Abstract
The epimorphic regeneration of zebrafish caudal fin is rapid and complete. We have analyzed the biomechanism of zebrafish caudal fin regeneration at various time points based on differential proteomics approaches. The spectrum of proteome changes caused by regeneration were analyzed among controls (0 h) and 1, 12, 24, 48, and 72 h postamputation involving quantitative differential proteomics analysis based on two-dimensional gel electrophoresis matrix-assisted laser desorption/ionization and differential in-gel electrophoresis Orbitrap analysis. A total of 96 proteins were found differentially regulated between the control nonregenerating and regenerating tissues of different time points for having at least 1.5-fold changes. 90 proteins were identified as differentially regulated for regeneration based on differential in-gel electrophoresis analysis between the control and regenerating tissues. 35 proteins were characterized for its expression in all of the five regenerating time points against the control samples. The proteins identified and associated with regeneration were found to be directly allied with various molecular, biological, and cellular functions. Based on network pathway analysis, the identified proteome data set for regeneration was majorly associated in maintaining cellular structure and architecture. Also the proteins were found associated for the cytoskeleton remodeling pathway and cellular immune defense mechanism. The major proteins that were found differentially regulated during zebrafish caudal fin regeneration includes keratin and its 10 isoforms, cofilin 2, annexin a1, skeletal α1 actin, and structural proteins. Annexin A1 was found to be exclusively undergoing phosphorylation during regeneration. The obtained differential proteome and the direct association of the various proteins might lead to a new understanding of the regeneration mechanism.
Regeneration is an important mechanism found among most of the animals including humans in various tissues and organs towards growth, regrowth, repair, reproduction, and survival. The biomechanism of regeneration has been widely studied but poorly understood because of its different extents in various animals. Understanding the basic molecular mechanism of regeneration in the wound environment is of high significance, because it can lead to an applied possibility of making nonregenerating to a regenerating system.
Tissue regeneration in vertebrates is found with extensive capabilities. Regeneration of limbs in urodele and caudal fin in zebrafish are the most projected regeneration studies among vertebrates. Zebrafish regenerates a wide variety of tissue structures including heart, fin, spinal cord, and optic nerve (1–3) based on the characteristic regeneration mechanism involving epithelialization, mesenchymal disorganization, blastema formation, regenerative outgrowth, and termination.
A great number of gene families, counting wnt, hox, fgf, fgfr, and msx genes, were shown to be differentially expressed for controlling the regeneration mechanism (4), without understanding a defiant pathway and biomechanism for the regeneration. In this study, we have analyzed the regeneration of zebrafish caudal fin tissue in normal conditions. The differential proteomic analyses were performed for the different time points of regeneration towards understanding and analyzing the differentially expressed genes or proteins.
EXPERIMENTAL PROCEDURES
Animals and Regeneration Experiments
Wild zebrafish obtained from local farmers were housed and maintained under standard conditions in sterile water (5, 6). Male and female adult zebrafish between the age of 6 months and 1 year were selected for the regeneration experiments. Caudal fin tissues of same size were amputated from the distal part of the caudal fin using sterile blades after anesthetizing the animals for 10 min in 0.1% Tricaine (Sigma). After recovering from anesthesia, the animals were allowed to regenerate the amputated caudal fin under normal conditions. Regenerating fin tissues were further collected by amputating the fins in the same way for different time points such as 1, 12, 24, 48, and 72 h postamputation.
Sample Preparation and Differential Proteomics Analysis
Fin tissues collected from each of the stages (0 (control), 1, 12, 24, 48, and 72 h postamputation (hpa))1 were pooled separately, and the total proteins were extracted after washing twice with Locke Ringer's solution (0.9% NaCl, 0.042% KCl, 0.024% CaCl2, 0.02% MgCl2, 0.05% NaHCO3, and 0.05% dextrose w/v in Milli-Q) and 1× phosphate-buffered saline. The total protein of the fin tissues were extracted upon homogenization and sonication in dissolving buffer (7 m urea, 2 m thiourea, 4% CHAPS, 18 mm Tris-HCl, 14 mm Trizmabase, two tablets of EDTA-free protease inhibitor cocktail, 0.2% Triton-X, 50 mm DTT). The proteins were further precipitated using trichloroacetic acid and quantified against bovine serum albumin standard using Amido Black assay (7). Differential proteomic analyses were performed between the regenerating samples and the nonregenerating control based on 2DE and DIGE analysis.
In 2DE analysis, the first dimensional separation based on pH was performed in a broad range 3–10 NL IPG strips using 400 μg of total protein under standard focusing conditions in duplicate. The second dimensional separation was performed in 12% SDS-PAGE for all of the six sample sets (8). The 2DE gels were colloidal stained, scanned, and quantified using IMP (GE Healthcare) software for the differential display of the spot pattern between the nonregenerating zero hour pattern against the five various regenerating patterns. The quantification of the protein spots for regeneration was analyzed by comparing the expression level of protein spots during regeneration against the nonregenerating control fixed as one fold.
DIGE were performed between the control (0 hpa) and the regenerating (1, 12, 24, 48, and 72 hpa) protein samples. DIGE were performed using the Ettan DIGE system (GE Healthcare) as per manufacturer's protocol using 50 μg of the control protein sample labeled with Cy3, 50 μg of regenerating protein sample labeled with Cy5, and 25 μg of each control and regenerating protein sample with Cy2, as internal control. First dimensional isoelectric focusing and second dimensional gel electrophoresis were performed as described for 2DE. The gels were then scanned in Typhoon TRIO scanner (Amersham Biosciences) at 100 dpi for all three fluorescent labels and analyzed in DeCyder software program (GE Healthcare) for estimating the spot quantitation. The expression levels of the protein spots were quantified by comparing the expression levels between the control nonregenerating zero hour and the regenerating time points.
MS AND MSMS Analysis
Protein spots having more than 1.5-fold change between the nonregenerating (zero hour sample) and regenerating tissues (all five time points) based on 2DE and DIGE gel analysis were selected for the MS and MSMS analyses. The protein spots were manually excised, destained, washed, dehydrated, and trypsin-digested (10) for the MS and MSMS analyses based on either MALDI MS/MS or Fourier transform mass spectrometer (FTMS) and ion trap tandem mass spectrometer (ITMSMS) analyses. MALDI MS/MS analyses were performed as described earlier (9). Tryptic digested peptides were estranged for FTMS and ITMSMS analysis using nanoflow LCMS analysis in the Orbitrap Nano analyzer (Thermo). The obtained MS/MS peak list were analyzed using SEQUEST (Thermo proteome Discover 1.1 version 1.1.0.263; Thermo Fisher Scientific) and MASCOT search engines against the Danio rerio database (IPI and Swissprot) with a mass tolerance of 10 ppm for the precursor ions and 0.2 dalton for fragment ions (10). The obtained protein details were tabulated and confirmed for its mass and pI against the experimental data.
One- and Two-dimensional Western Blot Analysis
Proteins obtained from the control and regenerating tissues were analyzed for its expression level based on both one-dimensional gel electrophoresis and 2DE Western blot analysis. For one-dimensional gel electrophoresis Western blot analysis, 40 μg of total proteins of each time point were electrophoresed on a 10% SDS-PAGE and transferred using wet transfer method. Immunoblot analyses were performed for keratin 5 (KRT5), keratin 17 (KRT17), cofilin 2 (CFL2), annexin A1 (ANXA1), and ornithine decarboxylase (ODC) targets using anti-keratin 5 polyclonal antibody (Pierce, Thermo Scientific), anti-keratin 17 monoclonal antibody (Pierce, Thermo Scientific), anti-cofilin polyclonal antibody (Pierce, Thermo Scientific), anti-ANXA1 polyclonal antibody (Abbiotec, LLC), and anti-ODC monoclonal antibody (Sigma) as primary antibodies and respective anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibodies (Pierce, Thermo Scientific). The immunoblots were scanned using ECL detection method for estimating the expression level of KRT5, KRT17, CFL2, and ANXA1 against the ODC housekeeping protein. The expression level of ANXA1 and its phosphorylation was confirmed based on Western blot analysis on 2DE gel. The 2DE gels were transferred using wet transfer method followed by immunodetection of ANXA1 and phosphorylation using the anti-ANXA1 polyclonal antibody (Abbiotec, LLC) and anti-pan phosphotyrosine monoclonal antibody (Cell Signaling Technology) as primary antibodies respectively, and horseradish peroxidase-conjugated secondary antibody as secondary antibody. The immunoblots were scanned for positive expressing spots using the ECL detection method.
Real Time PCR Analysis
Total RNA was extracted from the control and regenerating caudal fin tissues using TRI reagent (Sigma) according to the manufacturer's protocol. 1.0 μg of the total RNA was reverse transcribed using 200 units of reverse transcriptase (Superscript III; Invitrogen) and 75 ng of random hexamer at 50 °C for 90 min. Quantitative real time PCR was performed using MESA green Q-PCR master mix plus for SYBR assay (Eurogentec) in ABI 7900HT (Applied Biosystems). Real time PCR was performed for 10 different genes, which were found differentially regulated during regeneration using gene specific primers (see Table III). Zebrafish ODC was used as the reference housekeeping gene for normalization. PCR was performed in triplicate using the following conditions: one cycle of 50 °C for 5 min; one cycle of 95 °C for 5 min; and 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. Amplification of the single RT-PCR product was confirmed by monitoring the dissociation curve and loading the amplified product in 2% agarose gel against DNA ladder. The quantification of the PCR product was performed both using the Ct values and band quantification against the housekeeping gene.
Table III. Primers for quantitative real time PCR amplification.
| S No. | Gene name | Froward primer sequence (5′ to 3′) | Reverse primer sequence (5′ to 3′) |
|---|---|---|---|
| 1 | Keratin 5 | TGGAGGACTTCAAGAACAAATAT | CTTTGATCTGTGACTGGAGTTC |
| 2 | Keratin 17 | GGAGTGCGTGCTGGTAGTGTCT | GGTAGGAGGCCAGACGATCATT |
| 3 | Cofilin 2 | ATCCCTACCTTAAGTTTGTGAA | TGCTTAATACCTGTGAACTTCT |
| 4 | VDAC2 | CGGTATGGTGAAGCTCGATGTC | TGCGATCTGGTCTTCAATGTTG |
| 5 | VDAC3 | ATGAACGTGGGCTGTGATCTAG | CCGAACTCTGTGCCATCATTAAC |
| 6 | CTSB | GGATCTGCTAACCTGCTGTGAC | TCTCCACCCTCTCCTGAACATG |
| 7 | HSPA8 | AAGGAAACAGGACCACACCAAGT | GGACCTTGGGACGGGAATTG |
| 8 | SOD1 | TCGGAGACCTGGGTAATGTGAC | CGATCACTCCACAGGCCAGAC |
| 9 | ANXA1 | CCACAGTACAAGACATCACAGA | CTTGCTAGTGGCTTGCTGGTA |
| 10 | PRDX5 | GCAGGAGGAAGACCCGGGAAAC | CCAGGCGGACATAACAAACACATC |
| 11 | ODC | CAACATCATCGCCAAAAAGGTCATC | GCTCATCGGGCTTGGGTTTCTTGT |
Data Analysis
The proteins that were identified and found differentially displayed during the process of regeneration were mapped to their respective Uniprot accession ID, gene ID, and protein symbol based on Uniprot and DAVID (Database for Annotation Visualization and Integrated Discovery) analysis. All of the identified proteins were further analyzed for their cellular localization, biological processes, and molecular functions based on STRAP software program analysis (11). Also, the differentially displayed proteins were analyzed for different processes, networks and pathway maps using the GeneGo software (www.genego.com) analysis.
RESULTS
2DE and DIGE Analysis
From the six different broad range 2DE (supplemental Figs. 1–6) a total of 745, 685, 678, 691, 677, and 968 spots were detected by the software for the comparative differential analysis between nonregenerating and regenerating tissues. Based on the comparative analysis of spot volume and intensity, a total of 35 protein spots were selected from each of the time points for MALDI MS/MS and FTMS/ITMSMS analysis (Table I). The various protein spots selected for differential analysis were found to be either up or down-regulated by a minimum of 1.5-fold changes between the nonregenerating and any of the regenerating stages. From the DIGE analysis, a total of 58, 88, 39, 78, and 73 protein spots were selected for having at least 1.5-fold changes between the nonregenerating and regenerating 1, 12, 24, 48, and 72 hpa samples (Fig. 1). A total of 90 proteins were identified from the DIGE analysis based on either MALDIMS/MSMS or Orbitrap-based FTMS/ITMSMS analysis (Table II), which includes 41 and 49 down- and up-regulated proteins.
Table I. List of 35 differentially regulated proteins identified based on 2-DE MALDI MS/MS and FTMS/ITMSMS analysis.
This table provides the list of 35 proteins identified as differentially regulated proteins for caudal fin regeneration. The proteins are listed along with the ID, accession number, Uniprot ID, spot intensity indicating fold changes against 0 h, coverage, PSMs, peptides, number of amino acids, molecular mass, calculated (Calc.) pI, score, gene ID, description, and peptide sequences identified based on proteomics study.
| S No. | ID | Accession number | Uniprot ID | Spot intensity |
Coverage | No. PSMs | No. Peptides | No. amino acids | Molecular mass (kDa) | Calc. pI | Score | Gene ID | Description | Sequences | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1 hpa | 12 hpa | 24 hpa | 48 hpa | 72 hpa | ||||||||||||||
| 1 | 1 | IPI00933179 | E7EZL7 | 1 | 0.9 | 1.0 | 0.5 | 0.9 | 1.1 | 16.96 | 9 | 1 | 112 | 12.6 | 5.20 | 58.01 | AGR3 | PREDICTED: anterior gradient protein 3 homolog | mYTYEPDDIDFLAENmIK |
| 2 | 2 | IPI00503480 | B3DFP9 | 1 | 0.6 | 1.0 | 0.8 | 0.7 | 1.0 | 17.73 | 1 | 1 | 141 | 15.5 | 7.18 | 4.50 | SOD1 | Superoxide dismutase (Cu-Zn) | IEIEDAmLTLSGQHSIIGR |
| 3 | 3 | IPI00490384 | Q7ZUQ9 | 1 | 0.8 | 0.9 | 1.5 | 1.0 | 1.9 | 11.41 | 9 | 1 | 184 | 19.2 | 9.31 | 85.76 | cirbp | Cold inducible RNA-binding protein | LFIGGLSYDTTEQSLEEAFSK |
| 4 | 4 | IPI00512240 | A4VAK9 | 1 | 0.6 | 1.0 | 0.9 | 1.1 | 0.8 | 16.45 | 18 | 3 | 462 | 50.0 | 9.09 | 139.11 | eef1a | Elongation factor 1-α | SVEMHHESLTEATPGDNVGFNVK; NmITGTSQADcAVLIVAGGVGEFEAGISK; SVEMHHESLTEATPGDNVGFNVK |
| 5 | 5 | IPI00500568 | A5PMZ3 | 1 | 1.6 | 1.1 | 1.4 | 1.3 | 1.1 | 8.06 | 6 | 3 | 720 | 79.4 | 6.46 | 62.87 | gsn | Novel protein similar to gelsolin, like 1 | AVEVEPSAASLNTNDVFVLK; TYGQFFGGDcYLILYTYK; LVmVEEDAEPDALIQALGPK |
| 6 | 6 | IPI00852136 | Q90473 | 1 | 1.5 | 1.4 | 2.2 | 2.0 | 1.5 | 3.54 | 1 | 1 | 649 | 70.9 | 5.31 | 2.20 | hspa8 | Heat shock cognate 71-kDa protein | SINPDEAVAYGAAVQAAILSGDK |
| 7 | 7 | IPI00509935 | Q6PC82 | 1 | 0.3 | 1.1 | 0.6 | 1.0 | 0.5 | 7.76 | 1 | 1 | 245 | 27.9 | 4.78 | 21.31 | ywhab1 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, β polypeptide like | TAFDEAIAELDSLNEESYK |
| 8 | 8 | IPI00500949 | Q7T3A8 | 1 | 1.2 | 1.5 | 0.9 | 1.3 | 1.3 | 1.127 | 8 | 3 | 342 | 37.9 | 6.64 | 91.38 | anxa1a | Annexin A1a | GELEDVVLGLLmTPAQYDAFQLK; SLYQDIQDDTKGDYETILLALcGN; AIDLELKGDIEScLIAVVK |
| 9 | 9 | IPI00507097 | A7E2L4 | 1 | 0.8 | 1.0 | 1.3 | 1.0 | 1.0 | 13.88 | 5 | 2 | 317 | 35.1 | 7.69 | 44.69 | gnb2l1 | Guanine nucleotide-binding protein subunit β-2-like 1 | GHSGWVTQIATTPQFPDmILSASR; HLYTLDGGDTINALcFSPNR |
| 10 | 10 | IPI00496577 | Q6PFT7 | 1 | 1.1 | 0.6 | 0.9 | 0.8 | 0.8 | 44.37 | 44 | 6 | 142 | 15.9 | 5.40 | 8.77 | cotl1 | Coactosin-like 1 | YDGSTIVPGGHGSDYEEFK; EFmISDPKELEEEYLR; FTFITWIGENITGLQR; DDSSGIcWAcFK; ELEEEYLR; EFmISDPK |
| 11 | 11 | IPI00501058 | F1QCE3 | 1 | 1.1 | 1.8 | 1.2 | 1.4 | 1.3 | 35.19 | 14 | 3 | 162 | 17.1 | 5.53 | 9.75 | prdx5 | Peroxiredoxin 5 | AVDLVLNNAQLIPVLGNLR; LPAVEVQEEDPGNSLSmAELFScK; THLPGFIQMAGELR |
| 12 | 13 | IPI00503138 | Q6DC81 | 1 | 1.1 | 1.6 | 1.1 | 1.5 | 1.4 | 12.50 | 1 | 1 | 128 | 14.7 | 5.96 | 4.42 | nutf2 | Nuclear transport factor 2 | ADQDQVmGFQQVFLLK |
| 13 | 14 | IPI00960571 | Q4VBT1 | 1 | 2.2 | 2.2 | 1.9 | 1.7 | 2.0 | 49.22 | 47 | 6 | 128 | 14.5 | 6.57 | 31.34 | fabp1b | Fatty acid-binding protein 1b-like | VLNNSFTIGQDTEIQmLSGDK; TQFEIQENGNDYcLTTR; FELEKYEGWEEFMK; AIGHQELLK; YEGWEEFmK; VLNNSFTIGQDTEIQmLSGDKVK |
| 14 | 15 | IPI00507583 | Q9PTF3 | 1 | 0.9 | 1.1 | 1.4 | 1.2 | 1.5 | 11.24 | 5 | 1 | 169 | 19.3 | 7.83 | 24.59 | ndpkz3 | Nucleoside diphosphate kinase | YmSSGPVLAmVWEGLNVVK |
| 15 | 17 | IPI00483526 | Q6DRF3 | 1 | 0.4 | 0.7 | 0.6 | 0.5 | 0.5 | 24.05 | 13 | 4 | 237 | 26.1 | 6.79 | 89.75 | psmb1 | Proteasome subunit β type | SmTSGAIAAmLSTILYGR; FFPYYVYNIIGGLDEEGR; AGGSASAmLQPLLDNQIGFK; RFFPYYVYNIIGGLDEEGR |
| 16 | 18 | IPI00511345 | Q6TH32 | 1 | 0.4 | 0.6 | 0.5 | 0.8 | 0.7 | 12.73 | 5 | 1 | 165 | 18.8 | 7.33 | 14.67 | Cfl2 | Muscle cofilin 2 | HIIMEQGQEILQGDEGDPYLK |
| 17 | 20 | IPI00570286 | Q566W6 | 1 | 1.4 | 1.4 | 2.4 | 1.3 | 1.4 | 11.35 | 6 | 1 | 185 | 18.4 | 8.81 | 32.87 | cirbp | Cold inducible RNA binding protein (D. rerio) | LFIGGLSFDTTEQSLEDAFSK |
| 18 | 21 | IPI00497676 | Q4JHL7 | 1 | 1.1 | 1.0 | 0.9 | 1.5 | 1.2 | 29.33 | 148 | 5 | 283 | 30.3 | 8.82 | 632.12 | vdac2 | Voltage-dependent anion channel 2 | VSDKLETAVNLAWTAGSNSTR; TGDFQLHTNVNDGSEFGGSIYQK; WNTDNTLGTEINIEDQIAK; LETAVNLAWTAGSNSTR; VNNTSLVGVGYTQSLRPGIK |
| 19 | 22 | IPI00851926 | F1QJ11 | 1 | 2.1 | 1.5 | 1.8 | 1.6 | 2.0 | 6.49 | 1 | 1 | 308 | 32.8 | 8.91 | 10.23 | vdac3 | Voltage-dependent anion channel 3 | VNNASLVGVGYTQSLRPGVK |
| 20 | 23 | IPI00502668 | Q9DDU5 | 1 | 1.1 | 1.3 | 2.0 | 1.8 | 1.3 | 16.83 | 32 | 2 | 208 | 23.5 | 8.03 | 276.63 | Gstp1 | Glutathione S-transferase π | NDSEASLIDVmNDGVEDLR; FEDGDLVLFQSNAmLR |
| 21 | 24 | IPI00920104 | E9QDX8 | 1 | 1.7 | 2.2 | 2.0 | 2.3 | 1.7 | 6.36 | 1 | 1 | 283 | 31.0 | 6.93 | 8.71 | CTSB | Cathepsin B | NGPVEGAFTVYEDFLLYK |
| 22 | 25 | IPI00500865 | Q1LYC9 | 1 | 0.3 | 1.2 | 0.4 | 0.7 | 0.8 | 23.15 | 4 | 3 | 216 | 24.4 | 5.47 | 28.51 | capns1a | Calpain, small subunit 1a | VFNQLAGDDmEVSPTELmNILNK; KAIGGVIDVIGNIDPNQFVPSEPPPPR; AIGGVIDVIGNIDPNQFVPSEPPPPR |
| 23 | 26 | IPI00503919 | Q6PBY5 | 1 | 0.5 | 1.6 | 0.5 | 0.6 | 1.2 | 17.09 | 5 | 2 | 234 | 25.9 | 6.35 | 24.66 | psma2 | Proteasome subunit α type | YNEDLELEDAIHTAILTLK; LVQIEYALAAVAAGAPSVGIK |
| 24 | 28 | IPI00498656 | Q1LXJ1 | 1 | 0.4 | 0.5 | 0.8 | 0.7 | 0.7 | 19.86 | 27 | 4 | 423 | 46.3 | 5.49 | 337.74 | krt17 | Si:dkeyp-113d7.7 | SYSAGGGAGFGGGAGAGFNLSDGIDSSANEK; TLQSLQIELQSQLSmK; ILHATGVNAGIYLHIDNAK; SDLEmQIEGLKEELVFLK |
| 25 | 29 | IPI00504207 | Q9I8V1 | 1 | 0.6 | 0.6 | 0.6 | 0.7 | 0.9 | 10.34 | 2 | 2 | 377 | 41.9 | 5.39 | 8.64 | actc1b | Skeletal α1 actin | LcYVALDFENEmATAASSSSLEK; YPIEHGIITNWDDmEK |
| 26 | 30 | IPI00505088 | Q4V911 | 1 | 1.5 | 1.1 | 1.2 | 1.8 | 0.9 | 12.22 | 4 | 4 | 589 | 65.1 | 5.05 | 27.47 | ppp2r1b | Protein phosphatase 2 (formerly 2A), regulatory subunit A, β isoform | IGPVLESSALQAEVKPVLEK; LNSLcmAWLIDHVYAIR; DNTVEHLLPLFLAQLK; SDIIPLFTALASDEQDSVR |
| 27 | 31 | IPI00489480 | Q6DHB6 | 1 | 0.9 | 0.2 | 0.4 | 0.8 | 1.2 | 21.24 | 70 | 4 | 466 | 50.0 | 5.43 | 538.88 | krt12 | Zgc:92533 | SYSAGGGGGFGGGAGFGGGAGGGFGGGFGGGAGGDAFDVSANDK; GQMSGQVHVEVDAAPQEDLTK; SDLEMQIEGLKEELIFLK; TLQGLEIELQSQLSmK |
| 28 | 32 | IPI00483806 | Q803H5 | 1 | 1.4 | 1.0 | 1.2 | 1.3 | 1.5 | 25.00 | 8 | 5 | 340 | 37.3 | 6.00 | 30.63 | gnb1l | Guanine nucleotide binding protein (G protein), β polypeptide 1, like | KAcADATLSQITANIDPVGR; ADQELmVYSHDNIIcGITSVAFSK; VScLGVTDDGmAVATGSWDSFLK; LLLAGYDDFNcNVWDTLK; AcADATLSQITANIDPVGR |
| 29 | 33 | IPI00486878 | Q08BN3 | 1 | 0.9 | 0.6 | 0.9 | 1.1 | 1.3 | 7.02 | 1 | 1 | 242 | 27.2 | 5.24 | 2.33 | nudt14 | Si:dkey-77p13.2 protein | VPLHEAmTFAFDESIPK |
| 30 | 34 | IPI00492152 | Q6NSN6 | 1 | 1.0 | 0.9 | 1.7 | 0.9 | 0.9 | 11.18 | 4 | 2 | 331 | 37.3 | 6.54 | 24.05 | ppp1cab | Protein phosphatase 1, catalytic subunit, α | TFTDcFNcLPVAAIVDEK; IFccHGGLSPDLQSmEQVR |
| 31 | 35 | IPI00506729 | Q8JGM2 | 1 | 1.7 | 1.3 | 0.9 | 1.0 | 1.2 | 5.26 | 1 | 1 | 418 | 47.4 | 5.88 | 2.32 | arp3 | ARP3 actin-related protein 3-like protein | GVDDLDFYIGDEAIDKPTYATK |
| 32 | 37 | IPI00770145 | F1RBZ9 | 1 | 2.2 | 1.8 | 2.6 | 2.1 | 1.7 | 2.98 | 1 | 1 | 571 | 66.9 | 6.98 | 5.63 | msna | 67-kDa protein | FYPEDVSEELIQEATQR |
| 33 | 38 | IPI00500949 | Q7T3A8 | 1 | 1.5 | 1.0 | 1.8 | 1.2 | 1.2 | 1.33 | 1 | 1 | 342 | 37.9 | 6.64 | 11.37 | anxa1a | Annexin A1a (phosphorylated) | AAYQQATSKPLDVALK |
| 34 | 39 | IPI00492152 | Q6NSN6 | 1 | 0.7 | 0.8 | 0.6 | 1.1 | 1.1 | 5.44 | 1 | 1 | 331 | 37.3 | 6.54 | 6.68 | ppp1a | Protein phosphatase 1, catalytic subunit, α | TFTDcFNcLPVAAIVDEK |
| 35 | 40 | IPI00509268 | Q7SXV6 | 1 | 0.9 | 0.7 | 0.7 | 2.0 | 1.2 | 10.26 | 2 | 2 | 341 | 38.9 | 6.86 | 4.95 | sept | Sept2 protein (fragment) | LYPWGVVEVENPEHNDFLK; STLINSLFLTDLYPER |
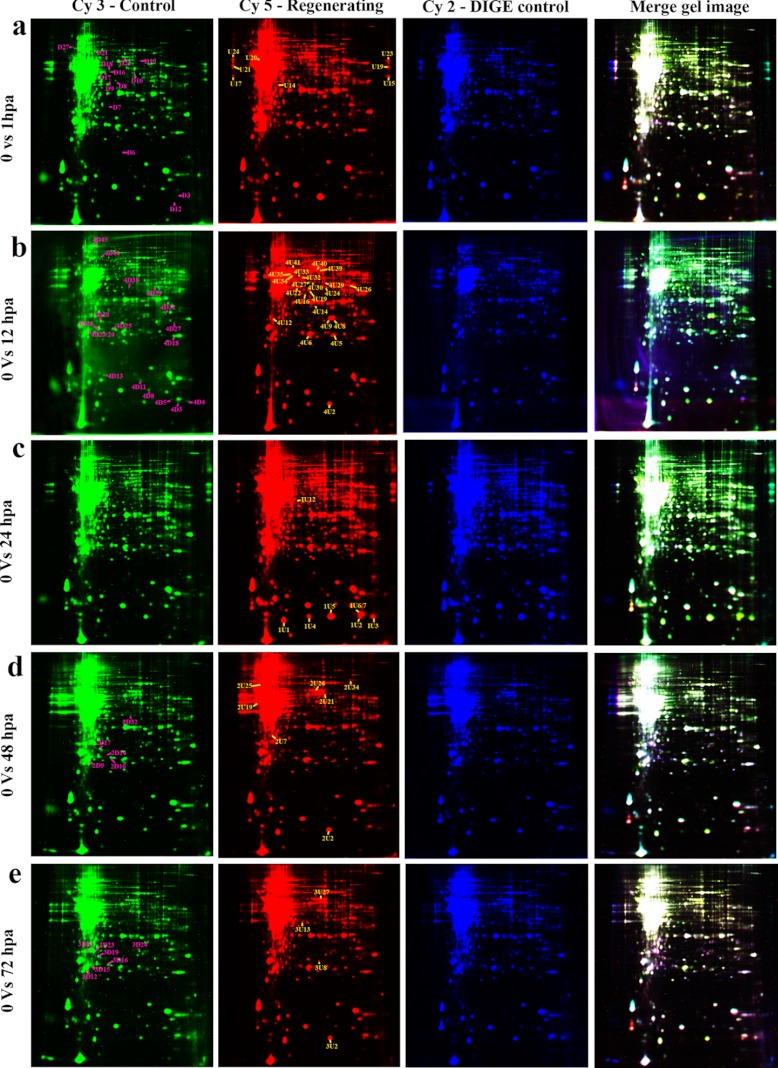
Fig. 1.
2DE DIGE map of zebrafish caudal fin. a, control (0 hpa) versus 1 hpa; b, control versus 12 hpa; c, control versus 24 hpa; d, control versus 48 hpa; e, control versus 72 hpa. Control (0 h) samples are labeled with Cy3 dye (green 2DE images), regenerating samples are labeled with Cy5 dye (red 2DE images), and internal standard combining the protein from control and regenerating tissues are labeled with Cy2 dye (blue 2DE images). The overlays of all three dyes are represented as multicolored 2DE images. The down-regulated proteins are represented in the green 2DE images with pink labeling, and up-regulated proteins are represented in the red 2DE images with yellow labeling.
Table II. List of proteins identified as differentially regulated during zebrafish caudal fin regeneration based on DIGE analysis.
This table provides the list of 90 proteins identified as differentially regulated proteins for caudal fin regeneration based on DIGE analysis. The proteins are listed along with the S numbers, accession numbers, Uniprot ID, fold changes, up- or down-regulation, Time points, coverage, PSMs, peptides, molecular mass, calculated (Calc.) pI, score, gene symbol, description, detection method, and peptide sequences identified based on proteomics study.
| S No. | Accession number | Uniprot ID | DIGE ID | Fold Change | U/D regulation | Time points | Coverage | No. PSMs | No. Peptides | Molecular mass (kDa) | Calc. pI | Score | Gene Symbol | Description | Detection method | Sequences |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | gI-32442452 | Q7SYH6 | D18 | -2.53 | D | 0/1 | 36 | NA | 14 | 51.849 | 5.86 | 102 | Pah | Pah | MALDI MS/MSMS | VLDEIVDGLR; TQIRGQVHELSR; QKDTVPWFPNDIQDLDR; DTVPWFPNDIQDLDR; FANQILSYGSELDADHPGFTDTVYR; EFADIAYNYR; ATWGTVFR; TLYPTHACR; EDNIPQLEDISHYLQSCTGFR; LRPVAGLLSSR; DFLAGLAFR; VFHSTQYIR; YNAYTQR; NLADSINGEISILCNALR |
| 2 | gI-18858329 | B3DG37 | D12 | -2.35 | D | 0/1 | 58 | NA | 7 | 16.606 | 7.7 | 62 | BA1 | bal globin | MALDI MS/MSMS | VEWTDAER; LNIDEIGPQALSR; CLIVYPWTQR; YFATFGNLSSPAAIMGNPK Oxidation (M); LHVDPDNFR; FGQAGFNADVQEAWQK; FLAVVVSALCR |
| 3 | gI-32442452 | Q7SYH6 | D16 | -2.11 | D | 0/1 | 27 | NA | 11 | 51.849 | 5.86 | 67 | Pah | Pah | MALDI MS/MSMS | VLDEIVDGLR; TQIRGQVHELSR; DTVPWFPNDIQDLDR; EFADIAYNYR; ATWGTVFR; TLYPTHACR; EHNRVFPLLEK; LRPVAGLLSSR; DFLAGLAFR; VFHSTQYIR; NLADSINGEISILCNALR |
| 4 | gI-189514813 | NA | D9 | -2.08 | D | 0/1 | 19 | NA | 14 | 109.563 | 8.97 | 55 | RIM1 | Predicted: similar to regulating synaptic membrane exocytosis protein 1 (Rab3 interacting molecule) | MALDI MS/MSMS | SQVPGANDTLLPNAGPGDHPKGADTMPASR Oxidation (M); SEPPREK; RPVSMHEQNGKVIGR; YPVKPQK; EEQEMRMHAK 2 Oxidation (M); MHAKVSK; EKAAEIMR; DSSLSSDQSESLRPPPPR; STMPREAGSLLGLK Oxidation (M); AGDEVLQWNGKALPGATK; MDRPSISVMSPTSPGMLR 2 Oxidation (M); MDRPSISVMSPTSPGMLRDLPLVLPGQLSVK Oxidation (M); ELPPRPDGRPRNPYVK; SRPAHIPMQR Oxidation (M) |
| 5 | gI-18858329 | Q90486 | D3 | -2.07 | D | 0/1 | 64 | NA | 9 | 16.606 | 7.7 | 75 | ba1 | bal globin | MALDI MS/MSMS | VEWTDAER; TAILGLWGK; LNIDEIGPQALSR; CLIVYPWTQR; YFATFGNLSSPAAIMGNPK; YFATFGNLSSPAAIMGNPK Oxidation (M); LHVDPDNFR; FGQAGFNADVQEAWQK; FLAVVVSALCR |
| 6 | gI-208019381 | B5WY15 | D21 | -1.83 | D | 0/1 | 18 | NA | 11 | 57.677 | 6.65 | 55 | ftr65 | finTRIM family protein | MALDI MS/MSMS | HHDTVSAAAER; HHDTVSAAAERTELESR; TELESRLR; ETQSRFQQR; SELTQMIR Oxidation (M); TAVSGAEER; LQQFCTEEMK Oxidation (M); SYWELEWSGAR; VFLSVSYK; GRGNECLFGR; VKLCDLTV |
| 7 | gI-55962880 | Q5SPF0 | D7 | -1.72 | D | 0/1 | 41 | NA | 8 | 28.89 | 6.36 | 61 | si:ch211–171o19.1 | Novel protein similar to vertebrate pim oncogene family | MALDI MS/MSMS | DEDTHIIEINSQHYKIGAELGK; QIAELLDWKVEAER; VEAERYIIVVER; LIPSMNLREFLIDNK Oxidation (M); EDTPEDLVRK; IMFNVTIAAHTCCQR Oxidation (M); IMFNVTIAAHTCCQRGVFHSDIK; LIDFGCSVLLTK |
| 8 | gI-189522994 | NA | D10 | -1.62 | D | 0/1 | 21 | NA | 13 | 87.212 | 9.71 | 57 | LOC569862 | Predicted: similar to cytoskeleton associated protein 2-like | MALDI MS/MSMS | TLSGVDENTSLK; AQPASHVANKSQK; IQSHNPVTNR; INQKPNSATNRSDVYQR; QHATLKASNAAASTK; SCVLPQTTSEHKELQQPVMR Oxidation (M); ELQQPVMR Oxidation (M); SNHTKPTVQFQTPK; SNHTKPTVQFQTPKSSICPSSTQGVR; KLQEWR; DVMSRVPMAQK Oxidation (M); SQQGAEPGQVDGHEIR; DDSQPQSSPLYVYRENEALR |
| 9 | gI-51230599 | rQ6AZV8 | D8 | −1.61 | D | 0/1 | 40 | NA | 11 | 44.853 | 5.84 | 67 | 4HPPD | 4-Hydroxyphenylpyruvate dioxygenase | MALDI MS/MSMS | FICFDHITFWVGNAK; LGFEPLAYR; IIYVFTSALNPGNK; DVAFTVENCDFLVEK; MAVVQTYGDTTHTFVER; LDFIDHVVGNQPDSEMVPIVEWYQR; LDFIDHVVGNQPDSEMVPIVEWYQR Oxidation (M); QLQTEYSALR; SQIQEYVEYYGGAGVQHIAMNTSDIISAIR; HNHQGFGAGNFK; SLFEAIEADQNAR |
| 10 | gI-39645428 | Q6P3L3 | D27 | −1.6 | D | 0/1 | 33 | NA | 21 | 72.117 | 5.04 | 130 | hsp5 | Heat shock protein 5 | MALDI MS/MSMS | VEIIANDQGNR; ITPSYVAFTTEGER; KVTHAVVTVPAYFNDAQR; VTHAVVTVPAYFNDAQR; QATKDAGTIAGLNVMR; IINEPTAAAIAYGLDKR; VMEHFIK; VMEHFIK Oxidation (M); VMEHFIKLYK; IEIESFFEGEDFSETLTR; AKFEELNMDLFR; AKFEELNMDLFR Oxidation (M); FEELNMDLFR; FEELNMDLFR Oxidation (M); VLEDSDLKKPDIDEIVLVGGSTR; KPDIDEIVLVGGSTR; VYEGERPLTK; DNHLLGTFDLTGIPPAPR; GVPQIEVTFEIDVNGILR; LTPEDIERMVNEAER; IEWLEAHQDADLEEFQAK |
| 11 | gI-32442452 | Q7SYH6 | D17 | -1.58 | D | 0/1 | 36 | NA | 13 | 51.849 | 5.86 | 91 | Pah | Pah | MALDI MS/MSMS | VLDEIVDGLR; TQIRGQVHELSR; DTVPWFPNDIQDLDR; FANQILSYGSELDADHPGFTDTVYR; EFADIAYNYR; ATWGTVFR; TLYPTHACR; EDNIPQLEDISHYLQSCTGFR; LRPVAGLLSSR; DFLAGLAFR; VFHSTQYIR; YNAYTQR; NLADSINGEISILCNALR |
| 12 | gI-125851812 | NA | D19 | −1.56 | D | 0/1 | 33 | NA | 13 | 59.925 | 6.39 | 79 | wu:fj78b06 | Predicted: wu:fj78b06 | MALDI MS/MSMS | SEVFGQLR; DGLLPEQTYFVGFAR; LAAFFSR; YVEESSFSDLNTHLLSLPGGAEANR; VIVEKPFGR; DLQSSEELSSHLSSLFTEEQIYR; IFGPIWNR; EPFGTQGR; GGYFDDFGIIR; WDGVPFILR; LQFTDVPGDIFSSQCR; KPGVYFSPEETELDLTYHSR; LILDVFCGSQMHFVR Oxidation (M) |
| 13 | gI-112180551 | Q6PHG2 | D22 | −1.53 | D | 0/1 | 38 | NA | 12 | 51.621 | 6.14 | 83 | Hpx | Hpx protein | MALDI MS/MSMS | GKPGGEGHKHELHHGAQLDR; HELHHGAQLDR; TFPELDDHHHLGHVDAAFR; GDEIYHFNMHTK; YMGHYYCFHGHQFSK; CPHFGSK; VHLDAITSDDAGNIYAFR; FHSDTIESEFK; ELHSEVDSVFSYDGHFYMIK; VGKPHTHLEGYPKPLK; DVLGIEGPVDAAFVCEDHHVVHIIK; IMPEQQK |
| 14 | gI-189514909 | NA | D6 | −1.52 | D | 0/1 | 43 | NA | 9 | 39.302 | 5.34 | 58 | LOC796672 | Predicted: similar to gap-protease | MALDI MS/MSMS | DPASASQISEFLTASNARIDR; SVKPCLPQPTCYAGEPHLCRSFLSK; VAFVITLLSGRAAQWGTTAWEK; SMADYSIEFR Oxidation (M); TLAAESGWNAEAQWDMFLHGLSDR; TLAAESGWNAEAQWDMFLHGLSDR Oxidation (M); LQDEIYSLDLPK; FLDPVPDSPVSPMAPEVGSVDPEPMQLGRSR 2 Oxidation (M); SRLSVEEK |
| 15 | gI-37595424 | Q6NWJ5 | U20 | 1.55 | U | 0/1 | 33 | NA | 13 | 50.687 | 4.93 | 77 | zTUBA6 | Tubulin α6 | MALDI MS/MSMS | AVFVDLEPTVIDEVR; QLFHPEQLITGK; EIIDLVLDR; NLDIERPTYTNLNR; LIGQIVSSITASLR; FDGALNVDLTEFQTNLVPYPR; YMACCLLYR; SIQFVDWCPTGFK; AVCMLSNTTAIAEAWAR; AVCMLSNTTAIAEAWAR Oxidation (M); AFVHWYVGEGMEEGEFSEAR; AFVHWYVGEGMEEGEFSEAR Oxidation (M); EDMAALEK |
| 16 | gI-18858425 | Q9PUB5 | U20 | 1.55 | U | 0/1 | 39 | NA | 29 | 58.551 | 5.34 | 218 | krt5 | Keratin 5 | MALDI MS/MSMS | NFSSMSTSAVPMGSR; FASFIDK; FLEQQNK; WSLLQEQTTTR; SNIDAMFEAYIANLR; SNIDAMFEAYIANLR Oxidation (M); SNIDAMFEAYIANLRR; NMQNLVEDFK; YEDEINKR; AAVENEFVLLK; VDSLQDEINFLR; AIFEEELR; AIFEEELRELQSQIK; DTSVVVEMDNSR; NLDMDAIVAEVR; NLDMDAIVAEVR Oxidation (M); AQYEDIANR; SRAEAESWYK; AEAESWYK; AEIADLNR; ANLEAQIAEAEER; ANLEAQIAEAEERGELAVK; IRDLEDALQR; DLEDALQR; EYQELMNVK; LALDIEIATYR; LALDIEIATYRK; KLLEGEESR; LLEGEESR |
| 17 | gI-55925273 | Q5U3E4 | U14 | 1.83 | U | 0/1 | 30 | NA | 9 | 37.009 | 5.54 | 60 | SGT1 | SGT1, suppressor of G2 allele of SKP1 | MALDI MS/MSMS | YSDSFIDEDPRR; ADNAEWLCQR; AYAYILLKEYTK; AQDLQPGLALPFLR; EGVIVSFGER; KTEAIQWEK; HFTASQYPSSSHYTR; LFQQIYSDGSDDVR; LFQQIYSDGSDDVRR |
| 18 | gI-62298523 | Q7ZVI7 | U15 | 11.66 | U | 0/1 | 33 | NA | 10 | 42.082 | 5.3 | 70 | actba | RecName: Full = actin, cytoplasmic 1; AltName: Full = β-actin-1; contains; RecName: Full = Actin, cyto | MALDI MS/MSMS | AVFPSIVGRPR; AVFPSIVGRPRHQGVMVGMGQK Oxidation (M); IWHHTFYNELR; VAPEEHPVLLTEAPLNPK; GYSFTTTAER; SYELPDGQVITIGNER; DLYANTVLSGGTTMYPGIADR Oxidation (M); EITSLAPSTMK Oxidation (M); EITSLAPSTMKIK Oxidation (M); QEYDESGPSIVHR |
| 19 | gI-94732413 | Q1LXJ0 | U19 | 12.68 | U | 0/1 | 28 | NA | 12 | 49.661 | 5.31 | 56 | zgc:92533 | Novel protein similar to type I cytokeratin, enveloping layer | MALDI MS/MSMS | VSGMYAGSVHGGAGGYGTR; VSGMYAGSVHGGAGGYGTR Oxidation (M); DYSAYYATISDLQNK; LAADDFR; QSVEADIAGLR; NHEEELLAAR; GQMSGQVHVEVDAAPQEDLTK; GQMSGQVHVEVDAAPQEDLTK Oxidation (M); DLEHWFQSK; TLQGLEIELQSQLSMK Oxidation (M); ASLEGTLADTQAR; TRLEMEIAEYR Oxidation (M) |
| 20 | gI-18858425 | Q9PUB5 | U23 | 12.68 | U | 0/1 | 30 | NA | 20 | 58.551 | 5.34 | 103 | krt5 | Keratin 5 | MALDI MS/MSMS | WSLLQEQTTTR; SNIDAMFEAYIANLR; SNIDAMFEAYIANLR Oxidation (M); QLDGLGNEKMK; MKLEGELK; MKLEGELK Oxidation (M); VDSLQDEINFLR; AIFEEELR; AIFEEELRELQSQIK; NLDMDAIVAEVR; NLDMDAIVAEVR Oxidation (M); AQYEDIANR; SRAEAESWYK; QKFEEMQSSAGK Oxidation (M); AEIADLNR; ANLEAQIAEAEER; ANLEAQIAEAEERGELAVK; IRDLEDALQR; LALDIEIATYR; SSIVSSQKR |
| 21 | gI-94732413 | Q1LXJ0 | U17 | 1.64, 2.39 | U | 0/1 | 25 | NA | 12 | 49.661 | 5.31 | 67 | zgc:92533 | Novel protein similar to type I cytokeratin, enveloping layer | MALDI MS/MSMS | ATMQNLNDR Oxidation (M); IRQFLDSK; LAADDFR; VKYENELSMR Oxidation (M); QSVEADIAGLR; KNHEEELLAAR; NHEEELLAAR; GQMSGQVHVEVDAAPQEDLTK; DLEHWFQSK; SEITELKR; ASLEGTLADTQAR; TRLEMEIAEYR Oxidation (M) |
| 22 | gI-94732413 | Q1LXJ0 | U21 | 1.68,4.87 | U | 0/1 | 46 | NA | 29 | 49.661 | 5.31 | 175 | zgc:92533 | Novel protein similar to type I cytokeratin, enveloping layer | MALDI MS/MSMS | MSSYSRSSFK; FSMSGSGGGGGR; FSMSGSGGGGGR Oxidation (M); VSGMYAGSVHGGAGGYGTR; VSGMYAGSVHGGAGGYGTR Oxidation (M); DYSAYYATISDLQNK; LAADDFR; VKYENELSMR; VKYENELSMR Oxidation (M); QSVEADIAGLR; QSVEADIAGLRK; VLDELTMTR; VLDELTMTR Oxidation (M); SDLEMQIEGLKEELIFLK Oxidation (M); NHEEELLAAR; GQMSGQVHVEVDAAPQEDLTK; GQMSGQVHVEVDAAPQEDLTK Oxidation (M); DLEHWFQSK; SEITELKR; TLQGLEIELQSQLSMK; TLQGLEIELQSQLSMK Oxidation (M); ASLEGTLADTQAR; QGQEYQMLLDIK; QGQEYQMLLDIK Oxidation (M); TRLEMEIAEYR; TRLEMEIAEYR Oxidation (M); LEMEIAEYR; LEMEIAEYRR; LEMEIAEYRR Oxidation (M) |
| 23 | gI-18858425 | Q9PUB5 | U24 | 2.88,4.27 | U | 0/1 | 35 | NA | 23 | 58.551 | 5.34 | 147 | krt5 | Keratin 5 | MALDI MS/MSMS | NFSSMSTSAVPMGSR Oxidation (M); FLEQQNK; WSLLQEQTTTR; SNIDAMFEAYIANLR; SNIDAMFEAYIANLR Oxidation (M); SNIDAMFEAYIANLRR; MKLEGELK Oxidation (M); AAVENEFVLLK; AAVENEFVLLKK; VDSLQDEINFLR; AIFEEELR; AIFEEELRELQSQIK; NLDMDAIVAEVR; NLDMDAIVAEVR Oxidation (M); AQYEDIANR; SRAEAESWYK; AEIADLNR; LQNEIEAVKGQR; ANLEAQIAEAEER; ANLEAQIAEAEERGELAVK; IRDLEDALQR; LALDIEIATYR; KLLEGEESR |
| 24 | IPI00482837 | Q90487 | 4D3 | -5.12 | D | 0/12 | 11.89 | 1 | 1 | 15.5 | 8.37 | 2.32 | hbaa1 | Hemoglobin subunit α | FTMS/ITMSMS | TYFSHWADLSPGSGPVK |
| 25 | IPI00493081 | Q90486 | 4D5 | -4.56 | D | 0/12 | 10.81 | 1 | 1 | 16.4 | 7.83 | 6.06 | bal1 | Hemoglobin subunit β-1 | FTMS/ITMSMS | FGQAGFNADVQEAWQK |
| 26 | IPI00481037 | A4QN86 | 4D38 | -4.13 | D | 0/12 | 10.34 | 2 | 2 | 45.4 | 6.89 | 8.47 | krt1–19d | Keratin, type 1, gene 19d | FTMS/ITMSMS | ATHSAGGFSmSGSADNSSIIGNEK; GGLHLSIDNTSLAmNDFK |
| 27 | IPI00491770 | Q6ZM17 | 4D4 | -3.51 | D | 0/12 | 25.17 | 2 | 2 | 15.6 | 8.85 | 10.68 | hba2 | α-Globin-like (D. rerio) | FTMS/ITMSMS | TYFSHWSDLSPGSGPVKK; IDDLVGGLAALSELHAFK |
| 28 | IPI00502952 | F1R3G8 | 4D28 | -3.25 | D | 0/12 | 13.15 | 2 | 2 | 32.8 | 5.54 | 7.17 | SRM | Spermidine synthase | FTMS/ITMSMS | TYGNVLILDGVIQcTER; HPLVESVVQcEIDEDVINVSK |
| 29 | IPI00832708 | Q6P3L0 | 4D45 | -2.91 | D | 0/12 | 1.91 | 1 | 1 | 93.9 | 5.60 | 2.21 | mvp | Isoform 2 of major vault protein | FTMS/ITMSMS | LAQDPFPLYPGEEIQK |
| 30 | IPI00864129 | F1Q8P5 | 4D23/24 | -2.66 | D | 0/12 | 4.41 | 1 | 1 | 40.3 | 5.16 | 6.07 | Si:dkeyp-113d7.4 | Similar to type I cytokeratin, enveloping layer (D. rerio) | FTMS/ITMSMS | TLQGLEIELQSQLSmK |
| 31 | IPI00497231 | Q5XJE1 | 4D26 | -2.47 | D | 0/12 | 21.12 | 3 | 3 | 27.2 | 5.88 | 9.01 | cutc | CutC copper transporter homolog | FTMS/ITMSMS | VLTSGcDSSALEGLPVIK; SQQADGLVFGALTEDGR; NSNVcmGGSLSVPEYAVK |
| 32 | IPI00507097 | A7E2L4 | 4D32 | -2.41 | D | 0/12 | 18.61 | 3 | 3 | 35.1 | 7.69 | 13.09 | gnb2l1 | Guanine nucleotide-binding protein subunit β-2-like 1 | FTMS/ITMSMS | YTIQDDSHTEWVScVR; TNHIGHTGYLNTVTVSPDGSLcASGGK; IIVDELRQDIITTNSK |
| 33 | IPI00501589 | A7YYB3 | 4D39 | -2.13 | D | 0/12 | 3.96 | 1 | 1 | 48.1 | 7.87 | 3.91 | uqcrc2 | Ubiquinol-cytochrome c reductase core protein II | FTMS/ITMSMS | LPSGLVIASLENYSPASR |
| 34 | IPI00500466 | F1RDA6 | 4D44 | -2.13 | D | 0/12 | 2.74 | 1 | 1 | 79.4 | 6.15 | 7.51 | ndufs1 | NADH-ubiquinone oxidoreductase 75-kDa subunit | FTMS/ITMSMS | VASQVAALDLGYKPGVDAIR |
| 35 | IPI00502304 | Q7ZUD3 | 4D13 | -1.96 | D | 0/12 | 12.24 | 1 | 1 | 17.5 | 5.47 | 2.21 | Gmfb | Gmfb protein | FTMS/ITMSMS | QLVILEEEHEDISPEDLK |
| 36 | IPI00490809 | O73872 | 4D11 | -1.94 | D | 0/12 | 23.38 | 3 | 2 | 15.9 | 6.61 | 25.11 | sod1cuzn | Superoxide dismutase (Cu-Zn) | FTMS/ITMSMS | IEIEDAmLTLSGQHSIIGR; GTGEVTGTVYFNQEGEK |
| 37 | IPI00773343 | A5PN62 | 4D25 | -1.71 | D | 0/12 | 6.99 | 2 | 1 | 31.0 | 5.52 | 6.24 | ddah2 | Nγ,Nγ-Dimethylarginine dimethylaminohydrolase 2 (D. rerio) | FTMS/ITMSMS | NIcSmGGPDTIIISNSDGAK |
| 38 | IPI00509360 | Q6PC90 | 4D8 | -1.64 | D | 0/12 | 11.36 | 1 | 1 | 14.5 | 7.24 | 13.14 | rps12 | 40 S ribosomal protein S12 | FTMS/ITMSMS | LVEALcAEHQINLIK |
| 39 | IPI00866470 | NA | 4U29 | 1.61 | U | 0/12 | 7.61 | 2 | 2 | 53.0 | 7.59 | 13.83 | FDXR | NADPH:adrenodoxin oxidoreductase, mitochondrial-like (D. rerio) | FTMS/ITMSMS | VcVVGGGPAGFYTAQQLLK; ETDITQNALEALTGSNVR |
| 40 | IPI00489824 | Q6PHG2 | 4U27 | 1.8 | U | 0/12 | 4.25 | 1 | 1 | 51.0 | 6.64 | 2.14 | hpx | Hemopexin | FTMS/ITMSMS | GIEFDAVAVNEEGVPYFFK |
| 41 | IPI00506057 | Q7T3L3 | 4U40 | 2 | U | 0/12 | 10.47 | 8 | 5 | 91.2 | 4.86 | 40.21 | GP96 | Tumor rejection antigen (Gp96) 1 | FTMS/ITMSMS | LLSLTNEDALAGNEELTIK; FQTSHSDTVLSSLEQYVER; LTNSPcALVASQYGWSGNMER; EEEAIQLDGLNTSQLK; TDDEVVQREEEAIQLDGLNTSQLK |
| 42 | IPI00931368 | E7EXC4 | 4U26 | 2.02 | U | 0/12 | 4.28 | 1 | 1 | 42.4 | 9.04 | 2.30 | SERPINB1 | Leukocyte elastase inhibitor isoform 1 | FTMS/ITMSMS | VNLTGmSSSNDLVLSK |
| 43 | IPI00482097 | Q6AZC1 | 4U24 | 2.03 | U | 0/12 | 13.05 | 3 | 3 | 45.6 | 7.55 | 20.19 | Psmc5 | Proteasome (prosome, macropain) 26 S subunit, ATPase, 5 | FTMS/ITMSMS | EELQLLQEQGSYVGEVVR; EHAPSIIFmDEIDSIGSSR; TmLELLNQLDGFEATK |
| 44 | IPI00503192 | O42271 | 4U34 | 2.1 | U | 0/12 | 4.45 | 1 | 1 | 49.9 | 5.10 | 2.17 | tuba1 | α-Tubulin | FTMS/ITMSMS | TIGGGDDSFNTFFSETGAGK |
| 45 | IPI00482295 | Q7ZVI7 | 4U16 | 2.1 | U | 0/12 | 5.60 | 2 | 1 | 41.7 | 5.48 | 8.50 | actba | Actin, cytoplasmic 1 | FTMS/ITMSMS | DLYANTVLSGGTTMYPGIADR |
| 46 | IPI00855614 | A7YT77 | 4U5 | 2.15 | U | 0/12 | 12.24 | 1 | 1 | 24.6 | 7.08 | 2.81 | qdpra | Quinoid dihydropteridine reductase a | FTMS/ITMSMS | SLAGENSGLPSGAVAVAILPVTLDTPmNR |
| 47 | IPI00505818 | Q7SY26 | 4U14 | 2.17 | U | 0/12 | 7.14 | 1 | 1 | 32.1 | 6.99 | 2.16 | SEPT2 | Septin-2-like | FTMS/ITMSMS | LTVVDTPGYGDAINSQDcFK |
| 48 | IPI00851815 | E9QBP0 | 4U12 | 2.19 | U | 0/12 | 11.11 | 1 | 1 | 17.3/23 | 4.9/5.28 | 2.55 | ARHGDIB | Rho GDP-dissociation inhibitor 2 | FTMS/ITMSMS | SLQEIQEmDKDDESLTK |
| 49 | IPI00960571 | Q4VBT1 | 4U2 | 2.3 | U | 0/12 | 16.41 | 20 | 1 | 14.5 | 6.57 | 73.99 | fabp1b | Fatty acid-binding protein 1b-like | FTMS/ITMSMS | VLNNSFTIGQDTEIQmLSGDK |
| 50 | IPI00612220 | F1QEW2 | 4U39/40 | 2.33 | U | 0/12 | 2.46 | 2 | 1 | 66.4 | 6.67 | 4.75 | wu:fb15e04 | Thread biopolymer filament subunit α isoform 1 (D. rerio) | FTMS/ITMSMS | QITQYGQEYQELLASK |
| 51 | IPI00488563 | F1Q986 | 4U19 | 2.38 | U | 0/12 | 4.33 | 1 | 1 | 44.6 | 6.25 | 13.18 | hpdb | 4-Hydroxyphenylpyruvate dioxygenase | FTMS/ITMSMS | MAVVQTYGDTTHTFVER |
| 52 | IPI00484294 | Q6P3K5 | 4U35 | 2.38 | U | 0/12 | 9.72 | 61 | 3 | 59.1 | 5.41 | 219.48 | krt5 | Krt5 protein | FTMS/ITMSMS | ANLEAQIAEAEERGELAVK; TFTSSGSMSGGmGGGMGGGIR; SNIDAmFEAYIANLR |
| 53 | IPI00504269 | A4FUP0 | 4U9 | 2.42 | U | 0/12 | 16.32 | 16 | 3 | 33.4 | 7.05 | 129.51 | sult2st3 | SULT2 ST3 | FTMS/ITMSMS | ILYVTYEEMLQDLR; mASFLEDPGTFDDFVNK; mSNYTmVPQEImDNNK |
| 54 | IPI00861872 | F1R5A5 | 4U33 | 2.71 | U | 0/12 | 9.32 | 22 | 3 | 58.6 | 5.41 | 143.96 | krt5 | Type II cytokeratin | FTMS/ITMSMS | ANLEAQIAEAEERGELAVK; SNIDAmFEAYIANLR; TFTSSGSMSGGMGGGGIR |
| 55 | IPI00834037 | A9JRS6 | 4U41 | 3.08 | U | 0/12 | 6.83 | 5 | 2 | 54.2 | 5.48 | 36.35 | krt4 | Type II basic cytokeratin | FTMS/ITMSMS | ANLEAQIAEAEERGELAVK; SNIDAMFEAYISNLR |
| 56 | IPI00817939 | E9QEA9 | 4U8 | 3.19 | U | 0/12 | 10.81 | 2 | 1 | 21.1/32 | 6.9/7.4 | 6.75 | nqo1 | NAD(P)H dehydrogenase (quinone) 1 isoform 1 | FTMS/ITMSMS | VLTQGFAFSmQNmYDNGIFK |
| 57 | IPI00503919 | Q6PBY5 | 4U6 | 3.54 | U | 0/12 | 8.97 | 1 | 1 | 25.9 | 6.35 | 6.26 | psma2 | Proteasome subunit α type | FTMS/ITMSMS | LVQIEYALAAVAAGAPSVGIK |
| 58 | IPI00486296 | Q24JW6 | 4U30 | 3.73 | U | 0/12 | 13.75 | 8 | 3 | 47.8 | 6.58 | 27.00 | serpina1 | Serine (or cysteine) proteinase inhibitor, clade A (α-1 antiproteinase, antitrypsin), member 1, like | FTMS/ITMSMS | NIFFSPVGISMALSLLAVGAK; GVDAcHLLAPHNADFAFSLYK; LDDILmDmGmTDAFDYK |
| 59 | IPI00504274 | Q6P2V4 | 4U30 | 3.73 | U | 0/12 | 4.06 | 1 | 1 | 49.9 | 6.65 | 9.62 | Hgd | Homogentisate 1,2-dioxygenase | FTMS/ITMSMS | KVDFVSGLHTVcGAGDSK |
| 60 | IPI00498656 | Q1LXJ1 | 4U22 | 3.86 | U | 0/12 | 3.78 | 1 | 1 | 46.3 | 5.49 | 33.27 | Si:dkeyp-113d7.7 | Similar to type I cytokeratin | FTMS/ITMSMS | TLQSLQIELQSQLSmK |
| 61 | IPI00503138 | Q6DC81 | 1U1 | 1.7 | U | 0/24 | 16.41 | 2 | 1 | 14.7 | 5.96 | 7.57 | nutf2 | Nuclear transport factor 2 | FTMS/ITMSMS | LADLYTDAScLTWEGEGFQGK |
| 62 | IPI00498656 | Q1LXJ1 | 1U12 | 2.23 | U | 0/24 | 11.58 | 3 | 2 | 46.3 | 5.49 | 30.03 | Si:dkeyp-113d7.7 | Similar to type I cytokeratin | FTMS/ITMSMS | SYSAGGGAGFGGGAGAGFNLSDGIDSSANEK; SDLEmQIEGLKEELVFLK |
| 63 | IPI00960571 | Q4VBT1 | 1U5 | 2.37 | U | 0/24 | 16.41 | 1 | 1 | 14.5 | 6.57 | 34.87 | fabp1b | Fatty acid-binding protein 1b-like | FTMS/ITMSMS | VLNNSFTIGQDTEIQmLSGDK |
| 64 | IPI00919918 | Q8JJ15 | 1U3 | 2.51 | U | 0/24 | 18.10 | 1 | 1 | 11.7 | 8.18 | 4.41 | ba1 | Ba1 globin | FTMS/ITMSMS | YFATFGNLSSPAAIMGNPK |
| 65 | IPI00919918 | Q8JJ15 | 1U6/7 | 3.5 | U | 0/24 | 18.10 | 1 | 1 | 11.7 | 8.18 | 2.71 | ba1 | Ba1 globin | FTMS/ITMSMS | YFATFGNLSSPAAIMGNPK |
| 66 | IPI00503480 | B3DFP9 | 1U2 | 4.64 | U | 0/24 | 17.73 | 2 | 2 | 15.5 | 7.18 | 4.53 | apo14 | 14-kDa apolipoprotein | FTMS/ITMSMS | TAVEPALEGSPTAGSLKDVVEELKK; TAVEPALEGSPTAGSLKDVVEELK |
| 67 | IPI00960571 | Q4VBT1 | 1U4 | 4.72 | U | 0/24 | 29.69 | 3 | 2 | 14.5 | 6.57 | 17.23 | fabp1b | Fatty acid-binding protein 1b-like | FTMS/ITMSMS | VLNNSFTIGQDTEIQmLSGDK; TQFEIQENGNDYcLTTR |
| 68 | IPI00507672 | Q6NY48 | 2D32 | -5.5 | D | 0/48 | 5.50 | 1 | 1 | 43.0 | 6.46 | 2.60 | serpinb5 | Serpin peptidase inhibitor, clade B (ovalbumin), member 5, like | FTMS/ITMSMS | ALGmTDVFGDScDLSGmASGK |
| 69 | IPI00484493 | Q6P5M2 | 2D17 | -2.86 | D | 0/48 | 6.23 | 1 | 1 | 33.3 | 5.71 | 2.19 | wdr61 | WD repeat-containing protein 61 | FTMS/ITMSMS | SmDAGPVDAWTVAFSPDSK |
| 70 | IPI00500865 | Q1LYC9 | 2D9 | -2.53 | D | 0/48 | 8.33 | 1 | 1 | 24.4 | 5.47 | 6.12 | capns1a | Calpain, small subunit 1a | FTMS/ITMSMS | RPLAYAVSNESAEEQQFR |
| 71 | IPI00495855 | Q9PVK4 | 2D10 | -2.27 | D | 0/48 | 5.99 | 1 | 1 | 36.2 | 6.89 | 5.85 | ldhba | l-Lactate dehydrogenase B chain | FTMS/ITMSMS | GYTNWAIGLSVADLTETLVK |
| 72 | IPI00504248 | Q801W1 | 2D14 | -2.12 | D | 0/48 | 7.47 | 1 | 1 | 30.3 | 6.67 | 2.19 | psma2 | Proteasome subunit β type | FTMS/ITMSMS | IHYIAPNIYccGAGTAADTEK |
| 73 | IPI00482681 | A4QP88 | 2U34 | 1.95 | U | 0/48 | 3.60 | 1 | 1 | 68.3 | 7.28 | 2.13 | khsrp | khsrp KH-type splicing regulatory protein (D. rerio) | FTMS/ITMSMS | mGGGGGGGGGGGGGGGGIEVPVPR |
| 74 | IPI00486027 | Q6NWF6 | 2U21 | 2 | U | 0/48 | 2.88 | 1 | 1 | 57.7 | 5.22 | 21.08 | krt2–8 | Keratin, type II cytoskeletal 8 | FTMS/ITMSMS | SNIDAmFEAYIANLR |
| 75 | IPI00962525 | NA | 2U7 | 2.03 | U | 0/48 | 5.71 | 1 | 1 | 34.0 | 5.26 | 2.31 | natt1 | Natterin-like protein-like | FTMS/ITMSMS | QEYPIDVGSGYcLGIVGR |
| 76 | IPI00963174 | Q7SXK0 | 2U26 | 2.03 | U | 0/48 | 1.16 | 1 | 1 | 183.1 | 6.57 | 3.89 | c3 | Complement C3 (D. rerio) | FTMS/ITMSMS | VTVNTQGNVLTQEITAQTK |
| 77 | IPI00488901 | Q6P972 | 2U25 | 2.24 | U | 0/48 | 4.43 | 1 | 1 | 50.0 | 5.02 | 4.51 | tuba2 | Tubulin, α2 | FTMS/ITMSMS | TVGGGDDSFNTFFSETGAGK |
| 78 | IPI00886613 | Q1LXJ9 | 2U19 | 2.44 | U | 0/48 | 9.30 | 6 | 2 | 49.9 | 5.02 | 26.15 | krt1 | Novel protein similar to type I cytokeratin, enveloping layer | FTMS/ITMSMS | YSmQLSGYQAQVSGmEGQLVQLR; DQmSGQVNVEVDAAPQEDLTK |
| 79 | IPI00503480 | B3DFP9 | 2U2 | 2.6 | U | 0/48 | 17.73 | 1 | 1 | 15.5 | 7.18 | 2.55 | apo14 | 14-kDa apolipoprotein | FTMS/ITMSMS | TAVEPALEGSPTAGSLKDVVEELKK |
| 80 | IPI00508445 | Q7SZQ6 | 3D12 | -3.48 | D | 0/72 | 39.22 | 9 | 6 | 23.4 | 5.29 | 4.90 | arhgdig | Rho GDP dissociation inhibitor (GDI) γ | FTMS/ITMSMS | DVPVVEDEDEPDLNYQPPAQK; EKDVPVVEDEDEPDLNYQPPAQK; LTLmcDQAPGPITmDLTGDLNALK; KDWDGPEERPF; QTLLGSGPVVADPTIPNVQVTR; DWDGPEERPF |
| 81 | IPI00487156 | A7MBX1 | 3D19 | -2.46 | D | 0/72 | 8.92 | 1 | 1 | 24.3 | 5.22 | 0.00 | capns1b | Capns1b protein | FTMS/ITMSMS | QFRKVFQQLAGDDmEVSPK |
| 82 | IPI00614220 | E7FE19 | 3D23/24 | -2.45 | D | 0/72 | 21.27 | 7 | 5 | 45.0 | 4.54 | 1.77 | CNP | 2′,3′-Cyclic nucleotide 3′-phosphodiesterase-like (D. rerio) | FTMS/ITMSMS | EGTQVEmDmGTLSYLSEGR; EAITADTTFSSFSEDKPVSDQGK; VGVAPTLLPGVESLPAGSR; VALTETQLNLWPEGEDK; TLDTLEAFK |
| 83 | IPI00923195 | B8JKP3 | 3D15 | -2.42 | D | 0/72 | 15.15 | 3 | 2 | 26.2 | 5.38 | 1.69 | TPMT | Probable thiopurine S-methyltransferase | FTMS/ITMSMS | YLLDTLEYNPELYK; TVFGGScNIDLLQSVDGFEEK |
| 84 | IPI00482295 | Q7ZVI7 | 3D23 | -2.32 | D | 0/72 | 9.87 | 2 | 2 | 41.7 | 5.48 | 0.00 | actba | Actin, cytoplasmic 1 | FTMS/ITMSMS | DLYANTVLSGGTTmYPGIADR; SYELPDGQVITIGNER |
| 85 | IPI00497231 | Q5XJE1 | 3D16 | -2.1 | D | 0/72 | 26.69 | 6 | 4 | 27.2 | 5.88 | 5.60 | cutc | CutC copper transporter homolog | FTMS/ITMSMS | VLTSGcDSSALEGLPVIK; SQQADGLVFGALTEDGR; NSNVcmGGSLSVPEYAVK; ENVEIPVFVmIRPR |
| 86 | IPI00490093 | Q6GMI7 | 3D21 | -2.03 | D | 0/72 | 23.37 | 9 | 5 | 30.0 | 5.25 | 1.70 | enoph1 | Enolase-phosphatase E1 | FTMS/ITMSMS | IGcQPEEImFLTDVTR; IKGEVYQDVVPAIR; IITSFNQLELIGNV; VFLLDIEGTTTPITFVK; GEVYQDVVPAIR |
| 87 | IPI00492298 | Q5XJU1 | 3U13 | 1.67 | U | 0/72 | 23.30 | 6 | 5 | 32.8 | 7.88 | 2.24 | CaiD | Carnitinyl-CoA dehydratase-like | FTMS/ITMSMS | ELAHGSDSLELEQDVSSGPGPmGPSR; LPQLIGLSR; VAEESSImGVFcR; FGVPLIDGGTVR; AHEALAFGLANR |
| 88 | IPI00500949 | Q7T3A8 | 3U8 | 2 | U | 0/72 | 25.44 | 11 | 7 | 37.9 | 6.64 | 0.00 | anxa1a | Annexin A1a | FTMS/ITMSMS | GVDEPTIIDTLVHR; GAGTTEDTLIEILASR; AAYQQATSKPLDVALK; GTDcSVFIDILTSR; GDIEScLIAVVK; NALLSLcK; AIDLELK |
| 89 | IPI00960571 | Q4VBT1 | 3U2 | 2.33 | U | 0/72 | 23.44 | 3 | 2 | 14.5 | 6.57 | 2.38 | fabp1b | fatty acid binding protein 1b-like | FTMS/ITMSMS | VLNNSFTIGQDTEIQmLSGDK; YEGWEEFmK |
| 90 | IPI00501165 | Q7ZUM5 | 3U27 | 2.37 | U | 0/72 | 1.90 | 2 | 1 | 94.7 | 5.16 | 1.63 | hspa4 | Heat shock protein 4 | FTMS/ITMSMS | LmNETTAVALAYGIYK |
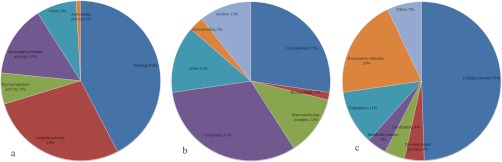
From both the 2DE and DIGE differential analysis, a total of 96 proteins were identified consensually as differentially expressed during the process of regeneration. Seprinb5 (−5.5) and act1ba (+11.6) are the most extremely down- and up-regulated proteins identified from this study, respectively, during regeneration. The proteins recognized from this study embodied a wide range of pI (4.5–9.7) and mass (11.7–183 kDa). Based on STRAP gene ontological analysis, the proteins identified for zebrafish caudal fin regeneration were found mainly associated with binding and catalytic activity as the major molecular functions (Fig. 2a) and cellular process as the major biological process (Fig. 2b). The differentially displayed proteins were majorly found localized in the cytoplasm and cytoskeleton followed by other organelles such as nucleus and mitochondria (Fig. 2c).
Fig. 2.
Pie chart distribution of proteins identified as differentially regulating during zebrafish caudal fin regeneration based on gene ontological molecular functions (a), biological process (b), and localization (c).
Significant Proteins Involved in Regeneration
Based on 2DE differential proteome analysis between the control and the five various regenerating time points, a total of 35 different proteins were analyzed for having at least 1.5-fold changes between them as either up- or down-regulated (Table I). Of the 35 proteins, ywhab1, capsn1a, psmb1, krtn17, krt12, cfl1, psma2, sod1, eef1a, actc1b, gnb2l1, cotl1, agr3, nudt14, anxa1a, and ppp1a were found to be down-regulated during regeneration. gstp1, nutf2, prdx5, vdac2, vdac3, cirbp, gnb1l, hspa8, ppp2r1b, gsn, arp3, ctsb, ndpkz3, ndpkz3, sept2, ppp1cab, msna, and gabp1b are the proteins that were found to be up-regulated during regeneration.
Differential Analysis
Based on DIGE analysis between the control, nonregenerating caudal fin and five various time points of regeneration, a total of 23, 37, 7, 12, and 11 proteins were identified (Fig. 1 and Table II) as differentially regulated. 14 of the 23 proteins identified between the 0 and 1 hpa were found to be down-regulated, which includes pah and ba1 globin as the major down-regulating proteins, and nine proteins were found up-regulated, which includes type 1 cytokeratin and Keratin 5. Between 0- and 12-hpa tissue samples, 15 and 22 proteins were found down- and up-regulated, which includes hemoglobin subunit α and cytokeratin type 1 as the most down- and up-regulated proteins, respectively. Differential proteome pattern between the control and the 24-hpa samples identified seven up-regulating proteins with fatty acid-binding protein 1b as the most up-regulated protein. Of the 12 proteins identified between the 0- and 48-hpa samples, five and seven proteins were found up- and down-regulated, which includes serpin peptidase and apo14 as the most down- and up-regulated proteins, respectively. ARHGDIG and hspa4 are the majorly down- and up-regulated proteins between the 0- and 72-hpa samples having seven and four down- and up-regulated proteins, respectively.
Regeneration and Down-regulation
During the caudal fin regeneration, almost 50% of identified proteins were found down-regulated, which majorly includes structural proteins such as Capsn1a, calcium-dependent cysteine proteinases, and muscle cofilin 2. The other major key structural proteins involved in regeneration through down-regulation are keratin 17, keratin 12, cfl2, actc1b, and cotl1. Keratin 17, the cytokeratin subfamily of intermediate filament protein, was found down-regulated right from the first hour after amputation till 3 days, whereas keratin 12 was found down-regulated from the 12th hour after postamputation and settling back to the basal level on the third day. The widely distributed intracellular actin-modulating protein cofilin 2, a muscle cofilin, was found down-regulated immediately after amputation and then maintained its state until 3 days postamputation. The ubiquitously distributed skeletal α1 actin, a major component of the cytoskeleton, undergoes an instant down-regulation for regeneration and reaches the normal level at the end of the 3rd day of regeneration.
Regeneration and Up-regulation
As with the down-regulation, an almost equivalent amount of protein undergoes up-regulation during the regeneration. The major proteins identified from this study that undergo up-regulation include PRDX5, voltage-dependent anion channel (VDAC) 2, VDAC3, and fatty acid-binding protein. PRDX5, an antioxidant protector during inflammation and nonpathological conditions, was found increased to almost 2-fold during regeneration at 12 hpa followed by decline in the level. The VDAC2 and VDAC3 proteins were found up-regulated during regeneration. In VDAC3, the mitochondrial membrane channel involved in translocation of adenine nucleotides was found undergoing more than 2-fold changes at the first hour of postamputation and maintaining the 2-fold changes during the other regeneration time points, whereas VDAC2 undergoes 1.5-fold change at the end of the 2nd day of regeneration. The heat shock cognate 71-kDa protein and orthologous of 70-kDa heat shock protein underwent up-regulation to nearly 2-fold between the first and second days postamputation. The 14-kDa fatty acid-binding protein 1b is the other major up-regulated protein during regeneration. ARP3 actin-related protein 3-like protein involved in control of actin polymerization in cells undergoes a short up-regulation during regeneration, reaching a maximum of 1.7-fold at the first hour of postamputation followed by down-regulation to reach the basal level.
Data Set Analysis
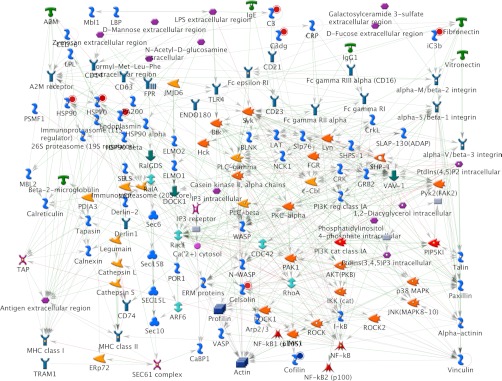
From the list of 90 and 35 proteins identified based on DIGE and 2DE analysis, respectively, 29 proteins were found commonly identified between the DIGE and 2DE analyses. The total list of 96 proteins consensually identified from both the differential display analyses were further analyzed for the distribution, process, and network pathway analysis. 62 proteins of the list were selected by the GeneGo software for analyses, which includes 42 in the pathway maps, 62 on the network pathways, 47 for various diseases, and 62 for gene ontological processes. The most significantly associated GeneGo pathway maps are the cytoskeleton remodeling pathway, the immune response-alternative complement pathway, and the oxidative stress role of ASK1 under oxidative stress pathway.
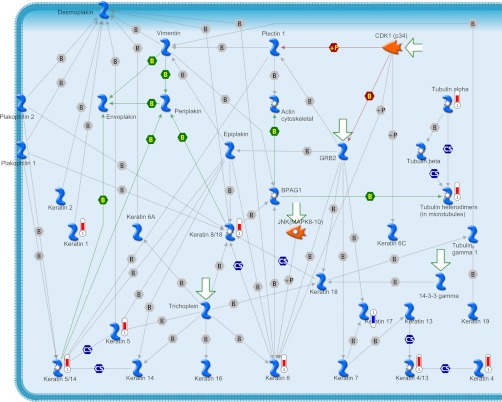
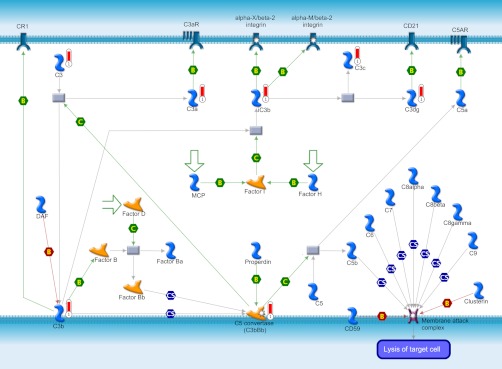
Cytoskeleton remodeling pathway (Fig. 3) was found as the most significantly associated pathway from the identified differentially expressed proteome data set for caudal fin regeneration. A total of 10 various proteins, mostly keratin isoforms, were directly associated in this pathway. Except keratin 17, all of the other keratin proteins such as keratin 8/18, 5, 1, 5/14, 8, 4/13, 4, α-tubulin and tubulin heterodimers involved in this pathway were found up-regulated during regeneration (Fig. 3). Immune response based on alternative complement pathway is the other major pathway found associated with the regeneration (Fig. 4). The proteins involved in this pathway include only up-regulating proteins such as c3, c3a, ic3b, c3c, c3dg, c3b, and c3bBb (Fig. 4). Down-regulated SOD1 and up-regulated HSP90 and HSP70 were found participating in the oxidative stress under the role of ASK1.
Fig. 3.
Cytoskeleton remodeling pathway map identified from the differentially expressed proteins of the zebrafish caudal fin. Proteins marked with red thermometer bars were identified as up-regulated, and those marked with blue color bars are down-regulated.
Fig. 4.
Immune response based on alternative complement pathway map identified from the differentially expressed proteins of the zebrafish caudal fin. Proteins with red thermometer bars were identified as up-regulated during the zebrafish caudal fin regeneration.
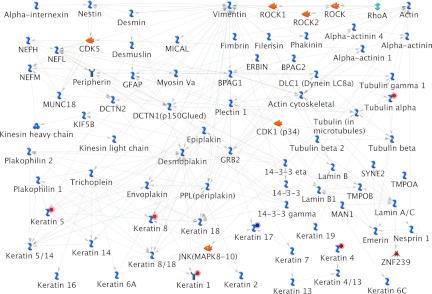
The various networks associated with the identified proteome data set include immune response to phagosome in antigen presentation (Fig. 5), cytoskeleton-mediated intermediate filament process (Fig. 6), protein folding response to unfolded proteins, inflammation associated by complement system, and cytoskeleton regulation-based cytoskeleton rearrangement. The immune response to phagosome in antigen presentation was found associated with up-regulating c3, c3dg, ic3b, gelsolin, hsp90, hsp70, and endoplasmin proteins and down-regulating cofilin 2 protein (Fig. 5). The cytoskeleton-mediated intermediate filament network pathway involved in specifying the cytoarchitecture and cytodynamics was found associated with up-regulating keratin 5, 8, 1, 4, α-tubulin proteins and down-regulating keratin 17 protein (Fig. 6). HSP70, HSP90, HSPA4, HSC70, and endoplasmin were found associated in the protein folding network.
Fig. 5.
Immune response to phagosome in antigen presentation network pathway map associated with the proteins identified as differentially regulated during zebrafish caudal fin regeneration. Proteins with red solid circles were identified as up-regulated, and those with blue solid circles are down-regulated.
Fig. 6.
Cytoskeleton-mediated intermediate filament process pathway map associated with the proteins identified as differentially regulated during zebrafish caudal fin regeneration. Proteins with red solid circles were identified as up-regulated, and those with blue solid circles are down-regulated.
Validation Based on RT-PCR
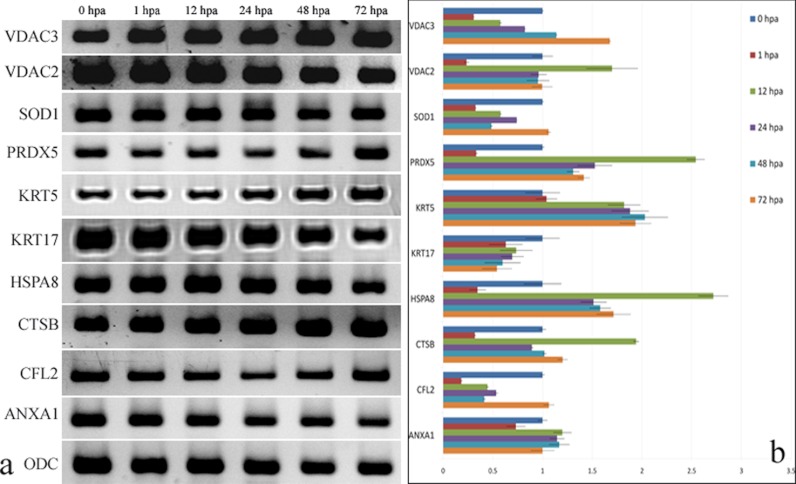
A total of 10 different genes (Table III), which were found differentially regulated during the caudal fin regeneration, were analyzed for its mRNA transcript level based on quantitative real time PCR. The expression patterns of these genes were analyzed between nonregenerating control (0 hpa) and the regenerating 1-, 12-, 24-, 48-, and 72-hpa tissues. It was found that the mRNA expression of the genes such as KRT5, HSPA8, VDAC3, VDAC2, PRDX5, and CTSB were found up-regulated, and genes such as KRT17, CFL2, and SOD1 were down-regulated during the course of regeneration (Fig. 7, a and b). Also, it was found that ANXA1 transcript remained unchanged majorly during regeneration against the control nonregenerating tissue. Thus, mRNA expression levels of the selected genes were found correlating to their protein expression levels as obtained from the 2DE and DIGE analysis (Fig. 7b).
Fig. 7.
Quantitative RT-PCR analyses of gene expression in regenerating zebrafish caudal fin. a, expression of genes for different regenerating time points. b, quantitative expression of genes for each regenerating time point calculated based on Ct value normalized against the housekeeping ODC gene.
Annexin A1 (ANXA1) and Regeneration
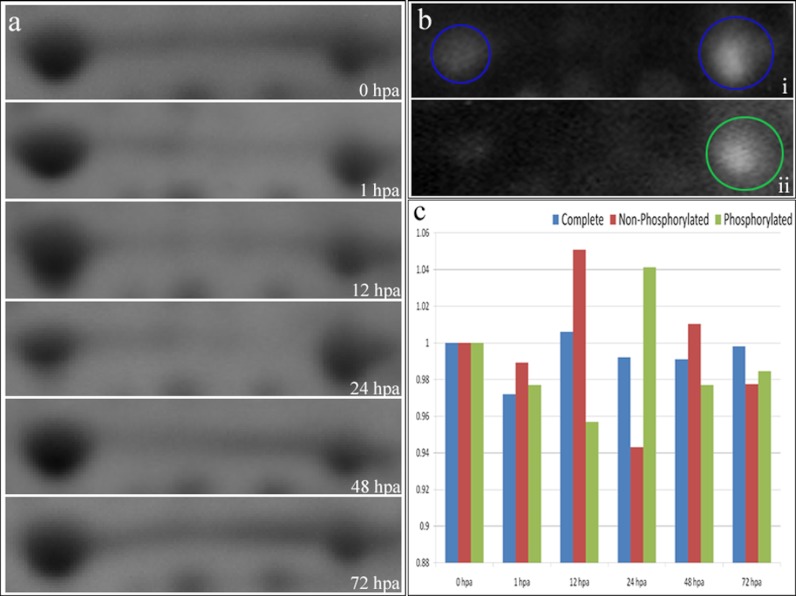
It was found from the 2DE analysis that the basal level of ANXA1 undergoes differential expressions because of phosphorylation. ANXA1, the Ca2+-dependent phospholipid-binding protein, was found expressed in two different forms (spots) of 38 kDa in the pI range of 6.5–6.7, with a 12-mm-long ANXA1 protein band streak running in between the two ANXA1 spots (Fig. 8a). The two different forms of annexin were confirmed as ANXA1 based on MS/MSMS and 2DE immunoblot analysis against ANXA1 antibody (Fig. 8b, panel i). The second form of ANXA1 after the streak was found immunolabeled to the anti-phosphorylation pan antibody specifically to be called the phosphorylated form of ANXA1 (Fig. 8b, panel ii) in comparison with the first acidic form. The two different forms of ANXA1, the nonphosphorylated and phosphorylated forms, were found to be undergoing differential expression during regeneration after amputation. The nonphosphorylated form of ANXA1 undergoes increase in its expression at 12 hpa, whereas the phosphorylated form of ANXA1 undergoes differential expression at the first hour onwards. Analysis of the ANXA1 streak showed that the ANXA1 decreases immediately after amputation, and it starts reappearing from the second day of amputation (Fig. 8c). Also it is interesting to note that the total amount of ANXA1 (the nonphosphorylated, phosphorylated, and the streak) always remained at the same level of expression, but the two different forms of ANXA1 caused by phosphorylation were undergoing differential expression (Fig. 8c) because of the regeneration mechanism. The total ANXA1 expression based on both real time PCR analysis (Fig. 7) and one-dimensional gel electrophoresis western blot analysis (Fig. 9) confirms that the total expression of ANXA1 remains the same all through the regenerating time points.
Fig. 8.
a, the 2DE spot pattern of ANXA1 for six various time points of regeneration. b, panel i, the 2DE Western blot pattern for ANXA1 antibody. The spots circled in blue represent the spots specifically labeling the ANXA1 protein. Panel ii, the 2DE Western blot for pan phosphorylation antibody. The spot circled in green represents the spot labeling the alkali form of ANXA1 exclusively. c, the distribution of the ANXA1 (the nonphosphorylated, phosphorylated and total ANXA1) as seen in the 2DE gel based on densitometry quantification. The blue bars represent the total ANXA1 protein, the red bars represent the nonphosphorylated form of ANXA1, and the green bars represent the phosphorylated form of ANXA1.
Fig. 9.
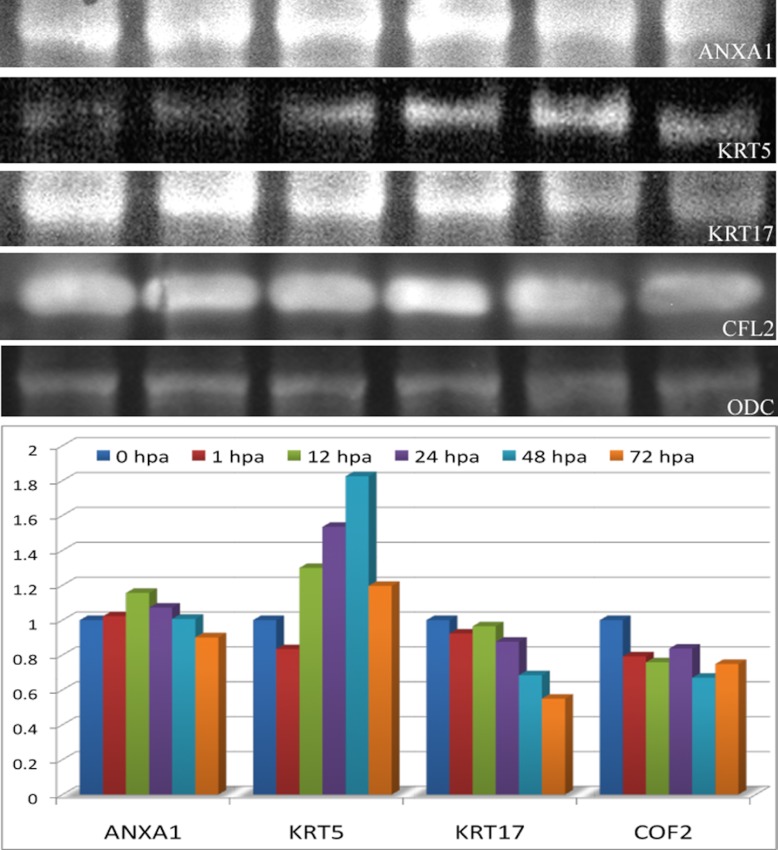
Western blot analysis showing the expression of ANXA1, KRT5, KRT17, CFL2, and ODC. The lower panel of the figure represents the relative expression of ANXA1, KRT5, KRT17, and CFL2 normalized against the ODC protein expression. The distribution was represented as a relative expression against the control 0 hpa.
Keratin and Regeneration
In this study we have identified an idiosyncratic association of keratin and its isoforms for regeneration. A total of 10 dissimilar keratin proteins identified during regeneration were found to be associated to the cytoskeleton remodeling pathway map (Fig. 3) and cytoskeleton intermediate filament network process (Fig. 5). Other than keratin 17, all of the other keratin proteins such as keratin 5, 8/18, 1, 5/14, 8, 4/13, 4, α-tubulin and tubulin heterodimers were found up-regulated during the caudal fin regeneration (Fig. 3). Real time and Western blot analysis of keratin 5 showed the up-regulation from the 12th hour of the regeneration when its expression level was compared against the housekeeping ODC expression (Figs. 7 and 9). In the same way, the expression of keratin 17 showed a down-regulation right from the first hour of regeneration based on both real time PCR and Western blot analysis (Figs. 7 and 9).
DISCUSSION
Regeneration of zebrafish caudal fin has been widely studied at the transcriptome level, such as differential transcriptome mapping (12, 13) and regulation of microRNAs (14). Proteomics-based analysis of regeneration has been extensively studied among amphibians for its blastema formation in regenerating axolotl limbs (15) and proteomic changes during onset of regeneration (16). For the first time in this study, the global proteome changes caused by the regeneration in zebrafish caudal fin were analyzed. The study was characterized by mapping of differential proteome changes caused by regeneration on gel-based sensitive DIGE and 2DE analyses. Based on this study, a total of 96 proteins were identified and found differentially expressed because of regeneration. The proteins were characterized for their identities based on the MALDI MS/MSMS analysis and Orbitrap based FTMS and ITMSMS analyses. The majorly identified and differentially expressed proteins were found to be directly and indirectly involved in various networks, pathways, and biological processes. 50% of the proteins identified from this study have also been reported from our previous work of understanding the zebrafish caudal fin proteome map (9). Because this study has identified the proteins involved in regeneration using the most sensitive DIGE method, proteins other than those mentioned in the caudal fin proteome map were identified as contributory target for regeneration. This study also validates the importance of DIGE-based quantification rather than the standard 2DE-based quantification for its significant identification of more proteins having 1.5-fold changes at different time points of regeneration. Comparison of this study against the differential transcriptome mapping (12, 13) showed many commonly identified targets as regeneration-specific, including prdx5, psmb1, SOD1, SEPT2, VDAC2, ywhab1, SERPINb1, HSP90, and CFL2. Differential analysis of the proteins involved in amphibian limb regeneration (16) also showed many common target proteins responsible for zebrafish caudal fin regeneration, such as ANXA1, KRT17, VDAC3, and CTSB.
Annexin A1 was found to directly regulate the regeneration of zebrafish caudal fin by differentially regulating the expression caused by phosphorylation. This study confirms the direct association of differential expression and phosphorylation of the ANXA1 during the regeneration (17). The ANXA1 expression and phosphorylation was shown as the factor to be involved directly for the liver regeneration and transformation through modulation of cPLA2 activity or EGF-R function (17).
In this differential proteomics analysis, several structural proteins were found to be directly associated and undergo differential expression for regeneration. The majorly identified structural proteins for caudal fin regeneration include keratin 5, 8/18, 1, 5/14, 8, 4/13, 4, α-tubulin tubulin heterodimers and cofilin 2. The identified proteins were also found to be directly associated as network partners for various pathways, such as upholding of the structure and architecture of the cells. Our study also validates the association of keratin 8 and 18 expressions for cell proliferation and differentiation in the mesenchymal progenitor cells of regenerating limb (18). It has been shown that the treatment of cultured blastemal cells with K8 and K18 antisense oligonucleotides significantly decreases DNA synthesis and induces differences in cell morphology (18). The inverse association of keratin 17 imparts a decisive role in regeneration because it is shown that the intermediate filament protein, keratin 17, has been involved in inducing the wounded stratified epithelia for regulation of cell growth (19), promoting epithelial proliferation and tumor growth by polarizing the immune response (20), and inducing tumor angiogenesis (21).
This study of understanding the zebrafish caudal fin regeneration based on proteomics approaches established the strong association of several structural components of the cells as direct biomarkers. The identified proteins were found playing a lead role in regeneration based on its differential expression. Association of the ANXA1 and keratin proteins might lead to a new role and insight in understanding the complexity of regeneration and its varied extent in different animals.
Acknowledgments
We thank Sruthi Purushothaman for help with the study and Noorul Fowzia for critically reviewing the manuscript.
Footnotes
* This work is supported by Council of Scientific and Industrial Research Network Project NWPO4 and by the Department of Biotechnology. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- hpa
- hour(s) postamputation
- 2DE
- two-dimensional gel electrophoresis
- FTMS
- Fourier transform mass spectrometer
- ITMSMS
- ion trap tandem mass spectrometer
- ANXA1
- annexin A1
- KRT
- keratin
- CFL2
- cofilin 2
- ODC
- ornithine decarboxylase
- VDAC
- voltage-dependent anion channel.
REFERENCES
- 1. Stoick-Cooper C. L., Moon R. T., Weidinger G. (2007) Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 21, 1292–1315 [DOI] [PubMed] [Google Scholar]
- 2. Johnson S. L., Weston J. A. (1995) Temperature-sensitive mutations that cause stage specific defects in zebrafish fin regeneration. Genetics 141, 1583–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poss K. D., Wilson L. G., Keating M. T. (2002) Heart regeneration in zebrafish. Science 298, 2188–2190 [DOI] [PubMed] [Google Scholar]
- 4. Poss K. D., Keating M. T., Nechiporuk A. (2003) Tales of regeneration in zebrafish. Dev. Dyn. 226, 202–210 [DOI] [PubMed] [Google Scholar]
- 5. Nasevicius A., Ekker S. C. (2000) Effective gene targeted “knockdown” in zebrafish. Nat. Genet. 26, 216–220 [DOI] [PubMed] [Google Scholar]
- 6. Brand M., Granato M., Nusslein-Volhard C. (2002) “Keeping and raising zebrafish,” in Zebrafish: A Practical Approach (Nusslein-Volhard C, Dahm R, eds.) pp. 7–38, Oxford University Press, New York [Google Scholar]
- 7. Schaffner W., Weissmann C. (1973) A rapid, sensitive, and specific method for the determination of proteins in dilute solution. Anal. Biochem. 56, 502–514 [DOI] [PubMed] [Google Scholar]
- 8. Singh S. K., Sundaram C. S., Shanbhag S., Idris M. M. (2010) Proteomic profile of zebrafish brain based on two-dimensional gel electrophoresis matrix-assisted laser desorption/ionization MS/MS analysis. Zebrafish 7, 169–177 [DOI] [PubMed] [Google Scholar]
- 9. Singh S. K., Lakshmi M. G., Saxena S., Swamy C. V., Idris M. M. (2011) Proteome profile of zebrafish caudal fin based on one-dimensional gel electrophoresis LCMS/MS and two-dimensional gel electrophoresis MALDI MS/MS. J. Sep. Sci. 34, 225–232 [DOI] [PubMed] [Google Scholar]
- 10. Sandeep S., Singh S. K., Meena Lakshmi M. G., Meghah V., Sundaram C. S., Swamy C. V., Idris M. M. (2011) Proteome profile of zebrafish kidney. J. Proteomics 74(12), 2937–2947 [DOI] [PubMed] [Google Scholar]
- 11. Bhatia V. N., Perlman D. H., Costello C. E., McComb M. E. (2009) Software tool for researching annotations of proteins: Open-source protein annotation software with data visualization. Anal. Chem. 81, 9819–9823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schebesta M., Lien C. L., Engel F. B., Keating M. T. (2006) Transcriptional profiling of caudal fin regeneration in zebrafish. Sci. World J. 6, (suppl. 1) 38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Padhi B. K., Joly L., Tellis P., Smith A., Nanjappa P., Chevrette M., Ekker M., Akimenko M. A. (2004) Screen for genes differentially expressed during regeneration of the zebrafish caudal fin. Dev. Dyn. 231, 527–541 [DOI] [PubMed] [Google Scholar]
- 14. Thatcher E. J., Paydar I., Anderson K. K., Patton J. G. (2008) Regulation of zebrafish fin regeneration by microRNAs. Proc. Natl. Acad. Sci. U.S.A. 105, 18384–18389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rao N., Jhamb D., Milner D. J., Li B., Song F., Wang M., Voss S. R., Palakal M., King M. W., Saranjami B., Nye H. L., Cameron J. A., Stocum D. L. (2009) Proteomic analysis of blastema formation in regenerating axolotl limbs. BMC Biol. 7, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. King M. W., Neff A. W., Mescher A. L. (2009) Proteomics analysis of regenerating amphibian limbs: Changes during the onset of regeneration. Int. J. Dev. Biol. 53, 955–969 [DOI] [PubMed] [Google Scholar]
- 17. de Coupade C., Gillet R., Bennoun M., Briand P., Russo-Marie F., Solito E. (2000) Annexin 1 expression and phosphorylation are upregulated during liver regeneration and transformation. Hepatology 31, 371–380 [DOI] [PubMed] [Google Scholar]
- 18. Corcoran J. P., Ferretti P. (1997) Keratin 8 and 18 expression in mesenchymal progenirot cells of regenerating limbs is associated with cell proliferation and differentiation. Dev. Dyn. 210, 355–370 [DOI] [PubMed] [Google Scholar]
- 19. Kim S., Wong P., Coulombe P. A. (2006) A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 441, 362–365 [DOI] [PubMed] [Google Scholar]
- 20. Depianto D., Kerns M. L., Dlugosz A. A., Coulombe P. A. (2010) Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat. Genet. 42, 910–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu Y., Zhang S. Z., Huang C. H., Liu X. Y., Zhong Z. H., Hou W. L., Su Z. F., Wei Y. Q. (2009) Keratin 17 identified by proteomic analysis may be involved in tumor angiogenesis. BMB Rep. 42, 344–349 [DOI] [PubMed] [Google Scholar]