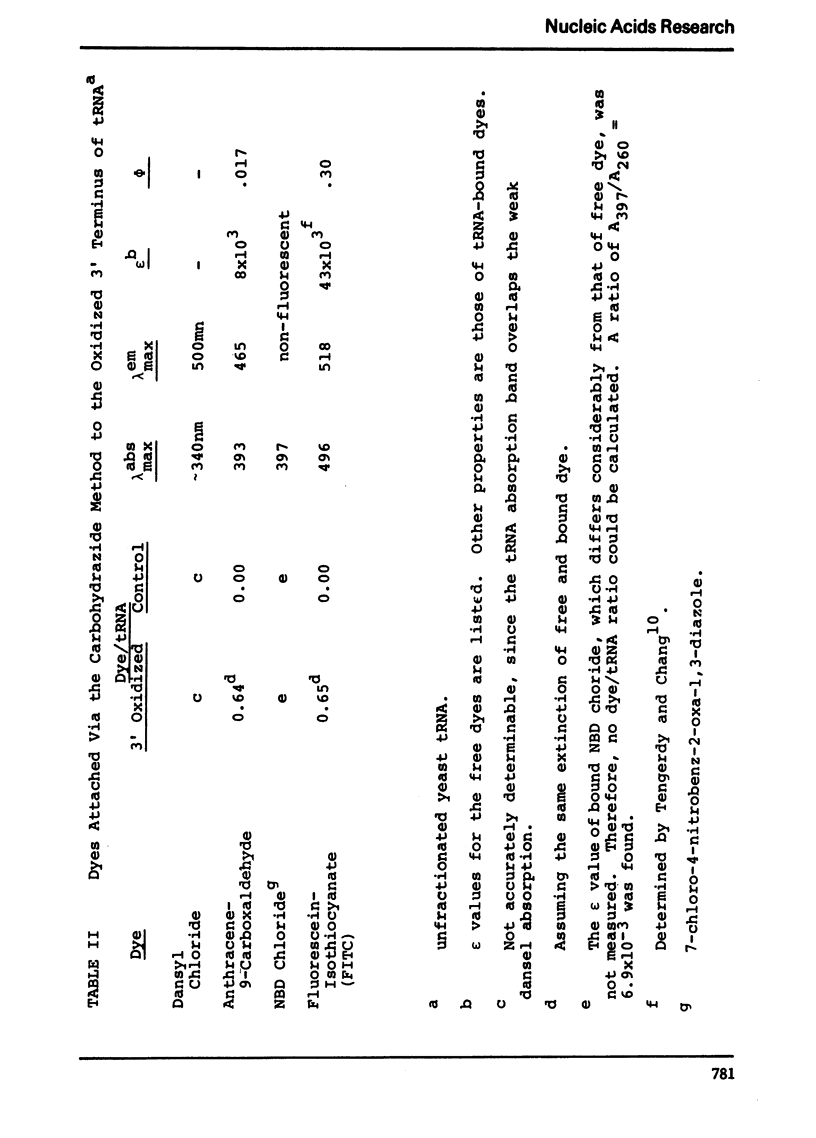

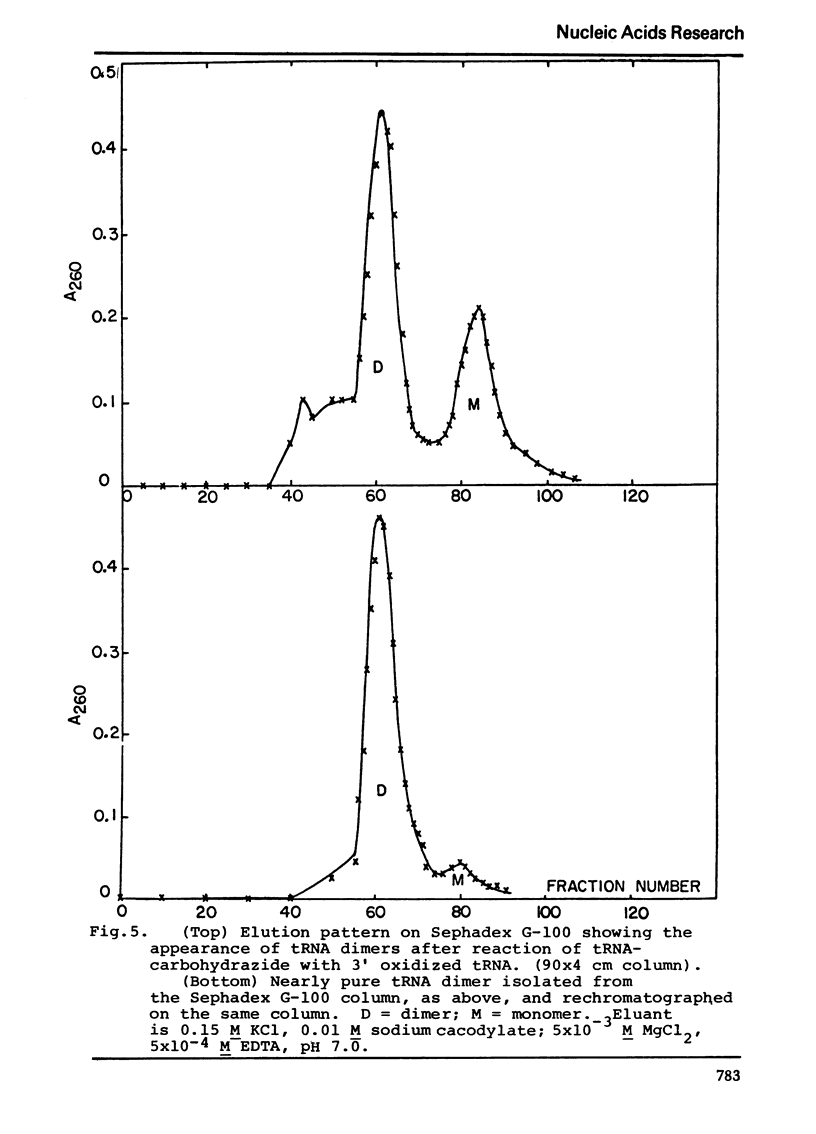
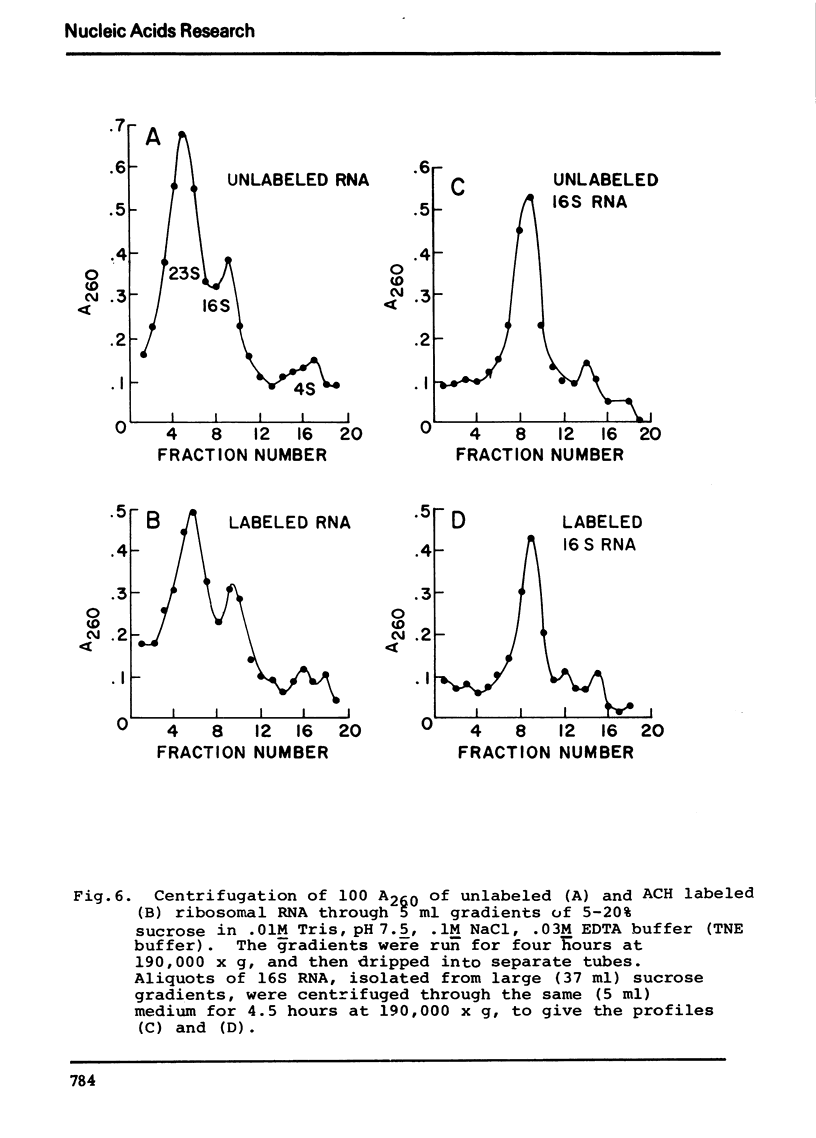


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abermann R. J., Yoshikami D. High-resolution shadowing of transfer RNA. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1587–1591. doi: 10.1073/pnas.69.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCHICH J. E. FLUORESCENCE STUDIES ON SOLUBLE RIBONUCLEIC ACID LABELLED WITH ACRIFLAVINE. Biochim Biophys Acta. 1963 Sep 24;75:274–276. doi: 10.1016/0006-3002(63)90608-5. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Quigley G. J., Suddath F. L., McPherson A., Sneden D., Kim J. J., Weinzierl J., Rich A. Three-dimensional structure of yeast phenylalanine transfer RNA: folding of the polynucleotide chain. Science. 1973 Jan 19;179(4070):285–288. doi: 10.1126/science.179.4070.285. [DOI] [PubMed] [Google Scholar]
- Millar D. B., Steiner R. F. The effect of environment on the structure and helix-coil transition of soluble ribonucleic acid. Biochemistry. 1966 Jul;5(7):2289–2301. doi: 10.1021/bi00871a018. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Cantor C. R., Chambers R. W. Effect of magnesium ions on the conformation of two highly purified yeast alanine transfer ribonucleic acids. Biochemistry. 1970 Sep 29;9(20):3993–4002. doi: 10.1021/bi00822a019. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L., Scott J. F. PARTIAL PURIFICATION OF SOLUBLE RNA. Proc Natl Acad Sci U S A. 1960 Jun;46(6):811–822. doi: 10.1073/pnas.46.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]


