Abstract
Prospective memory (PM) involves forming intentions, retaining those intentions, and later executing those intended responses at the appropriate time. Few studies have investigated this capacity in animals. Monkeys performed a computerized task that assessed their ability to remember to make a particular response if they observed a PM cue embedded within an ongoing learning-set (LS) task. At a break in the LS task, monkeys selected one of two icons indicating that they had or had not encoded the occurrence of the PM cue (the latter icon resumed the LS task). Critically, during this response period, the PM response icon appeared after a delay during which monkeys could self-initiate the PM response prior to receiving any external prompt. Monkeys selected the PM and LS icons when each was the optimal response, illustrating that they could encode, store, and respond appropriately to a stimulus event in the future. Critically, some monkeys self-initiated the PM response prior to that icon’s appearance, indicating that they could retrieve the PM and act on their intention to make that response without the aid of a prompt. These monkeys appeared capable of using PM in this task. Thus, this capacity appears not to be limited to humans.
Keywords: Prospective memory, rhesus monkey, capuchin monkey
Humans travel through time, although not in the way envisioned by science fiction writers. We remember past events, in some cases at an experiential level that recreates not just the details of the event itself, but also the subjective experience of living that event (Tulving, 1972, 1993). Humans travel forward in time as well and plan for events that are minutes to years in the future. The ability to anticipation future events allows an organism greater flexibility in its current behavior by allowing for responses now that are not solely responsive to present stimuli. In fact, some have suggested that it is more advantageous to anticipate the future than to remember the past (e.g., Suddendorf & Busby, 2003; Suddendorf, 2006).
Humans use prospective memory (hereafter PM) to take advantage of anticipated events and to aid in planning and decision-making by remembering things that must be done later (Einstein & McDaniel, 1990; March, Hicks, & Landau, 1998; Marsh, Hicks, & Cook, 2006; McDaniel & Einstein, 2007; Smith, 2003, 2008). Everyday life examples of PM range in importance from remembering to attach a file to an email before sending it to remembering to take one’s blood pressure medication before going to sleep. Although PM is easily disrupted and fragile, and PM failures can sometimes have devastating consequences, humans routinely use it as a tool to aid in planning and remembering future behavior (Smith, 2003, 2008; Kleigel, McDaniel, & Einstein, 2000; Kleigel, Mackinlay & Jager, 2008; McDaniel, Einstein, Graham, & Rall, 2004; Einstein, McDaniel, Smith, & Shaw, 1998; Einstein, McDaniel, Manzi, Cochran, & Baker, 2000; McDaniel, Einstein, Stout, & Morgan, 2003; Graf & Uttl, 2001).
PM refers specifically to the processes of encoding, storage, and delayed retrieval of a future response. Laboratory paradigms of human PM are designed so that the PM is embedded within ongoing activity (to prevent continuous rehearsal of the PM) and so that there is no explicit external prompt to make the future response (to prevent participants from searching their retrospective memory store at that time when somehow told to think back to what they had remembered; McDaniel & Einstein, 2007). In an example of a standard human PM paradigm, participants must remember to make a response (e.g., press a key) whenever a particular target item (e.g., the word “green”) occurs in the context of an unrelated task (e.g., rating words for pleasantness). These studies have focused on the ability to remember that a response is needed while other activities are ongoing (Kvavilashvili & Ellis, 1996; Thorpe, Jacova, & Wilkie, 2004) and have tended to deemphasize the difficulty of the retrospective memory component (i.e., the number of different target events and the complexity of the response) in order to isolate the processes that are involved in prospective remembering (McDaniel & Einstein, 2007; Riley, Cook, & Lamb, 1981). Thus, in the example given above, the retrospective memory challenge (remembering the action and the target word) is simple, and the real interest is in seeing whether participants will switch from seeing green as a word to be rated for pleasantness to seeing it as a cue for performing the PM action. Further, this is assessed without direct prompting to search memory for the significance of “green” when it occurs (e.g., by asking “is this the target word” each time a word is presented to the participant), so as to not make it a retrospective memory task (e.g., a cued recall task).
Few studies have directly investigated human-like PM in nonhuman animals (hereafter animals), despite the evolutionary advantage it could provide within the context of planning and decision-making. For example, it would be beneficial for monkeys to use PM to remember to visit a distant fruit tree at a later time as a result of just having encountered evidence that a particular fruit species is ripe (e.g., a discarded fruit pit or a similar fruit tree; see Menzel, 1991). Instead, animal studies typically focus on the ability to encode information for future use (often called prospective coding), without the added requirements of storage and retrieval of the PM within an ongoing task. For example, using a 12-arm radial maze, Cook, Brown, and Riley (1985) found evidence for within-trial flexible coding by rats. Each arm was baited and rats were allowed to visit the arms and collect food. At some point, rats were taken out of the test with food still remaining in some arms (after 2, 4, 6, 8, or 10 correct arm visits). After a delay, rats were given only two choices - an arm that had not yet been visited and one that had been visited. Inserting this delay early in trials, when only a few arms had been visited, or late in trials, when many arms had been visited, resulted in good choice behavior of the still-baited arm. However, when the delay occurred after an intermediate number of arms had been visited (e.g., 6), performance was much lower. These data seem to indicate that rats used a dual response strategy. Early in trials, they appeared to remember where they had already been in the trial, but late in trials they appeared to remember where they still had to go to find the remaining food. Zentall, Steirn, and Jackson-Smith (1990) also found evidence for this kind of prospective coding in pigeons using a radial maze analog. However, DiGian and Zentall (2007) did not find similar results, and Klein, Evans, and Beran (2011) reported that monkeys did not show evidence of a dual-coding strategy in a computerized analog of the radial arm maze, relying instead on retrospective coding alone. Thus, there is mixed evidence as to whether or not animals prospectively code information in that experimental paradigm. Even if animals do use prospective coding in that task, because their responses are not embedded within ongoing activity, the possibility remains that animals are continually rehearsing the goal locations, rather than storing that information for later use.
Another limitation of most animal PM studies is that they only assess whether animals remember the correct future response, but do not require spontaneous initiation of that response. Instead, a cue is given when it is time to make the delayed response, and this allows for the possibility that animals then rely on retrospective memory to recall what they were supposed to do. For example, during a sequential paired association task, monkeys first learn an association between two stimuli. Next, they view one member of the pair, and after a delay, they view the second stimulus. The monkeys must respond differently depending on whether or not the second stimulus is the one associated with the first or is not. Researchers have suggested that during the delay, monkeys are remembering what the associated (second) stimulus should be when it is presented later and not what the first stimulus was (e.g., Colombo & Graziano, 1994; Genovesio, Brasted, & Wise, 2006; also see Rainer, Rao, & Miller, 1999). Although this suggests that monkeys use PM, explicit prompting of animals to respond via presentation of the second stimulus (and the opportunity to continuously rehearse during the delay) has prevented such studies from directly assessing human-like PM (see also Murphy, 2009, for another prospective paradigm that allows for similar alternative explanations in a study of horse memory). For these reasons, Thorpe, Jacova, and Wilkie (2004) argued that most PM studies with animals actually show capacities that, in human memory research, would be identified as retrospective.
A more recent study has provided other suggestive evidence that animals can remember to make future responses. Wilson and Crystal (2012) reported that rats anticipating a future event exhibited reduced performance on an ongoing task, which is an effect that sometimes accompanies prospective memory (e.g., Smith, 2003). Rats that had learned that they would receive a meal if they poked their nose into a trough after a consistent interval showed decreasing sensitivity to time in an ongoing bisection test that took place during the delay interval. This suggested that the rats were exhibiting time-based prospective memory. However, rats made the nose poke response throughout the test interval (albeit at increasing frequency), and so their reduced performance on the ongoing task could have been attributed to the rats dividing their attention between the test trials and their responses to the food trough, rather than their actually memory for the future feeding. Nevertheless, this study demonstrated that rats would anticipate a future event, and so this methodology may lead to other insights with regard to future-oriented behavior in animals.
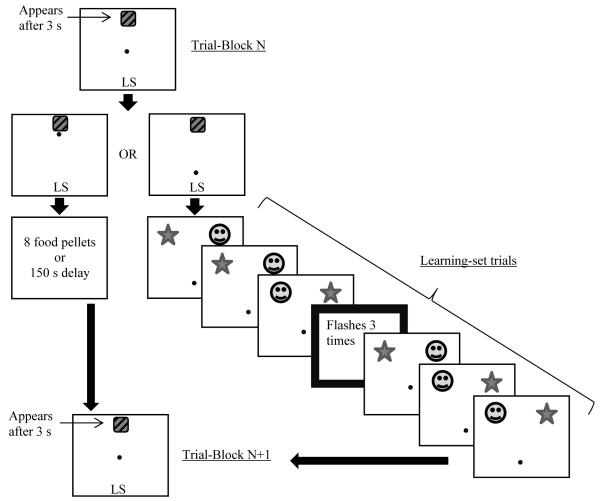
In the present study, to address the issues mentioned above, we designed a PM study to assess whether animals could encode, store, and spontaneously retrieve a future response within the context of an unrelated ongoing task. Within this paradigm, we specifically tested whether monkeys would remember to make future responses when the task made it unlikely that they could continuously rehearse (as could happen if they had nothing else to do during the delay), and when they were not provided with an external prompt as to when would be the appropriate time to make the response. We presented rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) with a computerized task in which they had to remember to make a particular future response if they observed a PM cue embedded within an ongoing learning set (LS) task (see Figure 1). The PM cue sometimes appeared in the inter-trial interval (ITI) between two LS trials within a block of six. Because we embedded the PM cue within the ongoing LS task, monkeys most likely could not continuously rehearse this information before making the future response because they were otherwise engaged in the ongoing task, which also required remembering the correct stimulus (S+) to be selected. In between LS blocks, monkeys had the opportunity to use their joystick to indicate whether or not they had seen the PM cue, and correctly doing so resulted in a large food reward. Incorrectly indicating that there was a recent PM cue where there had not been one resulted in a large timeout penalty in the task. Indicating that there had not been a PM cue had no consequence other than resuming the ongoing computer task. Critically, during this response period, the icon representing the PM response was delayed in its appearance relative to the cursor controlled by the monkey’s joystick and the icon that resumed the ongoing (LS) task. This allowed us to assess whether the monkeys would self-initiate a PM response prior to receiving a prompt or reminder to do so.
Figure 1.
A sample test trial block. The diagonally striped square is the prospective memory (PM) response icon and the letters “LS” are the icon for the learning-set task. The small circle is the cursor that monkeys control with the movement of the joystick. Contacting the LS icon with the cursor initiated a set of LS trials. The PM cue, a flashing thick border, could appear between any two of these trials. Contacting the PM icon resulted in either a large reward or large penalty, depending on whether a PM cue was presented in the previous LS trial-block.
This task is comparable to what is referred to as a delay-execute PM task in the human literature (e.g., Einstein et al., 2000; McDaniel et al., 2003; Rendell et al., 2009). In real world settings, PM cues often evoke intentions to implement an action, but that action cannot occur immediately, and people must delay the intention to yet another point in time. For instance, in the middle of lunch you see a colleague drinking a glass of milk and then remind yourself that you need to buy milk on the drive home later. Delay-execute PM tasks are designed to explore this situation. Using this and the more standard immediate-execute methodology, intriguing patterns of performance have been reported. For example, older adults show dramatic decline on delay-execute PM tasks compared to immediate-execute tasks, while younger adults perform equally well on both (Einstein et al., 2000; McDaniel et al., 2003). Also, for children, there is an impact of delaying the execution of a retrieved intention on PM performance, with improvements as children get older (e.g., Rendell et al., 2009). Thus, it is important to assess potential performance differences across other species and different human age groups using this paradigm.
We hypothesized that, within this task framework, members of both monkey species would encode, store, and retrieve the PM at the appropriate time, and we expected them to do so despite the inclusion of an unrelated ongoing task that most likely prevented continuous rehearsal of the future response. We also predicted that monkeys of both species would anticipate their next PM response by moving their cursor in the direction of the PM icon even before it appeared on the computer screen. This would indicate that they intended to make the PM response even before they were prompted as to whether they had seen the PM cue. This would mean that monkeys were not relying on the external prompt as a reminder to search their retrospective memory stores for an indication that they had observed the PM cue.
In addition to monkeys’ ability to remember to make the PM response, we also were interested in monkeys’ performance on the ongoing LS task as a function of whether or not they observed the PM cue. Adult humans may employ different strategies when engaging in PM tasks, such as rehearsing the intention, monitoring for the target, or relying on spontaneous retrieval. One way to investigate the use of these potential strategies is to examine performance on the concurrent task presented during the retention interval (McDaniel & Einstein, 2000; Smith, 2003). If PM requires effortful rehearsal or monitoring rather than occurring spontaneously, then performance in the concurrent task should suffer as a result of the additional cognitive load imposed by the PM task (Einstein et al., 2000). Because this effect only sometimes surfaces in the human literature, we did not have a specific prediction as to how remembering the PM response would affect monkeys’ performance in the ongoing task.
Method
Participants
We tested eight male rhesus monkeys: Obi (age 8), Han (age 9), Luke (age 12), Chewie (age 12), Murph (age 18), Lou (age 18), Gale (age 28), and Hank (age 28). Rhesus monkeys were individually housed with constant visual and auditory access to other monkeys. We also tested nine capuchin monkeys: Logan (age 6 male), Liam (age 7 male), Nala (age 8 female), Wren (age 8 female), Gabe (age 13 male), Lily (age 14 female), Griffin (age 14 male), and Drella (age 21 male). Capuchin monkeys were group housed but separated for testing. All monkeys had 24-hour access to water and frequent access to computerized testing systems, from which they could earn food pellets. All monkeys were fed manufactured chow, fruits, and vegetables daily between 1600 and 1800 hours. This study complied with protocols approved by the Georgia State University IACUC. All procedures were performed in full accordance with the USDA Animal Welfare Act and conformed to the “Guidelines for the use of laboratory animals.”
Materials
The monkeys were tested using the Language Research Center’s Computerized Test System comprising a personal computer, digital joystick, color monitor, and pellet dispenser (Evans, Beran, Chan, Klein, & Menzel, 2008; Richardson, Washburn, Hopkins, Savage-Rumbaugh, & Rumbaugh, 1990). Monkeys manipulated the joystick to produce isomorphic movements of a computer-graphic cursor on the screen. Contacting appropriate computer-generated stimuli with the cursor brought them a 45-mg (capuchins) or 94-mg (macaques) banana-flavored chow pellet (Bio-Serv, Frenchtown, NJ) using a pellet dispenser interfaced to the computer through a digitial I/O board (PDISO8A; Keithley Instruments, Cleveland, OH). All monkeys had previously participated in multiple psychological experiments involving this computerized test system.
Procedure
General procedure
The task began with the appearance of two choice icons on the computer screen (hereafter referred to as the menu screen; see Figure 1). One icon, hereafter called the PM icon, was a rounded square with black cross-hatching and it was centered at the top of the screen. The second icon, hereafter called the LS icon, was a pair of letters (“LS”) in 72-point font, and it was centered at the bottom of the screen. A small round cursor appeared in the center of the menu screen, and the monkeys could manipulate the joystick to bring the cursor into contact with either of the two icons. On this screen, the cursor moved rather slowly, and both responses required approximately ten seconds to complete.
If, on the menu screen, the monkey chose the LS icon, then the program would present a block of six two-choice discrimination (also called learning-set, LS) trials. In each of these 6-trial blocks, two clip-art stimuli were randomly chosen from an array of hundreds of images. Those two stimuli appeared at the top corners of the screen, with random assignment of the items to those locations (the same two stimuli appeared in all 6 trials in the block, randomly assigned between the left and right positions), and the cursor appeared near the bottom of the screen. If the monkey selected the positive stimulus (S+), it received a small reward (one food pellet), whereas if the monkey selected the negative stimulus (S-), it received a small penalty (10-s time-out, during which the screen remained blank). On trial one, the monkey could not know which stimulus was the S+ because this was randomly determined. However, information on trial one gave the monkey all the information it needed for all subsequent trials in the block if the monkey employed a win-stay, lose-shift strategy that is indicative of learning set (Harlow, 1949). Responses to either stimulus could be made in approximately three seconds, and each trial was followed by a five-second inter-trial interval. The LS trial-block was followed by a 1-s inter-block interval and then the presentation of the menu screen involving the PM and LS icons. The computer program alternated back and forth between these two screens for the entire test session.
In a certain percentage of LS trial-blocks, hereafter referred to as PM blocks, an additional stimulus event occurred. During one of the inter-trial intervals within the block of LS trials, a thick screen border would flash three times (hereafter referred to as the PM cue). The experimenter manually determined which trial number would be followed by the PM cue prior to each session. After presentation of the PM cue, the monkey completed the remaining LS trials in the block.
If, on the menu screen, the monkey chose the PM icon instead of the LS icon, then it received either a large reward (eight food pellets) or a large penalty (150-s time-out), depending on whether or not the previous trial block was a PM block that included the flashing cue. If the monkeys observed the PM cue while performing the LS trials, then selecting the PM icon on the next menu screen would lead to the large reward. But, if they selected that icon following an LS problem during which there was no PM cue, the large penalty was applied. Both the reward and penalty were followed by a one-second inter-block interval and then by the next menu screen. Monkeys could alternate between these menu selections and LS trial-blocks as often as they wanted until the end of the session.
Training
In this phase, 50% of LS trial-blocks presented the PM cue, and within these blocks, the PM cue was presented twice to ensure that the monkey observed it. The PM cue would flash three times between the selection of the LS icon and the appearance of the first pair of LS stimuli, as well as during one of the inter-trial intervals within the block, as described above. The experimenter manually determined which learning-set trial the PM cue would follow within these blocks. In the first session for every monkey, the PM cue flashed between the fifth and sixth LS trials (i.e., closest in time to the next opportunity to select the PM icon). Also, in the first session, 25% of trials forced the monkey to choose the appropriate icon at the end of the block (by only presenting that option). Subsequent sessions involved the same parameters until the monkey met a performance criterion of 66.67% selection of the optimal choice icon on the menu screen, depending on whether the previous LS trial-block presented the PM block or not (but, to provide a more conservative assessment of performance, the criterion was not applied to the first block of each session, any blocks involving a forced choice, or any blocks immediately following a PM response,). After meeting this criterion, the forced trials were removed from sessions and the same performance criterion was assessed. Once the monkey met the criterion within a session involving at least 30 blocks in each of the two categories (PM blocks and non-PM blocks) we moved the PM cue ahead one trial in the block (slightly farther in time from the next opportunity to select the PM icon). This was repeated until the monkey met the criterion when the PM cue appeared just after the first LS trial or the monkey completed ten consecutive sessions without reaching the criterion. Monkeys’ performance in the training phase is summarized in Table 1.
Table 1.
Prospective memory performance summary by individual.
| Earliest LS trial that PM cue followed and success criterion was still met |
Was the cursor closer to the PM Icon when it appeared in PM (vs. LS) responses? |
|||
|---|---|---|---|---|
| Monkey | Train (50% PM) | Test (50% PM) | Met criterion - 25% PM? | (independent samples t-tests; 10% PM) |
| Chewie | 1 | 1 | Yes | t(142) = 1.534, p = . 127u |
| Gale | 3 | 2 | No | t(832) = .622, p = .534 |
| Han | 1 | 1 | Yes | t(86) = −55.432, p < .001u |
| Hank | 1 | 1 | Yes | t(364) = 3.486, p = .001u |
| Lou | 2 | 1 | Yes | t(843) = 1.677, p = .094 |
| Luke | 2 | 4 | Yes | t(74) = −24.39, p < .001u |
| Murph | 1 | 1 | Yes | t(81) = 3.881, p < .001u |
| Obi | 1 | 1 | Yes | t(238) = 8.125, p < .001u |
|
| ||||
| Drella | 5 | N/A | N/A | N/A |
| Gabe | 5 | N/A | N/A | N/A |
| Griffin | 2 | 2 | No | t(413) = −25.982, p < .001u |
| Liam | 3 | 2 | Yes | t(56) = −6.387, p < .001u |
| Lily | 2 | 1 | Yes | t(392) = −25.041, p < .001u |
| Logan | 1 | 1 | Yes | t(83) = −32.043, p < .001u |
| Nala | 3 | 3 | Yes | t(651) = −9.928, p < .001 |
| Wren | 3 | 3 | Yes | t(506) = −3.482, p = .001 |
Note: Plain-text names are rhesus monkeys and italicized names are capuchin monkeys. Two capuchin monkeys (Drella and Gabe) failed to reach criterion on the first level of the task and were therefore removed from the study. Note that only the negative t values (also shown in bold) were significant in the predicted direction (three others were significant in the opposite direction). A superscript “u” next to an independent samples t result indicates that the unequal variances version of the test was required.
Testing
There were two key differences between the training and testing procedures. First, on the menu screen at the beginning of each block of testing, the PM icon was delayed in its appearance by three seconds, whereas the LS icon appeared immediately. Second, in PM blocks of the test phase, the PM cue appeared only once, during the ITI between two LS trials (and not immediately after selection of the LS icon on the choice screen).
As in training, we manually titrated how close in time the PM cue appeared relative to the availability of the next PM icon (i.e., following the fifth LS trial, then the fourth, etc.), and we did so by assessing the same performance criterion. In testing we also manipulated the percentage of trial-blocks in which the PM cue appeared throughout the session. Once a monkey met the criterion with PM cues appearing after the first LS trial, we reduced the percentage of these PM blocks from 50% to 25%, and then from 25% to 10% upon reaching the same performance criterion.
Each monkey completed 5 sessions involving 10% PM blocks, and each individual’s pooled data were analyzed with two-tailed binomial sign tests with an alpha level of .05 and a chance probability of .5 (one test each for PM blocks and non-PM blocks). We also analyzed these data at the group level using one independent sample t-test (two-tailed, expected value = 50%, alpha = .05) per trial-type per monkey species. Using these last 5 sessions of data involving 10% PM blocks, we also analyzed the position of each monkey’s cursor at the appearance of the PM icon. We used an independent sample t-test (two-tailed, alpha = .05) to determine whether each monkey’s cursor was closer to the PM icon when it appeared (after a delay of three seconds) in blocks in which they ultimately chose the PM icon in comparison to when they ended up choosing the LS icon. We excluded from all of these analyses, the first PM/LS response in each session, as well as all PM/LS responses that immediately followed a PM response, to provide a more conservative analysis.
The only exception to the progression described above occurred if a monkey failed to meet the performance criterion within ten consecutive sessions at a particular level of the test phase involving 50% PM blocks. In this case, the monkey was presented with the previous level until they reached the performance criterion for the second time at that level. Then, the monkey was presented with that same task, but with 25% PM blocks. Upon meeting the criterion with these task parameters (or upon completing ten consecutive sessions without meeting the criterion), the monkey would then complete five sessions of the same task level but with 10% of trial-blocks involving the PM cue. Thus, all monkeys were included in the final, most difficult test, giving us the best opportunity to assess the performance levels that were most interesting.
In addition to monkeys’ ability to remember to make the PM response, we also analyzed monkeys’ performance on LS trials as a function of whether or not they observed the PM cue. As noted in the human literature, if PM requires effortful rehearsal or monitoring rather than occurring spontaneously, then performance in the concurrent task should suffer as a result of the additional cognitive load imposed by the PM task (Einstein et al., 2000). We tested this possibility by conducting independent sample t-tests (two-tailed, alpha = .05) to compare each monkey’s accuracy and response time in the ongoing LS task within their last five sessions of the experiment (those involving 10% PM blocks). To create the most sensitive analyses, we analyzed whether monkeys were correct or incorrect and how long they took to complete a LS response in LS trials immediately following the presentation of the PM cue (trial 2 of 6 for most monkeys). To eliminate outliers in the analyses of response time, we excluded all values greater than 10 s (this excluded less than 3% of the trial count).
Results
Table 1 presents a summary of each monkey’s PM performance in this experiment as well as statistical results. In sessions in which the PM cue occurred in 50% of LS blocks, 6 of 8 rhesus monkeys and 2 of 6 capuchin monkeys met a 66.67% performance criterion at all sublevels of the task, including when the PM cue appeared just after the first LS trial of the block (furthest in time from the next opportunity to select the PM icon). The remaining monkeys stopped meeting this criterion when the PM cue was somewhat closer in time to the next PM response opportunity (rhesus monkeys: following the second or fourth trial; capuchin monkeys: following the second or third trial).
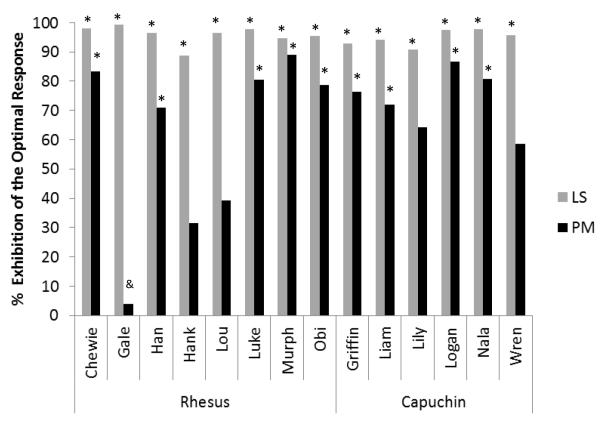
All but one monkey of each species continued to meet the success criterion when the proportion of trial-blocks presenting the PM cue was reduced from 50% to 25%. In sessions involving only 10% PM blocks, which were the most difficult trials presented in this experiment, all tested monkeys selected the LS icon in a statistically significant proportion of trial-blocks when that was the optimal response (capuchins: t(5) = 39.90, p < .001 ; rhesus: t(7) = 40.52, p < .001; individual binomial tests, all p < .001; Figure 2). However, not all monkeys selected the PM icon when it was the optimal response. As a group, capuchin monkeys selected the PM icon in a statistically significant proportion of trial blocks when it was the optimal response (t(5) = 5.42, p = .003), and 4 of 6 capuchin monkeys were successful when analyzed individually (binomial tests, p < .001; Figure 2). Rhesus monkeys, as a group, did not select the PM icon in a statistically significant proportion of trial blocks when it was the optimal response (t(7) = .89, p = .404), although 5 of 8 rhesus monkeys were successful in these trials when analyzed individually (binomial tests, p < .001; Figure 2).
Figure 2.
Test performance with 10% prospective memory (PM) blocks. The y-axis represents the percentage of total trial-blocks in which monkeys made a PM or learning-set (LS) icon selection when each was the optimal response. The two bar colors represent the different response types. An asterisk or ampersand above a given bar signifies that the optimal response was executed significantly more often or less often than chance (50%), respectively, as assessed by a two-tailed binomial test with an alpha level of .05.
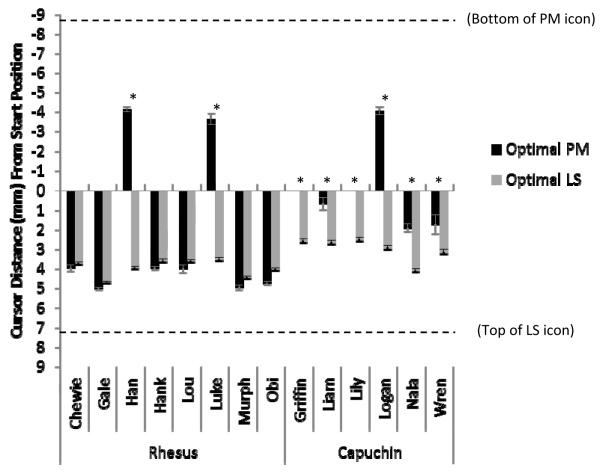
Critically, in 10% PM trial-blocks, 8 monkeys (2 of 8 rhesus monkeys and 6 of 6 capuchin monkeys) kept their cursor significantly closer to the PM icon on the computer screen prior to the icon’s appearance in cases in which they ultimately made the PM response in comparison to cases in which they ultimately selected the LS icon (when each of these was the optimal response; Figure 3; Table 1). There were some individual differences in how this variance in cursor position emerged for these eight monkeys. As shown in Figure 3, three of these individuals (two rhesus monkeys and one capuchin monkey) moved their cursor well into the half of the screen in which the PM icon was located by the time the PM icon appeared. Two other monkeys (both capuchin monkeys) left their cursor near its starting position in the middle of the screen until the PM icon appeared. The remaining three individuals (all capuchin monkeys) moved their cursors less toward the LS icon in these blocks in which they chose the PM icon in comparison to blocks in which they chose the LS icon.
Figure 3.
Cursor position when the prospective memory (PM) icon appeared in 10% PM test blocks. The y-axis represents the mean distance (in mm) between the cursor at start of trial and the cursor three seconds later when the PM icon appeared. The two different bar colors represent trials in which the monkeys ultimately selected the PM and learning-set (LS) response icons when each was the optimal response. The two dashed lines represent the vertical position of the two response icons. Error bars represent the standard error of the mean. An asterisk above a pair of bars signifies a significant difference (in the predicted direction) in cursor position between the two trial types as assessed by a two-tailed independent samples t-test with an alpha level of 0.05 (see also Table 1).
With regard to LS performance, one monkey exhibited lower accuracy in trials that directly followed the PM cue than in trials that did not follow a PM cue at all (see Table 2, the capuchin monkey Nala). This monkey was one of the individuals that exhibited above chance memory for the PM response and anticipated the appearance of the PM icon at the end of the LS trial-block. Also, one monkey exhibited slowed responding to LS trials directly following the PM cue, and this monkey was also one of the individuals that exhibited above chance memory for the PM response and anticipated the appearance of the PM icon at the end of the LS trial-block (see Table 2, the rhesus monkey Luke). However, six other monkeys showed the opposite effect with regard to response time (faster LS responses following the PM cue). Two of these monkeys (the capuchin monkeys Liam and Nala) belonged to the group that exhibited both above chance memory for the PM response and anticipation of the appearance of the PM icon at the end of the LS trial-block. Of the remaining four monkeys that showed this effect, two exhibited anticipation of the PM icon at the end of LS blocks (the capuchin monkeys Lily and Wren), and two did not (the rhesus monkeys Hank and Obi). None of the latter four monkeys remembered to select the PM icon significantly more often than chance in this experiment.
Table 2.
Learning-Set performance summary by individual.
| Accuracy (percent selection of the correct stimulus) | Response time (seconds to move the cursor to a stimulus) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Monkey | PM blocks |
Non-PM blocks |
independent samples t-test |
PM blocks |
Non-PM blocks |
independent samples t-test |
| Chewie | 76.19 | 87.09 | t(66) = −1.980, p = .052u | 2.08 | 2.04 | t(1101) = .349, p = .727 |
| Gale | 75.00 | 93.98 | t(3) = −.759, p = .503u | 1.71 | 1.56 | t(808) = .742, p = .458 |
| Han | 83.93 | 89.78 | t(59) = −1.193, p = .238u | 1.81 | 1.93 | t(176) = −1.760, p = .08u |
| Hank | 86.29 | 83.30 | t(652) = .924, p = .356 | 2.09 | 2.35 | t(407) = −3.889, p < .001u |
| Lou | 89.66 | 89.06 | t(842) = .141, p = .888 | 1.98 | 2.07 | t(94) = −1.266, p = .209u |
| Luke | 98.48 | 97.50 | t(704) = .496, p = .620 | 1.73 | 1.54 | t(699) = 2.412, p = .016 |
| Murph | 92.50 | 94.18 | t(327) = −.400, p = .689 | 2.37 | 2.22 | t(319) = 1.226, p = .221 |
| Obi | 95.19 | 96.46 | t(1119) = −.656, p = .512 | 1.64 | 1.84 | t(184) = 3.521, p = .001u |
|
| ||||||
| Drella | N/A | N/A | N/A | N/A | N/A | N/A |
| Gabe | N/A | N/A | N/A | N/A | N/A | N/A |
| Griffin | 94.12 | 90.77 | t(62) = 1.346, p = .183u | 2.03 | 2.07 | t(475) = −.104, p = .917 |
| Liam | 89.13 | 94.26 | t(49) = −1.421, p = .161u | 1.58 | 2.08 | t(151) = −5.975, p < .001u |
| Lily | 77.78 | 80.1 | t(30) = −1.385, p = .176u | 1.74 | 2.45 | t(158) = −8.821, p < .001u |
| Logan | 96.61 | 98.38 | t(675) = −.831, p = .406 | 1.92 | 1.99 | t(669) = −.508, p = .611 |
| Nala | 81.36 | 88.89 | t(66) = −2.161, p = .034u | 1.64 | 1.99 | t(117) = −5.306, p < .001u |
| Wren | 94.16 | 92.62 | t(35) = −1.386, p = .122u | 1.76 | 2 | t(71) = −2.665, p = .010u |
Note: Plain-text names are rhesus monkeys and italicized names are capuchin monkeys. Two capuchin monkeys (Drella and Gabe) failed to reach criterion on the first level of the task and were therefore removed from the study. Note that only the t-values shown in bold were significant in the predicted direction (six others were significant in the opposite direction). A superscript “u” next to an independent samples t result indicates that the unequal variances version of the test was required.
Discussion
In this study, we hypothesized that monkeys would exhibit evidence of prospective memory by remembering to indicate, at an appropriate time, that a specific stimulus event had occurred within the ongoing task. Most monkeys of both species provided evidence to support this hypothesis, and they did so even when the PM cues were infrequent, occurring only 10% of the time. This was important because it reduced the likelihood that monkeys could make a rewarded PM response on the basis of chance alone. What is really compelling about the monkeys’ performance is the fact that monkeys never really needed to make the PM response. They could have responded only to the LS icon, and then immediately moved into a relatively easy discrimination task in which they were rewarded at high levels. The fact that they would instead risk a very long timeout period to indicate that the PM cue had been seen is impressive when considered from the perspective of what a monkey might do if it was simply attempting to avoid timeouts and consistently earn pellets.
There are several criteria that researchers use to qualify human task performance as prospective memory (McDaniel & Einstein, 2007) and these are useful criteria to apply to nonhuman performance as well (Thorpe et al., 2004). First, it is important that the retrieval and execution of the PM not occur immediately (i.e., the information must be stored in memory). The design of our task did not allow monkeys to immediately execute the PM response because monkeys had to complete the remaining LS trials between presentation of the PM cue and availability of the PM icon. On average, rhesus monkeys were able to complete 4.5 LS trials before making a successful PM response, which involved durations of approximately 55 seconds between the appearance of the PM cue and the selection of the PM icon (assuming only one error in the LS trial-block). Capuchin monkeys completed an average of 4 LS trials before making a successful PM response (requiring approximately 50 seconds).
Another important aspect of human prospective memory tasks is to prevent continuous rehearsal of the encoded information. The task used in the present study did so by having monkeys complete an ongoing LS task that required attention and working memory to determine and then store the identity of the positive stimulus. This should have disrupted efforts to continually rehearse the appearance of the PM cue, although monkeys may still have been able to use additional working memory resources to rehearse the encoded information. Evidence of the involvement of working memory sometimes surfaces in tests given to humans as lowered performance in the ongoing task as a result of retaining the prospective memory (e.g., see Smith, 2010). In the present study, monkeys did not show a clear effect of having to remember to make the PM response on their ongoing task performance (LS accuracy or response time) and some monkeys were near ceiling in their performance of this task. Therefore, either monkeys were spontaneously retrieving the intention to select the PM icon at the end of trial blocks, or the LS task was not sensitive enough to detect whatever concurrent load the PM imposed upon monkeys’ executive functioning. Future modifications to this task or the use of an entirely different ongoing task will be needed to better indicate just how likely or unlikely rehearsal and monitoring are for these monkeys.
As in human PM tasks, to qualify monkeys’ memory performance in the present task as prospective, and not retrospective, monkeys needed to anticipate an upcoming opportunity to execute the PM response and initiate this response before the occurrence of an explicit visual prompt. Some monkeys of each species exhibited such behavior. Only two rhesus monkeys seemed to anticipate the PM response opportunity, but those that did initiated the response well before the prompt occurred so that their cursor was nearly halfway to the PM icon when it appeared. All six capuchin monkeys seemed to anticipate the PM response opportunity. Five of them exhibited this effect by refraining from making any response or making less of a response towards the LS icon prior to the appearance of the PM icon. Since these patterns were only seen when monkeys ultimately made a PM response (when it was the optimal response), they indicate that monkeys were hesitant to resume the ongoing LS task when they had a recent memory of the PM cue, and thus, that monkeys were anticipating the opportunity to make the PM response before it was possible to do so.
Interestingly, three rhesus monkeys (Hank, Murph, and Obi) showed the opposite pattern than what was predicted with respect to cursor position on the screen when the PM icon appeared. Their cursors were actually closer to the LS icon in trials in which it was more appropriate to select the PM icon than in trials in which the LS icon was the appropriate response. A reviewer of this manuscript suggested that this may actually be a psychologically meaningful effect. Prospective memory researchers often distinguish between prospective memory (remembering to do something) and retrospective memory (remembering what has to be done) components (e.g., McDaniel & Einstein, 2007; Riley, Cook, & Lamb, 1981; Smith, 2008). Thus, it is possible that these three monkeys are successfully exhibiting prospective memory (by remembering that an important response is needed) but are demonstrating a retrospective memory failure (by quickly moving their cursor to the LS icon instead of the PM icon). This is an intriguing possibility and is worth considering further in future studies.
These data suggest that the monkeys were engaging in simple, but perhaps human-like prospective memory. Previous studies have examined prospective coding of information for later use, such as which arms in a radial maze have yet to be visited (e.g., Riley et al., 1981; Cook et al.; 1985; Roberts & Roberts, 2002), or what the second stimulus should be in a sequential paired associate test (e.g., Colombo & Graziano, 1994; Rainer et al., 1999), but such studies do not preclude the possibility of rehearsal or prompted responding. In contrast, the present study suggests that monkeys can encode, store, retrieve, and execute a future response when the PM cue is embedded in ongoing activity, and they can execute that response before a prompt is provided that would remind the animal of when to do so. The present task also has value as a new tool for investigating such memory processes in nonhuman animals, and perhaps young children as the game-like nature of the task is readily engaging but allows for interesting variations to determine just what kinds of PM cues might be salient and well-remembered. Future studies using this and other paradigms should examine this capacity in additional nonhuman species and possibly on a greater temporal scale to assess the width and breadth of PM capacities in nonhuman animals and provide key data in understanding the evolutionary foundations of this important human capacity.
Highlights.
We assessed the existence of prospective memory in monkeys using a computerized task.
Monkeys encoded and stored stimuli for future use while performing an unrelated task.
Monkeys retrieved and acted upon those stimuli at appropriate times, sometimes without prompting.
Prospective memory appears not to be limited to humans.
Acknowledgments
This research was supported by National Science Foundation grant BCS-0924811 and National Institute of Child Health and Development grant HD060563. The authors thank Jessica Bramlett, Betty Chan, and Joseph McIntyre for assistance with data collection, as well as Bonnie Perdue, Megan Hoffman, and Charles Menzel for comments on an earlier draft of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Colombo M, Graziano M. Effects of auditory and visual interference on auditory-visual delaying matching to sample in monkeys (Macaca fascicularis) Behavioral Neuroscience. 1994;108:636–639. doi: 10.1037//0735-7044.108.3.636. [DOI] [PubMed] [Google Scholar]
- Cook RG, Brown MF, Riley DA. Flexible memory processing by rats: Use of prospective and retrospective information in a radial maze. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:453–469. [PubMed] [Google Scholar]
- DiGian KA, Zentall TR. Pigeons may not use dual coding in the radial maze analog task. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:262–272. doi: 10.1037/0097-7403.33.3.262. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Manzi M, Cochran B, Baker M. Prospective memory and aging: Forgetting intentions over short delays. Psychology and Aging. 2000;671:683. doi: 10.1037//0882-7974.15.4.671. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Smith RE, Shaw P. Habitual prospective memory and aging: Remembering intentions and forgetting actions. Psychological Science. 1998;9:284–288. [Google Scholar]
- Einstein GO, McDaniel MD. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:716–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Chan B, Klein ED, Menzel CR. An efficient computerized testing method for the capuchin monkey (Cebus apella): Adaptation of the LRC-CTS to a socially housed nonhuman primate species. Behavior Research Methods, Instruments, & Computers. 2008;40:590–596. doi: 10.3758/brm.40.2.590. [DOI] [PubMed] [Google Scholar]
- Genovesio A, Brasted PJ, Wise SP. Representation of future and previous spatial goals by separate neural populations in prefrontal cortex. The Journal of Neuroscience. 2006;26:7305–7316. doi: 10.1523/JNEUROSCI.0699-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf P, Uttl B. Prospective memory: A new focus for research. Consciousness and Cognition. 2001;10:437–450. doi: 10.1006/ccog.2001.0504. [DOI] [PubMed] [Google Scholar]
- Harlow HH. The formation of learning sets. Psychological Review. 1949;56:51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- Klein ED, Evans TA, Beran MJ. An investigation of prospective and retrospective coding in capuchin monkeys and rhesus monkeys. Zeitschrift für Psychologie. 2011;219:85–91. doi: 10.1027/2151-2604/a000052. [Google Scholar]
- Kliegel M, Mackinlay R, Jager T. Complex prospective memory: Development across the lifespan and the role of task interruption. Developmental Psychology. 2008;44:612–617. doi: 10.1037/0012-1649.44.2.612. [DOI] [PubMed] [Google Scholar]
- Kliegel M, McDaniel MD, Einstein GO. Plan formation, retention, and execution in prospective memory: A new approach and age related effects. Memory and Cognition. 2000;28:1041–1049. doi: 10.3758/bf03209352. [DOI] [PubMed] [Google Scholar]
- Kvavilashvili L, Ellis JA. Varieties of intention: Some distinctions and classifications. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Lawrence Erlbaum; Mahwah, NJ: 1996. pp. 23–51. [Google Scholar]
- Marsh RJ, Hicks JL, Cook GI. Task interference from prospective memory covaries with contextual associations of fulfilling them. Memory & Cognition. 2006;34:1037–1045. doi: 10.3758/bf03193250. [DOI] [PubMed] [Google Scholar]
- Marsh RJ, Hicks JL, Landau JD. An investigation of everyday prospective memory. Memory & Cognition. 1998;26:633–643. doi: 10.3758/bf03211383. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: a multiprocess framework. Applied Cognitive Psychology. 2000;14:S127–S144. [Google Scholar]
- McDaniel MA, Einstein GO. Prospective Memory. Sage Publications; Los Angeles: 2007. [Google Scholar]
- McDaniel MA, Einstein GO, Graham T, Rall E. Delaying execution of intentions: Overcoming the costs of interruptions. Applied Cognitive Psychology. 2004;18:533–547. [Google Scholar]
- McDaniel MA, Einstein GO, Stout AC, Morgan Z. Aging and maintaining intention over delays: Use it or lose it. Psychology and Aging. 2003;18:807–822. doi: 10.1037/0882-7974.18.4.823. [DOI] [PubMed] [Google Scholar]
- Menzel CR. Cognitive aspects of foraging in Japanese monkeys. Animal Behaviour. 1991;41:397–402. [Google Scholar]
- Murphy J. Assessing equine prospective memory in a Y-maze apparatus. The Veterinary Journal. 2009;181:24–28. doi: 10.1016/j.tvjl.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Rainer G, Rao SC, Miller EK. Prospective coding for objects in primate prefrontal cortex. The Journal of Neuroscience. 1999;19:5493–5505. doi: 10.1523/JNEUROSCI.19-13-05493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell PG, Vella MJ, Kleigel M, Terrett G. Effect of delay on children’s delay-execute prospective memory performance. Cognitive Development. 2009;24:156–168. [Google Scholar]
- Richardson WK, Washburn DA, Hopkins WD, Savage-Rumbaugh SE, Rumbaugh DM. The NASA/LRC computerized test system. Behavior Research Methods, Instruments, & Computers. 1990;22(2):127–131. doi: 10.3758/bf03203132. [DOI] [PubMed] [Google Scholar]
- Riley DA, Cook RG, Lamb MR. A classification and analysis of short-term retention codes. Academic Press; New York: 1981. [Google Scholar]
- Roberts WA, Roberts S. Two tests of the stuck-in-time hypothesis. Journal of General Psychology. 2002;129:415–426. doi: 10.1080/00221300209602105. [DOI] [PubMed] [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Smith RE. Attention, memory, and delayed intentions. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental and applied perspectives. Lawrence Erlbaum; New York: 2008. pp. 29–52. [Google Scholar]
- Smith RE. What costs do reveal and moving beyond the cost debate: Reply to Einstein and McDaniel. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36(4):1089–1095. doi: 10.1037/a0019183. (2010) doi: 10.1037/a0019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T. Foresight and evolution of the human mind. Science. 2006;312:1006–1007. doi: 10.1126/science.1129217. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Busby J. Mental time travel in animals? Trends in Cognitive Sciences. 2003;7(9):391–396. doi: 10.1016/s1364-6613(03)00187-6. doi: 10.1016/s1364-6613(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Thorpe CM, Jacova C, Wilkie DM. Some pitfalls in measuring memory in animals. Neuroscience and Biobehavioral Reviews. 2004;28:711–718. doi: 10.1016/j.neubiorev.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson E, editors. Organization of memory. Academic Press; San Diego, CA: 1972. pp. 381–403. [Google Scholar]
- Tulving E. What is episodic memory? Current Directions in Psychological Science. 1993;2:67–70. [Google Scholar]
- Wilson AG, Crystal JD. Prospective memory in the rat. Animal Cognition. 2012;15:349–358. doi: 10.1007/s10071-011-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall TR, Steirn JN, Jackson-Smith P. Memory strategies in pigeons’ performance of a radial-arm-maze analog task. Journal of Experimental Psychology: Animal Behavior Processes. 1990;20:390–402. [Google Scholar]