Abstract
The membranous labyrinth of the inner ear establishes a precise geometrical topology so that it may subserve the functions of hearing and balance. How this geometry arises from a simple ectodermal placode is under active investigation. The placode invaginates to form the otic cup, which deepens before pinching off to form the otic vesicle. By the vesicle stage many genes expressed in the developing ear have assumed broad, asymmetrical expression domains. We have been exploring the possibility that these domains may reflect developmental compartments that are instrumental in specifying the location and identity of different parts of the ear. The boundaries between compartments are proposed to be the site of inductive interactions required for this specification. Our work has shown that sensory organs and the endolymphatic duct each arise near the boundaries of broader gene expression domains, lending support to this idea. A further prediction of the model, that the compartment boundaries will also represent lineage-restriction compartments, is supported in part by fate mapping the otic cup. Our data suggest that two lineage-restriction boundaries intersect at the dorsal pole of the otocyst, a convergence that may be critical for the specification of endolymphatic duct outgrowth. We speculate that the patterning information necessary to establish these two orthogonal boundaries may emanate, in part, from the hindbrain. The compartment boundary model of ear development now needs to be tested through a variety of experimental perturbations, such as the removal of boundaries, the generation of ectopic boundaries, and/or changes in compartment identity.
Inner Ear Morphogenesis and Gene Expression Patterns
The generation of the vertebrate inner ear requires coordination between morphogenesis and cell fate specification. Morphogenesis will convert a flat epithelial patch (the otic placode) into a hollow sphere (the otic vesicle or otocyst) and finally into a labyrinth of cavities, ducts, and tubules (Fig. 1). It is accompanied by an enormous increase in cell numbers and overall size of the ear, as well focal regions of programmed cell death (for discussion see ref. 1). Cell fate specification will give rise to the neurons of the associated sensory ganglia, the sensory organs for hearing and balance, and nonsensory tissues with various degrees of specialization (2, 3). During the course of these changes, more than 40 different genes are expressed in the developing ear, often in spatially and temporally complex patterns (4, 5). How does this intricate patterning of genes and tissues come about? Can we define some of the rules that govern pattern formation of the inner ear so as to provide a framework for understanding what goes wrong with mutations that lead to congenital defects in the ear?
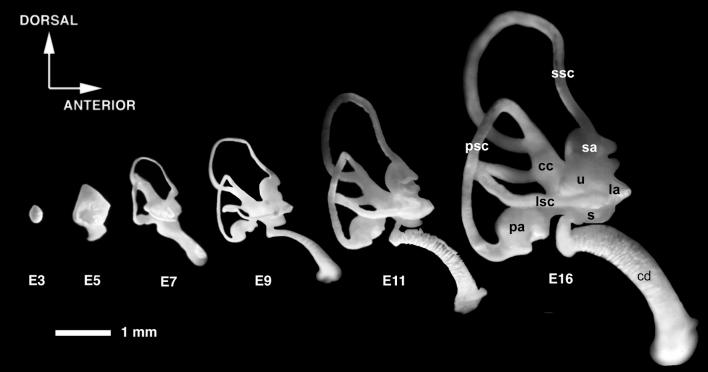
Figure 1.
Morphogenesis of the chicken inner ear viewed by filling the inner ears with opaque paint. Lateral views are shown. The labyrinth develops from a simple ovoid vesicle. Between embryonic day 3 (E3) and E7, the major structural parts of the ear make their appearance, whereas growth, cell fate specification, and tissue differentiation continue for a longer period. cc, Common crus; la, lateral ampulla; lsc, lateral semicircular canal; psc, posterior semicircular canal; sa, superior ampulla; ssc, superior semicircular canal; u, utricle. Modified from Bissonnette and Fekete (58). Both lateral and posterior views of paint-filled ears have been morphed with Elastic Reality (version 1.3; now owned by Avid) to generate a QuickTime movie that can be found as supplementary material on the PNAS website, www.pnas.org.
Several reviews have recently summarized the genes that are expressed early during inner ear development, primarily in mouse, chicken, zebra fish, and Xenopus (4–7). With the exception of those that appear to mark the incipient sensory organs, by the otic vesicle stage most of these genes have relatively broad expression domains, although they rarely encompass the entire otic epithelium. That is, they are usually expressed asymmetrically. Highly schematic views of some of these expression patterns are indicated in Fig. 2. Here, we raise the possibility that the domains may abut each other rather abruptly, giving rise to precise boundaries, although we emphasize that this has rarely been rigorously demonstrated. In fact, data from our lab are consistent with some abrupt boundaries, while also showing evidence for partially overlapping expression domains (see below). Gene overlap has also been noted at the ventral pole of the mouse otocyst on the basis of the expression of two transcription factor genes, Nkx5.1 (Hmx3) and Pax2, and one receptor tyrosine kinase gene, EphA4 (formerly called sek1) (8). A separate study reported that three members of the Iroquois family of transcription factors are partially overlapping in this same region, at least transiently (9). Clearly, the number and location of the various gene expression domains will continue to undergo further refinement.
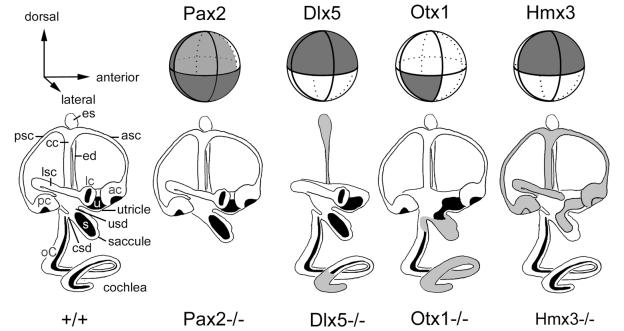
Figure 2.
Schematic representation of the mouse otic vesicle, showing the expression domains of several genes (top row) and the phenotypes that result when the gene is knocked out (bottom row). Dark lines encircling the vesicle indicate theoretical boundaries that segregate the vesicle into compartments. In the bottom row, structures reported missing in the knockout are left off the drawings, while those that have been reported to have a variable phenotype either by a single group or by two different groups are shaded gray. See text for discussion. Data were derived from the following sources: Pax2 expression, ref. 22; pax2−/−, ref. 23; Hmx3 expression, refs. 8 and 59; Hmx3−/−, refs. 20 and 53; Otx1 expression, refs. 55 and 57; Otx1−/−, refs. 54, 55, and 60; Dlx5 expression and Dlx5−/−, ref. 52. asc, Anterior semicircular canal; ac, anterior crista; cc, common crus; csd, cochlear-saccular duct; ed, endolymphatic duct; es, endolymphatic sac; lc, lateral crista; lsc, lateral semicircular canal; oC, organ of Corti; pc, posterior crista; psc, posterior semicircular canal; s, saccular macula; u, utricular macula; usd, utricular-saccular duct.
Compartment and Boundary Model of Inner Ear Development
Recently, we proposed a model for a global underlying mechanism for pattern formation in the ear that was based on its segregation into separate compartments (2, 10). The model can be stated succinctly as follows:
(i) The ear is segregated into compartments defined by broad gene expression domains, and it is predicted that these will represent lineage-restriction compartments.
(ii) Compartment boundaries may specify the location of the sensory organs, endolymphatic duct outgrowth, or emigration of neuronal precursors, by means of short-range signals sent across the boundaries.
(iii) Compartment identity, perhaps defined by a combination of transcription factors, will specify the structural parts of the ear (semicircular canal, utricle, saccule, cochlea, endolymphatic duct).
(iv) Compartment identity will also dictate the type of sensory organ (crista, macula, organ of Corti) that forms within each compartment.
Using the Drosophila wing and leg imaginal discs as inspiration (11, 12), we reasoned that the compartment boundaries of the ear could be used to pinpoint specific places within this three-dimensional space where key signaling events would occur. In a direct analogy, we would expect the compartment boundaries to also be lineage restriction compartments that resist cell mixing (13). The compartment boundaries would allow for short-range signaling molecules, such as diffusible factors or cell surface molecules, to signal between the cells of adjacent compartments (14, 15). To take the analogy further, we might expect the result of this inductive signaling to be the secretion of a morphogen or other longer-range signaling molecule that could direct pattern formation on either side of the boundary. However, it should be noted that the distance over which diffusion is likely to act has been difficult to assess in vertebrate systems, and it may be on the order of only a couple hundred micrometers (16). In the ear, the consequences of either short- or long-range inductive signaling might be to dictate the positions of the sensory organs or the outgrowth of the endolymphatic duct, for example. This is shown schematically in Fig. 3.
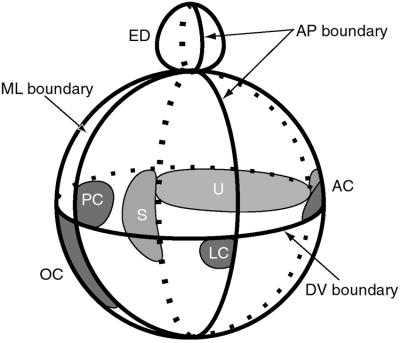
Figure 3.
Model showing one possible arrangement of sensory primordia with respect to the theoretical compartment boundaries defined in Fig. 2. Both chicken and mouse data were used to derive the model, which is viewed from the side with the same orientation as Fig. 2. Several sensory organ primordia arise on the edge of a SOHo boundary (10). The lateral crista primordium is located on the edge of the Otx2 domain (55). The endolymphatic duct also arises on one side of the medial–lateral (ML) boundary at the dorsal pole of the otocyst (see text and Fig. 5). AC, anterior crista; ED, endolymphatic duct; LC, lateral crista; OC, organ of Corti; PC, posterior crista; S, saccular macula; U, utricular macula.
Note that the model predicts that morphologically elongated sensory organs would require an intersection of only two compartments to define their spatial location. Such organs would include the organ of Corti (in mammals), the basilar papilla (in reptiles and birds), or the vestibular maculae. In contrast, morphologically punctate sensory organs, such as the cristae, would require an intersection of at least three compartments to pinpoint location. Note also that the model allows two sensory organs to form adjacent to one other, but separated by a boundary. This review will summarize the current evidence in favor of the compartment boundary model, and suggest future experimental approaches that might offer tests of the model's validity.
Sensory Organs Arise at the Boundaries of Broader Gene Expression Domains
One hypothesis suggested by the compartment boundary model is that sensory organs will arise at or adjacent to the boundaries defined by large gene expression domains (Fig. 3). This possibility is now supported by circumstantial evidence in the chicken otocyst. In situ hybridization was used to map the spatial domains of two homeobox-containing genes, SOHo-1 (hereafter called SOHo) and GH6. These two chicken genes were among the first members of the Hmx (Nkx5) family of putative transcription factor genes that were reported to be expressed in the developing ear (17, 18). Others include Hmx2 (Nkx5.2) in the mouse and Hmx3 (Nkx5.1) in the mouse and chicken (8, 19, 20). SOHo was initially thought to be expressed exclusively in nonsensory tissues of the ear (17), but with the discovery of Bmp4 as a marker of incipient sensory organs (21), this is now thought to be incorrect. To fully explore the expression domains of SOHo and GH6, and their relationship to Bmp4, we teamed up with F. Nunes and D. K. Wu to hybridize adjacent sections through ears at various developmental stages. The results showed that the two Hmx genes seemed to define a lateral “compartment,” eventually becoming confined to the territory of the forming semicircular canals (10). At an earlier stage of morphogenesis, several sensory organs formed at the boundaries of this lateral compartment, either just within it (two cristae of the semicircular canals) or just outside of it (the utricular macula and the basilar papilla/lagena anlagen). Fig. 4 shows the expression patterns of SOHo and Bmp4 at a stage of development when only the anlagen of the anterior and posterior cristae are expressing Bmp4. Note that both of these sensory anlagen share a boundary with the SOHo expression domain (arrows, Fig. 4). The expression of SOHo is somewhat weaker in the Bmp4-expressing loci, possibly a prelude to the later stages of sensory organ development, when SOHo expression is lost altogether in the hair cell-bearing patches (10).
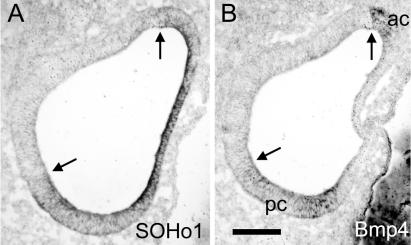
Figure 4.
Sensory organ primordia first arise adjacent to the boundary of a gene, SOHo-1, that identifies a putative lateral compartment. Adjacent horizontal sections through the center of the stage 19 chicken otocyst were probed for SOHo1 mRNA (A) or Bmp4 mRNA (B). At this stage, Bmp4 marks the sensory anlagen of the anterior crista (ac) and the posterior crista (pc) (21). Arrows indicate the places where the two genes appear to share the same boundary. Lateral is to the left and anterior is up. (Scale bar = 100 μm.)
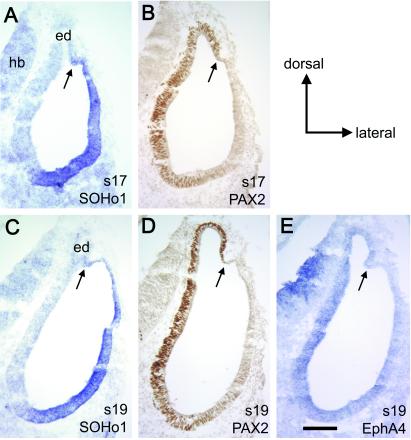
The Endolymphatic Duct Arises at a Putative Medial–Lateral (M-L) Boundary
While SOHo and GH6 appear to identify a lateral compartment in the chick, there is evidence that GATA3, an unrelated transcription factor, maps to a similar place in the mouse otocyst (M. Holley, personal communication). One notable feature of the three genes, SOHo, GH6, and GATA3, is that they each stop just beyond the base of the endolymphatic duct (Fig. 5 A and C). We were interested in comparing these to medial genes to ascertain whether they also ended abruptly at the dorsal pole, perhaps forming a boundary with the lateral genes. We mapped two molecules in the chick that were interesting in this regard, PAX2 protein and EphA4 mRNA. The messages for both genes had previously been described as being expressed in the medial part of the chick or mouse otocyst (8, 22, 23). We have used a commercial antibody against PAX2 to map the expression of the protein in the ear of the chicken embryo at stages 17–26 (24). Alternate sections were subjected to in situ hybridization using probes to SOHo or EphA4. The results (Fig. 5 B, D, and E) show that PAX2 and EphA4 appear to map to the same locations: the medial wall of the vesicle, including the endolymphatic duct, and nearly all of the enlarging cochlear duct region (24). Interestingly, the endolymphatic duct arises at the limit of the PAX2/EphA4 expression domain at the dorsal pole of the vesicle. Immediately adjacent to this domain, but later separated by a small gap, SOHo expression begins. That is, our data indicate that the endolymphatic duct arises just medial to a M-L boundary defined by the juxtaposition of PAX2/EphA4 and SOHo near the dorsal pole (arrows, Fig. 5).
Figure 5.
A putative compartment boundary separating medial and lateral gene expression domains can be found near the dorsal pole of the otocyst in the chicken embryo. Adjacent transverse sections through the middle of the chicken otocyst were probed for SOHo1 mRNA (A and C), PAX2 protein (B and D), or EphA4 mRNA (E). The expression of EphA4 is generally weak, but appears to map to the same location as PAX2 at stage 19 (s19), as well as all stages from 18 to 27 (data not shown). Note that all three genes appear to overlap in the ventral but not the dorsal otocyst. Arrows indicate the position of a putative M-L boundary just lateral to the developing endolymphatic duct (ed). (Scale bar = 100 μm.) hb, Hindbrain.
Although it was initially reported that Pax2 mRNA was not expressed in the endolymphatic duct of the mouse (8, 22), this finding has recently been called into question. From studies using PAX2 antibodies (M. Holley, personal communication) or Pax2 mRNA probes (D. K. Wu, personal communication), it now appears that both the protein and the mRNA are detected in the endolymphatic duct at early stages of duct outgrowth in the mouse. Thus, in both the mouse and the chick, PAX2 defines a medial compartment that includes the endolymphatic duct. Furthermore, this compartment abuts a lateral gene expression domain at the dorsal pole of the otocyst, thus providing circumstantial support in favor of a compartment boundary. It is intriguing that a key morphological structure, the endolymphatic duct, makes its appearance immediately adjacent to this proposed compartment boundary.
While PAX2/EphA4 and SOHo never seem to overlap in the dorsal part of the otocyst, they clearly overlap in the ventral pole (Fig. 5) (24). This is yet another example of partially overlapping domains in the ventral vesicle, leading to the conclusion that unraveling the number, identity, and timing of gene expression compartments in this part of the vesicle will be a challenge.
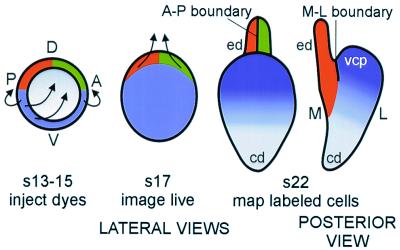
Lineage-Restriction Compartments in the Dorsal Otocyst Revealed by Fate Mapping the Chicken Otic Cup
To reveal whether gene expression compartments also represent lineage-restriction compartments, one can mark individual clones of cells and determine whether they mingle freely with unrelated clones. If relatively large clones of cells generate abrupt, linear borders, then a lineage compartment is indicated. Although clonal marking is ideal for addressing this issue, fate mapping slightly larger pools of cells can also reveal lineage compartments. In addition to being useful in detecting boundaries, fate mapping can also provide information about the range of cell movements that accompany morphogenesis. In the ear, this is particularly critical for understanding the topology of otic vesicle closure and how this might aid in the establishment of axial polarity in the otocyst. Without fate mapping, it is difficult to envision how the three major axes of the ear (anterior–posterior, dorsal–ventral, and medial–lateral) arise from a two-dimensional palette, the otic placode. The process of otic cup closure appears to cinch together the outer edges, or rim, of the cup through a purse-string type of closure mechanism. The question arises as to how cells move to accomplish otic cup closure, and how genes with asymmetric expression patterns at the cup stage might be related through these stages of development. That is, it is worthwhile to consider whether a gene expressed at both stages is marking a stable pool of progenitor cells, or whether some cells are down-regulating while others are up-regulating a particular gene. This knowledge is critical for understanding how these stages (placode to vesicle) are related in terms of positional information.
To address the question of what morphogenetic movements of cells accompany cup closure, and to gain further insight into the possible generation of compartments and boundaries, we have performed fate mapping of the cells located on the rim of the otic cup. This was accomplished by targeting small focal injections of fluorescent carbocyanine dyes to the rim of the otic cup. The location of the labeled cells was assessed 24 h later, after cup closure, and 48 h later, in the early stages of morphogenesis. Although the data will be presented in detail elsewhere (44), a schematic summary of the pertinent results is shown in Fig. 6. Fate mapping the otic cup revealed that the dorsal half of the rim gave rise to the endolymphatic duct. Surprisingly, there appeared to be a rather abrupt border that bisected the endolymphatic duct, whereby cells arising from the anterior half did not mix with cells arising from the posterior half. These data suggested that there may be an A-P lineage restriction boundary at this location (Fig. 6). At the present time, there have been no reports of genes whose expression domains clearly respect this putative A-P boundary, bisecting the endolymphatic duct.
Figure 6.
A schematic of the results of fate mapping the chick otic cup. The data suggest that two lineage-restriction boundaries intersect at the dorsal pole of the otocyst. The M-L lineage-restriction boundary corresponds to the same location as the boundary defined by SOHo1 and PAX2 expression domains (compare with Fig. 5). The anterior–posterior (A-P) lineage-restriction boundary appears to bisect the endolymphatic duct (ed). Arrows indicate directions of growth seen at subsequent stages. This schematic is based on data from ref. 44. D, dorsal; V, ventral; cd, cochlear duct; s, stage; vcp, vertical canal pouch.
Cells from the posteroventral third of the cup gave rise to the entire lateral half of the closed vesicle. Much of this region will go on to form the three semicircular canals. At the time of vesicle closure, these cells came into contact with those that will form the base of the endolymphatic duct, but did not appear to mingle with them. That is, another lineage-restriction boundary is suggested that would prevent the medial (dorsally derived) and lateral (posteroventrally derived) progenitors from mixing. We call this the M-L boundary (Fig. 6). This cell lineage boundary is located at the same place as the gene expression boundary defined by SOHo and PAX2 (arrows, Fig. 5). Thus, the M-L boundary would appear to fulfil both the gene expression and the lineage-restriction criteria needed to represent an authentic compartment boundary.
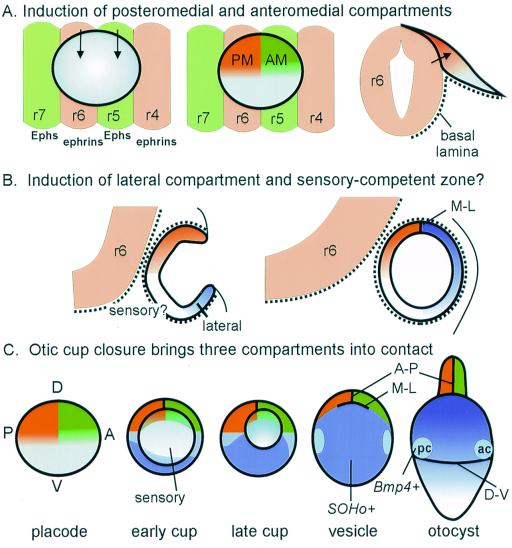
Three Compartments Appear to Intersect at the Dorsal Pole of the Vesicle
As predicted by the compartment-boundary model, there appears to be an intersection of three compartments (anteromedial, posteromedial, and lateral) at the dorsal pole of the vesicle that is ideally positioned to provide the information needed to specify the location of endolymphatic duct outgrowth. We predict that the lateral compartment may be sending an inductive signal to the two adjacent compartments, which then act or interact together to generate another signal directing cells near the boundary intersection to evaginate dorsally. The lateral compartment is not drawn up into this elongating duct, but remains a distinct compartment.
Within a few stages, the lateral compartment will independently begin to enlarge dorsally to form the vertical canal pouch (Fig. 6, vcp), which eventually becomes the anterior and posterior canals. Perhaps canal outgrowth is controlled by a signal sent in the opposite direction to those specifying duct outgrowth. That is, a signal might originate in both medial compartments that crosses all along the M-L boundary to direct canal outgrowth as a thin plate oriented in the A-P direction. The formation of the vertical pouch causes the former “dorsal” pole of the vesicle (where A-P and M-L boundaries originally intersected) to lie in a deepening crevasse between the endolymphatic duct and the vertical canal pouch.
Is the Hindbrain a Source of Patterning Information for Inner Ear A-P Compartments?
If, as suggested by the fate mapping, there are two intersecting compartment boundaries formed as the cup closes, then the implication is that the cells constituting the three compartments must have distinct identities that keep them from mixing. One possibility is that the ear first acquires general axial polarity, such as A-P or dorsal-ventral polarity. Because the ear is a relatively late-developing embryonic field, it should be possible for it to obtain information about its axial polarity from adjacent tissues. This positional information could take the form of broad gradients, or it could be imposed more directly as absolute differences between neighboring cells, depending on the source(s) of the putative inductive signal(s). An ideal candidate for a source of positional information is the hindbrain (25–30). Consider first how the hindbrain might contribute information to specify the anterior and posterior lineage-restriction compartments that appear to bisect the dorsomedial otocyst. It is noteworthy that the otic cup forms adjacent to two hindbrain compartments, rhombomeres 5 and 6 (r5 and r6). The boundary between the two rhombomeres is aligned almost precisely with the middle of the otic cup. Therefore, the A-P boundary of the otic cup is aligned with the r5-r6 boundary (Fig. 7A). This seems unlikely to be a chance arrangement. The r5-r6 boundary is one of the earliest to make its appearance; it is formed by early stage 9 (31), when the otic placode is just beginning to form (32–34). We speculate that r5 and r6 may be sending different signals to the anterior and posterior otocyst, respectively. We further suggest that these signals might be transferred most effectively or even exclusively to the dorsal half of the vesicle, where the neural tube and otic placode cells come into direct contact because of the absence of a basal lamina between them (34). This would generate, from the start, separate anteromedial (AM) and posteromedial (PM) compartments that are detectable by fate mapping (Fig. 7A).
Figure 7.
A schematic showing possible inductive influences of hindbrain on the development of putative compartments in the otic vesicle. (A) The r5-r6 boundary in the hindbrain is aligned with the A-P lineage-restriction boundary in the otic cup. Thus, r5 and r6 are appropriately positioned to send different inductive signals (arrows) to anterior and posterior otic placode, respectively. Because only the dorsal half of the placode makes intimate contact with the hindbrain cells because of the absence of a basal lamina between the two structures (34), inductive signals that require direct cell contact may not extend to the ventral rim of the otic field, as shown. (B) It is proposed that the ventral tissue does not acquire lateral identity until the cup stage, and is separated from the dorsomedial tissue by a region that acquires sensory competence. The signaling sources for either lateral or sensory identity are unknown, but they could be influenced by distance from the hindbrain and/or proximity to lateral ectoderm or mesoderm. (C) A schematic of otic vesicle formation, combining information from fate mapping and gene expression domains of SOHo and Bmp4. The gray region in the center of the field is proposed to correspond to a sensory-competent region that will intersect with the broader gene expression domains to form the sensory patches and perhaps the ganglion cells. Only the formation of the anterior crista (ac) and posterior crista (pc) is shown. The timing of appearance and location of a putative dorsal–ventral (D-V) boundary, although predicted to be present by stage 22 to place the cristae at the right location, has not yet been confirmed by fate mapping. cd, Cochlear duct; ed, endolymphatic duct; r, rhombomere.
One cell–cell signaling system that could potentially be involved in sending differential positional information from r5 and r6 to the adjacent otic field is the Eph/ephrin system (35, 36). r6 cells express the ligands, ephrin B2 and B3, at high levels but express the receptor tyrosine kinase, EphA7, only weakly; in contrast, r5 cells do not express the ephrins but express the receptors, EphA4, -A7, -B2, and -B3 at high levels (28, 37, 38). Thus, the posteromedial otic cells in direct contact with r6 are positioned to receive an ephrin-mediated signal that those adjacent to r5 would not. Do the otic placode cells express the appropriate receptors? To date, there are no systematic studies at the appropriate stages of development. By the vesicle stage, the medial side expresses at least two receptors, EphA4 and EphA7 (8, 28). One of these, EphA4, is reportedly able to bind not only to the ephrin A ligands but also to ephrin B2 and B3 (37, 39), which are expressed by r6. Clearly, more information is needed about the timing and full range of Eph/ephrin expression in the otic cup to fully explore this potential signaling system. Of course, other types of signals, including diffusible molecules such as bone morphogenic proteins (BMPs) are also candidates for inductive interactions between neural tube and otic epithelium.
The proposed requirement of r5 and/or r6 for inducing A-P differences in the otocyst raises an interesting prediction: that the A-P boundary of the dorsomedial otocyst would be compromised in mutants where r5 and/or r6 fails to form or where the otic epithelium arises far from the hindbrain. According to our model, defective outgrowth of the endolymphatic duct should be one result of all such mutants. Mutants that fulfill one or both of these criteria and are missing the endolymphatic duct include knockouts of Hoxa1 (26, 40–43) and the kreisler mouse mutant (27–29, 45). Mice lacking two retinoic acid receptors, RARα and RARβ, provide an informative counterexample (46). These mice have defects in hindbrain patterning near the otic field, including an expanded r5 and a combined r6/7 rhombomere. However, the r5/r6 boundary is still present with an expanded otic placode arising next to it. Despite the alterations in r5 and r6, the otic vesicle closes, the endolymphatic duct forms, and the inner ear displays no abnormalities by 14.5 days post coitus. This may be explained by the presence of the r5/r6 boundary at the otic placode stage.
Consider next the medial versus lateral positional information. Again it seems plausible for the neural tube cells to interact directly with the medial placode, but not the lateral placode, to provide it with general medial positional information. One possibility is that the lateral cells acquire a distinct identity from the medial cells, either because they do not receive a medializing signal from the neural tube or because they fail to receive an inhibitory signal from the neural tube that prevents them from becoming lateral. However, we suspect that specification of M-L identity may not be this simple, and instead speculate that a lateral compartment may arise later and spatially nonadjacent to the dorsomedial compartments at the placode stage (Fig. 7B). There are several reasons for making this suggestion. First, if a lateral compartment (L) were to form immediately adjacent to the two dorsomedial compartments (AM and PM), then the three compartments would already intersect as early as the placode stage, in the center of the placode. This situation is both temporally and spatially inappropriate for the specification of endolymphatic duct outgrowth according to our model. Second, if we use SOHo as an indicator of lateral identity, we find that it is not expressed until the otic cup stage in the chick, and then only along the posteroventral rim of the cup that is destined to become the lateral compartment (10). Third, there is additional evidence from gene expression data that the center (the ventromedial part) of the invaginating cup may differ from surrounding tissues in the expression of Lmx1, a gene whose expression levels are down-regulated by the adjacent ventral (but not dorsal) neural tube (47). So we are suggesting that lateral identity is acquired or becomes fixed slightly later than medial identity, and only by the lateral-most part of the invaginating cup (as shown in Fig. 7B). The nature of such signal(s) is unclear; it may involve specification and/or maintenance from the adjacent ectoderm. Assuming that M-L differences do arise by the otic cup stage, we would predict that these compartments would differ in their adhesive properties. In this way, when the two groups of cells first meet at the dorsal pole of the otocyst at the time of cup closure, they would resist mixing.
Perturbations that cause abnormal separation between the neural tube and the otic placode would be predicted to cause insufficient or abnormal M-L distinctions within the otic epithelium. In this regard, it is interesting to consider the kreisler mutant phenotype (27–30, 45). In these mice, the otic cup develops abnormally far from the hindbrain, and the expression domain of at least one otic gene, Fgf3, is likewise displaced from lateral to medial (48). As a corollary, endolymphatic duct outgrowth is often severely truncated or absent. A second example is the case of the retinaldehyde dehydrogenase 2 knockout mice (Raldh2−/−), which are unable to synthesize retinoic acid. This defect causes severe alterations in hindbrain patterning and gene expression (49, 50), including a failure of r5 and r6 to be properly specified. The hindbrain defects are accompanied by abnormal expression of M-L genes in the otocyst: Pax2 transcripts are absent and Nkx5.1 (Hmx3) transcripts are expressed throughout the otocyst at 9.5 days post coitus (dpc). This suggests that the otocyst is completely lateralized and the M-L boundary is missing. The endolymphatic duct does not appear to form by 10.5 dpc, when the Raldh2−/− mice die. The Raldh2−/− phenotype suggests that the hindbrain participates in establishing asymmetric M-L gene expression in the otocyst, although a direct effect of retinoids on the ear (51) cannot be ruled out.
Testing a Compartment Boundary Model of Ear Morphogenesis
Ultimately, the utility of a model lies in its predictive value. In the case of the inner ear, it should be possible to predict how the system will respond to different kinds of perturbations, including those that should disrupt compartment boundaries, or those that would be expected to change compartment identity. The generation of knockout mice lacking one or more of the genes expressed in the inner ear is one experimental approach (6). If the genes showing broad expression domains are providing information to specify the identity of the compartments, then eliminating an individual gene might be equivalent to changing the compartment identity. In addition to this, or as an alternative consequence, the gene knockout might disrupt or eliminate one or more of the compartment boundaries defined by the gene of interest. One can look carefully at the phenotypes (shown schematically in Fig. 2B) and try to make sense of them in light of a compartment-boundary model. The phenotypes generated by Pax2 and Dlx5 seem to come closest to suggesting that these two genes each specify a compartment that will develop into the cochlea or the vertical canals, respectively. However, this interpretation is complicated by the fact that the expression domain of Pax2 (and possibly Dlx5) includes tissue fated to form the endolymphatic duct in the chick (J.V.B and D.M.F., unpublished observations), and yet the duct is not always reported to be defective in the mutants (23, 52).
The lateral canal and its sensory organ are of particular interest with respect to the mutant phenotypes. The crista ampullaris has been reported missing in both Hmx3 and Otx1 knockout mice (53, 54). In the schematic, these two genes are mapped to adjacent compartments, and it is known that the lateral crista arises within the Otx1 domain at its dorsal border (55). Although the nonoverlapping domains of Hmx3 and Otx1 must be verified by careful study of adjacent sections, it remains possible that interfering with the expression domains on either side of a compartment boundary can result in the specific loss of the lateral crista. This result would be predicted by the compartment boundary model, assuming that the boundary could not be maintained in the absence of either one of the two genes.
Our hypothesis is that the convergence of three compartments, AM, PM, and L, is a necessary prerequisite for endolymphatic duct outgrowth; one of the most obvious tests would be to generate an ectopic convergence of these boundaries elsewhere in the otic epithelium. The model makes a very specific prediction about what should happen if an ectopic L compartment were created on the A-P boundary in the medial domain: two extra (supernumerary) endolymphatic ducts should arise in this instance. It will be a challenge to perform such an experiment, in view of the fact that some genes appear to regulate (e.g., Hmx3) whereas others (e.g., Pax2) do not (20, 56, 57) in situations where the otic field is rotated or transplanted ectopically. Nonetheless, if properly executed, the result of creating ectopic compartment boundaries could well prove to be as intriguing, and enlightening, as the classical studies in developing and regenerating limbs.
Supplementary Material
Acknowledgments
We thank J. P. Bissonnette for the paint-fill images used to prepare Fig. 1 and the ear morph movie. Contact D.M.F. to download a higher-resolution version of the movie. We thank Connie Cepko for providing probes. This work was supported by grants from the National Institutes of Health (to D.M.F.), the March of Dimes Birth Defects Foundation (to D.M.F.), and the National Organization for Hearing Research (to J.V.B.).
Abbreviations
- M-L
medial–lateral
- A-P
anterior–posterior
Footnotes
This paper was presented at the National Academy of Sciences colloquium “Auditory Neuroscience: Development, Transduction, and Integration,” held May 19–21, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Lang H, Bever M M, Fekete D M. J Comp Neurol. 2000;417:205–220. doi: 10.1002/(sici)1096-9861(20000207)417:2<205::aid-cne6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Fekete D M. Curr Opin Neurobiol. 1996;6:533–541. doi: 10.1016/s0959-4388(96)80061-4. [DOI] [PubMed] [Google Scholar]
- 3.Whitfield T, Haddon C, Lewis J. Semin Cell Dev Biol. 1997;8:239–247. doi: 10.1006/scdb.1997.0143. [DOI] [PubMed] [Google Scholar]
- 4.Torres M, Giraldez F. Mech Dev. 1998;71:5–21. doi: 10.1016/s0925-4773(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 5.Rivolta M N. Audiol Neurootol. 1997;2:36–49. doi: 10.1159/000259228. [DOI] [PubMed] [Google Scholar]
- 6.Fekete D M. Trends Neurosci. 1999;22:263–269. doi: 10.1016/s0166-2236(98)01366-6. [DOI] [PubMed] [Google Scholar]
- 7.Ryan A F. Semin Cell Dev Biol. 1997;8:249–256. doi: 10.1006/scdb.1997.0146. [DOI] [PubMed] [Google Scholar]
- 8.Rinkwitz-Brandt S, Arnold H H, Bober E. Hear Res. 1996;99:129–138. doi: 10.1016/s0378-5955(96)00093-7. [DOI] [PubMed] [Google Scholar]
- 9.Bosse A, Zülch A, Becker M-B, Torres M, Gómez-Skarmeta J L, Modellell J, Gruss P. Mech Dev. 1997;69:169–181. doi: 10.1016/s0925-4773(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 10.Kiernan A E, Nunes F, Wu D K, Fekete D M. Dev Biol. 1997;191:215–229. doi: 10.1006/dbio.1997.8716. [DOI] [PubMed] [Google Scholar]
- 11.Meinhardt H. J Embryol Exp Morphol. 1983;76:115–137. [PubMed] [Google Scholar]
- 12.Meinhardt H. Dev Biol. 1983;96:375–385. doi: 10.1016/0012-1606(83)90175-6. [DOI] [PubMed] [Google Scholar]
- 13.Dahmann C, Basler K. Trends Genet. 1999;15:320–326. doi: 10.1016/s0168-9525(99)01774-6. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence P A, Struhl G. Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- 15.Shubin N, Tabin C, Carroll S. Nature (London) 1997;388:639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Drossopoulou G, Chuang P T, Duprez D, Marti E, Bumcrot D, Vargesson N, Clarke J, Niswander L, McMahon A, et al. Development (Cambridge, UK) 1997;124:4393–4404. doi: 10.1242/dev.124.21.4393. [DOI] [PubMed] [Google Scholar]
- 17.Deitcher D L, Fekete D M, Cepko C L. J Neurosci. 1994;14:486–498. doi: 10.1523/JNEUROSCI.14-02-00486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stadler H S, Solursh M. Dev Biol. 1994;161:251–262. doi: 10.1006/dbio.1994.1025. [DOI] [PubMed] [Google Scholar]
- 19.Bober E, Baum C, Braun T, Arnold H H. Dev Biol. 1994;162:288–303. doi: 10.1006/dbio.1994.1086. [DOI] [PubMed] [Google Scholar]
- 20.Herbrand H, Guthrie S, Hadrys T, Hoffmann S, Arnold H, Rinkwitz-Brandt S, Bober E. Development (Cambridge, UK) 1998;125:645–654. doi: 10.1242/dev.125.4.645. [DOI] [PubMed] [Google Scholar]
- 21.Wu D K, Oh S-H. J Neurosci. 1996;16:6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nornes H O, Dressler G R, Knapik E W, Deutsch U, Gruss P. Development (Cambridge, UK) 1990;109:797–809. doi: 10.1242/dev.109.4.797. [DOI] [PubMed] [Google Scholar]
- 23.Torres M, Gomez-Pardo E, Gruss P. Development (Cambridge, UK) 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- 24.Fekete D M, Gao X. In: Molecular and Cellular Biology of the Ear. Lim D, editor. New York: Plenum; 2000. pp. 99–112. [Google Scholar]
- 25.Mansour S L, Goddard J M, Capecchi M R. Development (Cambridge, UK) 1993;117:13–28. doi: 10.1242/dev.117.1.13. [DOI] [PubMed] [Google Scholar]
- 26.Mark M, Lufkin T, Vonesch J L, Ruberte E, Olivo J C, Dolle P, Gorry P, Lumsden A, Chambon P. Development (Cambridge, UK) 1993;119:319–338. doi: 10.1242/dev.119.2.319. [DOI] [PubMed] [Google Scholar]
- 27.Deol M S. J Embryol Exp Morphol. 1964;12:475–490. [PubMed] [Google Scholar]
- 28.Manzanares M, Trainor P A, Nonchev S, Ariza-McNaughton L, Brodie J, Gould A, Marshall H, Morrison A, Kwan C T, Sham M H, et al. Dev Biol. 1999;211:220–237. doi: 10.1006/dbio.1999.9318. [DOI] [PubMed] [Google Scholar]
- 29.Cordes S P, Barsh G S. Cell. 1994;79:1025–1034. doi: 10.1016/0092-8674(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 30.Frohman M A, Martin G R, Cordes S P, Halamek L P, Barsh G. Development (Cambridge, UK) 1993;117:925–936. doi: 10.1242/dev.117.3.925. [DOI] [PubMed] [Google Scholar]
- 31.Fraser S, Keynes R, Lumsden A. Nature (London) 1990;344:431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- 32.Meier S. Anat Rec. 1978;191:447–458. doi: 10.1002/ar.1091910405. [DOI] [PubMed] [Google Scholar]
- 33.Hilfer S R, Esteves R A, Sanzo J F. J Exp Zool. 1989;251:253–264. doi: 10.1002/jez.1402510213. [DOI] [PubMed] [Google Scholar]
- 34.Mayordomo R, Rodriguez-Gallardo L, Alvarez I S. J Anat. 1998;193:35–48. doi: 10.1046/j.1469-7580.1998.19310035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Q, Mellitzer G, Robinson V, Wilkinson D G. Nature (London) 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- 36.Mellitzer G, Xu Q, Wilkinson D G. Curr Opin Neurobiol. 2000;10:400–408. doi: 10.1016/s0959-4388(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 37.Bergemann A D, Zhang L, Chiang M K, Brambilla R, Klein R, Flanagan J G. Oncogene. 1998;16:471–480. doi: 10.1038/sj.onc.1201557. [DOI] [PubMed] [Google Scholar]
- 38.Gale N W, Flenniken A, Compton D C, Jenkins N, Copeland N G, Gilbert D J, Davis S, Wilkinson D G, Yancopoulos G D. Oncogene. 1996;13:1343–1352. [PubMed] [Google Scholar]
- 39.Gale N W, Holland S J, Valenzuela D M, Flenniken A, Pan L, Ryan T E, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson D G, et al. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter E M, Goddard J M, Osamu C, Manley N R, Capecchi M R. Development (Cambridge, UK) 1993;118:1063–1075. doi: 10.1242/dev.118.4.1063. [DOI] [PubMed] [Google Scholar]
- 41.Chisaka O, Musci T S, Capecchi M R. Nature (London) 1992;355:516–520. doi: 10.1038/355516a0. [DOI] [PubMed] [Google Scholar]
- 42.Lufkin T, Dierich A, LeMeur M, Mark M, Chambon P. Cell. 1991;66:1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- 43.Rossel M, Capecchi M R. Development (Cambridge, UK) 1999;126:5027–5040. doi: 10.1242/dev.126.22.5027. [DOI] [PubMed] [Google Scholar]
- 44.Brigande, J. V., Iten, L. E. & Fekete, D. M. (2000) Dev. Biol., in press. [DOI] [PubMed]
- 45.McKay I J, Muchamore I, Krumlauf R, Maden M, Lumsden A, Lewis J. Development (Cambridge, UK) 1994;120:2199–2211. doi: 10.1242/dev.120.8.2199. [DOI] [PubMed] [Google Scholar]
- 46.Dupe V, Ghyselinck N B, Wendling O, Chambon P, Mark M. Development (Cambridge, UK) 1999;126:5051–5059. doi: 10.1242/dev.126.22.5051. [DOI] [PubMed] [Google Scholar]
- 47.Giraldez F. Dev Biol. 1998;203:189–200. doi: 10.1006/dbio.1998.9023. [DOI] [PubMed] [Google Scholar]
- 48.McKay I J, Lewis J, Lumsden A. Dev Biol. 1996;174:370–378. doi: 10.1006/dbio.1996.0081. [DOI] [PubMed] [Google Scholar]
- 49.Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Development (Cambridge, UK) 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- 50.Niederreither K, Subbarayan V, Dolle P, Chambon P. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 51.Choo D, Sanne J L, Wu D K. Dev Biol. 1998;204:136–150. doi: 10.1006/dbio.1998.9095. [DOI] [PubMed] [Google Scholar]
- 52.Acampora D, Merlo G R, Paleari L, Zerega B, Postiglione M P, Mantero S, Bober E, Barbieri O, Simeone A, Levi G. Development (Cambridge, UK) 1999;126:3795–3809. doi: 10.1242/dev.126.17.3795. [DOI] [PubMed] [Google Scholar]
- 53.Wang W, Van De Water T, Lufkin T. Development (Cambridge, UK) 1998;125:621–634. doi: 10.1242/dev.125.4.621. [DOI] [PubMed] [Google Scholar]
- 54.Acampora D, Mazan S, Avantaggiato V, Barone P, Tuorto F, Lallemand Y, Brulet P, Simeone A. Nat Genet. 1996;14:218–222. doi: 10.1038/ng1096-218. [DOI] [PubMed] [Google Scholar]
- 55.Morsli H, Tuorto F, Choo D, Postiglione M P, Simeone A, Wu D K. Development (Cambridge, UK) 1999;126:2335–2343. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- 56.Hutson M R, Lewis J E, Nguyen-Luu D, Lindberg K H, Barald K F. J Neurocytol. 1999;28:795–807. doi: 10.1023/a:1007057719025. [DOI] [PubMed] [Google Scholar]
- 57.Wu D K, Nunes F D, Choo D. Development (Cambridge, UK) 1998;125:11–20. doi: 10.1242/dev.125.1.11. [DOI] [PubMed] [Google Scholar]
- 58.Bissonnette J P, Fekete D M. J Comp Neurol. 1996;368:620–630. doi: 10.1002/(SICI)1096-9861(19960513)368:4<620::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 59.Rinkwitz-Brandt S, Justus M, Oldenettel I, Arnold H-H, Bober E. Mech Dev. 1995;52:371–381. doi: 10.1016/0925-4773(95)00414-v. [DOI] [PubMed] [Google Scholar]
- 60.Acampora D, Avantaggiato V, Tuorto F, Barone P, Reichert H, Finkelstein R, Simeone A. Development (Cambridge, UK) 1998;125:1691–1702. doi: 10.1242/dev.125.9.1691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.